Abstract
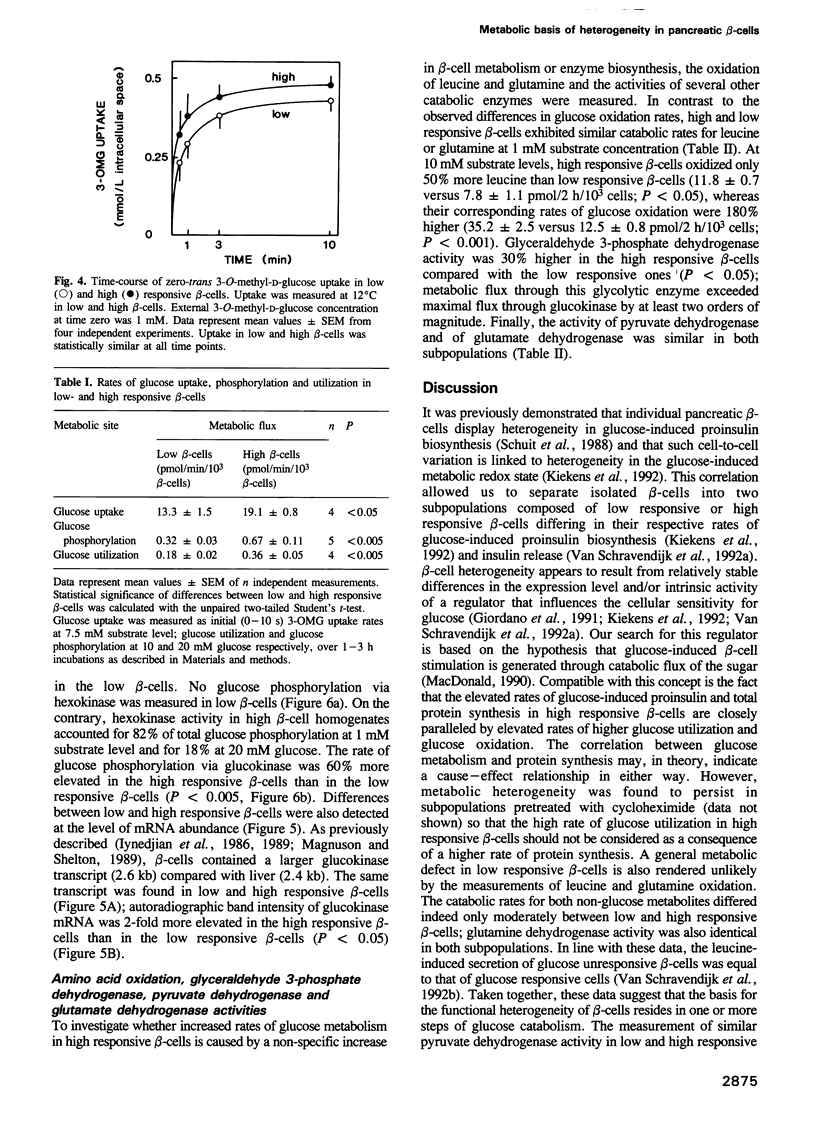
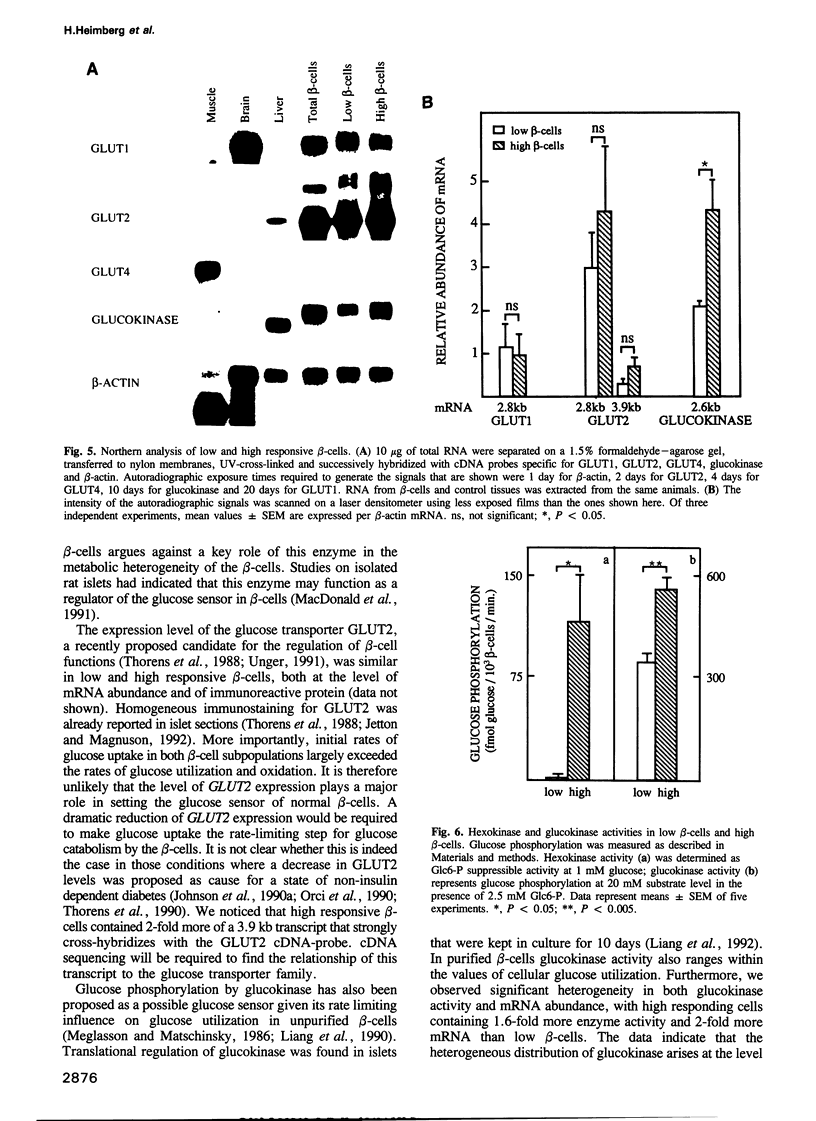
Rat beta-cells differ in their individual rates of glucose-induced insulin biosynthesis and release. This functional heterogeneity has been correlated with intercellular differences in metabolic redox responsiveness to glucose. The present study compares glucose metabolism in two beta-cell subpopulations that have been separated on the basis of the presence (high responsive) or absence (low responsive) of a metabolic redox shift at 7.5 mM glucose. Mean rates of glucose utilization and glucose oxidation in high responsive beta-cells were 2- to 4-fold higher than in low responsive beta-cells, whereas their leucine and glutamine oxidation was only 10-50% higher. This heterogeneity in glucose metabolism cannot be attributed to differences in GLUT2 mRNA levels or in glucose transport. In both cell subpopulations, the rates of glucose transport (13-19 pmol/min/10(3) beta-cells) were at least 50-fold higher than corresponding rates of glucose utilization. On the other hand, rates of glucose phosphorylation (0.3-0.7 pmol/min/10(3) beta-cells) ranged within those of total glucose utilization (0.2-0.4 pmol/min/10(3) beta-cells). High responsive beta-cells exhibited a 60% higher glucokinase activity than low responsive beta-cells and their glucokinase mRNA level was 100% higher. Furthermore, glucose phosphorylation via low Km hexokinase was detected only in the high responsive beta-cell subpopulation. Heterogeneity in glucose sensitivity among pancreatic beta-cells can therefore be explained by intercellular differences in glucose phosphorylation rather than in glucose transport.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosco D., Meda P. Actively synthesizing beta-cells secrete preferentially after glucose stimulation. Endocrinology. 1991 Dec;129(6):3157–3166. doi: 10.1210/endo-129-6-3157. [DOI] [PubMed] [Google Scholar]
- De Vos A., Schuit F. C., Malaisse W. J. Preferential stimulation by glucose of its oxidation relative to glycolysis in purified insulin-producing cells. Biochem Int. 1991 May;24(1):117–121. [PubMed] [Google Scholar]
- Epstein P. N., Boschero A. C., Atwater I., Cai X., Overbeek P. A. Expression of yeast hexokinase in pancreatic beta cells of transgenic mice reduces blood glucose, enhances insulin secretion, and decreases diabetes. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12038–12042. doi: 10.1073/pnas.89.24.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRODSKY G. M., BATTS A. A., BENNETT L. L., VCELLA C., MCWILLIAMS N. B., SMITH D. F. EFFECTS OF CARBOHYDRATES ON SECRETION OF INSULIN FROM ISOLATED RAT PANCREAS. Am J Physiol. 1963 Oct;205:638–644. doi: 10.1152/ajplegacy.1963.205.4.638. [DOI] [PubMed] [Google Scholar]
- Giordano E., Bosco D., Cirulli V., Meda P. Repeated glucose stimulation reveals distinct and lasting secretion patterns of individual rat pancreatic B cells. J Clin Invest. 1991 Jun;87(6):2178–2185. doi: 10.1172/JCI115251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorus F. K., Malaisse W. J., Pipeleers D. G. Differences in glucose handling by pancreatic A- and B-cells. J Biol Chem. 1984 Jan 25;259(2):1196–1200. [PubMed] [Google Scholar]
- Hiriart M., Ramirez-Medeles M. C. Functional subpopulations of individual pancreatic B-cells in culture. Endocrinology. 1991 Jun;128(6):3193–3198. doi: 10.1210/endo-128-6-3193. [DOI] [PubMed] [Google Scholar]
- Hughes S. D., Johnson J. H., Quaade C., Newgard C. B. Engineering of glucose-stimulated insulin secretion and biosynthesis in non-islet cells. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):688–692. doi: 10.1073/pnas.89.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iynedjian P. B., Möbius G., Seitz H. J., Wollheim C. B., Renold A. E. Tissue-specific expression of glucokinase: identification of the gene product in liver and pancreatic islets. Proc Natl Acad Sci U S A. 1986 Apr;83(7):1998–2001. doi: 10.1073/pnas.83.7.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iynedjian P. B., Pilot P. R., Nouspikel T., Milburn J. L., Quaade C., Hughes S., Ucla C., Newgard C. B. Differential expression and regulation of the glucokinase gene in liver and islets of Langerhans. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7838–7842. doi: 10.1073/pnas.86.20.7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetton T. L., Magnuson M. A. Heterogeneous expression of glucokinase among pancreatic beta cells. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2619–2623. doi: 10.1073/pnas.89.7.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. H., Newgard C. B., Milburn J. L., Lodish H. F., Thorens B. The high Km glucose transporter of islets of Langerhans is functionally similar to the low affinity transporter of liver and has an identical primary sequence. J Biol Chem. 1990 Apr 25;265(12):6548–6551. [PubMed] [Google Scholar]
- Johnson J. H., Ogawa A., Chen L., Orci L., Newgard C. B., Alam T., Unger R. H. Underexpression of beta cell high Km glucose transporters in noninsulin-dependent diabetes. Science. 1990 Oct 26;250(4980):546–549. doi: 10.1126/science.2237405. [DOI] [PubMed] [Google Scholar]
- Kiekens R., In 't Veld P., Mahler T., Schuit F., Van De Winkel M., Pipeleers D. Differences in glucose recognition by individual rat pancreatic B cells are associated with intercellular differences in glucose-induced biosynthetic activity. J Clin Invest. 1992 Jan;89(1):117–125. doi: 10.1172/JCI115551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Najafi H., Matschinsky F. M. Glucose regulates glucokinase activity in cultured islets from rat pancreas. J Biol Chem. 1990 Oct 5;265(28):16863–16866. [PubMed] [Google Scholar]
- Liang Y., Najafi H., Smith R. M., Zimmerman E. C., Magnuson M. A., Tal M., Matschinsky F. M. Concordant glucose induction of glucokinase, glucose usage, and glucose-stimulated insulin release in pancreatic islets maintained in organ culture. Diabetes. 1992 Jul;41(7):792–806. doi: 10.2337/diab.41.7.792. [DOI] [PubMed] [Google Scholar]
- MacDonald M. J. Elusive proximal signals of beta-cells for insulin secretion. Diabetes. 1990 Dec;39(12):1461–1466. doi: 10.2337/diab.39.12.1461. [DOI] [PubMed] [Google Scholar]
- MacDonald M. J., Kaysen J. H., Moran S. M., Pomije C. E. Pyruvate dehydrogenase and pyruvate carboxylase. Sites of pretranslational regulation by glucose of glucose-induced insulin release in pancreatic islets. J Biol Chem. 1991 Nov 25;266(33):22392–22397. [PubMed] [Google Scholar]
- Magnuson M. A., Shelton K. D. An alternate promoter in the glucokinase gene is active in the pancreatic beta cell. J Biol Chem. 1989 Sep 25;264(27):15936–15942. [PubMed] [Google Scholar]
- Meglasson M. D., Matschinsky F. M. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2(3-4):163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Baetens D., Inman L., Amherdt M., Peterson R. G., Newgard C. B., Johnson J. H., Unger R. H. Evidence that down-regulation of beta-cell glucose transporters in non-insulin-dependent diabetes may be the cause of diabetic hyperglycemia. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9953–9957. doi: 10.1073/pnas.87.24.9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permutt M. A., Kipnis D. M. Insulin biosynthesis. I. On the mechanism of glucose stimulation. J Biol Chem. 1972 Feb 25;247(4):1194–1199. [PubMed] [Google Scholar]
- Pipeleers D. G. Heterogeneity in pancreatic beta-cell population. Diabetes. 1992 Jul;41(7):777–781. doi: 10.2337/diab.41.7.777. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Brenner C. A., Schultz R., Mark D., Werb Z. Developmental expression of PDGF, TGF-alpha, and TGF-beta genes in preimplantation mouse embryos. Science. 1988 Sep 30;241(4874):1823–1825. doi: 10.1126/science.3175624. [DOI] [PubMed] [Google Scholar]
- Schuit F. C., In't Veld P. A., Pipeleers D. G. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3865–3869. doi: 10.1073/pnas.85.11.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit F. C., Kiekens R., Pipeleers D. G. Measuring the balance between insulin synthesis and insulin release. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1182–1187. doi: 10.1016/0006-291x(91)91017-7. [DOI] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Thorens B., Weir G. C., Leahy J. L., Lodish H. F., Bonner-Weir S. Reduced expression of the liver/beta-cell glucose transporter isoform in glucose-insensitive pancreatic beta cells of diabetic rats. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6492–6496. doi: 10.1073/pnas.87.17.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trus M. D., Zawalich W. S., Burch P. T., Berner D. K., Weill V. A., Matschinsky F. M. Regulation of glucose metabolism in pancreatic islets. Diabetes. 1981 Nov;30(11):911–922. doi: 10.2337/diab.30.11.911. [DOI] [PubMed] [Google Scholar]
- Unger R. H. Diabetic hyperglycemia: link to impaired glucose transport in pancreatic beta cells. Science. 1991 Mar 8;251(4998):1200–1205. doi: 10.1126/science.2006409. [DOI] [PubMed] [Google Scholar]
- Van De Winkel M., Pipeleers D. Autofluorescence-activated cell sorting of pancreatic islet cells: purification of insulin-containing B-cells according to glucose-induced changes in cellular redox state. Biochem Biophys Res Commun. 1983 Jul 29;114(2):835–842. doi: 10.1016/0006-291x(83)90857-4. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E. A protein from rat liver confers to glucokinase the property of being antagonistically regulated by fructose 6-phosphate and fructose 1-phosphate. Eur J Biochem. 1989 Jan 15;179(1):179–184. doi: 10.1111/j.1432-1033.1989.tb14538.x. [DOI] [PubMed] [Google Scholar]
- Van Schravendijk C. F., Kiekens R., Pipeleers D. G. Pancreatic beta cell heterogeneity in glucose-induced insulin secretion. J Biol Chem. 1992 Oct 25;267(30):21344–21348. [PubMed] [Google Scholar]
- Vionnet N., Stoffel M., Takeda J., Yasuda K., Bell G. I., Zouali H., Lesage S., Velho G., Iris F., Passa P. Nonsense mutation in the glucokinase gene causes early-onset non-insulin-dependent diabetes mellitus. Nature. 1992 Apr 23;356(6371):721–722. doi: 10.1038/356721a0. [DOI] [PubMed] [Google Scholar]



