Abstract
Objectives
Variation in care within and across geographic areas remains poorly understood. The goal of this paper is to examine whether physician social networks—as defined by shared patients—are associated with rates of complications following radical prostatectomy.
Methods
In five cities, we constructed networks of physicians based on their shared patients in 2004–2005 SEER-Medicare data. From these networks, we identified subgroups of urologists who most frequently shared patients with one another. Among men with localized prostate cancer who underwent radical prostatectomy, we used multilevel analysis with generalized linear mixed effect models to examine whether physician network structure—along with specific characteristics of the network subgroups—was associated with rates of 30-day and late urinary complications, and long term incontinence after accounting for patient-level sociodemographic, clinical factors, and urologist patient volume.
Results
Networks included 2677 men in 5 cities who underwent radical prostatectomy. The unadjusted rate of 30-day surgical complications varied across network subgroups from an 18.8 percentage point difference in the rate of complications across network subgroups in City 1 to 26.9 percentage point difference in City 5. Large differences in unadjusted rates of late urinary complications and long term incontinence across subgroups were similarly found. Network subgroup characteristics—average urologist centrality and patient racial composition—were significantly associated with rates of surgical complications.
Conclusions
Analysis of physician networks of SEER-Medicare data provides insight into observed variation in rates of complications for localized prostate cancer. If validated, such approaches may be used to target future quality improvement interventions.
Keywords: cancer, claims data, health services, outcomes research
INTRODUCTION
While variation in care across different geographic areas has been widely described since the 1970s,1 there has been increased recognition of variation within particular locales.2 The potential mechanisms underlying this variation between and across areas remain poorly understood. Physician networks based on shared patients may be one tool to help better delineate variation in care. In patient-sharing networks, physicians are considered connected to one another if they provide care to the same patient.3 Patient-sharing networks signal connections between physicians such as those due to practice structure and hospital affiliation.4–6 Importantly, they also represent informal connections between physicians including referral patterns and advice seeking.3 By reflecting both formal and informal connections that may shape clinical practice, physician patient-sharing networks may provide insight into variation in care.
Physician patient-sharing networks have been associated with the costs and intensity of medical care within geographic areas.7 In the setting of prostate cancer, physician patient-sharing networks have been associated with the likelihood of receiving a radical prostatectomy for localized disease within 3 cities.6 We seek to extend previous work by exploring whether physician patient-sharing networks are associated with variation in complications following radical prostatectomy for prostate cancer.
Complications following radical prostatectomy are an important case study. In the US, an estimated 238,590 men will receive a diagnosis of prostate cancer in 2013.8 The decision to undergo radical prostatectomy—a common treatment modality for men with localized disease9—is preference-sensitive. The surgery is associated with surgical complications in the month after surgery as well as longer term urinary incontinence and erectile dysfunction.10–12 Though research has demonstrated that men who undergo radical prostatectomy by high volume surgeons and at high volume institutions are less likely to have complications,13–15 relatively little is known about the reasons underlying variations in the rates of complications.16,17
Within five cities, we construct patient-sharing networks comprised of urologists, primary care providers, and radiation oncologists who care for prostate cancer patients. We then examine whether the network structure is associated with different rates of complications following radical prostatectomy. Our underlying hypothesis is that patients seen by providers who more frequently share patients with one another may have similar rates of complications, after adjusting for patient clinical and sociodemographic characteristics.
We further explore whether particular characteristics of these network subgroups are associated with differences in rates of complications. We focus on two network characteristics—the average importance (centrality) of the doctors and the proportion of minority patients in the network subgroups. Doctors who are important in the network structure may have achieved their prestige by providing higher quality of care and may play an important role in shaping local norms and behaviors.18 We hypothesized that patients treated by network subgroups with higher levels of importance would have lower likelihood of complications. The disparities literature has shown that health care providers19 and institutions20,21 who treat a disproportionate share of minority patients may have greater difficulty providing high quality care. Thus, patients treated by network subgroups with a high proportion of minority patients were hypothesized to have lower quality of care.
METHODS
Study Design
The study was a retrospective, observational cohort study using registry and administrative claims data from the Surveillance, Epidemiology and End Results (SEER)-Medicare database. The study was approved by the institutional review boards at the University of Pennsylvania and Johns Hopkins University School of Medicine.
Data Sources
The SEER-Medicare database links patient demographic and tumor-specific data collected by SEER cancer registries to longitudinal health care claims for Medicare enrollees.22 Data on physicians’ specialties was available from the Medicare Physician Identification and Eligibility Registry (MPIER file) linked through Unique Provider Identification Numbers (UPINs).
Study Population
We identified men age 65 years or older living in five cities with prostate cancer diagnosed between January 1, 2004 and December 31, 2005 in SEER with follow up through December 31, 2006 in Medicare. Two years of data were analyzed to allow for adequate connectivity of the networks based on our preliminary analyses. Cities were defined based on US Census definitions of Combined Statistical Areas (CSAs), which refer to cities and the surrounding areas that are linked by economic and social activity (http://www.census.gov/population/www/metroareas/metrodef.html). CSAs represent a larger geographic region than health referral regions (HRRs), as preliminary analyses revealed that urologists cared for high numbers of patients from multiple, adjacent HRRs. Cities were included if they had at least 200 patients who underwent prostatectomy across the two years and SEER-Medicare included the great majority of the geographic area. We do not present city names to ensure patient and doctor confidentiality.
Data on patients with incomplete Medicare records (i.e. those enrolled in health maintenance organizations or not enrolled in fee-for-service Medicare program) were excluded. For the construction of the patient-sharing networks and definition of network subgroups (see below), we included all men without metastatic disease (N=13,465). We then used these network subgroups in analyses that examined prostatectomy complications for a more homogeneous patient cohort. Specifically, we limited the sample to men with AJCC 6th edition stage 2 and 3 disease who underwent radical prostatectomy (N=2,974). Prostatectomy was identified from Medicare inpatient, outpatient, and physician/supplier component files as described previously.23 We excluded those with node positive disease (N=59), unknown Gleason grade (N=13), men who could not be matched to their surgical urologist (N=225), and, due to their small sample size, men with stage 1 disease (N=107). The final analytic sample size was 2,677.
Definition of Variables
Complications
Complications were defined according to the work of Begg and colleagues using ICD-9 diagnosis and procedural codes.13 Thirty-day surgical complications included cardiac, respiratory, vascular, wound, genitourinary, miscellaneous medical, miscellaneous surgical, and blood transfusion complications. Late urinary complications were defined as occurring from 31 to 365 days following surgery and included bladder neck obstruction, uretheral stricture, intestinal fistula, lymphocele, cystitis/bleeding, and definitive incontinence repair. Long term incontinence was defined as occurring 18 months or more after the surgery.24
Explanatory variables
Gleason grade was categorized as <7, 7, and 8 to 10. PSA at the time of diagnosis was classified as 4 ng/ml or less, >4 to <10, 10 or greater, or unknown. Patient comorbidities were identified by classifying all available inpatient and outpatient Medicare claims for the 90 day interval preceding prostate cancer diagnosis into 46 categories,25 and, for clarity, reported as 0, 1, or ≥2. Race was classified from both SEER and Medicare sources, and individuals were classified as white if they did not have a classification of black, Hispanic, or Asian in either data file; or non-white. Area-level U.S. Census information was used as a proxy for individual measures of socioeconomic status. Men were linked to their Census tract and, when not available, ZIP Code to determine median income, which was categorized into quartiles based on the sample distribution in each city. Urologist surgical volume was defined as the number of radical prostatectomies performed over the two year period, with high volume defined as the top quartile in a given city (cut-point for high volume ranged from 16 to 41).13
Network creation
We created networks for each city in which doctors were connected to one another via shared patients. From each network, we then sought to identify subgroups of doctors who frequently share patients with one another (as defined below).
Provider definitions
We focused on following doctors who are most likely to be involved in the patients’ prostate cancer care and thus most likely to directly or indirectly interact. These include the surgical urologist (defined as the urologist who billed for the prostatectomy), diagnosing urologist (defined as the urologist who billed for a claim on or closest to the date of the patient’s diagnosis), primary care provider,26 plurality provider (the doctor, regardless of specialty, who billed for the greatest number of claims in the 12 months prior to diagnosis),26 and radiation oncologist (among men who underwent external beam or brachytherapy, the provider who performed the clinical planning and simulation). Primary care providers were included in the network structure based on their role in prostate cancer screening (typically through prostate specific antigen testing) and referral for diagnosis which may help shape the network structure.
Network construction
Networks were constructed using data from all patients without metastatic disease and all of their providers as described above. In these networks, providers (represented by vertices or nodes) were linked to one another by shared patients (termed edges). The strength of connection between doctors (called edge weight) was the number of shared patients. Based on our preliminary analyses and published data,3 we required that providers be connected by at least 2 shared patients. Network construction was performed in R version 2.12.0 using the igraph software package.27
Network subgroup definition
From these networks, we identified network subgroups. Subgroups define doctors who are more densely connected with one another via shared patients than to doctors outside the subgroup. Specifically, we used the Girvan-Newman algorithm to define network subgroups (also called ‘community-structures’).28 In this approach, a betweenness score for each edge was calculated. The betweenness score was proportional to the number of shortest paths that ran along each edge, where the shortest path represented the most direct route between a pair of providers and accounted for edge weight. The algorithm then removed the edge with the highest betweenness, thereby separating the network into 2 smaller subgroups. Betweenness scores were then recalculated and the process continued iteratively until a goodness-of-fit test (modularity) was optimized.29 Each doctor was assigned to a single, mutually exclusive subgroup. Patients, however, may have doctors that were assigned to multiple subgroups. For our analyses, patients were assigned to the network subgroup of the urologist who performed their prostatectomy. We focused our investigation on network subgroups with at least 20 patients in order to highlight the potential importance of larger subgroups. Patients in network subgroups with fewer than 20 patients were retained in the analyses in a dummy category.
Network subgroup characteristics
For each network subgroup, we averaged the degree of the treating urologists where degree is defined as the number of other physicians with whom he/she shares patients. This measure was dichotomized for interpretability with network subgroups defined as having a high average degree if they were in the top 75% of the distribution in each city. The proportion of non-white patients was calculated for each network subgroup. Network subgroups were classified as having a high percentage of non-white patients if the percentage was greater than the average percent of non-white patients in the city.
Hospital assignment
Patients were assigned to the hospital where they received their radical prostatectomy.
Statistical analyses
After presenting univavariate analyses, we determined whether network subgroup was associated with complications in multivariable analyses. To account for the correlation structure of patients being nested within urologists and urologists being nested within network subgroups, we used generalized linear mixed effect models for binary outcomes,30 where complication was the dependent variable and the network subgroups was an independent variable. We included fixed effects for patient-level covariates (age, comorbidity, Gleason grade, tumor stage, PSA, area-level median income, race classified as either white or non-white), for the urologist-level covariate (provider volume), and for subgroups, and random effects for urologists. Analyses were performed separately for each city and for each type of complication. To facilitate interpretation of these models, we generated the predicted probability and 95% confidence interval for each complication and in each subgroup. We used the Bonferroni correction to adjust for multiple testing in the five cities; thus tests were considered statistical significant at P< 0.01. To examine the percent of variation explained by subgroups, we estimated the intra-subgroup correlation coefficients (ICC) based on the logit scale of the complication rate.30
In the second series of analyses, we examined whether network subgroup characteristics were associated with odds of complications. For each complication, all cities were included in a single model, and we used a generalized linear mixed effects model with fixed effects for patient-level covariates, the urologist-level covariate, network subgroup characteristics, and cities and random effects for urologists and network subgroups. The subgroup level predictors of interest were high average degree of urologists in the subgroup and a high percent of non-white patients.
In sensitivity analyses we sought to determine whether network subgroups were significantly associated with complications after accounting for hospital assignment. In these models, we considered hospital as a random effect. Data analysis was performed using SAS (version 9.3, Cary, NC).
RESULTS
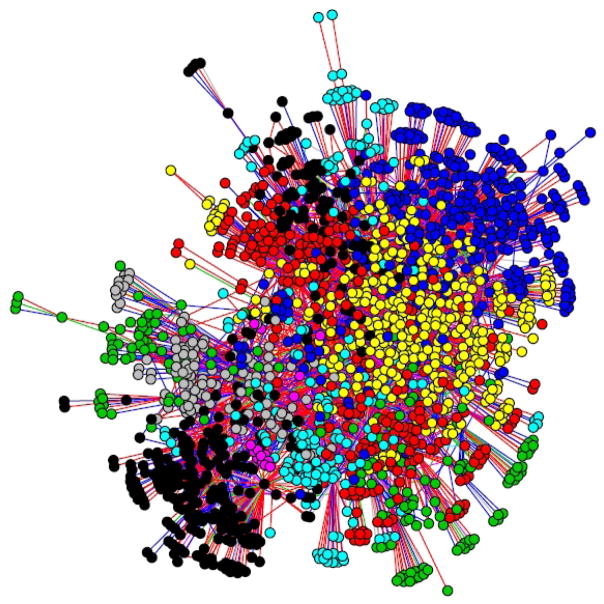
There were 2677 men in 5 cities with localized prostate cancer that underwent radical prostatectomy in the analyses. The sample in each city ranged in size from 271 to 12224 men. The sociodemographic characteristics of the sample are presented in Table 1. Across the cities, we observed 30-day surgical complications in 23.4% of men; 43.8% had late urinary complications, and 8.3% had long-term urinary incontinence. Figure 1 presents an example of a network formed by doctors who share patients with one another.
Table 1.
Descriptive statistics for overall sample and each city, 2004–2005
| Total | City 1 | City 2 | City 3 | City 4 | City 5 | |
|---|---|---|---|---|---|---|
|
| ||||||
| N(%) | 2677(100) | 448 | 1224 | 270 | 356 | 379 |
| Age | ||||||
| 65–69 | 1397 (52.2) | 273(60.9) | 620(50.7) | 131(48.5) | 190(53.4) | 183(48.3) |
| 70–74 | 901(33.7) | 129(28.8) | 427(35.0) | 87(32.2) | 120(33.7) | 138(36.4) |
| 75+ | 379(14.2) | 46(10.2) | 177(14.4) | 52(19.3) | 46(12.9) | 58(15.3) |
| Comorbidity | ||||||
| 0 | 841(31.4) | 117(26.1) | 351(28.7) | 97(35.9) | 135(37.9) | 141(37.2) |
| 1 | 846(31.6) | 143(31.9) | 347(28.4) | 106(39.3) | 122(34.3) | 128(32.8) |
| 2+ | 990(37.0) | 188(42.0) | 526(43.0) | 67(24.8) | 99(27.8) | 110(29.0) |
| Year of diagnosis | ||||||
| 2004 | 1453(54.3) | 235(52.5) | 661(54.0) | 155(57.4) | 215(60.4) | 187(49.3) |
| 2005 | 1224(45.7) | 213(47.5) | 563(46.0) | 115(42.6) | 141(39.6) | 192(50.7) |
| Gleason grade | ||||||
| <7 | 1096(40.9) | 178(39.7) | 519(42.4) | 137(50.7) | 127(35.7) | 135(35.6) |
| 7 | 1265(47.3) | 233(52.0) | 556(45.4) | 111(41.1) | 176(49.4) | 191(49.9) |
| 8 to 10 | 316(11.8) | 37(8.3) | 149(12.2) | 22(8.2) | 53(14.9) | 55(14.5) |
| Tumor stage | ||||||
| 2 | 2189(81.8) | 364(81.3) | 998(81.5) | 226(83.7) | 300(84.3) | 301(79.4) |
| 3 | 488(18.2) | 84(18.8) | 226(18.5) | 44(16.3) | 56(15.7) | 78(20.6) |
| PSA status | ||||||
| 10+ | 419(15.7) | 54(12.1) | 193(15.8) | 40(14.8) | 69(19.4) | 63(16.6) |
| 4.1 to 9.9 | 1521(56.8) | 244(54.5) | 706(57.7) | 154(57.0) | 206(57.9) | 211(55.7) |
| <=4 | 361(13.5) | 73(16.3) | 155(12.7) | 39(14.4) | 41(11.5) | 53(14.0) |
| Unknown | 376(14.1) | 77(17.2) | 170(13.9) | 37(15.7) | 40(11.2) | 52(13.7) |
| Marital status | ||||||
| Married | 2093(78.2) | 268(59.8) | 986(80.6) | 231(85.6) | 293(82.3) | 315(83.1) |
| Unmarried | 438(16.4) | 69(15.4) | 226(18.5) | 25(9.3) | >52(>14) | >54(>14) |
| Unknown | 146(5.5) | 111(24.8) | 12(1.0) | 14(5.2) | <11(<4)* | <11(<3)* |
| 30-day surgical complication | 625(23.4) | 84(18.8) | 310(25.3) | 51(18.9) | 78(21.9) | 102(26.9) |
| Late urinary complication | 1173(43.8) | 154(34.4) | 631(51.6) | 102(37.8) | 113(31.7) | 173(45.6) |
| Long-term incontinence | 222(8.3) | 24(5.4) | 124(10.1) | 18(6.7) | 29(8.2) | 27(7.1) |
Cell sizes masked due to small sample sizes and SEER-Medicare restrictions regarding confidentiality. Racial and income composition of subgroups not shown in table due to confidentiality restrictions
FIGURE 1.
An example patient-sharing network for City 1. The 1904 physicians are represented by circles; they are connected by shared patients represented by lines and including all patients without metastatic cancer regardless of whether or not they received surgery. Network subgroups are shaded different colors. The location of physicians is determined by social distance using the Fruchterman-Reingold layout.
In each city, subgroups of doctors who were densely connected to one another were identified (Table 2). Cities had from 4 to 17 large network subgroups (with large defined as at least 20 patients). The mean number of patients per large network subgroup ranged from 34 in City 4 to 68 patients in City 2. The mean number of urologists per network subgroup ranged from 3.8 urologists in City 3 to 10.5 in City 2. On average, there were between 2.8 (City 3) to 6.8 (City 2) hospitals represented in each network subgroup.
Table 2.
Descriptive characteristics of the network structure in 5 cities, 2004–2005
| City 1 | City 2 | City 3 | City 4 | City 5 | |
|---|---|---|---|---|---|
|
| |||||
| N patients | 448 | 1224 | 270 | 356 | 379 |
| N urologists | 80 | 206 | 29 | 92 | 64 |
| N large network subgroups* | 7 | 17 | 4 | 9 | 9 |
| Average number of patients per large network subgroup (range) | 57.4 (21–148) | 68.4 (20–203) | 44.3 (30–56) | 34.0 (20–49) | 37.6 (21–57) |
| Average number of urologists per large network subgroup (range) | 8.7(3–22) | 10.5(2–25) | 3.8(1–8) | 7.2(4–12) | 5.9(3–13) |
| Average number of hospitals per large network subgroup (range) | 4.7(1–9) | 6.8(1–15) | 2.8(1–6) | 4.1(2–7) | 3.2(1–6) |
Large network subgroups are defined as having at least 20 patients.
Table 3 presents the results of the unadjusted rates of complications across network subgroups. The unadjusted rate of 30-day surgical complications varied across network subgroups and by city from a 20.7 percentage point difference in the rate of complications across network subgroups in City 4 to 31.2 percentage point difference in City 5. Differences in the rates of late urinary complications ranged from 32.1 to 72.5 percentage points across network subgroups within cities, and differences in the rates of long term incontinence ranged from 6.7 to 36.8 percentage points. These reached statistical significance for 30-day surgical complications in City 3, for late urinary complications in Cities 2, 3, and 5, and for long-term incontinence in City 2.
Table 3.
Average rates of complications and chi-squared test for variation across network subgroups within cities, 2004–2005
| City 1 | City 2 | City 3 | City 4 | City 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| P-value | P-value | P-value | P-value | P-value | ||||||
| 30-day surgical Complications | 18.8 % | 25.3 % | 18.9 % | 21.9 % | 26.9 % | |||||
| Range* | 8.8–31.7 | 0.049 | 11.1–37.7 | 0.307 | 5.0–26.9 | 0.005 | 12.0–32.7 | 0.489 | 14.3–45.5 | 0.047 |
| Late urinary Complications | 34.4 | 51.6 | 37.8 | 31.7 | 45.7 | |||||
| Range* | 25.0–57.1 | 0.242 | 31.3–91.2 | <0.001 | 10.0–82.5 | <0.001 | 15.8–48.8 | 0.031 | 17.1–71.4 | <0.001 |
| Long-term incontinence | 5.4 | 10.1 | 6.7 | 8.2 | 7.1 | |||||
| Range* | 0.0–19.5 | 0.023 | 0.0–36.8 | <0.001 | 3.3–10.0 | 0.803 | 0.0–12.2 | 0.413 | 0.0–14.3 | 0.609 |
Indicates range across subgroups, P<0.01 is considered significant after correcting for 5 different cities.
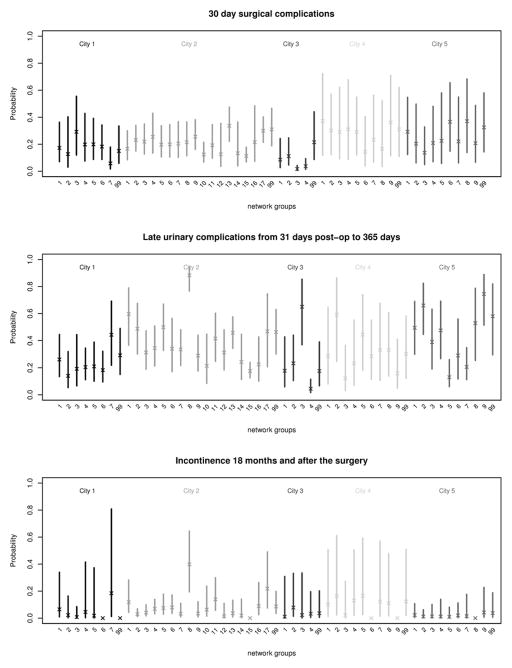
Figure 2 presents the estimated mean complication rates for men receiving their prostatectomy from urologists in the different network subgroups, controlling for patient-level characteristics and provider volumes. The results show varying rates of complications across network subgroups within each city, though with overlapping confidence intervals. From the regression models, the overall network subgroup term was significant (P<0.001) for 30-day surgical complications in Cities 2 and 3; there were significant differences in rates of late urinary complications among network subgroups in Cities 2, 3 and 5; and for long term incontinence, there were significant differences among network subgroups in Cities 1, 2, 4, and 5 (ICC of 14.7, 13.3, and 0 and 0%, respectively). The ICCs indicate that network subgroups explained up to 13.5% in 30-day complications (City 3), 31.3% in late urinary complications (City 3), and 14.7% of long term incontinence (City 1).
FIGURE 2.
Estimated mean rates of complications with 95% confidence intervals by network subgroup across 5 cities. The subgroups are ordered by size (number of patients) from biggest to smallest. Subgroup number 99 in each city represents patients who were assigned to subgroups with less than 20 patients; these patients were retained as a dummy category. Marginal probabilities were estimated based on the population-average model49 given the following covariates: age=65–69 yrs, comorbidity=2+, Gleason grade=7, tumor stage =2, PSA =4.1–9.9, median area income=second lowest quartile, white race, marital status=married, and provider surgical volume=low.
In models that examined characteristics of network subgroups, average urologist degree was significantly associated with 30-day surgical complications and long term incontinence (p-values for overall subgroup term 0.010 and <0.0001 respectively), and the proportion of non-white patients in a network subgroup was significantly associated with long term incontinence (p-value <0.001). Not all associations with network characteristics, however, were in the same directions. For example, average urologist degree was associated with significantly lower 30-day complications in City 3 but higher long term incontinence in City 4 (Appendix Table 1 in Supplemental Materials at: XXX).
In analyses where we adjusted for clustering by hospital, we continued to observe significant associations between network subgroups and complications. In these models, hospitals accounted for up to 11.2% of variations in 30-day surgical complications (City 3), 13.8% for late urinary complications (City 3), and 12.3% for long-term incontinence (City 2).
DISCUSSION
Within each city, it is possible to use network analytic techniques to identify subgroups of doctors who are more likely to share patients with one another. These network subgroups may explain a portion of the observed variation in surgical complications following radical prostatectomy, and, despite the small sample sizes, comparisons among some subgroups remained significant after accounting for patient-level characteristics and adjusting for clustering by urologist. The findings, although exploratory in nature, raise a number of important questions.
First, what might network subgroups represent in terms of health care delivery? Prior work on quality of cancer care has tended to focus on the individual provider (e.g. provider volume)13–15 or on features of the hospital system (e.g. percent minority patients,31 status as a National Cancer Institute designated cancer center,32,33 and hospital volume14). In contrast, network subgroups are defined by doctors who are tightly ‘connected’ to one another through shared patients and may represent doctors who are formally connected to one (e.g. through practice structure, hospital affiliation) as well as informally (e.g. by referral patterns and advice seeking).3,6
Although associated with practice structure, network subgroups typically encompass doctors from multiple practices.6 SEER-Medicare data remove tax identification numbers which are sometimes used to group physicians into practices,26 thus having alternative techniques to group providers may be useful. Similarly, we find that while patients who received their surgery at the same hospital tended to be in the same subgroup, subgroups often encompass patients seen at multiple hospitals. Moreover, with a large proportion of cancer care occurring in the outpatient setting, it may be challenging to group providers into hospital-based networks.34 The current strategy may represent a promising approach to delineating naturally occurring networks of physicians, reflecting the ways that health care is delivered in practice.4 As discussed further in the limitation section, however, the optimal approach to constructing patient sharing networks has not been determined; the approach may vary across cancer types, datasets and outcomes, meriting future validation.
Second, why might network subgroups be associated with rates of surgical complications? One explanation may be that doctors of higher or lower quality tend to be connected to one another.35 Prior work has suggested that ‘high status’ doctors may be most likely to refer to one another.36 Thus, high quality doctors may be more likely to share patients with other high quality doctors and be placed in the same subgroup.
An overlapping explanation is that the practice style of one physician in a network subgroup may impact (either positively or negatively) the style of another physician in the subgroup.37 Literature on the diffusion of innovation suggests that doctors who are important in the network structure may have a large impact on medical adoption.38,39 This diffusion may occur through direct care of a particular patient or through informal consultations.40 It is plausible that these may affect technical aspects of care and specific surgical techniques that are employed. We would expect this effect to be most pronounced for urologists who are directly connected to one another. Urologists who are connected through PCPs or other providers, however, may also induce behavior change through cooperation (e.g., through institutional structures) or competition (e.g. for referrals from PCPs).
A related explanation is, to the extent that network subgroups reflect practice structure and hospital affiliation, rates of complications may stem from the underlying context of differing institutional resources and practices. The context of network subgroups may further be shaped by participation in different insurance networks and by other area-level factors.
Selection effects may occur at multiple levels and help explain the observed associations between network subgroups and complications. Patients may select physicians (and network subgroups) with varying propensities to screen for prostate cancer, diagnose the disease, and operate on surgical candidates.6 A large portion of patients with prostate cancer who undergo radical prostatectomy have a different diagnosing and treating urologist;41 it is uncertain the extent to which this represents patient choice, provider referrals patterns, and/or other factors.
Third, why were rates of complications not consistent among the subgroups? Within each city, specific subgroups did not invariably have high rates of complications across the three complications, and the proportion of variance explained by subgroups was relatively modest. The results suggest that the importance of subgroups in explaining radical prostatectomy complications may be one of many factors (e.g. technical skill of the surgeon), and the potential mechanisms through which networks are associated with health may vary depending on the particular type of complication. The findings were not consistent across the cities, potentially indicating that other aspects of the local health care delivery and/or network structure may help shape these associations.
Fourth, what characteristics of specific subgroups are associated with quality of care? We found some evidence that average urologist degree, a marker of importance, was associated with complications as was the subgroup racial composition; however, within cities, not all associations were in the expected directions. The reasons for these divergent findings are uncertain and suggest the need for additional research into the characteristics of physician patient-sharing networks. Examining the association between physician-specific measures of his/her network position and complications may also be a promising line of research.
Finally, how might this type of approach be deployed when trying to improve quality? If validated, the ability to identify subgroups may help target interventions to groups of physicians with the highest rates of specific types of complications. As noted above, these subgroups may be related to albeit distinct from approaches that target specific practices or hospitals.4 Within each subgroup, quality improvement initiatives may be facilitated by working with specific providers within the network structure.37 This builds on and complements the tradition of identifying key opinion leaders from surveys to promote the adoption of best practices.42,43 Interventions may focus on doctors that are central in a subgroup to facilitate the spread of norms and quality standards or, alternatively on doctors on the periphery who may be early adopters of new technology.39,44,45
This work has several limitations. The optimal approach to constructing physician patient-sharing networks and identifying network subgroups has not been developed. By limiting our analyses to specific cities, we create a “boundary specification problem” in which patients nearby the city’s boundaries may alter the network structure.46 We considered providers to be connected to one another if they shared two patients. Network structure and associations with complications were largely consistent when lowering the threshold to one patient; increasing the threshold to 3 patients resulted in many fewer physicians being connected to one another (Appendix Table 2 in Supplemental Materials at: XXX). Adding more years of data to test the stability and evolution of networks over time and including patients who do not have cancer but are nonetheless shared by providers may prove useful in generating more robust depictions of health care delivery.
Additional limitations stem from the use of claims data. First, Medicare claims have been shown to have a high sensitivity and specificity for surgical47,48 and late urinary complications.13 In contrast, ascertainment of incontinence has a 39% sensitivity compared to self-report but high specificity (92%).13 Although we substantially underestimate the incidence of long-term incontinence, our goal was not to determine the complication rate, but to assess how the rate of complications differs according to subgroup. There is no evidence that the ascertainment of complications would be differential between subgroups. Second, we were unable to include patients younger than 65, those without a diagnosis of prostate cancer, and men enrolled in HMOs in our construction of patient-sharing networks. Though Medicare urologist volume is highly correlated with total patient volume,13 the exclusion of these patients may alter the network structure by reducing the number of shared patients between doctors. Third, we were unable to match all patients to their diagnosing urologist. Patients who did not match were similar to those who did on observable characteristics; however, the proportion of patients who did not match ranged from 3.9 to 9.5% across the cities. The impact of this on our network structure remains uncertain. Fourth, though we adjust for surgical volume, incomplete adjustment for surgical technique and surgeon’s experience may bias our findings. Fifth, though we test models that include hospital structure, we recognize that the models are unable to account for practice structure which may be an important determinant of complication rates. Sixth, this observational analysis is unable to determine causality, and, as noted above, selection effects may be an important contributor our findings. Finally, the small sample sizes in some network subgroups may have led to unstable estimates in some instances and limited our ability to detect clinically meaningful differences.
In summary, physician networks based on shared patients are associated with variation in rates of complications following radical prostatectomy for prostate cancer. Physician patient-sharing networks may be useful tools to better understand cancer care variation within and across geographic regions.
Supplementary Material
Acknowledgments
Funding: Dr. Pollack’s salary is supported by the National Cancer Institute and Office of Behavioral and Social Sciences (K07 CA151910). We thank the support of the Maryland Cigarette Restitution Fund Research Grant to the Johns Hopkins Institutions.
We would like to thank Beth Ann Griffin for her statistical advice and Robert Sunderland for his programming assistance.
Footnotes
Disclaimers: There are no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wennberg J, Gittelsohn A. Small area variations in health care delivery: A population-based health information system can guide planning and regulatory decision-making. Science. 1973;182:1102–8. doi: 10.1126/science.182.4117.1102. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Baik SH, Fendrick AM, Baicker K. Comparing local and regional variation in health care spending. N Engl J Med. 2012;367:1724–31. doi: 10.1056/NEJMsa1203980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett ML, Landon BE, O’Malley AJ, et al. Mapping Physician Networks with Self-Reported and Administrative Data. Health Serv Res. 2011;46:1592–609. doi: 10.1111/j.1475-6773.2011.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landon BE, Onnela J-P, Keating NL, et al. Using Administrative Data to Identify Naturally Occurring Networks of Physicians. Med Care. 2013;51:715–21. doi: 10.1097/MLR.0b013e3182977991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landon BE, Keating NL, Barnett ML, et al. Variation in patient-sharing networks of physicians across the United States. JAMA. 2012;308:265–73. doi: 10.1001/jama.2012.7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollack CE, Weissman G, Bekelman JE, et al. Physician social networks and variation in prostate cancer treatment in three cities. Health Serv Res. 2012;47:380–403. doi: 10.1111/j.1475-6773.2011.01331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnett ML, Christakis NA, O’Malley J, et al. Physician Patient-sharing Networks and the Cost and Intensity of Care in US Hospitals. Med Care. 2012;50:152–60. doi: 10.1097/MLR.0b013e31822dcef7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 9.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eton DT, Lepore SJ. Prostate cancer and health-related quality of life: a review of the literature. Psycho-Oncol. 2002;11:307–26. doi: 10.1002/pon.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potosky AL, Davis WW, Hoffman RM, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: The Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2004;96:1358–67. doi: 10.1093/jnci/djh259. [DOI] [PubMed] [Google Scholar]
- 12.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 13.Begg C, Riedel E, Bach P, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;347:693–6. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 14.Gooden KM, Howard DL, Carpenter WR, et al. The effect of hospittal and surgeon colume on racial differences in recurrence-free survival after radical prostatectomy. Med Care. 2008;46:1170–6. doi: 10.1097/MLR.0b013e31817d696d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu JC, Gold KG, Pashos CL, et al. Role of surgeon volume in radical prostatectomy outcomes. J Clin Oncol. 2003;21:401–5. doi: 10.1200/JCO.2003.05.169. [DOI] [PubMed] [Google Scholar]
- 16.Bianco FJ, Jr, Scardino PT, Stephenson AJ, Diblasio CJ, Fearn PA, Eastham JA. Long-term oncologic results of salvage radical prostatectomy for locally recurrent prostate cancer after radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:448–53. doi: 10.1016/j.ijrobp.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 17.The Center for Evaluative Clinical Sciences. The Dartmouth Atlas of Health Care: The Quality of Medical Care in the United States. Hanover, NH: 1999. [Google Scholar]
- 18.Wasserman S, Faust K. Social Network Analysis: Methods and Applications. New York: Cambridge University Press; 1999. [Google Scholar]
- 19.Bach PB, Pham HH, Schrag D, et al. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351:575–84. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 20.Hasnain-Wynia R, Baker DW, Nerenz D, et al. Disparities in health care are driven by where minority patients seek care. Arch Intern Med. 2007;167:1233–9. doi: 10.1001/archinte.167.12.1233. [DOI] [PubMed] [Google Scholar]
- 21.Jha AK, Orav EJ, Li Z, Epstein AM. Concentration and quality of hospitals that care for elderly black patients. Arch Intern Med. 2007;167:1177–82. doi: 10.1001/archinte.167.11.1177. [DOI] [PubMed] [Google Scholar]
- 22.Potosky AL. Potential for cancer related health services research using a linked Medicare-tumre registry database. Med Care. 1993;31:732–48. [PubMed] [Google Scholar]
- 23.Bekelman JE, Zelefsky MJ, Jang TL, et al. Variation in adherence to external beam radiotherapy quality measures among elderly men with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007;69:1456–66. doi: 10.1016/j.ijrobp.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu JC, Gu X, Lipsitz SR, et al. Comparative Effectiveness of Minimally Invasive vs Open Radical Prostatectomy. JAMA. 2009;302:1557–64. doi: 10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- 25.Elixhauser A, Steiner C, Harris D, Coffey R. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Pham HH, Schrag D, O’Malley AS, et al. Care patterns in medicare and their implications for pay for performance. N Engl J Med. 2007;356:1130–9. doi: 10.1056/NEJMsa063979. [DOI] [PubMed] [Google Scholar]
- 27.Csardi G, Nepusz T. The igraph software package for complex network research. Paper presented at: International Conference on Complex Systems; 2006; Boston, MA. [Google Scholar]
- 28.Girvan M, Newman MEJ. Community structure in social and biological networks. P Natl Acad Sci. 2002;99:7821–6. doi: 10.1073/pnas.122653799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman MEJ. Modularity and community structure in networks. P Natl Acad Sci. 2006;103:8577–82. doi: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo G, Zhao H. Multilevel modeling for binary data. Ann Rev Sociol. 2000;26:441–62. [Google Scholar]
- 31.Pollack CE, Bekelman JE, Liao KJ, Armstrong K. Hospital racial composition and the treatment of localized prostate cancer. Cancer. 2011;117:5569–78. doi: 10.1002/cncr.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birkmeyer NJO, Goodney PP, Stukel TA, et al. Do cancer centers designated by the national cancer institute have better surgical outcomes? Cancer. 2005;103:435–41. doi: 10.1002/cncr.20785. [DOI] [PubMed] [Google Scholar]
- 33.Onega T, Duell EJ, Shi X, et al. Influence of NCI cancer center attendance on mortality in lung, breast, colorectal, and prostate cancer patients. Med Care Res Rev. 2009;66:542–60. doi: 10.1177/1077558709335536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bynum JPW, Bernal-Delgado E, Gottlieb D, Fisher E. Assigning ambulatory patients and their physicians to hospitals: A method for obtaining population-based provider performance measurements. Health Serv Res. 2007;42:45–62. doi: 10.1111/j.1475-6773.2006.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McPherson M, Smith-Lovin L, Cook JM. Birds of a feather: Homophily in social networks. Ann Rev Sociol. 2001;27:415–44. [Google Scholar]
- 36.Shortell SM. Patterns of referral among internists in private practice: A social exchange model. J Health Soc Behav. 1973;14:335–48. [PubMed] [Google Scholar]
- 37.Valente TW. Network interventions. Science. 2012;337:49–53. doi: 10.1126/science.1217330. [DOI] [PubMed] [Google Scholar]
- 38.Coleman J, Katz E, Menzel H. The Diffusion of an innovation among physicians. Sociometry. 1957;20:253–70. [Google Scholar]
- 39.Rogers EM. Diffusion of Innovation. New York: The Free Press; 1995. [Google Scholar]
- 40.Keating N, Zaslavsky AM, Ayanian JZ. PHysicians’ experiences and beliefs regarding informal consultation. JAMA. 1998;280:900–4. doi: 10.1001/jama.280.10.900. [DOI] [PubMed] [Google Scholar]
- 41.Pollack CE, Bekelman JE, Epstein AJ, et al. Racial disparities in changing to a high-volume urologist among men with localized prostate cancer. Med Care. 2011;49:999–1006. doi: 10.1097/MLR.0b013e3182364019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lomas J, Enkin M, Anderson GM, et al. Opinion leaders vs audit and feedback to implement practice guidelines: Delivery after previous cesarean section. JAMA. 1991;265:2202–7. [PubMed] [Google Scholar]
- 43.Soumerai SB, McLaughlin TJ, Gurwitz JH, et al. Effect of local opinion leaders on quality of care for acute myocardial infarction: A randomized controlled trial. JAMA. 1998;279:1358–63. doi: 10.1001/jama.279.17.1358. [DOI] [PubMed] [Google Scholar]
- 44.Anderson JG, Jay SJ. The diffusion of medical technology: Social network analysis and policy research. Sociol Q. 1985;26:49–64. [Google Scholar]
- 45.Coleman JS, Katz E, Menzel H. Medical Innovation: A Diffusion Study. New York: Bobbs Merrill; 1966. [Google Scholar]
- 46.Laumann EO, Marsden PV, Prensky D. The boundary specification problem in network analysis. In: Burt RS, Minor MJ, editors. Applied Network Analysis. London: Sage Publications; 1983. [Google Scholar]
- 47.Lawthers AG, McCarthy EP, Davis RB, et al. Identification of In-Hospital Complications from Claims Data: Is it Valid? Med Care. 2000;38:785–95. doi: 10.1097/00005650-200008000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Potosky AL, Warren JL, Riedel ER, et al. Measuring complications of cancer treatment using the SEER-Medicare Data. Med Care. 2002;40(Suppl):62–8. doi: 10.1097/00005650-200208001-00009. [DOI] [PubMed] [Google Scholar]
- 49.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.