Summary
Iron is a critical nutrient for the growth and survival of most bacterial species. Accordingly, much attention has been paid to the mechanisms by which host organisms sequester iron from invading bacteria and how bacteria acquire iron from their environment. However, under oxidative stress conditions such as those encountered within phagocytic cells during the host immune response, iron is released from proteins and can act as a catalyst for Fenton chemistry to produce cytotoxic reactive oxygen species. The transitory efflux of free intracellular iron may be beneficial to bacteria under such conditions. The recent discovery of putative iron efflux transporters in Salmonella enterica serovar Typhimurium is discussed in the context of cellular iron homeostasis.
Introduction
All organisms require transition metals for growth and survival. Approximately one third of all proteins and nearly half of all enzymes that have been structurally characterized contain one or more metal ions (Waldron et al., 2009; Andreini et al., 2008). Iron is the most common redox active metal found in proteins, typically within heme or iron-sulfur prosthetic groups (Beinert et al., 1997; Andreini et al., 2008). Iron is also found in regulatory proteins, which in the enteric bacterium Salmonella enterica includes Fur, Fnr, NorR, SoxR, IscR and NsrR (Ernst et al., 1978; Bagg and Neilands, 1987; Green et al., 1991; Fink et al., 2007; D’Autreaux et al., 2005; Hidalgo and Demple, 1994; Pomposiello and Demple, 2000; Schwartz et al., 2001; Tucker et al., 2008; Karlinsey et al., 2012). While iron-containing proteins are essential for fundamental physiological processes such as respiration, central metabolism and DNA repair, free iron is able to catalyze biomolecular damage to DNA, proteins and lipids via Fenton chemistry. In the Haber-Weiss cycle of reactions, free ferrous iron reacts with hydrogen peroxide (H2O2) to produce hydroxyl radicals and ferric iron. Ferric iron can then react with H2O2 to produce superoxide and regenerate the original ferrous iron catalyst. To protect against the damage that would result from oxyradical production, iron must be carefully handled within cells to maintain free intracellular iron at low levels.
Iron Withholding and Acquisition in Host-Pathogen Interactions
Most research with respect to iron and infection has focused on mechanisms by which mammalian hosts and pathogens compete for transition metals (Zaharik et al., 2004; Schaible and Kaufmann, 2005). Studies have also indicated the presence of extracellular iron-storage protein homologs in invertebrates such as crabs, worms and insects (Ong et al., 2005; Simonsen et al., 2011; Charlesworth et al., 1997), which have been suggested to play a role in immunity (Simonsen et al., 2011; Beck et al., 2002). The concept of metal withholding in nutritional immunity of higher organisms has been the subject of many recent reviews (Diaz-Ochoa et al., 2014; Cassat and Skaar, 2013; Cerasi et al., 2013; Hood and Skaar, 2012). To acquire necessary iron, bacteria express a variety of uptake systems and produce siderophores to chelate and acquire iron within the host (Figure 1). FeoAB is an uptake system for ferrous iron that consists of a cytosolic protein and inner membrane transporter (Cartron et al., 2006). SitABCD is an ABC family transporter that allows the acquisition of both ferrous iron and manganese (Zhou et al., 1999; Boyer et al., 2002). Iron-chelating siderophores are synthesized by bacteria and secreted using an active efflux mechanism involving inner membrane transporters such as EntS and IroC and the outer membrane protein TolC (Furrer et al., 2002; Crouch et al., 2008; Bleuel et al., 2005). Uptake of iron-bound siderophores also requires specialized proteins such as IroN and the Fep system (Hantke et al., 2003; Crouch et al., 2008). An additional system for iron uptake utilized by some gram negative bacteria but not found in Salmonella is the ferric citrate iron acquisition system Fec (Mahren et al., 2005; Wagegg and Braun, 1981).
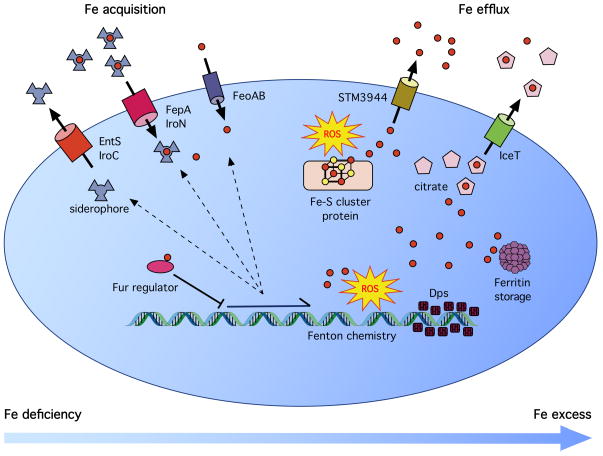
Figure 1. Maintenance of Cellular Iron Homeostasis under Deficient and Replete Conditions.
When intracellular iron levels are low (left), cells express siderophores and other iron import systems to acquire iron from the environment. Under iron replete conditions, the iron-sensing transcriptional regulator Fur represses the expression of iron acquisition genes. Available iron is incorporated into iron-sulfur cluster and mononuclear iron proteins, and excess iron is sequestered by ferritin (right). Additional iron can be sequestered by the DNA-binding protein Dps. Under stress conditions in which iron is mobilized from proteins, iron exporters, predicted to be localized in the inner membrane, promote iron efflux to mitigate DNA damage from Fenton chemistry.
Mammalian hosts use the inflammation-induced hormone hepcidin to restrict absorption of dietary iron and degrade ferroportin, which blocks the release of recycled iron stored in macrophages (Ganz, 2013). Host organisms also minimize the availability of extracellular free iron by expressing iron-binding proteins such as transferrin and lactoferrin (Theurl et al., 2005; Weiss and Schett, 2013). Macrophages use the Nramp1 (Slc11A1) metal transporter to restrict iron availability in the phagosomal environment by exporting iron from the phagosome (Atkinson and Barton, 1999; Barton et al., 1999; Blackwell and Searle, 1999; Cellier et al., 2007). The extent of the host-pathogen arms race over iron acquisition is exemplified by the host protein lipocalin-2, which binds the siderophore enterobactin (Flo et al., 2004) but is unable to bind a glucosylated derivative called salmochelin, which then allows Salmonella and certain other enteric bacteria to obtain iron during infection (Neilands, 1995; Fischbach et al., 2005; Crouch et al., 2008; Raffatellu et al., 2009). Lipocalin-2 also promotes iron export from macrophages and modulates macrophage activation (Fritsche et al., 2012; Warszawska et al., 2013).
Studies have shown that iron availability is an important determinant of virulence. Mice with systemic iron deficiency are more resistant to infection (Puschmann and Ganzoni, 1977), whereas acute iron overload in mouse tissues results in enhanced bacterial outgrowth (Sawatzki et al., 1983). Nramp1+ mice are more resistant to infection than Nramp1− organisms (Vidal et al., 1995a; Vidal et al., 1995b). Murine macrophages expressing Nramp1 are better able to restrict the growth of intracellular bacteria (Nairz et al., 2009a; Fritsche et al., 2012). Humans carrying a missense C282Y mutation in the HFE gene develop an iron overload condition known as hemochromatosis due to reduced surface expression and accelerated degradation of the HFE protein (Waheed et al., 1997; Parkkila et al., 2000). The iron overload resulting from this condition confers enhanced susceptibility to extracellular pathogens such as Vibrio vulnificus and Yersinia spp. (Weinberg, 2000; Bullen et al., 1991; Wright et al., 1981; Quenee et al., 2012). Similar iron overload phenotypes have been observed in C282Y and HFE null mice from various genetic backgrounds, providing a model system for the study of this condition and allowing identification of additional genes that might modify the clinical expression of hemochromatosis (Zhou et al., 1998; Levy et al., 1999; Fleming et al., 2001; Levy et al., 2000). Macrophages lacking HFE are paradoxically better able to limit the growth of intracellular pathogens such as Salmonella and Mycobacterium tuberculosis (Olakanmi et al., 2007; Nairz et al., 2009b), and this is most likely attributable to lipocalin-2-mediated redistribution of iron away from the intracellular environment (Nairz et al., 2009b). Thus, HFE controls iron compartmentalization, and an HFE mutation lowers iron availability within macrophages but increases it elsewhere. It has been suggested that typhoid and tuberculosis may have selected for the high prevelance of HFE mutations in some populations (Moalem et al., 2004).
Fenton Chemistry and Iron Toxicity
While it is clear that access to sufficient iron is critical for successful microbial replication within the host, during the course of infection pathogens may encounter stress conditions that raise intracellular free iron levels and promote Fenton chemistry, presenting an entirely different challenge to survival. The Fenton reaction proceeds relatively rapidly at physiological pH and temperature, and small alterations in the concentrations of H2O2 or free iron can have a dramatic impact on the amount of radical production and resulting damage (Park et al., 2005). DNA is the most critical target of hydroxyl radical damage, although proteins and lipids can be affected as well.
Intracellular pathogens encounter reactive oxygen species (ROS) and reactive nitrogen species (RNS) inside host macrophages. The macrophage NADPH oxidase NOX2 generates superoxide (O2·), which is rapidly converted to H2O2. Local H2O2 concentrations in phagocytes can reach 100 μM (Park et al., 2005). H2O2 is capable of readily diffusing across membranes and into bacterial cells where it can exert a significant physiological impact (Seaver and Imlay, 2001). While bacteria produce multiple catalases and peroxidases to detoxify the H2O2 produced as a result of normal metabolism, these defenses can be overwhelmed by the phagocytic oxidative burst with H2O2 levels rising to toxic micromolar levels (Gort and Imlay, 1998; Seaver and Imlay, 2001). Both humans and mice lacking a functional NADPH oxidase are significantly more susceptible to infection (Felmy et al., 2013).
H2O2 and other reactive oxygen species can harm cells in a variety of ways. One of these is by attacking solvent-exposed iron-sulfur clusters or mononuclear iron centers to result in enzyme inhibition and the release of free iron (Jang and Imlay, 2007; Sobota and Imlay, 2011; Anjem and Imlay, 2012). While iron-sulfur cluster enzymes have been well characterized, the number of mononuclear iron containing enzymes has likely been underestimated due to the challenge of studying these labile proteins under aerobic in vitro conditions (Anjem and Imlay, 2012). Damage to iron-sulfur cluster- and mononuclear iron center-containing proteins leads to a measurable increase in free iron levels within the cell (Keyer and Imlay, 1996). In addition to damaging DNA, proteins and lipids, oxyradicals can mobilize additional free iron.
Immune cells also produce nitric oxide (NO·) as an antimicrobial defense. While NO· is not as bactericidal as H2O2, NO· congeners such as peroxynitrite (ONOO−) can release iron from metalloproteins and accelerate damage from H2O2 (Woodmansee and Imlay, 2003). Inhibition of respiration by NO· leads to increased NADH levels, which in turn can reduce free flavins, leading to reduction of the free iron pool and enhanced Fenton chemistry (Woodmansee and Imlay, 2003). Organisms lacking both nitric oxide synthase and the phagocyte oxidase are exquisitely sensitive to infection compared to those lacking only one of the two defenses, suggesting they perform complementary functions in vivo (Shiloh et al., 1999).
It is therefore clear that while iron is an essential nutrient for nearly all bacterial species, it can also exert cytotoxic effects under conditions of oxidative and nitrosative stress, such as those encountered within host immune cells. ROS- and RNS-mediated damage to iron-containing proteins leads to transient increases in intracellular free iron, and cells must be able to adapt to changing iron conditions as they move from iron-limited to iron-replete or iron-overload conditions.
Control of iron within cells
Since free intracellular iron is capable of catalyzing damaging chemistry, cells have evolved mechanisms to limit iron toxicity. At the center of this response network is the iron-binding regulator Fur, which acts as an iron sensor to maintain cellular iron at sufficient levels while restricting the presence of free iron. Under iron-replete conditions Fur represses the expression of iron acquisition systems (Hantke, 1981; Ernst et al., 1978) (Figure 1). This repression is relieved during iron starvation when insufficient iron is available to bind to the regulator. Fur also plays a role in regulating iron storage within bacterial cells. Production of the bacterial ferritins FtnA and Bfr is indirectly stimulated by Fur through the actions of the small RNA ryhB (Masse and Gottesman, 2002). The specialized ferritin Dps is capable of both sequestering iron and binding to DNA to shield it from the effects of reactive oxygen species (Almiron et al., 1992; Zhao et al., 2002) (Figure 1). In Salmonella, dps is repressed by Fur under iron-replete conditions, while in other bacteria dps expression has been shown to be RpoS- and OxyR-dependent (Velayudhan et al., 2007; Altuvia et al., 1994). Mutants lacking dps are highly sensitive to H2O2 (Velayudhan et al., 2007). Fur plays a critical role in the control of iron acquisition, use and storage, and fur mutants display high rates of mutation and are highly sensitive to oxidative damage from iron overload (Touati et al., 1995). The induction of fur expression by OxyR and SoxRS during oxidative stress enhances the ability of cells to mount a protective response to oxidative stress by modifying iron metabolism (Zheng et al., 1999).
While cells can compensate for elevated free iron levels by reducing iron uptake, upregulating iron storage proteins, and increasing intracellular concentrations of magnesium and manganese to protect against oxidative damage, these responses are likely to be less rapid than the immediate rise in free iron that follows damage to iron-containing proteins. The acute danger presented by the release of intracellular free iron due to ROS and Fenton chemistry has led us to consider alternative defense strategies that bacteria might employ under these conditions. The transient efflux of free iron from the cell could conceivably be of benefit in the presence of ROS and RNS. Removing iron as a Fenton catalyst could prevent further damage until the iron can be safely reacquired and stored, or used to repair damaged iron centers.
Iron Efflux Transporters in Salmonella
We have recently identified two proteins in Salmonella Typhimurium, STM3944 and IceT, whose expression leads to reduced levels of total intracellular iron (Frawley et al., 2013; Velayudhan et al., 2014). Since the storage form of cellular iron is not available to chelators and transporters, it is likely that the reduction in total iron mediated by STM3944 and IceT reflects a reduction in the free iron pool. Both proteins are expressed under stress conditions and appear to exert a protective effect against ROS and RNS.
The STM3944 protein is encoded by the first gene in an operon with a gene encoding the bacterial frataxin homolog CyaY, an iron chaperone that supports iron-sulfur cluster biosynthesis and repair. STM3944 is predicted to encode a four transmembrane domain-containing inner membrane protein in the major facilitator superfamily with homology to the Mn2+/Fe2+ Nramp1 transporter. Inactivation of stm3944 dramatically enhances the H2O2 susceptibility of a cyaY mutant, and this phenotype is catalase-independent (Velayudhan et al., 2014). STM3944 expression leads to reduced free and total iron levels under conditions in which iron homeostasis is disrupted (Velayudhan et al., 2014). These observations suggest that STM3944 plays a role in the efflux of free intracellular iron under stress conditions in which iron storage and [Fe-S] cluster repair are disrupted.
The IceT (Iron citrate efflux Transporter) protein is also a member of the MFS (major facilitator) superfamily with homologs among other γ-proteobacteria as well as certain α- and β-proteobacteria (Frawley et al., 2013). IceT is co-regulated with the MdtABC multidrug efflux transporter and two-component regulator BaeSR (Baranova and Nikaido, 2002; Nishino et al., 2005). Expression of these genes is induced by NO· or by disrupted iron homeostasis (Frawley et al., 2013). IceT expression protects cells from death caused by iron overload and leads to reduced levels of total cellular iron. Expression also leads to secretion of an iron-chelating molecule that has been identified as citrate by biochemical and genetic analyses. Secretion of citrate occurs in excess of iron secretion, thus IceT appears to efflux both iron-bound and free citrate (Frawley et al., 2013).
The efflux of iron and citrate by IceT is an intriguing defense strategy against ROS and RNS, as citrate is both an iron chelator and a key intermediate in the TCA cycle. Elevated levels of citrate appear to be toxic to cells, as bacteria with mutations in aconitase face selective pressure to acquire secondary mutations in citrate synthase and will even secrete citrate by an uncharacterized mechanism (Baumgart et al., 2011; Gruer et al., 1997; Viollier et al., 2001; Varghese et al., 2003). Iron toxicity resulting from deletion of the yeast frataxin YFH1 is exacerbated by overexpression of citrate synthase and can be ameliorated by deletion of the CIT2 citrate synthase (Chen et al., 2002). Elevated citrate levels also exacerbate instability of mitochondrial DNA in an iron-dependent fashion (Farooq et al., 2013). Although the intracellular interactions of iron and citrate have not been precisely characterized, it is clear that elevated levels of citrate and free iron can be detrimental to cells. Secretion of citrate and iron-citrate by IceT removes a Fenton catalyst from the cell, prevents citrate from accumulating intracellularly, and restricts growth. This response confers resistance not only to iron toxicity and H2O2 but also to various antibiotics whose actions may be potentiated by Fenton chemistry (Frawley et al., 2013). Unlike STM3944, IceT has no homology to known MFS metal transporters such as Nramp1, nor is it homologous to metal-citrate importers such as those in the CitMHS family despite its function in transport of a citrate-chelated metal ion. IceT and its homologs may therefore represent a novel class of MFS transporters, since the protein appears to be both the first iron excretory system and the first citrate efflux pump to be described in bacteria.
Conclusions
Iron plays a critical role in bacterial physiology as an essential component of metabolic enzymes and regulatory proteins. The ability of iron to transfer electrons at physiological pH makes it both useful and dangerous for cells. As a result, bacteria have evolved a variety of mechanisms to both actively acquire iron from the environment under iron-limiting conditions and to control the availability of intracellular free iron under stress or iron-replete conditions (Figure 1). Much research has focused on mechanisms by which iron and other transition metals are acquired within the host and the global regulation of bacterial iron metabolism by Fur. However, most biologically important metals can be toxic at certain concentrations, and mechanisms of protection against metal toxicity are increasingly appreciated. Efflux transporters for other transition metals, with the exception of magnesium, have been described (Liesegang et al., 1993; Rensing et al., 1997; Stahler et al., 2006; Chao and Fu, 2004; Worlock and Smith, 2002; Long et al., 2012; Waters et al., 2011; Hoch et al., 2012; Guilhen et al., 2013; Delmar et al., 2013; Patel et al., 2014; Padilla-Benavides et al., 2014), and the recent characterization of STM3944 and IceT now adds iron efflux transporters to this list. The efflux of intracellular free iron during stresses that damage iron-containing proteins represents an important and underappreciated aspect of iron homeostasis in bacteria.
Acknowledgments
The authors would like to thank Joyce Karlinsey for helpful suggestions. F.C.F. received support from grants AI39557, AI44486, and AI77629, and E.R.F. is the recipient of fellowship AI112101 from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
References
- Almiron M, Link AJ, Furlong D, Kolter R. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 1992;6:2646–2654. doi: 10.1101/gad.6.12b.2646. [DOI] [PubMed] [Google Scholar]
- Altuvia S, Almiron M, Huisman G, Kolter R, Storz G. The dps promoter is activated by OxyR during growth and by IHF and sigma S in stationary phase. Mol Microbiol. 1994;13:265–272. doi: 10.1111/j.1365-2958.1994.tb00421.x. [DOI] [PubMed] [Google Scholar]
- Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM. Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem. 2008;13:1205–1218. doi: 10.1007/s00775-008-0404-5. [DOI] [PubMed] [Google Scholar]
- Anjem A, Imlay JA. Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J Biol Chem. 2012;287:15544–15556. doi: 10.1074/jbc.M111.330365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson PG, Barton CH. High level expression of Nramp1G169 in RAW264.7 cell transfectants: analysis of intracellular iron transport. Immunology. 1999;96:656–662. doi: 10.1046/j.1365-2567.1999.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagg A, Neilands JB. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987;26:5471–5477. doi: 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- Baranova N, Nikaido H. The baeSR two-component regulatory system activates transcription of the yegMNOB (mdtABCD) transporter gene cluster in Escherichia coli and increases its resistance to novobiocin and deoxycholate. J Bacteriol. 2002;184:4168–4176. doi: 10.1128/JB.184.15.4168-4176.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton CH, Biggs TE, Baker ST, Bowen H, Atkinson PG. Nramp1: a link between intracellular iron transport and innate resistance to intracellular pathogens. J Leukoc Biol. 1999;66:757–762. doi: 10.1002/jlb.66.5.757. [DOI] [PubMed] [Google Scholar]
- Baumgart M, Mustafi N, Krug A, Bott M. Deletion of the aconitase gene in Corynebacterium glutamicum causes strong selection pressure for secondary mutations inactivating citrate synthase. J Bacteriol. 2011;193:6864–6873. doi: 10.1128/JB.05465-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck G, Ellis TW, Habicht GS, Schluter SF, Marchalonis JJ. Evolution of the acute phase response: iron release by echinoderm (Asterias forbesi) coelomocytes, and cloning of an echinoderm ferritin molecule. Dev Comp Immunol. 2002;26:11–26. doi: 10.1016/s0145-305x(01)00051-9. [DOI] [PubMed] [Google Scholar]
- Beinert H, Holm RH, Munck E. Iron-sulfur clusters: nature’s modular, multipurpose structures. Science. 1997;277:653–659. doi: 10.1126/science.277.5326.653. [DOI] [PubMed] [Google Scholar]
- Blackwell JM, Searle S. Genetic regulation of macrophage activation: understanding the function of Nramp1 (=Ity/Lsh/Bcg) Immunol Lett. 1999;65:73–80. doi: 10.1016/s0165-2478(98)00127-8. [DOI] [PubMed] [Google Scholar]
- Bleuel C, Grosse C, Taudte N, Scherer J, Wesenberg D, Krauss GJ, Nies DH, Grass G. TolC is involved in enterobactin efflux across the outer membrane of Escherichia coli. J Bacteriol. 2005;187:6701–6707. doi: 10.1128/JB.187.19.6701-6707.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer E, Bergevin I, Malo D, Gros P, Cellier MF. Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect Immun. 2002;70:6032–6042. doi: 10.1128/IAI.70.11.6032-6042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen JJ, Spalding PB, Ward CG, Gutteridge JM. Hemochromatosis, iron and septicemia caused by Vibrio vulnificus. Arch Intern Med. 1991;151:1606–1609. [PubMed] [Google Scholar]
- Cartron ML, Maddocks S, Gillingham P, Craven CJ, Andrews SC. Feo--transport of ferrous iron into bacteria. Biometals. 2006;19:143–157. doi: 10.1007/s10534-006-0003-2. [DOI] [PubMed] [Google Scholar]
- Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13:509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellier MF, Courville P, Campion C. Nramp1 phagocyte intracellular metal withdrawal defense. Microbes Infect. 2007;9:1662–1670. doi: 10.1016/j.micinf.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Cerasi M, Ammendola S, Battistoni A. Competition for zinc binding in the host-pathogen interaction. Front Cell Infect Microbiol. 2013;3:108. doi: 10.3389/fcimb.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y, Fu D. Kinetic study of the antiport mechanism of an Escherichia coli zinc transporter, ZitB. J Biol Chem. 2004;279:12043–12050. doi: 10.1074/jbc.M313510200. [DOI] [PubMed] [Google Scholar]
- Charlesworth A, Georgieva T, Gospodov I, Law JH, Dunkov BC, Ralcheva N, Barillas-Mury C, Ralchev K, Kafatos FC. Isolation and properties of Drosophila melanogaster ferritin--molecular cloning of a cDNA that encodes one subunit, and localization of the gene on the third chromosome. Eur J Biochem. 1997;247:470–475. doi: 10.1111/j.1432-1033.1997.00470.x. [DOI] [PubMed] [Google Scholar]
- Chen OS, Hemenway S, Kaplan J. Genetic analysis of iron citrate toxicity in yeast: implications for mammalian iron homeostasis. Proc Natl Acad Sci U S A. 2002;99:16922–16927. doi: 10.1073/pnas.232392299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch ML, Castor M, Karlinsey JE, Kalhorn T, Fang FC. Biosynthesis and IroC-dependent export of the siderophore salmochelin are essential for virulence of Salmonella enterica serovar Typhimurium. Mol Microbiol. 2008;67:971–983. doi: 10.1111/j.1365-2958.2007.06089.x. [DOI] [PubMed] [Google Scholar]
- D’Autreaux B, Tucker NP, Dixon R, Spiro S. A non-haem iron centre in the transcription factor NorR senses nitric oxide. Nature. 2005;437:769–772. doi: 10.1038/nature03953. [DOI] [PubMed] [Google Scholar]
- Delmar JA, Su CC, Yu EW. Structural mechanisms of heavy-metal extrusion by the Cus efflux system. Biometals. 2013;26:593–607. doi: 10.1007/s10534-013-9628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Ochoa VE, Jellbauer S, Klaus S, Raffatellu M. Transition metal ions at the crossroads of mucosal immunity and microbial pathogenesis. Front Cell Infect Microbiol. 2014;4:2. doi: 10.3389/fcimb.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst JF, Bennett RL, Rothfield LI. Constitutive expression of the iron-enterochelin and ferrichrome uptake systems in a mutant strain of Salmonella typhimurium. J Bacteriol. 1978;135:928–934. doi: 10.1128/jb.135.3.928-934.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq MA, Pracheil TM, Dong Z, Xiao F, Liu Z. Mitochondrial DNA instability in cells lacking aconitase correlates with iron citrate toxicity. Oxid Med Cell Longev. 2013;2013:493536. doi: 10.1155/2013/493536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmy B, Songhet P, Slack EM, Muller AJ, Kremer M, Van Maele L, Cayet D, Heikenwalder M, Sirard JC, Hardt WD. NADPH oxidase deficient mice develop colitis and bacteremia upon infection with normally avirulent, TTSS-1-and TTSS-2-deficient Salmonella Typhimurium. PLoS One. 2013;8:e77204. doi: 10.1371/journal.pone.0077204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink RC, Evans MR, Porwollik S, Vazquez-Torres A, Jones-Carson J, Troxell B, Libby SJ, McClelland M, Hassan HM. FNR is a global regulator of virulence and anaerobic metabolism in Salmonella enterica serovar Typhimurium (ATCC 14028s) J Bacteriol. 2007;189:2262–2273. doi: 10.1128/JB.00726-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach MA, Lin H, Liu DR, Walsh CT. In vitro characterization of IroB, a pathogen-associated C-glycosyltransferase. Proc Natl Acad Sci U S A. 2005;102:571–576. doi: 10.1073/pnas.0408463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RE, Holden CC, Tomatsu S, Waheed A, Brunt EM, Britton RS, Bacon BR, Roopenian DC, Sly WS. Mouse strain differences determine severity of iron accumulation in Hfe knockout model of hereditary hemochromatosis. Proc Natl Acad Sci U S A. 2001;98:2707–2711. doi: 10.1073/pnas.051630898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- Frawley ER, Crouch ML, Bingham-Ramos LK, Robbins HF, Wang W, Wright GD, Fang FC. Iron and citrate export by a major facilitator superfamily pump regulates metabolism and stress resistance in Salmonella Typhimurium. Proc Natl Acad Sci U S A. 2013;110:12054–12059. doi: 10.1073/pnas.1218274110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche G, Nairz M, Libby SJ, Fang FC, Weiss G. Slc11a1 (Nramp1) impairs growth of Salmonella enterica serovar typhimurium in macrophages via stimulation of lipocalin-2 expression. J Leukoc Biol. 2012;92:353–359. doi: 10.1189/jlb.1111554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furrer JL, Sanders DN, Hook-Barnard IG, McIntosh MA. Export of the siderophore enterobactin in Escherichia coli: involvement of a 43 kDa membrane exporter. Mol Microbiol. 2002;44:1225–1234. doi: 10.1046/j.1365-2958.2002.02885.x. [DOI] [PubMed] [Google Scholar]
- Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93:1721–1741. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- Gort AS, Imlay JA. Balance between endogenous superoxide stress and antioxidant defenses. J Bacteriol. 1998;180:1402–1410. doi: 10.1128/jb.180.6.1402-1410.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Trageser M, Six S, Unden G, Guest JR. Characterization of the FNR protein of Escherichia coli, an iron-binding transcriptional regulator. Proc Biol Sci. 1991;244:137–144. doi: 10.1098/rspb.1991.0062. [DOI] [PubMed] [Google Scholar]
- Gruer MJ, Bradbury AJ, Guest JR. Construction and properties of aconitase mutants of Escherichia coli. Microbiology. 1997;143:1837–1846. doi: 10.1099/00221287-143-6-1837. [DOI] [PubMed] [Google Scholar]
- Guilhen C, Taha MK, Veyrier FJ. Role of transition metal exporters in virulence: the example of Neisseria meningitidis. Front Cell Infect Microbiol. 2013;3:102. doi: 10.3389/fcimb.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182:288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Hantke K, Nicholson G, Rabsch W, Winkelmann G. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc Natl Acad Sci U S A. 2003;100:3677–3682. doi: 10.1073/pnas.0737682100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo E, Demple B. An iron-sulfur center essential for transcriptional activation by the redox-sensing SoxR protein. EMBO J. 1994;13:138–146. doi: 10.1002/j.1460-2075.1994.tb06243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch E, Lin W, Chai J, Hershfinkel M, Fu D, Sekler I. Histidine pairing at the metal transport site of mammalian ZnT transporters controls Zn2+ over Cd2+ selectivity. Proc Natl Acad Sci U S A. 2012;109:7202–7207. doi: 10.1073/pnas.1200362109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Imlay JA. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J Biol Chem. 2007;282:929–937. doi: 10.1074/jbc.M607646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlinsey JE, Bang IS, Becker LA, Frawley ER, Porwollik S, Robbins HF, Thomas VC, Urbano R, McClelland M, Fang FC. The NsrR regulon in nitrosative stress resistance of Salmonella enterica serovar Typhimurium. Mol Microbiol. 2012;85:1179–1193. doi: 10.1111/j.1365-2958.2012.08167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyer K, Imlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci U S A. 1996;93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JE, Montross LK, Andrews NC. Genes that modify the hemochromatosis phenotype in mice. J Clin Invest. 2000;105:1209–1216. doi: 10.1172/JCI9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JE, Montross LK, Cohen DE, Fleming MD, Andrews NC. The C282Y mutation causing hereditary hemochromatosis does not produce a null allele. Blood. 1999;94:9–11. [PubMed] [Google Scholar]
- Liesegang H, Lemke K, Siddiqui RA, Schlegel HG. Characterization of the inducible nickel and cobalt resistance determinant cnr from pMOL28 of Alcaligenes eutrophus CH34. J Bacteriol. 1993;175:767–778. doi: 10.1128/jb.175.3.767-778.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long F, Su CC, Lei HT, Bolla JR, Do SV, Yu EW. Structure and mechanism of the tripartite CusCBA heavy-metal efflux complex. Philos Trans R Soc Lond B Biol Sci. 2012;367:1047–1058. doi: 10.1098/rstb.2011.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahren S, Schnell H, Braun V. Occurrence and regulation of the ferric citrate transport system in Escherichia coli B, Klebsiella pneumoniae, Enterobacter aerogenes, and Photorhabdus luminescens. Arch Microbiol. 2005;184:175–186. doi: 10.1007/s00203-005-0035-y. [DOI] [PubMed] [Google Scholar]
- Masse E, Gottesman S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 2002;99:4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moalem S, Weinberg ED, Percy ME. Hemochromatosis and the enigma of misplaced iron: implications for infectious disease and survival. Biometals. 2004;17:135–139. doi: 10.1023/b:biom.0000018375.20026.b3. [DOI] [PubMed] [Google Scholar]
- Nairz M, Fritsche G, Crouch ML, Barton HC, Fang FC, Weiss G. Slc11a1 limits intracellular growth of Salmonella enterica sv. Typhimurium by promoting macrophage immune effector functions and impairing bacterial iron acquisition. Cell Microbiol. 2009a;11:1365–1381. doi: 10.1111/j.1462-5822.2009.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M, Theurl I, Schroll A, Theurl M, Fritsche G, Lindner E, Seifert M, Crouch ML, Hantke K, Akira S, Fang FC, Weiss G. Absence of functional Hfe protects mice from invasive Salmonella enterica serovar Typhimurium infection via induction of lipocalin-2. Blood. 2009b;114:3642–3651. doi: 10.1182/blood-2009-05-223354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands JB. Siderophores: structure and function of microbial iron transport compounds. J Biol Chem. 1995;270:26723–26726. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- Nishino K, Honda T, Yamaguchi A. Genome-wide analyses of Escherichia coli gene expression responsive to the BaeSR two-component regulatory system. J Bacteriol. 2005;187:1763–1772. doi: 10.1128/JB.187.5.1763-1772.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olakanmi O, Schlesinger LS, Britigan BE. Hereditary hemochromatosis results in decreased iron acquisition and growth by Mycobacterium tuberculosis within human macrophages. J Leukoc Biol. 2007;81:195–204. doi: 10.1189/jlb.0606405. [DOI] [PubMed] [Google Scholar]
- Ong DS, Wang L, Zhu Y, Ho B, Ding JL. The response of ferritin to LPS and acute phase of Pseudomonas infection. J Endotoxin Res. 2005;11:267–280. doi: 10.1179/096805105X58698. [DOI] [PubMed] [Google Scholar]
- Padilla-Benavides T, George Thompson AM, McEvoy MM, Arguello JM. Mechanism of ATPase-Mediated Cu+ Export and Delivery to Periplasmic Chaperones: The Interaction of Escherichia coli CopA and CusF. J Biol Chem. 2014 doi: 10.1074/jbc.M114.577668. pii: jbc.M114.577668, epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, You X, Imlay JA. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx-mutants of Escherichia coli. Proc Natl Acad Sci U S A. 2005;102:9317–9322. doi: 10.1073/pnas.0502051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkila S, Parkkila AK, Waheed A, Britton RS, Zhou XY, Fleming RE, Tomatsu S, Bacon BR, Sly WS. Cell surface expression of HFE protein in epithelial cells, macrophages, and monocytes. Haematologica. 2000;85:340–345. [PubMed] [Google Scholar]
- Patel SJ, Padilla-Benavides T, Collins JM, Arguello JM. Functional diversity of five homologous Cu+-ATPases present in Sinorhizobium meliloti. Microbiology. 2014;160:1237–1251. doi: 10.1099/mic.0.079137-0. [DOI] [PubMed] [Google Scholar]
- Pomposiello PJ, Demple B. Identification of SoxS-regulated genes in Salmonella enterica serovar typhimurium. J Bacteriol. 2000;182:23–29. doi: 10.1128/jb.182.1.23-29.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puschmann M, Ganzoni AM. Increased resistance of iron-deficient mice to Salmonella infection. Infect Immun. 1977;17:663–664. doi: 10.1128/iai.17.3.663-664.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenee LE, Hermanas TM, Ciletti N, Louvel H, Miller NC, Elli D, Blaylock B, Mitchell A, Schroeder J, Krausz T, Kanabrocki J, Schneewind O. Hereditary hemochromatosis restores the virulence of plague vaccine strains. J Infect Dis. 2012;206:1050–1058. doi: 10.1093/infdis/jis433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, Mak TW, Tsolis RM, Bevins CL, Solnick JV, Dandekar S, Baumler AJ. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 2009;5:476–486. doi: 10.1016/j.chom.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing C, Mitra B, Rosen BP. The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc Natl Acad Sci U S A. 1997;94:14326–14331. doi: 10.1073/pnas.94.26.14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawatzki G, Hoffmann FA, Kubanek B. Acute iron overload in mice: pathogenesis of Salmonella typhimurium infection. Infect Immun. 1983;39:659–665. doi: 10.1128/iai.39.2.659-665.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible UE, Kaufmann SH. A nutritive view on the host-pathogen interplay. Trends Microbiol. 2005;13:373–380. doi: 10.1016/j.tim.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Schwartz CJ, Giel JL, Patschkowski T, Luther C, Ruzicka FJ, Beinert H, Kiley PJ. IscR, an Fe-S cluster-containing transcription factor, represses expression of Escherichia coli genes encoding Fe-S cluster assembly proteins. Proc Natl Acad Sci U S A. 2001;98:14895–14900. doi: 10.1073/pnas.251550898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaver LC, Imlay JA. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J Bacteriol. 2001;183:7182–7189. doi: 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- Simonsen KT, Moller-Jensen J, Kristensen AR, Andersen JS, Riddle DL, Kallipolitis BH. Quantitative proteomics identifies ferritin in the innate immune response of C. elegans. Virulence. 2011;2:120–130. doi: 10.4161/viru.2.2.15270. [DOI] [PubMed] [Google Scholar]
- Sobota JM, Imlay JA. Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc Natl Acad Sci U S A. 2011;108:5402–5407. doi: 10.1073/pnas.1100410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahler FN, Odenbreit S, Haas R, Wilrich J, Van Vliet AH, Kusters JG, Kist M, Bereswill S. The novel Helicobacter pylori CznABC metal efflux pump is required for cadmium, zinc, and nickel resistance, urease modulation, and gastric colonization. Infect Immun. 2006;74:3845–3852. doi: 10.1128/IAI.02025-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurl I, Fritsche G, Ludwiczek S, Garimorth K, Bellmann-Weiler R, Weiss G. The macrophage: a cellular factory at the interphase between iron and immunity for the control of infections. Biometals. 2005;18:359–367. doi: 10.1007/s10534-005-3710-1. [DOI] [PubMed] [Google Scholar]
- Touati D, Jacques M, Tardat B, Bouchard L, Despied S. Lethal oxidative damage and mutagenesis are generated by iron in delta fur mutants of Escherichia coli: protective role of superoxide dismutase. J Bacteriol. 1995;177:2305–2314. doi: 10.1128/jb.177.9.2305-2314.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker NP, Hicks MG, Clarke TA, Crack JC, Chandra G, Le Brun NE, Dixon R, Hutchings MI. The transcriptional repressor protein NsrR senses nitric oxide directly via a [2Fe-2S] cluster. PLoS One. 2008;3:e3623. doi: 10.1371/journal.pone.0003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese S, Tang Y, Imlay JA. Contrasting sensitivities of Escherichia coli aconitases A and B to oxidation and iron depletion. J Bacteriol. 2003;185:221–230. doi: 10.1128/JB.185.1.221-230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayudhan J, Castor M, Richardson A, Main-Hester KL, Fang FC. The role of ferritins in the physiology of Salmonella enterica sv. Typhimurium: a unique role for ferritin B in iron-sulphur cluster repair and virulence. Mol Microbiol. 2007;63:1495–1507. doi: 10.1111/j.1365-2958.2007.05600.x. [DOI] [PubMed] [Google Scholar]
- Velayudhan J, Karlinsey JE, Frawley ER, Becker LA, Nartea M, Fang FC. Distinct Roles of the Salmonella enterica Serovar Typhimurium CyaY and YggX Proteins in the Biosynthesis and Repair of Iron-Sulfur Clusters. Infect Immun. 2014;82:1390–1401. doi: 10.1128/IAI.01022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S, Gros P, Skamene E. Natural resistance to infection with intracellular parasites: molecular genetics identifies Nramp1 as the Bcg/Ity/Lsh locus. J Leukoc Biol. 1995a;58:382–390. doi: 10.1002/jlb.58.4.382. [DOI] [PubMed] [Google Scholar]
- Vidal S, Tremblay ML, Govoni G, Gauthier S, Sebastiani G, Malo D, Skamene E, Olivier M, Jothy S, Gros P. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J Exp Med. 1995b;182:655–666. doi: 10.1084/jem.182.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollier PH, Nguyen KT, Minas W, Folcher M, Dale GE, Thompson CJ. Roles of aconitase in growth, metabolism, and morphological differentiation of Streptomyces coelicolor. J Bacteriol. 2001;183:3193–3203. doi: 10.1128/JB.183.10.3193-3203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagegg W, Braun V. Ferric citrate transport in Escherichia coli requires outer membrane receptor protein fecA. J Bacteriol. 1981;145:156–163. doi: 10.1128/jb.145.1.156-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A, Parkkila S, Zhou XY, Tomatsu S, Tsuchihashi Z, Feder JN, Schatzman RC, Britton RS, Bacon BR, Sly WS. Hereditary hemochromatosis: effects of C282Y and H63D mutations on association with beta2-microglobulin, intracellular processing, and cell surface expression of the HFE protein in COS-7 cells. Proc Natl Acad Sci U S A. 1997;94:12384–12389. doi: 10.1073/pnas.94.23.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron KJ, Rutherford JC, Ford D, Robinson NJ. Metalloproteins and metal sensing. Nature. 2009;460:823–830. doi: 10.1038/nature08300. [DOI] [PubMed] [Google Scholar]
- Warszawska JM, Gawish R, Sharif O, Sigel S, Doninger B, Lakovits K, Mesteri I, Nairz M, Boon L, Spiel A, Fuhrmann V, Strobl M, Müller M, Schenk P, Weiss G, Knapp S. Lipocalin 2 deactivates macrophages and worsens pneumonia outcomes. J Clin Invest. 2013;123:3363–3372. doi: 10.1172/JCI67911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters LS, Sandoval M, Storz G. The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis. J Bacteriol. 2011;193:5887–5897. doi: 10.1128/JB.05872-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg ED. Microbial pathogens with impaired ability to acquire host iron. Biometals. 2000;13:85–89. doi: 10.1023/a:1009293500209. [DOI] [PubMed] [Google Scholar]
- Weiss G, Schett G. Anaemia in inflammatory rheumatic diseases. Nat Rev Rheumatol. 2013;9:205–215. doi: 10.1038/nrrheum.2012.183. [DOI] [PubMed] [Google Scholar]
- Woodmansee AN, Imlay JA. A mechanism by which nitric oxide accelerates the rate of oxidative DNA damage in Escherichia coli. Mol Microbiol. 2003;49:11–22. doi: 10.1046/j.1365-2958.2003.03530.x. [DOI] [PubMed] [Google Scholar]
- Worlock AJ, Smith RL. ZntB is a novel Zn2+ transporter in Salmonella enterica serovar Typhimurium. J Bacteriol. 2002;184:4369–4373. doi: 10.1128/JB.184.16.4369-4373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AC, Simpson LM, Oliver JD. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect Immun. 1981;34:503–507. doi: 10.1128/iai.34.2.503-507.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharik ML, Cullen VL, Fung AM, Libby SJ, Kujat Choy SL, Coburn B, Kehres DG, Maguire ME, Fang FC, Finlay BB. The Salmonella enterica serovar typhimurium divalent cation transport systems MntH and SitABCD are essential for virulence in an Nramp1G169 murine typhoid model. Infect Immun. 2004;72:5522–5525. doi: 10.1128/IAI.72.9.5522-5525.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Ceci P, Ilari A, Giangiacomo L, Laue TM, Chiancone E, Chasteen ND. Iron and hydrogen peroxide detoxification properties of DNA-binding protein from starved cells. A ferritin-like DNA-binding protein of Escherichia coli. J Biol Chem. 2002;277:27689–27696. doi: 10.1074/jbc.M202094200. [DOI] [PubMed] [Google Scholar]
- Zheng M, Doan B, Schneider TD, Storz G. OxyR and SoxRS regulation of fur. J Bacteriol. 1999;181:4639–4643. doi: 10.1128/jb.181.15.4639-4643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Hardt WD, Galan JE. Salmonella typhimurium encodes a putative iron transport system within the centisome 63 pathogenicity island. Infect Immun. 1999;67:1974–1981. doi: 10.1128/iai.67.4.1974-1981.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XY, Tomatsu S, Fleming RE, Parkkila S, Waheed A, Jiang J, Fei Y, Brunt EM, Ruddy DA, Prass CE, Schatzman RC, O’Neill R, Britton RS, Bacon BR, Sly WS. HFE gene knockout produces mouse model of hereditary hemochromatosis. Proc Natl Acad Sci U S A. 1998;95:2492–2497. doi: 10.1073/pnas.95.5.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]