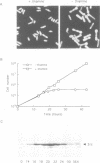
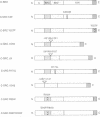
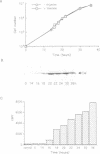
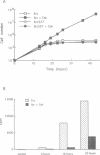
Abstract
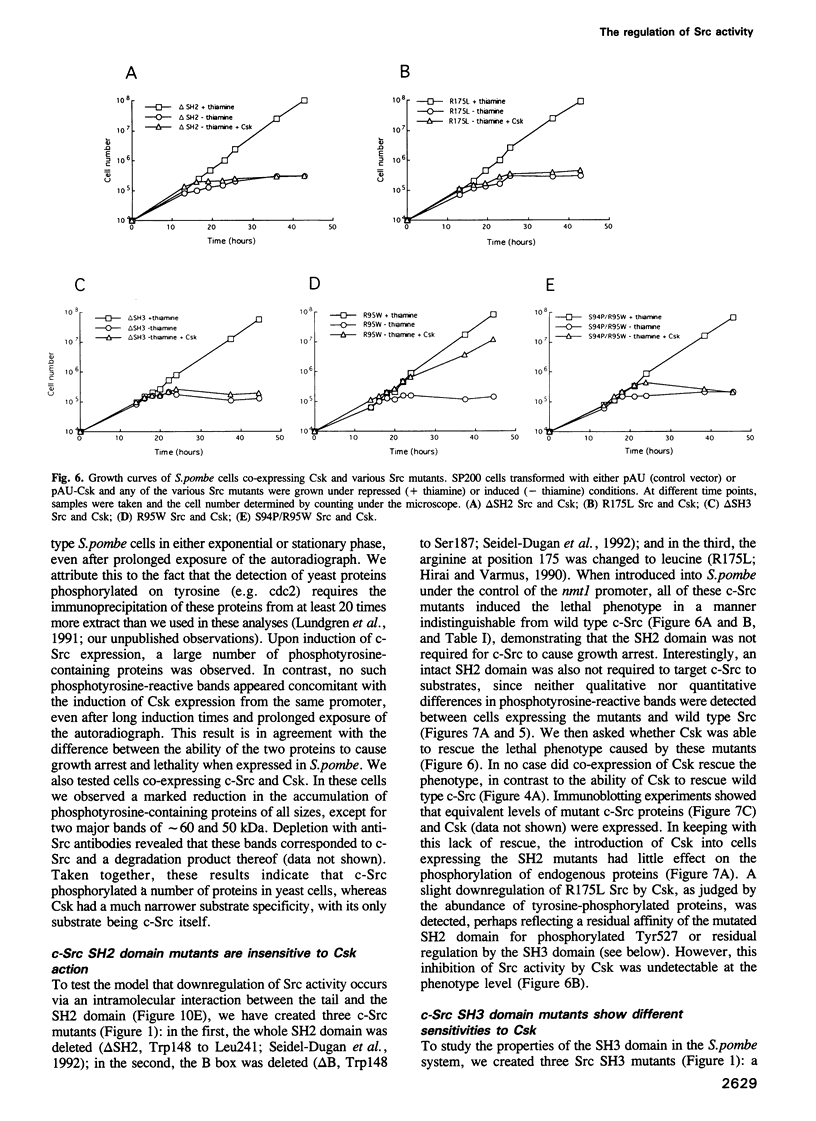
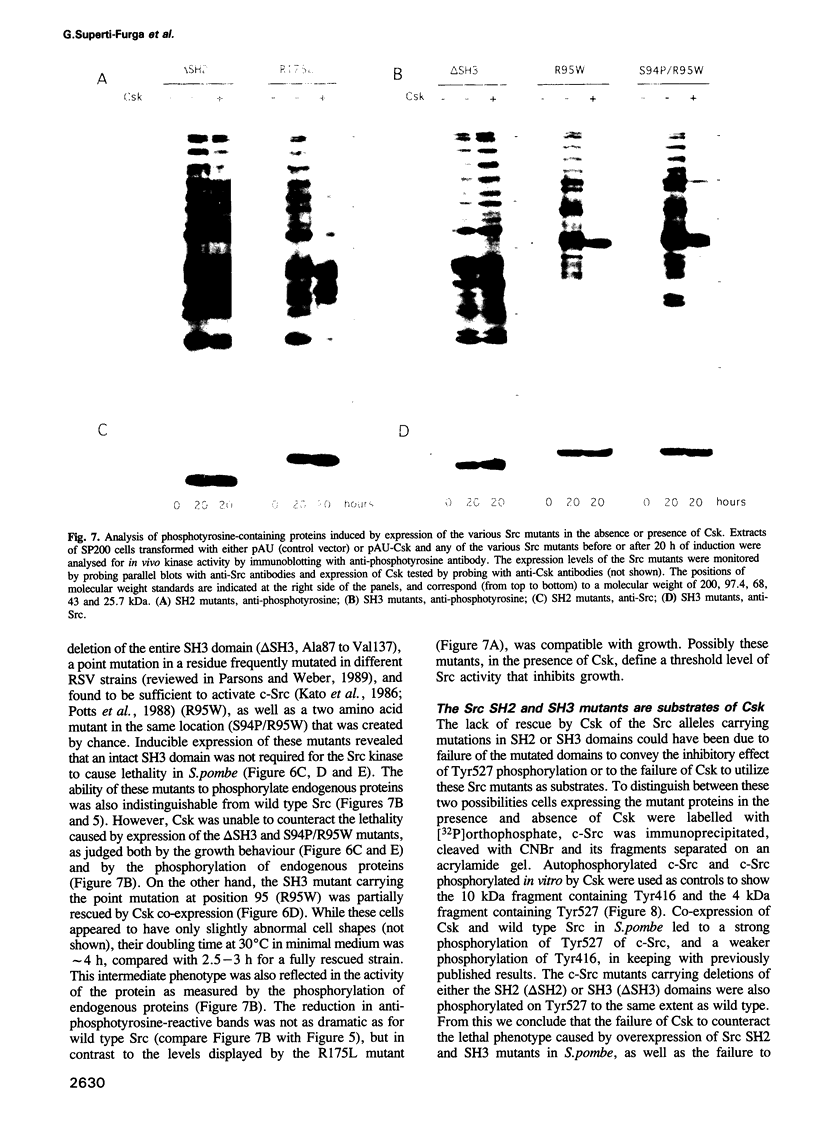
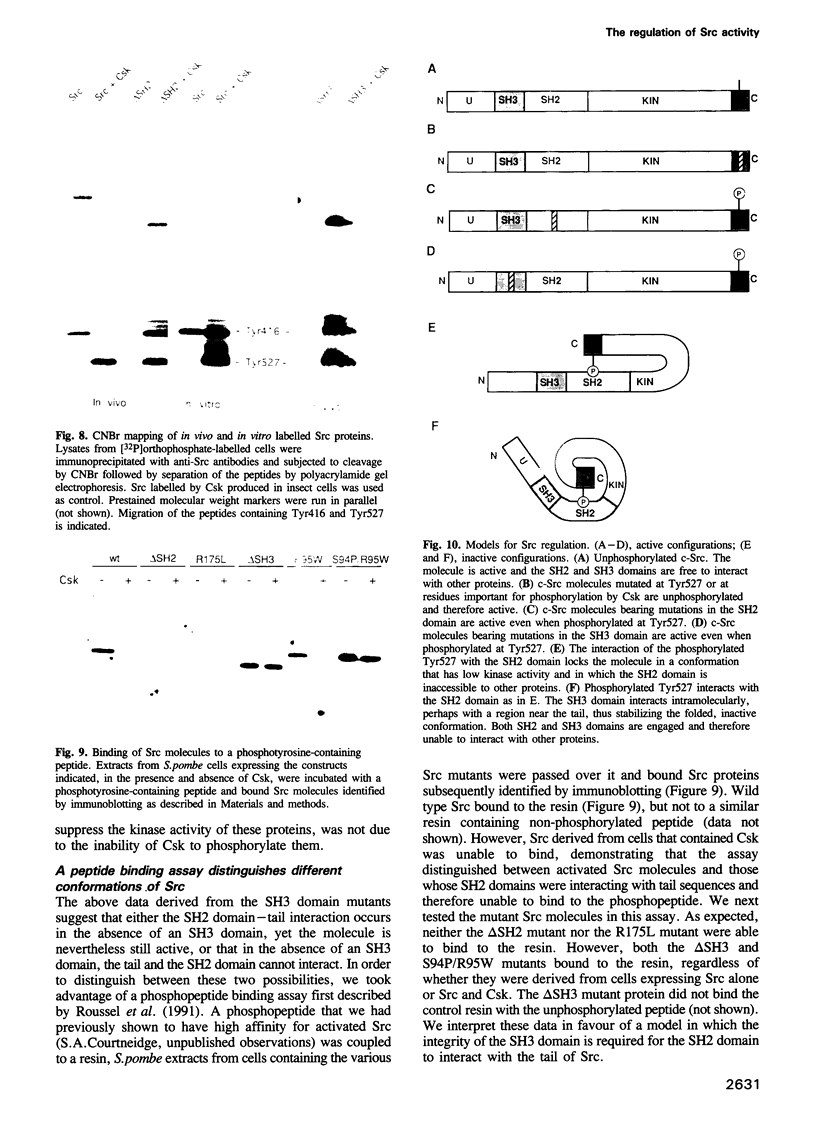
The protein tyrosine kinase c-Src is negatively regulated by phosphorylation of Tyr527 in its carboxy-terminal tail. A kinase that phosphorylates Tyr527, called Csk, has recently been identified. We expressed c-Src in yeast to test the role of the SH2 and SH3 domains of Src in the negative regulation exerted by Tyr527 phosphorylation. Inducible expression of c-Src in Schizosaccharomyces pombe caused cell death. Co-expression of Csk counteracted this effect. Src proteins mutated in either the SH2 or SH3 domain were as lethal as wild type c-Src, but were insensitive to Csk, even though they were substrates for Csk in vivo. Peptide binding experiments revealed that Src proteins with mutant SH3 domains adopted a conformation in which the SH2 domain was not interacting with the tail. These data support the model of an SH2 domain-phosphorylated tail interaction repressing c-Src activity, but expand it to include a role for the SH3 domain. We propose that the SH3 domain contributes to the maintenance of the folded, inactive configuration of the Src molecule by stabilizing the SH2 domain-phosphorylated tail interaction. Moreover, the system we describe here allows for further study of the regulation of tyrosine kinases in a neutral background and in an organism amenable to genetic analysis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D., Koch C. A., Grey L., Ellis C., Moran M. F., Pawson T. Binding of SH2 domains of phospholipase C gamma 1, GAP, and Src to activated growth factor receptors. Science. 1990 Nov 16;250(4983):979–982. doi: 10.1126/science.2173144. [DOI] [PubMed] [Google Scholar]
- Basi G., Schmid E., Maundrell K. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene. 1993 Jan 15;123(1):131–136. doi: 10.1016/0378-1119(93)90552-e. [DOI] [PubMed] [Google Scholar]
- Beach D., Rodgers L., Gould J. ran1+ controls the transition from mitotic division to meiosis in fission yeast. Curr Genet. 1985;10(4):297–311. doi: 10.1007/BF00365626. [DOI] [PubMed] [Google Scholar]
- Bergman M., Mustelin T., Oetken C., Partanen J., Flint N. A., Amrein K. E., Autero M., Burn P., Alitalo K. The human p50csk tyrosine kinase phosphorylates p56lck at Tyr-505 and down regulates its catalytic activity. EMBO J. 1992 Aug;11(8):2919–2924. doi: 10.1002/j.1460-2075.1992.tb05361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chenevert J., Corrado K., Bender A., Pringle J., Herskowitz I. A yeast gene (BEM1) necessary for cell polarization whose product contains two SH3 domains. Nature. 1992 Mar 5;356(6364):77–79. doi: 10.1038/356077a0. [DOI] [PubMed] [Google Scholar]
- Cicchetti P., Mayer B. J., Thiel G., Baltimore D. Identification of a protein that binds to the SH3 region of Abl and is similar to Bcr and GAP-rho. Science. 1992 Aug 7;257(5071):803–806. doi: 10.1126/science.1379745. [DOI] [PubMed] [Google Scholar]
- Clark S. G., Stern M. J., Horvitz H. R. C. elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature. 1992 Mar 26;356(6367):340–344. doi: 10.1038/356340a0. [DOI] [PubMed] [Google Scholar]
- Cobb B. S., Payne D. M., Reynolds A. B., Parsons J. T. Regulation of the oncogenic activity of the cellular src protein requires the correct spacing between the kinase domain and the C-terminal phosphorylated tyrosine (Tyr-527). Mol Cell Biol. 1991 Dec;11(12):5832–5838. doi: 10.1128/mcb.11.12.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., King C. S. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol Cell Biol. 1986 Dec;6(12):4467–4477. doi: 10.1128/mcb.6.12.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Runge K. Avian pp60c-src is more active when expressed in yeast than in vertebrate fibroblasts. Oncogene Res. 1987 Sep-Oct;1(4):297–310. [PubMed] [Google Scholar]
- Drubin D. G., Mulholland J., Zhu Z. M., Botstein D. Homology of a yeast actin-binding protein to signal transduction proteins and myosin-I. Nature. 1990 Jan 18;343(6255):288–290. doi: 10.1038/343288a0. [DOI] [PubMed] [Google Scholar]
- Espino P. C., Harvey R., Schweickhardt R. L., White G. A., Smith A. E., Cheng S. H. The amino-terminal region of pp60c-src has a modulatory role and contains multiple sites of tyrosine phosphorylation. Oncogene. 1990 Mar;5(3):283–293. [PubMed] [Google Scholar]
- Fantl W. J., Escobedo J. A., Martin G. A., Turck C. W., del Rosario M., McCormick F., Williams L. T. Distinct phosphotyrosines on a growth factor receptor bind to specific molecules that mediate different signaling pathways. Cell. 1992 May 1;69(3):413–423. doi: 10.1016/0092-8674(92)90444-h. [DOI] [PubMed] [Google Scholar]
- Grandori C. Regulation of kinase activity. Nature. 1989 Apr 6;338(6215):467–467. doi: 10.1038/338467a0. [DOI] [PubMed] [Google Scholar]
- Harvey R., Hehir K. M., Smith A. E., Cheng S. H. pp60c-src variants containing lesions that affect phosphorylation at tyrosines 416 and 527. Mol Cell Biol. 1989 Sep;9(9):3647–3656. doi: 10.1128/mcb.9.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H., Varmus H. E. Site-directed mutagenesis of the SH2- and SH3-coding domains of c-src produces varied phenotypes, including oncogenic activation of p60c-src. Mol Cell Biol. 1990 Apr;10(4):1307–1318. doi: 10.1128/mcb.10.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D. A., Fukui Y., Yamamoto M. Homologous activators of ras in fission and budding yeast. Nature. 1990 Mar 22;344(6264):355–357. doi: 10.1038/344355a0. [DOI] [PubMed] [Google Scholar]
- Hunter T. A tail of two src's: mutatis mutandis. Cell. 1987 Apr 10;49(1):1–4. doi: 10.1016/0092-8674(87)90745-8. [DOI] [PubMed] [Google Scholar]
- Kanner S. B., Reynolds A. B., Wang H. C., Vines R. R., Parsons J. T. The SH2 and SH3 domains of pp60src direct stable association with tyrosine phosphorylated proteins p130 and p110. EMBO J. 1991 Jul;10(7):1689–1698. doi: 10.1002/j.1460-2075.1991.tb07693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J. Y., Takeya T., Grandori C., Iba H., Levy J. B., Hanafusa H. Amino acid substitutions sufficient to convert the nontransforming p60c-src protein to a transforming protein. Mol Cell Biol. 1986 Dec;6(12):4155–4160. doi: 10.1128/mcb.6.12.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Kussick S. J., Cooper J. A. Phosphorylation and regulatory effects of the carboxy terminus of a Drosophila src homolog. Oncogene. 1992 Aug;7(8):1577–1586. [PubMed] [Google Scholar]
- Kypta R. M., Goldberg Y., Ulug E. T., Courtneidge S. A. Association between the PDGF receptor and members of the src family of tyrosine kinases. Cell. 1990 Aug 10;62(3):481–492. doi: 10.1016/0092-8674(90)90013-5. [DOI] [PubMed] [Google Scholar]
- Lundgren K., Walworth N., Booher R., Dembski M., Kirschner M., Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991 Mar 22;64(6):1111–1122. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- Luo K., Hurley T. R., Sefton B. M. Transfer of proteins to membranes facilitates both cyanogen bromide cleavage and two-dimensional proteolytic mapping. Oncogene. 1990 Jun;5(6):921–923. [PubMed] [Google Scholar]
- MacAuley A., Cooper J. A. Acidic residues at the carboxyl terminus of p60c-src are required for regulation of tyrosine kinase activity and transformation. New Biol. 1990 Sep;2(9):828–840. [PubMed] [Google Scholar]
- MacAuley A., Cooper J. A. The carboxy-terminal sequence of p56lck can regulate p60c-src. Mol Cell Biol. 1988 Aug;8(8):3560–3564. doi: 10.1128/mcb.8.8.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Mayer B. J., Fukui Y., Hanafusa H. Binding of transforming protein, P47gag-crk, to a broad range of phosphotyrosine-containing proteins. Science. 1990 Jun 22;248(4962):1537–1539. doi: 10.1126/science.1694307. [DOI] [PubMed] [Google Scholar]
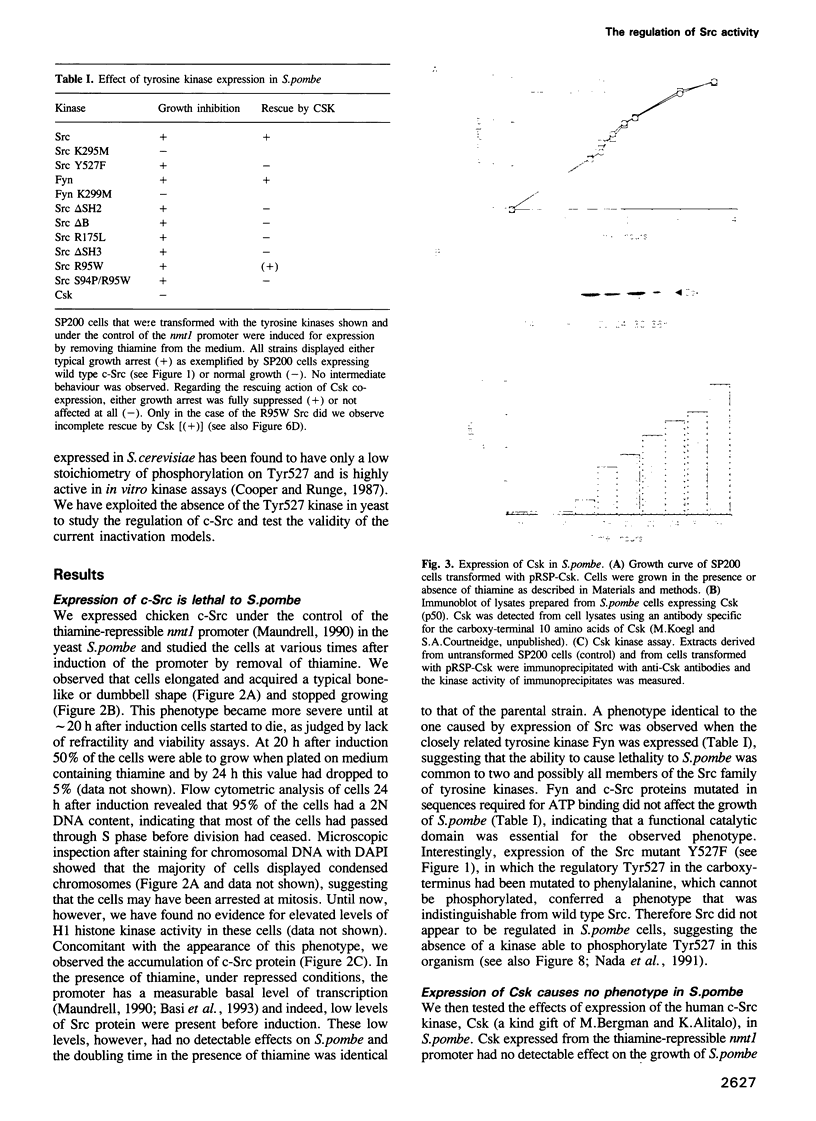
- Maundrell K. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J Biol Chem. 1990 Jul 5;265(19):10857–10864. [PubMed] [Google Scholar]
- Mayer B. J., Baltimore D. Signalling through SH2 and SH3 domains. Trends Cell Biol. 1993 Jan;3(1):8–13. doi: 10.1016/0962-8924(93)90194-6. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Hanafusa H. Association of the v-crk oncogene product with phosphotyrosine-containing proteins and protein kinase activity. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2638–2642. doi: 10.1073/pnas.87.7.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B. J., Jackson P. K., Baltimore D. The noncatalytic src homology region 2 segment of abl tyrosine kinase binds to tyrosine-phosphorylated cellular proteins with high affinity. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):627–631. doi: 10.1073/pnas.88.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B. J., Jackson P. K., Van Etten R. A., Baltimore D. Point mutations in the abl SH2 domain coordinately impair phosphotyrosine binding in vitro and transforming activity in vivo. Mol Cell Biol. 1992 Feb;12(2):609–618. doi: 10.1128/mcb.12.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod M., Stein M., Beach D. The product of the mei3+ gene, expressed under control of the mating-type locus, induces meiosis and sporulation in fission yeast. EMBO J. 1987 Mar;6(3):729–736. doi: 10.1002/j.1460-2075.1987.tb04814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nada S., Okada M., MacAuley A., Cooper J. A., Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991 May 2;351(6321):69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- O'Brien M. C., Fukui Y., Hanafusa H. Activation of the proto-oncogene p60c-src by point mutations in the SH2 domain. Mol Cell Biol. 1990 Jun;10(6):2855–2862. doi: 10.1128/mcb.10.6.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M., Nada S., Yamanashi Y., Yamamoto T., Nakagawa H. CSK: a protein-tyrosine kinase involved in regulation of src family kinases. J Biol Chem. 1991 Dec 25;266(36):24249–24252. [PubMed] [Google Scholar]
- Okada M., Nakagawa H. A protein tyrosine kinase involved in regulation of pp60c-src function. J Biol Chem. 1989 Dec 15;264(35):20886–20893. [PubMed] [Google Scholar]
- Okada M., Nakagawa H. Identification of a novel protein tyrosine kinase that phosphorylates pp60c-src and regulates its activity in neonatal rat brain. Biochem Biophys Res Commun. 1988 Jul 29;154(2):796–802. doi: 10.1016/0006-291x(88)90210-0. [DOI] [PubMed] [Google Scholar]
- Okada M., Nakagawa H. Protein tyrosine kinase in rat brain: neonatal rat brain expresses two types of pp60c-src and a novel protein tyrosine kinase. J Biochem. 1988 Aug;104(2):297–305. doi: 10.1093/oxfordjournals.jbchem.a122461. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Weber M. J. Genetics of src: structure and functional organization of a protein tyrosine kinase. Curr Top Microbiol Immunol. 1989;147:79–127. doi: 10.1007/978-3-642-74697-0_3. [DOI] [PubMed] [Google Scholar]
- Partanen J., Armstrong E., Bergman M., Mäkelä T. P., Hirvonen H., Huebner K., Alitalo K. cyl encodes a putative cytoplasmic tyrosine kinase lacking the conserved tyrosine autophosphorylation site (Y416src). Oncogene. 1991 Nov;6(11):2013–2018. [PubMed] [Google Scholar]
- Pawson T., Gish G. D. SH2 and SH3 domains: from structure to function. Cell. 1992 Oct 30;71(3):359–362. doi: 10.1016/0092-8674(92)90504-6. [DOI] [PubMed] [Google Scholar]
- Pawson T. Non-catalytic domains of cytoplasmic protein-tyrosine kinases: regulatory elements in signal transduction. Oncogene. 1988 Nov;3(5):491–495. [PubMed] [Google Scholar]
- Potts W. M., Reynolds A. B., Lansing T. J., Parsons J. T. Activation of pp60c-src transforming potential by mutations altering the structure of an amino terminal domain containing residues 90-95. Oncogene Res. 1988;3(4):343–355. [PubMed] [Google Scholar]
- Ren R., Mayer B. J., Cicchetti P., Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993 Feb 19;259(5098):1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- Reynolds P. J., Hurley T. R., Sefton B. M. Functional analysis of the SH2 and SH3 domains of the lck tyrosine protein kinase. Oncogene. 1992 Oct;7(10):1949–1955. [PubMed] [Google Scholar]
- Roussel R. R., Brodeur S. R., Shalloway D., Laudano A. P. Selective binding of activated pp60c-src by an immobilized synthetic phosphopeptide modeled on the carboxyl terminus of pp60c-src. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10696–10700. doi: 10.1073/pnas.88.23.10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P. R., Hall B. D. The primary structure of the alcohol dehydrogenase gene from the fission yeast Schizosaccharomyces pombe. J Biol Chem. 1983 Jan 10;258(1):143–149. [PubMed] [Google Scholar]
- Sabe H., Okada M., Nakagawa H., Hanafusa H. Activation of c-Src in cells bearing v-Crk and its suppression by Csk. Mol Cell Biol. 1992 Oct;12(10):4706–4713. doi: 10.1128/mcb.12.10.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh S. M., Brugge J. S. Investigation of factors that influence phosphorylation of pp60c-src on tyrosine 527. Mol Cell Biol. 1988 Jun;8(6):2465–2471. doi: 10.1128/mcb.8.6.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel-Dugan C., Meyer B. E., Thomas S. M., Brugge J. S. Effects of SH2 and SH3 deletions on the functional activities of wild-type and transforming variants of c-Src. Mol Cell Biol. 1992 Apr;12(4):1835–1845. doi: 10.1128/mcb.12.4.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. E., Soriano P., Brugge J. S. Phosphorylation of c-Src on tyrosine 527 by another protein tyrosine kinase. Science. 1991 Oct 25;254(5031):568–571. doi: 10.1126/science.1719633. [DOI] [PubMed] [Google Scholar]
- Veillette A., Caron L., Fournel M., Pawson T. Regulation of the enzymatic function of the lymphocyte-specific tyrosine protein kinase p56lck by the non-catalytic SH2 and SH3 domains. Oncogene. 1992 May;7(5):971–980. [PubMed] [Google Scholar]
- Wages D. S., Keefer J., Rall T. B., Weber M. J. Mutations in the SH3 domain of the src oncogene which decrease association of phosphatidylinositol 3'-kinase activity with pp60v-src and alter cellular morphology. J Virol. 1992 Apr;66(4):1866–1874. doi: 10.1128/jvi.66.4.1866-1874.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman G., Kominos D., Robertson S. C., Pant N., Baltimore D., Birge R. B., Cowburn D., Hanafusa H., Mayer B. J., Overduin M. Crystal structure of the phosphotyrosine recognition domain SH2 of v-src complexed with tyrosine-phosphorylated peptides. Nature. 1992 Aug 20;358(6388):646–653. doi: 10.1038/358646a0. [DOI] [PubMed] [Google Scholar]