Abstract
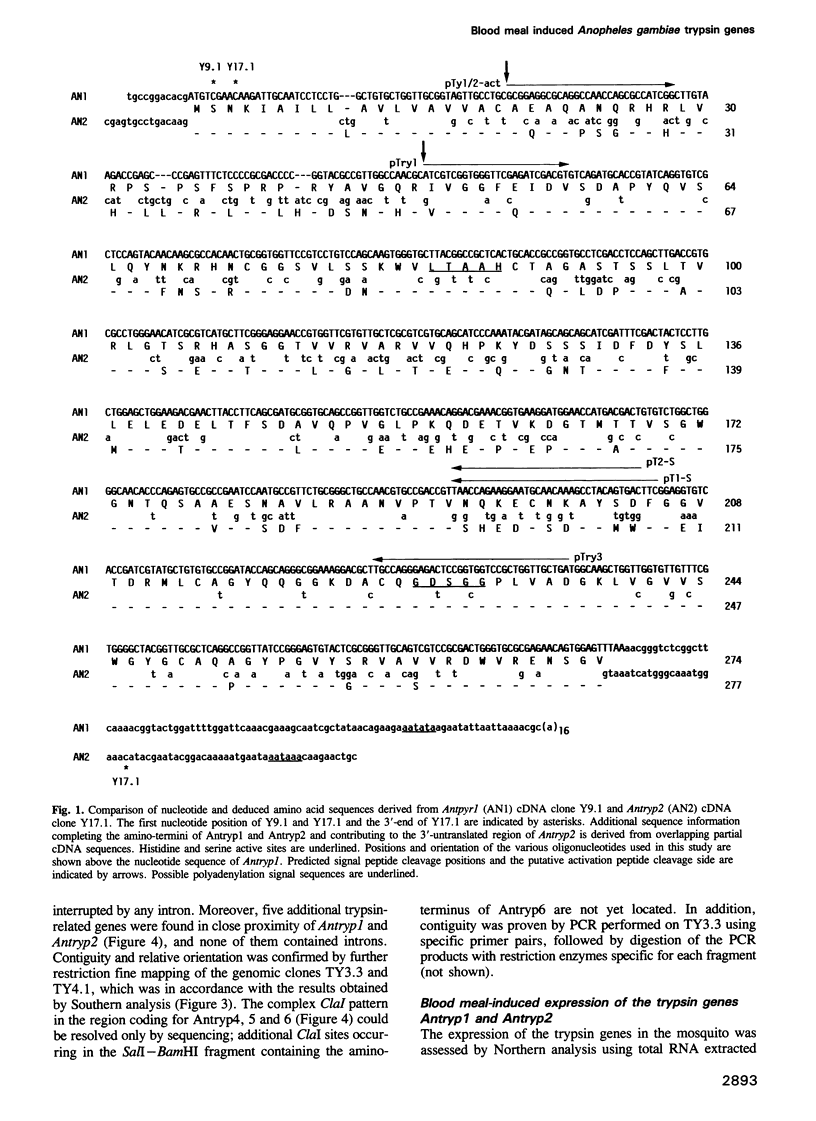
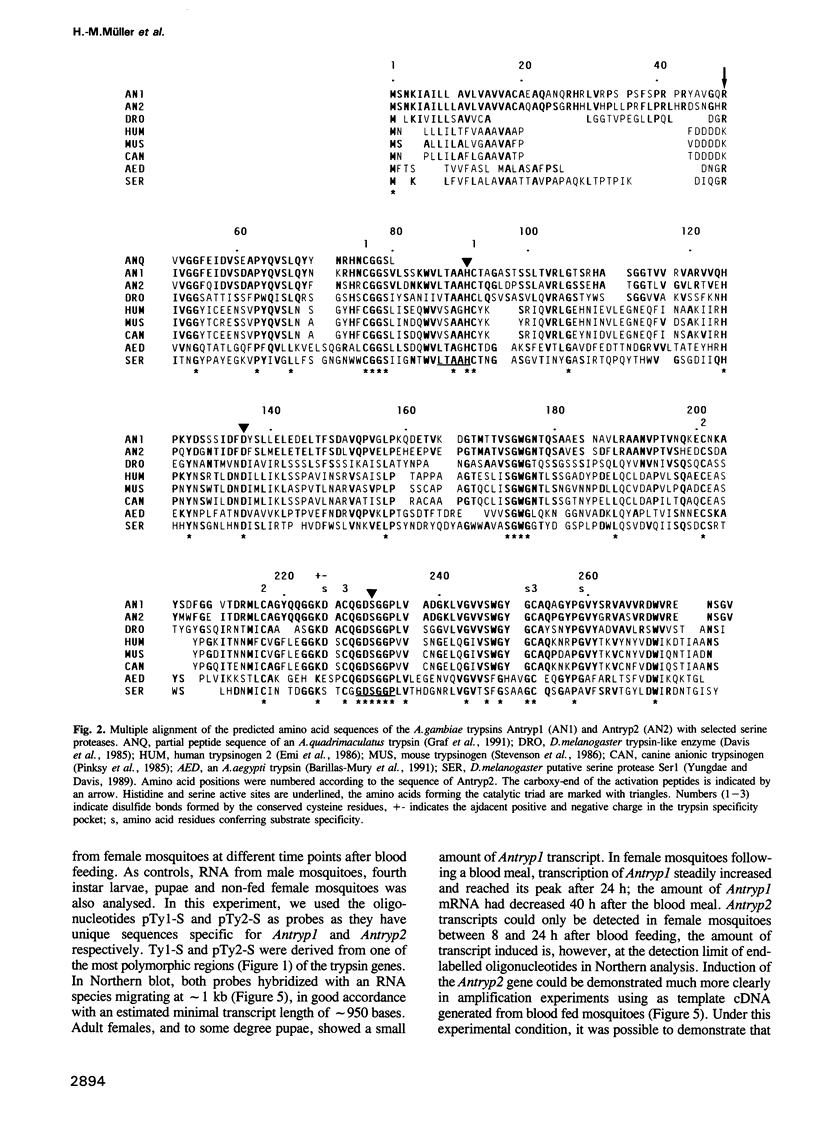
Serine proteases are among the enzymes that play a crucial role during the digestion of the blood meal in the gut of mosquitoes. The identification of the corresponding genes would have important implications for the control of mosquitoes and mosquito-borne diseases. Analysis of the genomic organization of these genes may lead to the isolation of a gut-specific, inducible promoter for the expression of anti-parasitic agents in transgenic mosquitoes. Moreover, specific inhibitors could be designed on the basis of the structural properties of the enzymes. We report here on the identification of a trypsin gene family in Anopheles gambiae, the mosquito vector of malaria in Africa. Mosquito trypsin-related sequences were amplified by PCR using as template cDNA derived from RNA of blood fed mosquitoes. Cloning of the PCR product revealed two distinct sequences. Corresponding full-length cDNA clones were obtained and sequenced. Antryp1 and Antryp2 code for proteins of 274 and 277 amino acids respectively, showing 75% homology at the amino acid level. The deduced amino acid sequences clearly identify them as trypsins. Five additional trypsin sequences were found in overlapping genomic clones. The genes identified are tightly clustered within 11 kb and sequencing indicates that no introns are present. Northern and PCR analysis indicated that the transcription of both Antryp1 and Antryp2 is induced by blood feeding. Moreover, the Antryp1 protein was detected among the proteins of a midgut lysate of blood fed mosquitoes using antisera against recombinant Antryp1. In addition, the recombinant polypeptides derived from Antryp1 and Antryp2 expressed in Escherichia coli showed a strong proteolytic activity against different sets of blood proteins. We conclude that the products of Antryp1 and Antryp2 play an important role in the breakdown of the proteins during the digestion of the blood meal in the mosquito gut.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Quinto C., Quiroga M., Valenzuela P., Craik C. S., Rutter W. J. Isolation and sequence of a rat chymotrypsin B gene. J Biol Chem. 1984 Nov 25;259(22):14265–14270. [PubMed] [Google Scholar]
- Besansky N. J., Powell J. R. Reassociation kinetics of Anopheles gambiae (Diptera: Culicidae) DNA. J Med Entomol. 1992 Jan;29(1):125–128. doi: 10.1093/jmedent/29.1.125. [DOI] [PubMed] [Google Scholar]
- Billingsley P. F., Hecker H. Blood digestion in the mosquito, Anopheles stephensi Liston (Diptera: Culicidae): activity and distribution of trypsin, aminopeptidase, and alpha-glucosidase in the midgut. J Med Entomol. 1991 Nov;28(6):865–871. doi: 10.1093/jmedent/28.6.865. [DOI] [PubMed] [Google Scholar]
- Brodrick J. W., Largman C., Hsiang M. W., Johnson J. H., Geokas M. C. Structural basis for the specific activation of human enteropeptidase. J Biol Chem. 1978 Apr 25;253(8):2737–2742. [PubMed] [Google Scholar]
- Brodrick J. W., Largman C., Johnson J. H., Geokas M. C. Human cationic trypsinogen. Purification, characterization, and characteristics of autoactivation. J Biol Chem. 1978 Apr 25;253(8):2732–2736. [PubMed] [Google Scholar]
- Coluzzi M. Heterogeneities of the malaria vectorial system in tropical Africa and their significance in malaria epidemiology and control. Bull World Health Organ. 1984;62 (Suppl):107–113. [PMC free article] [PubMed] [Google Scholar]
- Davis C. A., Riddell D. C., Higgins M. J., Holden J. J., White B. N. A gene family in Drosophila melanogaster coding for trypsin-like enzymes. Nucleic Acids Res. 1985 Sep 25;13(18):6605–6619. doi: 10.1093/nar/13.18.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emi M., Nakamura Y., Ogawa M., Yamamoto T., Nishide T., Mori T., Matsubara K. Cloning, characterization and nucleotide sequences of two cDNAs encoding human pancreatic trypsinogens. Gene. 1986;41(2-3):305–310. doi: 10.1016/0378-1119(86)90111-3. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Graf R., Boehlen P., Briegel H. Structural diversity of trypsin from different mosquito species feeding on vertebrate blood. Experientia. 1991 Jun 15;47(6):603–609. doi: 10.1007/BF01949885. [DOI] [PubMed] [Google Scholar]
- Grant G. A., Henderson K. O., Eisen A. Z., Bradshaw R. A. Amino acid sequence of a collagenolytic protease from the hepatopancreas of the fiddler crab, Uca pugilator. Biochemistry. 1980 Sep 30;19(20):4653–4659. doi: 10.1021/bi00561a018. [DOI] [PubMed] [Google Scholar]
- Jany K. D., Bekelar K., Pfleiderer G., Ishay J. Amino acid sequence of an insect chymotrypsin from the larvae of the hornet, Vespa orientalis. Biochem Biophys Res Commun. 1983 Jan 14;110(1):1–7. doi: 10.1016/0006-291x(83)91251-2. [DOI] [PubMed] [Google Scholar]
- Kraut J. Serine proteases: structure and mechanism of catalysis. Annu Rev Biochem. 1977;46:331–358. doi: 10.1146/annurev.bi.46.070177.001555. [DOI] [PubMed] [Google Scholar]
- Lecroisey A., Gilles A. M., De Wolf A., Keil B. Complete amino acid sequence of the collagenase from the insect Hypoderma lineatum. J Biol Chem. 1987 Jun 5;262(16):7546–7551. [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Quinto C., Swain W., Pictet R. L., Nikovits W., Rutter W. J. Primary structure of two distinct rat pancreatic preproelastases determined by sequence analysis of the complete cloned messenger ribonucleic acid sequences. Biochemistry. 1982 Mar 16;21(6):1453–1463. doi: 10.1021/bi00535a053. [DOI] [PubMed] [Google Scholar]
- Pinsky S. D., LaForge K. S., Scheele G. Differential regulation of trypsinogen mRNA translation: full-length mRNA sequences encoding two oppositely charged trypsinogen isoenzymes in the dog pancreas. Mol Cell Biol. 1985 Oct;5(10):2669–2676. doi: 10.1128/mcb.5.10.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Stevenson B. J., Hagenbüchle O., Wellauer P. K. Sequence organisation and transcriptional regulation of the mouse elastase II and trypsin genes. Nucleic Acids Res. 1986 Nov 11;14(21):8307–8330. doi: 10.1093/nar/14.21.8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Sasagawa T., Woodbury R. G., Ericsson L. H., Dörsam H., Kraemer M., Neurath H., Zwilling R. Amino acid sequence of crayfish (Astacus fluviatilis) trypsin If. Biochemistry. 1983 Mar 15;22(6):1459–1465. doi: 10.1021/bi00275a021. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. B. Anopheles gambiae complex and disease transmission in Africa. Trans R Soc Trop Med Hyg. 1974;68(4):278–301. doi: 10.1016/0035-9203(74)90035-2. [DOI] [PubMed] [Google Scholar]
- Willadsen P., Riding G. A., McKenna R. V., Kemp D. H., Tellam R. L., Nielsen J. N., Lahnstein J., Cobon G. S., Gough J. M. Immunologic control of a parasitic arthropod. Identification of a protective antigen from Boophilus microplus. J Immunol. 1989 Aug 15;143(4):1346–1351. [PubMed] [Google Scholar]
- Yun Y., Davis R. L. Levels of RNA from a family of putative serine protease genes are reduced in Drosophila melanogaster dunce mutants and are regulated by cyclic AMP. Mol Cell Biol. 1989 Feb;9(2):692–700. doi: 10.1128/mcb.9.2.692. [DOI] [PMC free article] [PubMed] [Google Scholar]