Abstract
Rationale
Global tuberculosis (TB) control may require mass vaccination with a new TB vaccine, such as a recombinant bacille Calmette Guerin (BCG) or attenuated Mycobacterium tuberculosis (MTB). The safety profile of live mycobacterial vaccines in latently infected adults with prior infant BCG vaccination is unknown.
Objectives
Evaluate safety and reactogenicity of BCG revaccination, with or without isoniazid (INH) pretreatment, in adults with latent MTB infection (LTBI).
Methods
Eighty-two healthy, HIV uninfected, South African adults, with a BCG scar and tuberculin skin test (TST) diameter ≥15mm, were randomized to receive 6 months of INH, starting either before, or 6 months after, intradermal revaccination with BCG Vaccine SSI (Statens Serum Institut, Copenhagen). Safety and reactogenicity data are reported through 3 months post BCG revaccination.
Results
Baseline characteristics were similar between treatment arms. Mean baseline TST diameter was 20 ± 4mm. Seventy-two subjects received BCG revaccination. Injection site erythema (68%) and induration (86%) peaked 1 week after revaccination. Ulceration (76%) peaked at 2 weeks, and resolved by 3 months in all but 3 subjects. Diameter of ulceration was >10mm in only 8%, but a residual scar was common (85%). No regional lymphadenitis or serious morbidity related to BCG was seen. Reactogenicity was not affected by INH pretreatment.
Conclusion
BCG revaccination of MTB infected adults is safe, well tolerated, and reactogenicity is similar to that of primary BCG vaccination. Clinical trials of live recombinant BCG or attenuated MTB vaccines may be considered in latently infected adults, with or without INH pretreatment (ClinicalTrials.gov identifier: NCT01119521).
Keywords: BCG, revaccination, safety, LTBI, isoniazid
INTRODUCTION
The World Health Organization (WHO) estimates one third of the global population is infected with Mycobacterium tuberculosis (MTB). Many of these individuals have been vaccinated with bacille Calmette Guerin (BCG) vaccine in infancy[1], yet these latently infected persons remain at risk for later development of TB disease[2, 3]. Mass vaccination of adults in high TB burden countries with a new, effective tuberculosis (TB) vaccine will be key to global elimination of TB[4, 5]. Candidate TB vaccines in clinical trials among persons without MTB infection include live mycobacterial vaccines, such as recombinant bacille Calmette Guerin (BCG) and attenuated MTB vaccines[6–8]. However, many persons in high TB burden countries are already exposed and infected with MTB by early adulthood[2, 3]. Latent MTB infection (LTBI) might prevent optimal immune responses to live TB vaccines[9, 10, 11–13]. Therapeutic interventions, such as pre-vaccination isoniazid (INH) treatment, might allow manipulation of this immune response, but the overall risk-benefit ratio must be considered. In HIV infected individuals with LTBI, a multiple-dose series of an inactivated whole cell TB vaccine was shown to be safe and effective when concurrently administered with INH[14]. Theoretically, live TB vaccines, including BCG, might develop increased reactogenicity and morbidity in HIV uninfected persons with LTBI (Koch phenomenon)[15, 16], but objective data are lacking, since the safety profile of live mycobacterial vaccines in this population has not been described[16].
The current BCG vaccine provides an opportunity to study the safety, reactogenicity, and immunogenicity of a live mycobacterial vaccine, in preparation for efficacy trials of novel TB vaccines in MTB infected adults[6, 8, 17]. However, since most high TB burden countries employ universal infant BCG vaccination, such studies in adults involve BCG revaccination[1]. BCG revaccination does not confer additional protection against development of TB disease[18], [19, 20], and this strategy is not endorsed by the World Health Organization[21]. BCG revaccination of 170,000 Brazilian schoolchildren did not show efficacy against TB disease in the REVAC trial[22], although extended follow-up suggests that BCG revaccination might offer limited protection in selected sub-populations[22, 23].
The safety and reactogenicity of BCG revaccination in adults with LTBI has not been described. In infants, the usual reaction to primary BCG vaccination is characterized by local skin induration that resolves over several weeks, followed by a small superficial ulcer, which spontaneously heals leaving a small scar[24] [25]. Minor, transient enlargement of regional lymph nodes is common. Rarely, suppurative lymphadenitis and fistulae may develop 3–6 months after vaccination, more frequently in HIV infected persons. Although limited safety data are available for BCG vaccination of adults in high TB burden settings, no serious adverse effects were reported following primary BCG vaccination of TST positive persons in a large Indian BCG trial[26].
The effects of pre-vaccination treatment of LTBI on protective immune responses to live mycobacterial vaccines, including BCG, are not known. Isoniazid (INH) preventive therapy (IPT) has been shown to increase TB-specific immune responses, including frequency of interferon (IFN)-gamma-producing T cells[27]. The primary risk of INH treatment is reversible hepatotoxicity, particularly in older persons, pregnant and post-partum women, persons who consume ethanol on a daily basis, and those with chronic liver disease[28][28][28][27]. Established LTBI, as opposed to recent MTB infection, is thought to be 60–80% protective against subsequent TB disease in persons who have not received BCG vaccination[29]. Therefore, although the benefit of established LTBI in BCG-vaccinated populations is unknown[30], the protection offered by any novel vaccine in combination with INH should exceed that provided by pre-existing LTBI[29, 30]. South African guidelines currently recommend IPT only for children younger than 5 years who are close contacts of persons with active TB, and for HIV infected TST positive adults, but not for HIV uninfected adults with LTBI[31].
We hypothesized that INH pre-clearance of latent MTB bacilli results in a more optimal immunologic response following BCG revaccination. However, risk-benefit analysis must consider the safety of both BCG revaccination and INH treatment in MTB-infected persons. We report safety and reactogenicity data from a randomized clinical trial designed to test whether INH pretreatment enhances the immunogenicity of BCG revaccination in MTB infected adults.
METHODS
Study design
The study was a phase 1, single-center, open label, randomized clinical trial to assess the safety and immunogenicity of pre-clearance of LTBI by treatment with 6 months of INH before BCG revaccination, in healthy adults with strongly positive TST. Eighteen to 40 year-old, healthy, HIV-uninfected adults, with a BCG scar and TST reaction ≥ 15 mm, were randomly assigned to receive either (a) a 6-month course of INH treatment to be completed within no more than 7 months, followed within 7–8 weeks by revaccination with BCG Vaccine SSI (Danish 1331 strain, Statens Serum Institut, Copenhagen, Denmark); followed by 12 months of observation; or (b) 7 months of observation, followed by BCG revaccination; followed, after a further 6 months of observation by 6 months of INH treatment. Thus, all subjects would receive BCG revaccination at approximately the same time after study entry and all subjects would receive INH treatment for LTBI. Subjects in both study arms were followed for immunogenicity for 12 months after BCG revaccination. Here we report the safety and reactogenicity data through 3 months post BCG revaccination for all subjects.
The protocol was approved by the Medicines Control Council (MCC) of South Africa, the Human Research Ethics Committee of the University of Cape Town (387/2008) and the University Hospitals Case Medical Center Institutional Review Board. Written informed consent was obtained from all participants. The trial was conducted according to International Conference on Harmonisation (ICH) Good Clinical Practice (GCP) Guidelines and overseen by an independent, chartered Safety Monitoring Committee (SMC). The trial was registered in a clinical trials database (ClinicalTrials.gov identifier: NCT01119521).
Recruitment
Healthy volunteers were recruited from Worcester, near Cape Town, South Africa. This rural town (population 92,000) has a sputum smear positive TB incidence of 815 per 100,000, and all-forms TB incidence of 1,030 per 100,000 person years (unpublished data, Regional Health Department, Western Cape Province, South Africa, 2010). We have shown previously that 19% of healthy adolescents and 52% of healthy HIV uninfected adults in this region have a TST ≥15mm[32, 33]. We performed a TST using 2TU intradermal PPD-RT23 (Statens Serum Institut) and read transverse induration diameter by palpation 48 – 72 hours later. Subjects with a BCG scar and strongly positive TST ≥15mm were eligible for enrollment. HIV-infected persons; those with active TB disease; abnormal hepatic or renal function, anemia or leucopenia; active severe dermatologic disease; receiving chronic immunosuppressive medication; pregnant or lactating females; and persons who received any live vaccine within 60 days of screening, were ineligible. Active TB at baseline was excluded by clinical evaluation, chest radiography, and sputum smear and culture if indicated. Persons found to be HIV infected at baseline, on the basis of two independent rapid tests, were referred for HIV care.
Eligible subjects were assigned by central randomization using a permuted block design, to either (a) INH pretreatment, followed within 7–8 weeks by intradermal BCG revaccination, followed by 12 months of observation; or (b) 7 months of observation, followed by intradermal BCG revaccination, followed, after a further 6 months of observation, by INH treatment. Laboratory staff who performed safety and immunologic tests were unaware of treatment assignment, but subjects and clinicians were not blinded to study arm.
Adherence and Safety of INH Pretreatment of LTBI
Subjects were treated with daily INH 5 mg/kg rounded to the nearest 100 mg (maximum 300 mg daily) (Westward Pharmaceutical Corporation, Eatontown, NJ, USA), supplemented with pyridoxine 25 mg/day, under supervision of a healthcare worker or trained lay supervisor, at least 5 days each week. Adherence was monitored by review of dispensing records and urine INH metabolite testing (BBL Taxo INH Test Strips, Becton-Dickinson) at scheduled and unscheduled study visits. Treatment completion was defined as receipt of 180 doses of INH within 210 days. Subjects were questioned about symptoms of INH hepatotoxicity and other side effects during each visit and hepatic enzymes and total bilirubin were routinely checked after one month on treatment for all subjects.
Follow-up for safety and immunological evaluation
Subjects assigned to receive IPT before BCG revaccination were evaluated monthly for safety, including screening for incident TB disease, while receiving IPT. Subjects were evaluated for safety, reactogenicity, and immunogenicity at baseline, before starting IPT, before BCG revaccination, and at 7 and 14 days, and 1 and 3 months after BCG revaccination.
Subjects assigned to receive BCG revaccination before IPT were evaluated monthly, including screening for incident TB disease, during the 7 months of observation before BCG revaccination. Subjects were evaluated for safety, reactogenicity, and immunogenicity at baseline, when starting observation, before receiving BCG, and at 7 and 14 days, and 1 and 3 months after BCG revaccination.
Safety evaluation included a clinical history and examination; complete blood count (CBC) with differential and platelets; serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin; serum glucose, urea (BUN), creatinine; urine testing for glucose and protein; and pregnancy testing before receiving BCG revaccination and before starting IPT. Subjects receiving IPT were specifically evaluated for symptoms and signs of hepatotoxicity, including nausea, vomiting, loss of appetite, malaise, stool or urine color change, itching, rash, or jaundice.
Screening for incident TB disease was performed at each visit. A QuantiFERON-TB Gold In-Tube (QFT-GIT) (Cellestis Inc) test was performed at baseline and prior to BCG revaccination. A chest radiograph was performed and sputum was collected for smear and culture if symptoms compatible with TB were detected. Additional safety evaluations were at the discretion of the attending clinician.
Immunologic endpoints were selected to assess the effect of INH preclearance on the kinetics and characteristics of MTB-specific cellular immune responses following BCG revaccination. These assays are ongoing and results will be described in subsequent publications.
BCG Revaccination
Subjects were revaccinated with BCG Vaccine SSI, derived from the Danish BCG strain 1331 (Statens Serum Institut, Copenhagen, Denmark), reconstituted with Sauton Diluent. BCG Vaccine SSI, the vaccine used in the South African national immunization program, is supplied in 10-dose vials containing 0.75 mg (2 to 8 × 105 CFU) lyophilized BCG. BCG was administered intradermally at a dose of 0.1 mL in the left deltoid, according to the manufacturer’s instructions. Subjects were monitored for 30 minutes post revaccination and received a thermometer and memory aid to record daily oral body temperatures and adverse reactions for 2 weeks after vaccination. Following BCG revaccination, subjects were specifically evaluated for fever, myalgia, fatigue, chills, night sweats, weight loss, rhinorrhea, cough, wheezing, or shortness of breath. Reactogenicity evaluation included assessment of injection site pain, and presence and transverse diameter (mm) of local erythema, induration, ulceration, and scarring. Regional lymph nodes were evaluated for evidence of lymphadenitis. Local, regional, and systemic adverse events were graded by severity, relationship to INH and/or BCG revaccination, expectedness, and outcome.
Data are reported as n (%) and mean (standard deviation, SD), or median (interquartile range) for non-parametric distributions. Comparisons between study arms were done using Fisher’s exact or chi-square tests for categorical data and the Mann-Whitney test for continuous data. Relative risks and their 95% confidence intervals were computed.
RESULTS
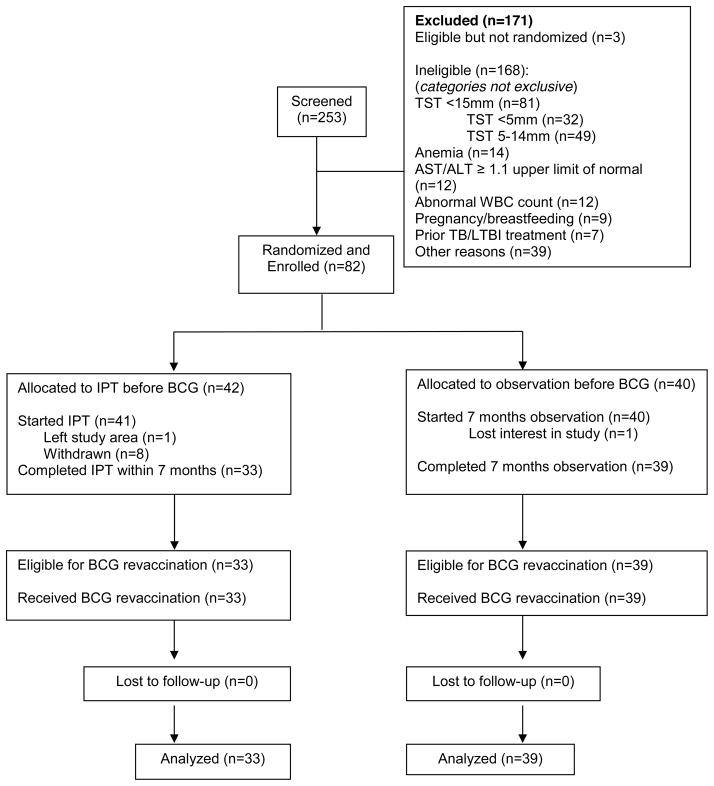
A total of 253 subjects were screened between October 2010 – September 2011 and 82 subjects were enrolled. Subject flow is shown in Figure 1. The most frequent reasons for ineligibility were TST <15 mm, anemia, abnormal serum AST or ALT or total bilirubin, or white blood cell count ≤ 3,500/mm3 or ≥ 10,800/mm3. Baseline demographic, clinical and laboratory characteristics of enrolled subjects are summarized in Table 1. The mean participant age was 27 (SD 6) years, 33% of subjects were male, and mean baseline TST was 20 (SD 4) mm. Baseline characteristics did not differ between treatment arms.
Figure 1.
Participant Flow
Table 1.
Baseline characteristics of all enrolled subjects by study arm
| Baseline Characteristics | INH before BCG (n=42) | Observation before BCG (n=40) |
|---|---|---|
| Age, mean (s.d.), years | 26 (6) | 27 (6) |
| Female, n (%) | 29 (69) | 26 (65) |
| BMI, mean (s.d.), kg/m2 | 26 (6) | 26 (6) |
| WBC count, mean (s.d.), x 103/mm3 | 7.4 (1.7) | 7.1 (1.2) |
| Hemoglobin, mean (s.d.), gm/dL | 14.1 (1.2) | 14.1 (1.3) |
| TST, mean (s.d.), mm | 20 (5) | 20 (4) |
INH = Isoniazid; BCG = Bacillus Calmette-Guerin; BMI = body mass index, weight was measured in kilograms (kg) and height in meters (m); s.d. = standard deviation; TST = Tuberculin skin test; WBC = white blood cell.
INH Pretreatment of LTBI
Thirty-four urine INH metabolite tests were performed in subjects receiving IPT before BCG revaccination, of which 25 (74%) were positive, including 12 of 18 unscheduled tests (67%) and 13 of 16 scheduled tests (81%). Systemic adverse events prior to BCG revaccination were more common among the 41 subjects in the INH pretreatment arm, although the majority of adverse events were mild (Table 2). Overall, 13 subjects (32%) developed Grade 1 hepatotoxocity (defined as 1.1 – 2.5 times the upper limit of the normal laboratory reference range for ALT or AST). One subject developed asymptomatic Grade 3 hepatotoxicity and was withdrawn without sequelae after discontinuation of IPT. No subjects developed a rash related to INH. One subject had a grand mal seizure after an overdose of INH as a suicidal gesture in the setting of ethanol intoxication. This subject was discharged without sequelae and withdrawn. No cases of peripheral neuropathy related to INH were observed. Thus, in total, two subjects required discontinuation of IPT due to related adverse events.
Table 2.
Cumulative adverse events prior to BCG revaccination by body system, severity, and study arm
| Body System | INH before BCG (n=41α)* | Observation before BCG (n=40)* | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| All n (%)** |
Grade 3 n |
Grade 4 n |
All n (%)** |
Grade 3 n |
Grade 4 n |
Total Adverse Events N (%)*** |
|
| Body as a Whole | 5 (12) | 0 | 0 | 1 (3) | 0 | 0 | 6 (7) |
| Cardiovascular | 0 (0) | 0 | 0 | 0 (0) | 0 | 0 | 0 (0) |
| Digestive | 11 (27) | 0 | 0 | 5 (13) | 0 | 0 | 16 (20) |
| Endocrine | 1 (2) | 1a | 0 | 0 (0) | 0 | 0 | 1 (1) |
| Hemic/Lymphatic | 2 (5) | 0 | 0 | 3 (8) | 0 | 0 | 5 (6) |
| Hepatic | 10 (24) | 1b | 0 | 3 (8) | 0 | 0 | 13 (16) |
| Metabolic/Nutritional | 0 (0) | 0 | 0 | 0 (0) | 0 | 0 | 0 (0) |
| Musculoskeletal | 3 (7) | 0 | 0 | 1 (3) | 1d | 0 | 4 (5) |
| Nervous | 3 (7) | 2c | 0 | 0 (0) | 0 | 0 | 3 (4) |
| Respiratory | 12 (29) | 0 | 0 | 18 (45) | 0 | 0 | 30 (37) |
| Skin and Appendages | 15 (37) | 0 | 0 | 4 (10) | 0 | 0 | 19 (23) |
| Special Senses | 0 (0) | 0 | 0 | 0 (0) | 0 | 0 | 0 (0) |
| Urogenital | 4 (10) | 0 | 0 | 1 (3) | 0 | 0 | 5 (6) |
| Total | 66 | 4 | 0 | 36 | 1 | 0 | 102 |
One subject moved out of the study area before starting INH;
All subjects who started INH or Observation before BCG revaccination per protocol;
Denominator is total number of subjects in each arm;
Denominator is total number of subjects in both arms
1 diabetes mellitus;
1 INH-associated hepatotoxicity;
1 generalized seizure associated with INH overdose and 1 extra-medullary hematoma;
1 mandibular fracture.
Safety of BCG Revaccination in Adults with LTBI
Local Adverse Reactions
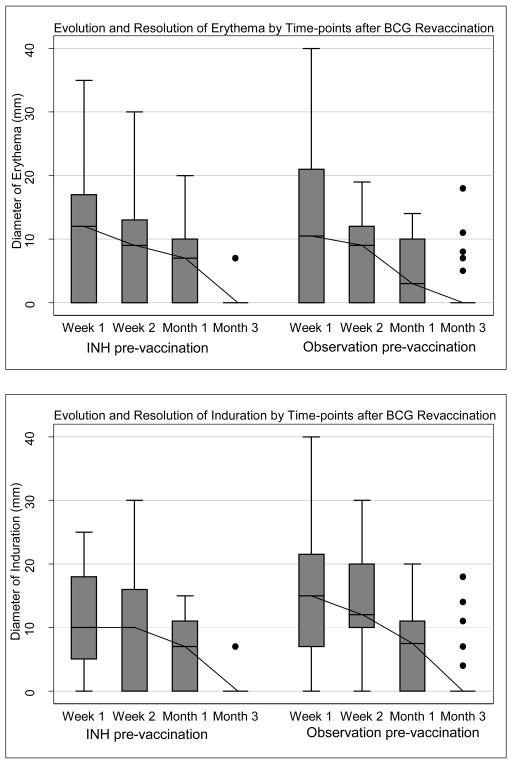
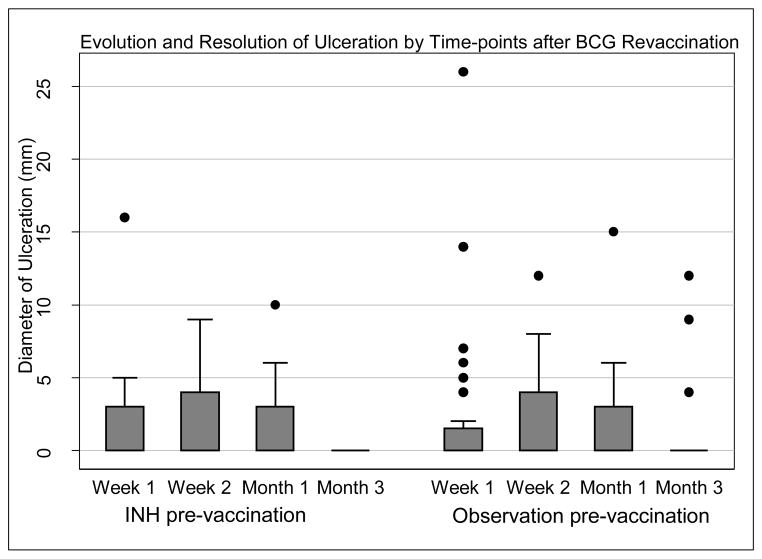
Seventy-two subjects received BCG revaccination. The rate of local reactogenicity events, including erythema, induration, and ulcer formation and resolution, did not differ between the study arms (Table 3). In total, 35% of subjects (n=25) reported injection site pain; 68% (n=49) showed erythema; 86% (n=62) induration; and 76% (n=55) ulceration. Thirty-five subjects (49%) demonstrated the triad of erythema, induration, and ulceration. Transient axillary lymphadenopathy was noted in 10 subjects. Erythema and induration diameter peaked at week one (median erythema diameter 11mm, IQR 0–18, and median induration diameter 12mm, IQR 5.5–20), whereas ulceration peaked later at week 2 (Figure 2). Among subjects who developed an ulcer, median diameter was 5mm (IQR 4.0–6.0). Six subjects (8%) demonstrated an ulcer >10mm in diameter (>15mm in two subjects and >25mm in one subject). Ulceration resolved by month 3 in all but two subjects, in whom ulceration had resolved by month 6, and was associated with a small scar in 61 subjects (85%). Maximum ulcer diameter did not correlate with QFT-GIT quantitative result (IU/mL) prior to BCG revaccination, either for subjects receiving IPT pre-treatment (Spearman correlation coefficient r=0.16; p = 0.74), or for subjects undergoing observation before BCG revaccination (r=0.12; p=0.71).
Table 3.
New local reactogenicity adverse events after BCG revaccination, by study arm
| Adverse Event | INH before BCG (n=33) # |
Observation before BCG (n=39) # |
RR | 95%CI |
|---|---|---|---|---|
| Pain | 10 | 17 | 0.70 | 0.34–1.37 |
| Redness | 26 | 28 | 1.19 | 0.81–1.42 |
| Swelling | 2 | 4 | 0.59 | 0.08–3.57 |
| Induration | 28 | 36 | 0.92 | 0.80–1.10 |
| Ulcer | 30 | 33 | 1.07 | 0.88–1.23 |
| Lymphadenopathy | 3 | 7 | 0.51 | 0.11–2.00 |
| Pain + Redness | 8 | 17 | 0.56 | 0.24–1.17 |
| Induration + Ulcer | 25 | 33 | 0.90 | 0.71–1.15 |
| Redness + Induration + Ulcer | 20 | 26 | 0.91 | 0.61–1.33 |
n = all subjects that received BCG vaccination
RR=relative risk
Figure 2.
(a – c) Evolution and resolution of local reactogenicity (erythema, induration, and ulceration) over three months following BCG revaccination in the INH arm and observation arm respectively. Error bars are interquartile range, minimum-maximum range, with median line and outliers.
Systemic reactions
Systemic adverse events were uncommon following BCG revaccination (n=14). Eleven systemic adverse events were deemed related. The most frequent systemic adverse event following BCG revaccination was headache, which was reported in six subjects. Two subjects demonstrated anemia. Fever, nausea/vomiting, diarrhea, leucopenia, neutrophilia, and neutropenia were each noted in single subjects.
DISCUSSION
We have shown that BCG revaccination of MTB infected adults was safe and well-tolerated, with acceptable local and systemic reactions that were similar to primary BCG vaccination, in a randomized clinical trial designed to test whether INH pretreatment enhances immunogenicity of BCG revaccination in adults with LTBI[25]. The time profile of reactogenicity at the BCG revaccination site was characterized by erythema and induration, peaking at one week. Early erythema and induration were followed by a small ulcer, which peaked at two weeks post revaccination, and healed leaving a small scar in most subjects. Although half of MTB infected adults showed this triad of erythema and induration followed by a small ulcer at the injection site, these lesions, including ulceration, had almost all resolved within three months after BCG revaccination.
Our results are consistent with the REVAC trial in Brazilian school children, in which BCG revaccination was not associated with more severe adverse events than those known to occur after primary BCG vaccination [35]. However, since tuberculin skin testing was not performed in the REVAC trial, it is unknown how many subjects were MTB infected. Earlier studies of BCG revaccination in 2,997 Swedish school children reported open vaccination site ulcers (mean diameter 4mm) in 4% of children receiving the Danish BCG Vaccine SSI used in our trial [36]. Our findings in MTB infected adults show a higher rate of ulceration, which was greater than 10 mm in a minority of subjects, yet these lesions had almost all healed within three months after revaccination. Our data are also similar to those reported for primary intradermal BCG vaccination of TST unreactive US adults, 98% of whom showed ulceration (mean diameter 4.9 mm and duration 4.3 weeks)[25].
Previous reports have concluded that BCG revaccination is not associated with a higher rate of serious adverse events than primary BCG vaccination [37, 38]. The absence of serious adverse events related to BCG revaccination in this trial is consistent with those findings. Disseminated BCG infection and death are rare and occur almost exclusively in children with congenital immune deficiency or persons with advanced AIDS[39]. Since we excluded individuals at increased risk for BCG disease, the absence of regional lymphadenitis and disseminated BCG disease is not unexpected. There is a theoretical concern that revaccination with a TB vaccine might result in severe Koch reactions associated with significant morbidity[15]. However, the Koch phenomenon has not been observed in this or other BCG revaccination trials, or in other trials of novel TB vaccines, conducted in previously BCG vaccinated and MTB infected subjects at our site[40–42].
We did not expect the safety profile of subjects receiving INH pretreatment before BCG revaccination to differ from the known complications of IPT in MTB infected persons 25. Since persons with risk factors for hepatotoxicity were excluded, our finding that IPT had to be stopped due to hepatotoxicity in only one subject is not surprising. The rate of local reactogenicity events was unaffected by prior receipt of IPT. Thus, based on the known safety profile for INH in MTB infected persons and our findings for local and systemic adverse events following BCG revaccination, the use of IPT prior to BCG revaccination appears safe and well tolerated.
These findings regarding the safety of BCG revaccination in latently infected adults lend support to the advancement of novel live mycobacterial vaccines, including recombinant BCG and attenuated MTB vaccines[6, 17, 43], into large-scale clinical trials in high TB burden countries, where the majority of adults are likely to be MTB infected[3]. Adults are the primary target population for future mass TB vaccination campaigns, in an effort to interrupt MTB transmission[4]. The potential role of INH pre-clearance in combination with new live mycobacterial vaccines, although safe and well tolerated, will be informed by the results of our ongoing immunologic assays.
Our study has several limitations. These findings may not be generalizable to individuals with LTBI who demonstrate lesser magnitude TST reactions. Although TST reactions >15mm are not likely to result from childhood BCG immunization, or infection with non-tuberculous mycobacteria, the possibility of false positives is acknowledged. Interferon-gamma release assays, which are thought to be more specific than TST, were positive at baseline in 94% of individuals with strongly positive TST in this study population[44]. Sample size was calculated based on immunologic endpoints and therefore, this study had limited power to detect rare adverse reactions. Study treatment was open-label and participants and clinical staff were aware of whether patients received INH treatment before or after BCG revaccination. However, laboratory staff were blinded to treatment arm assignment. Strengths of the trial include use of a TST cut-point of 15mm induration to assure a greater likelihood of true latent TB infection; documentation of adherence to INH therapy; and use of standardized questionnaires to record detailed safety and reactogenicity data.
Highlights.
The safety profile of live mycobacterial vaccines in TB infected adults is unknown.
BCG revaccination of adults with latent TB infection was safe and well tolerated.
Reactogenicity of BCG revaccination was unaffected by isoniazid pretreatment.
Trials of live mycobacterial vaccines may be considered in TB infected adults.
Acknowledgments
Angelique Kany Kany Luabeya; Michele Tameris; Marwou de Kock; and the SATVI and TBRU DMID 07-0083 trial teams.
Sources of Support: Funded by the Tuberculosis Research Unit at Case Western Reserve University, established with funds from the United States National Institutes of Allergy and Infectious Diseases, National Institutes of Health and Human Services, under Contract No. NO1-AI95383 and HHSN266200700022C/NO1-AI-70022
Footnotes
Author Contributions: Conceived and designed the study (DFH, WHB, WAH, JLJ); collected and analyzed data (MH, HG, BP, SS, PC, SMD); wrote the first draft manuscript (MH, HG, PC, SMD, JLJ); revised the final manuscript (All Authors).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zwerling A, et al. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8(3):e1001012. doi: 10.1371/journal.pmed.1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Control: WHO Report. Geneva: S.W.H.O.R.N.W.H.T; 2011. [Google Scholar]
- 3.Mahomed H, et al. The tuberculin skin test versus QuantiFERON TB Gold(R) in predicting tuberculosis disease in an adolescent cohort study in South Africa. PLoS One. 2011;6(3):e17984. doi: 10.1371/journal.pone.0017984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dye C, Williams BG. Eliminating human tuberculosis in the twenty-first century. J R Soc Interface. 2008;5(23):653–62. doi: 10.1098/rsif.2007.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu-Raddad LJ, et al. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci U S A. 2009;106(33):13980–5. doi: 10.1073/pnas.0901720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbues A, et al. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine. 2013;31(42):4867–73. doi: 10.1016/j.vaccine.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 7.Grode L, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J Clin Invest. 2005;115(9):2472–9. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grode L, et al. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine. 2013;31(9):1340–8. doi: 10.1016/j.vaccine.2012.12.053. [DOI] [PubMed] [Google Scholar]
- 9.Hougardy JM, et al. Regulatory T cells depress immune responses to protective antigens in active tuberculosis. Am J Respir Crit Care Med. 2007;176(4):409–16. doi: 10.1164/rccm.200701-084OC. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong JA, HP Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971;134:713–40. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vergne I, et al. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2005;102(11):4033–8. doi: 10.1073/pnas.0409716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walburger A, et al. Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science. 2004;304(5678):1800–4. doi: 10.1126/science.1099384. [DOI] [PubMed] [Google Scholar]
- 13.Xu S, et al. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J Immunol. 1994;153(6):2568–78. [PubMed] [Google Scholar]
- 14.von Reyn CF, et al. Prevention of tuberculosis in Bacille Calmette-Guerin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. Aids. 2010;24(5):675–85. doi: 10.1097/QAD.0b013e3283350f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rook GA, al Attiyah R, Filley E. New insights into the immunopathology of tuberculosis. Pathobiology. 1991;59(3):148–52. doi: 10.1159/000163633. [DOI] [PubMed] [Google Scholar]
- 16.Rook GA, Stanford JL. The Koch phenomenon and the immunopathology of tuberculosis. Curr Top Microbiol Immunol. 1996;215:239–62. doi: 10.1007/978-3-642-80166-2_11. [DOI] [PubMed] [Google Scholar]
- 17.Walker KB, et al. The second Geneva Consensus: Recommendations for novel live TB vaccines. Vaccine. 2010;28(11):2259–70. doi: 10.1016/j.vaccine.2009.12.083. [DOI] [PubMed] [Google Scholar]
- 18.Leung CC, et al. Efficacy of the BCG revaccination programme in a cohort given BCG vaccination at birth in Hong Kong. Int J Tuberc Lung Dis. 2001;5(8):717–23. [PubMed] [Google Scholar]
- 19.Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Karonga Prevention Trial Group. Lancet. 1996;348(9019):17–24. [PubMed] [Google Scholar]
- 20.Ponnighaus JM, et al. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet. 1992;339(8794):636–9. doi: 10.1016/0140-6736(92)90794-4. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. BCG Vaccine. WHO Position paper. Wkly Epidemiol Rec. 2004:27–38. [PubMed] [Google Scholar]
- 22.Rodrigues LC, et al. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet. 2005;366(9493):1290–5. doi: 10.1016/S0140-6736(05)67145-0. [DOI] [PubMed] [Google Scholar]
- 23.Barreto ML, et al. Evidence of an effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: second report of the BCG-REVAC cluster-randomised trial. Vaccine. 2011;29(31):4875–7. doi: 10.1016/j.vaccine.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 24.FitzGerald JM. Management of adverse reactions to bacille Calmette-Guerin vaccine. Clin Infect Dis. 2000;31(Suppl 3):S75–6. doi: 10.1086/314074. [DOI] [PubMed] [Google Scholar]
- 25.Hoft DF, et al. Clinical reactogenicity of intradermal bacille Calmette-Guerin vaccination. Clin Infect Dis. 1999;28(4):785–90. doi: 10.1086/515201. [DOI] [PubMed] [Google Scholar]
- 26.Baily GV. Tuberculosis prevention Trial, Madras. Indian J Med Res. 1980;72(Suppl):1–74. [PubMed] [Google Scholar]
- 27.Wilkinson KA, et al. Effect of treatment of latent tuberculosis infection on the T cell response to Mycobacterium tuberculosis antigens. J Infect Dis. 2006;193(3):354–9. doi: 10.1086/499311. [DOI] [PubMed] [Google Scholar]
- 28.Kopanoff DE, Snider DE, Jr, Caras GJ. Isoniazid-related hepatitis: a U.S. Public Health Service cooperative surveillance study. Am Rev Respir Dis. 1978;117(6):991–1001. doi: 10.1164/arrd.1978.117.6.991. [DOI] [PubMed] [Google Scholar]
- 29.Andrews JR, et al. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis. 2012;54(6):784–91. doi: 10.1093/cid/cir951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brooks-Pollock E, et al. Epidemiologic inference from the distribution of tuberculosis cases in households in Lima, Peru. J Infect Dis. 2011;203(11):1582–9. doi: 10.1093/infdis/jir162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Tuberculosis Control Programme (NTCP) NDoH, S.A; D.o.H. RoSA, editor. The South African Tuberculosis Control Programme Practical Guidelines 2000. Department of Health; 2000. [Google Scholar]
- 32.Mahomed H, et al. Comparison of mantoux skin test with three generations of a whole blood IFN-gamma assay for tuberculosis infection. Int J Tuberc Lung Dis. 2006;10(3):310–6. [PubMed] [Google Scholar]
- 33.Mahomed H, et al. Predictive factors for latent tuberculosis infection among adolescents in a high-burden area in South Africa. Int J Tuberc Lung Dis. 2011;15(3):331–6. [PubMed] [Google Scholar]
- 34.Rothman KJ G.S.M.e.r.e.P.L.W.W. 2008 [Google Scholar]
- 35.Dourado I, et al. Rates of adverse reactions to first and second doses of BCG vaccination: results of a large community trial in Brazilian schoolchildren. Int J Tuberc Lung Dis. 2003;7(4):399–402. [PubMed] [Google Scholar]
- 36.Bottiger M, et al. A comparative study of Danish (Statens Seruminstitut), Glaxo and Behringwerke vaccines--revaccination of schoolchildren. J Biol Stand. 1983;11(1):1–12. doi: 10.1016/s0092-1157(83)80040-7. [DOI] [PubMed] [Google Scholar]
- 37.Global Tuberculosis Programme and Global Programme on Vaccines, Statement on BCG revaccination for the prevention of tuberculosis. Wkly Epidemiol Rec. 1995:229–31. [PubMed] [Google Scholar]
- 38.Karonga Prevention Trial Group. Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet. 1996;348:17–24. [PubMed] [Google Scholar]
- 39.Hesseling AC, et al. Consensus statement on the revised World Health Organization recommendations for BCG vaccination in HIV-infected infants. Int J Tuberc Lung Dis. 2008;12(12):1376–9. [PubMed] [Google Scholar]
- 40.Hawkridge T, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J Infect Dis. 2008;198(4):544–52. doi: 10.1086/590185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scriba TJ, et al. Modified vaccinia Ankara-expressing Ag85A, a novel tuberculosis vaccine, is safe in adolescents and children, and induces polyfunctional CD4+ T cells. Eur J Immunol. 2010;40(1):279–90. doi: 10.1002/eji.200939754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scriba TJ, et al. A phase IIa trial of the new tuberculosis vaccine, MVA85A, in HIV- and/or Mycobacterium tuberculosis-infected adults. Am J Respir Crit Care Med. 2012;185(7):769–78. doi: 10.1164/rccm.201108-1548OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gray DM, Zar H, Cotton M. Impact of tuberculosis preventive therapy on tuberculosis and mortality in HIV-infected children. Cochrane Database Syst Rev. 2009;1:CD006418. doi: 10.1002/14651858.CD006418.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Johnson JL, et al. Effect of Isoniazid Therapy for Latent TB Infection on QuantiFERON-TB Gold In-Tube Responses in Adults With Positive Tuberculin Skin Test Results in a High TB Incidence Area: A Controlled Study. Chest. 2014;145(3):612–7. doi: 10.1378/chest.13-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]