Abstract
Background: Sugar-sweetened soda consumption is consistently associated with an increased risk of several chronic inflammatory diseases such as type 2 diabetes and cardiovascular diseases. Whether it plays a role in the development of rheumatoid arthritis (RA), a common autoimmune inflammatory disease, remains unclear.
Objective: The aim was to evaluate the association between sugar-sweetened soda consumption and risk of RA in US women.
Design: We prospectively followed 79,570 women from the Nurses’ Health Study (NHS; 1980–2008) and 107,330 women from the NHS II (1991–2009). Information on sugar-sweetened soda consumption (including regular cola, caffeine-free cola, and other sugar-sweetened carbonated soda) was obtained from a validated food-frequency questionnaire at baseline and approximately every 4 y during follow-up. Incident RA cases were validated by medical record review. Time-varying Cox proportional hazards regression models were used to calculate HRs after adjustment for confounders. Results from both cohorts were pooled by an inverse-variance–weighted, fixed-effects model.
Results: During 3,381,268 person-years of follow-up, 857 incident cases of RA were documented in the 2 cohorts. In the multivariable pooled analyses, we found that women who consumed ≥1 serving of sugar-sweetened soda/d had a 63% (HR: 1.63; 95% CI: 1.15, 2.30; P-trend = 0.004) increased risk of developing seropositive RA compared with those who consumed no sugar-sweetened soda or who consumed <1 serving/mo. When we restricted analyses to those with later RA onset (after age 55 y) in the NHS, the association appeared to be stronger (HR: 2.64; 95% CI: 1.56, 4.46; P-trend < 0.0001). No significant association was found for sugar-sweetened soda and seronegative RA. Diet soda consumption was not significantly associated with risk of RA in the 2 cohorts.
Conclusion: Regular consumption of sugar-sweetened soda, but not diet soda, is associated with increased risk of seropositive RA in women, independent of other dietary and lifestyle factors.
INTRODUCTION
Rheumatoid arthritis (RA)5 is the most common autoimmune inflammatory disease, characterized by persistent synovitis, systemic inflammation, and autoantibodies [rheumatoid factor (RF) and anticitrullinated peptide antibodies (ACPAs)], and affects ∼1% of adults, mostly women (1, 2). Although the etiology is not completely elucidated, RA is generally considered to be a multifactorial disease, caused by both genetic factors and environmental exposures including lifestyle and dietary risk factors (1, 3–5). Cross-sectional studies have documented a high prevalence of metabolic syndrome and associated factors such as obesity, dyslipidemia, or impaired glucose metabolism in patients with RA (6). The incidence of type 2 diabetes (T2D) and cardiovascular disease (CVD) is greater among patients with RA (7, 8), suggesting that the etiology of RA may have some overlap with the development of these chronic inflammatory conditions. Although many dietary risk factors have been identified for T2D and CVD, whether these dietary factors are also related to RA remains unclear (5, 9–11). Sugar-sweetened sodas are the primary source of added sugar in the American diet, which may contribute to the development of T2D and CVD by inducing obesity, insulin resistance, and inflammation (12), all of which are also in the etiologic pathway of RA. Therefore, we examined the association between sugar-sweetened soda consumption and risk of RA.
To our knowledge, no previous study has explored whether soda consumption is associated with the risk of development of RA. We therefore investigated the relations between sugar-sweetened and diet soda with RA risk in 2 well-established large cohorts of young and middle-aged women, with a series of lifestyle and dietary factors controlled for.
SUBJECTS AND METHODS
Study population
The Nurses’ Health Study (NHS) was initiated in 1976 and comprised 121,700 female registered nurses, aged 30–55 y at inception. The NHS II was established in 1989 and consisted of 116,671 female registered nurses, aged 25–42 y at the study's initiation. The participants in both cohorts responded to a baseline questionnaire about their lifestyles and medical histories and were followed biennially through validated questionnaires that obtained updated information on their medical history, lifestyle, and occurrence of chronic diseases. Follow-up was complete for >90% of participants for every 2-y period in the 2 cohorts (13, 14). The baseline for this analysis was 1980 for the NHS and 1991 for the NHS II when dietary information was first collected with the use of the validated food-frequency questionnaire (FFQ).
In this analysis, we included women who completed the 1980 FFQ for NHS and the 1991 FFQ for NHS II with <70 missing items and total energy intake between 500 and 3500 kcal/d. We censored all women at the date of self-reported psoriasis, psoriatic arthritis, and connective tissue disease that was not subsequently confirmed as RA at the self-reported date. Women lost to follow-up were censored at their last response to questionnaires because incident cases could not subsequently be identified. In addition, cancer or its treatments may alter the immune system, and the secondary symptoms (eg, joint pain from paraneoplastic syndromes) may change the diet and physical activity. We therefore excluded participants with a history of cancer at baseline and censored incident cancer cases at the self-reported date in the analysis. After exclusions, there were a total of 79,570 NHS participants and 107,330 NHS II participants for this analysis. All aspects of this study were approved by the Partners Health Care Institutional Review Board.
RA case identification
The ascertainment of RA cases in the NHS and NHS II was a 2-step process. The connective tissue disease screening questionnaire was mailed to participants who self-reported a new physician's diagnosis of RA (15). Two board-certified rheumatologists trained in chart abstraction conducted independent medical record reviews according to the 1987 American College of Rheumatology classification criteria of RA (16). The serostatus of RA was determined by positive RFs or ACPAs in the medical record. Detailed RA assessment procedures have been described elsewhere (17, 18).
Assessment of soda consumption
Since 1980 and 1991, the FFQ has been administered to NHS and NHS II participants, respectively, to collect information on their usual intakes of foods and beverages over the previous year. Starting in 1980, participants were asked how often during the previous year they had consumed sugar-sweetened soda (“Coke, Pepsi, or other cola with sugar” and “other carbonated beverages with sugar”) and diet sodas (“low-calorie cola with caffeine,” “low-calorie caffeine-free cola,” and “other low-calorie beverages”) with the use of standard portion sizes (1 standard serving, cup, glass, can, or bottle). There were 9 possible responses that ranged from never or less than once a month to ≥6 times/d. Total energy and nutrient intakes were calculated by summing energy or nutrient intakes from all foods. We summed the intakes of single items to create a total of sugar-sweetened soda and diet soda. A previous validation study in participants in the NHS found reasonably high correlation coefficients for most of the dietary factors. For example, the correlation coefficients between the FFQ and multiple dietary records were 0.84 for Coke (Coca-Cola Company) and Pepsi (PepsiCo) colas and 0.36 for artificially sweetened beverages (19). We used cumulative average estimates of soda consumption to reflect long-term dietary habits and to reduce measurement error (20). Previous studies suggested that inflammatory biomarkers of RA are elevated before the development of RA (17), and patients with preclinical RA typically have early symptoms such as joint pain, which may lead them to change their usual diet. To reduce the possibility of such reverse causation, our primary analysis was a lag analysis in which soda intake was used to predict incident RA that occurred at least 2 y later. For example, to predict RA incidence during the 1992–1994 time period, we used cumulative soda intake that was obtained by averaging the daily consumption reported between 1980 and 1990 (excluding the 1992 measure, the most recent exposure).
Assessment of covariates
In the biennial follow-up questionnaires, we used time-varying information on a participant's age, body weight, smoking status, menopausal status, use of postmenopausal hormone therapy, multivitamin use, oral contraceptive use, and history of chronic diseases. Information on alcohol consumption, physical activity, census tract family median income, parity, breastfeeding status, and age at menarche was also collected in both cohorts. All information on covariates was self-reported on the mailed questionnaires administered every 2 y since 1976 in the NHS and since 1989 in the NHS II. BMI was calculated as weight in kilograms divided by height in meters squared. In the NHS, recreational physical activity was measured biennially beginning in 1986 with a validated questionnaire that asked about the average time spent on 10 common activities. In the NHS II, similar measures were performed in 1989, 1991, 1997, 2001, and 2005. The information was summed and calculated as weekly energy expenditure in metabolic equivalent hours weighting each activity by its intensity level (21). We used an Alternate Healthy Eating Index, a score that measures adherence to a diet pattern on the basis of 11 foods and nutrients most predictive of reduced disease risk in the literature, to adjust for healthy dietary pattern (22).
Statistical analyses
Time-varying Cox proportional hazards models were used to examine the associations between sugar-sweetened soda consumption and risk of all RA, seropositive RA, and seronegative RA. Separate models were used to examine the associations between diet soda consumption and risk of all RA, seropositive RA, and seronegative RA. Baseline characteristics were presented as means ± SDs for continuous variables, and percentages were used for categorical variables. Cumulative average soda consumption was included in the model as a time-varying exposure and updated until 2 questionnaires before RA diagnosis (excluding the most recent questionnaire); similarly, age, smoking status, physical activity, alcohol consumption, postmenopausal hormone use, dietary variables, and BMI were included in the multivariate model as time-varying covariates. Soda consumption was first included in the model with 4 groups (<1 serving/mo, 1–4 servings/mo, 2–6 servings/wk, or ≥1 servings/d) on the basis of previous studies (13, 14, 23), and then the median value of soda consumption within each group was used as a continuous variable to calculate the P value for trend. Each multivariable model adjusted for a series of potential confounders and RA risk factors including age, census tract median family income (quartiles), cigarette smoking status (never; past; current, 1–14 cigarettes/d; or current, ≥15 cigarettes/d), cumulative average alcohol consumption (<5.0, 5.0–15.0, or >15 g/d), age at menarche (<12, 12, or >12 y), parity and breastfeeding (nulliparous, parous/no breastfeeding, parous/1–12 mo breastfeeding, parous/>12 mo breastfeeding), hormone use (premenopausal, postmenopausal with never use, current use, and past use), physical activity (0–2.9, 3–8.9, 9–17.9, 18–26.9, or ≥27 metabolic equivalent task-hours/wk), BMI (in kg/m2; <20, 20–22.9, 23–24.9, 25–29.9, or ≥30), diabetes history, multivitamin use, Alternate Healthy Eating Index (quartiles), and total energy (kcal, quintiles). To examine whether the association between soda consumption and RA was mediated by weight gain, we further adjusted for weight change in the model. The weight change was calculated as the difference between current weight and weight in 1978 for the NHS and weight in 1989 for the NHS II and was categorized into the following 5 categories: −2 kg or less, −1.9 to 1.9 kg, 2–9.9 kg, 10–19.9 kg, or ≥20 kg. Because the participants in the NHS were older than participants in the NHS II in which RA cases mostly developed before age 55 y, we developed separate models for the RA cases diagnosed at or before age 55 y and after age 55 y in the NHS to evaluate whether the association between soda consumption and risk of RA varied between early- and late-onset RA. A test of heterogeneity (Q statistics) was performed to evaluate whether the association was different for the cohort assuming a linear relation between soda consumption and risk of RA. We also assessed the interactions of soda consumption with smoking pack-years (cutoff of 10 pack-years) to evaluate the potential effect modifications of smoking status. The log-likelihood ratio test was used to calculate P values for interaction. The proportional hazards assumption was tested by including an interaction term between soda consumption and the follow-up time in the Cox proportional model. The proportional hazards assumption was valid in both cohorts (P-interaction = 0.79 in the NHS and 0.94 in the NHS II). Similarly, we also did not find the proportional hazards assumption violation with other covariates in the model.
We performed several sensitivity analyses. First, we used updated time-varying soda consumption (excluding the most recent diet assessment) instead of the cumulative average to assess the association of short-term soda consumption with RA risk. Second, we analyzed soda consumption without a one-questionnaire cycle lag to evaluate the robustness of the primary lagged analysis. Another sensitivity analysis was performed for fruit punch and noncarbonated sweetened beverages instead of only soda as a sugar-sweetened beverage analysis. We also assessed the association between fruit juice consumption and risk of RA. In addition, we tested whether including participants with self-reported cancer in the analysis would affect the interaction by smoking. Finally, we evaluated the substitution effect of several other beverages (diet soda, fruit juice, tea, whole milk, and skim milk) for soda on risk of RA. We estimated the association of substituting a serving of one beverage for another by including both as continuous variables in the same multivariable model. Differences in their β coefficients were used to estimate the HRs for the substitution association, and their variances and covariance matrix were used to derive the 95% CI (20).
The inverse-variance–weighted, fixed-effects model was used to combine the results from 2 cohorts. All statistical tests were 2-sided and performed by using SAS 9.2 for UNIX (SAS Institute).
RESULTS
During 3,381,267 person-years of follow-up (28 y for the NHS and 20 y for the NHS II), we documented 857 cases of RA (559 cases in the NHS, 298 in the NHS II). The age-standardized baseline characteristics of the study population by amounts of sugar-sweetened soda consumption are shown in Table 1 (24). For both cohorts, women who consumed more soda tended to have lower census tract median family income, lower levels of physical activity, lower alcohol and artificially sweetened beverage consumption, higher total energy intake, and poorer diet quality and were less likely to start menarche before age 12 y. Cigarette smoking, oral contraceptive use, and parity, breastfeeding, and postmenopausal status tended to be similar across soda categories. Although higher soda consumption was associated with lower multivitamin and hormone use in the NHS, a similar relation was not observed in the NHS II.
TABLE 1.
Age-adjusted baseline characteristics of study population by frequency of sugar-sweetened soda consumption1
| NHS (n = 79,570) |
NHS II (n = 107,330) |
|||||||
| <1 serving/mo | 1–4 servings/mo | 2–6 servings/wk | ≥1 servings/d | <1 serving/mo | 1–4 servings/mo | 2–6 servings/wk | ≥1 servings/d | |
| No. of participants | 36,100 | 9814 | 25,915 | 7741 | 64,336 | 8570 | 25,237 | 9187 |
| Age (y) | 49.6 ± 7.12 | 49.1 ± 7.1 | 47.5 ± 7.1 | 46.3 ± 7.1 | 38.9 ± 4.6 | 38.6 ± 4.7 | 38.3 ± 4.7 | 38.1 ± 4.8 |
| BMI (kg/m2) | 24.5 ± 4.5 | 24.3 ± 4.3 | 24.6 ± 4.5 | 25.2 ± 5.1 | 25.6 ± 5.7 | 25.0 ± 5.4 | 24.8 ± 5.4 | 25.5 ± 6.4 |
| Census tract median family income ($ ×1000) | 67.2 ± 27.1 | 64.2 ± 25.6 | 62.6 ± 24.5 | 61.4 ± 24.3 | 63.4 ± 23.5 | 61.8 ± 22.8 | 61.0 ± 21.9 | 58.2 ± 20.8 |
| Physical activity (MET-h/wk) | 15.6 ± 22.0 | 13.4 ± 20.3 | 13.0 ± 18.4 | 11.7 ± 17.8 | 23.0 ± 29.2 | 19.6 ± 25.1 | 18.6 ± 24.9 | 16.8 ± 24.1 |
| AHEI score | 50.5 ± 10.9 | 47.7 ± 10.1 | 45.0 ± 9.7 | 42.3 ± 10.1 | 46.9 ± 10.5 | 43.8 ± 9.9 | 40.1 ± 9.4 | 34.2 ± 8.8 |
| Alcohol consumption (g/d) | 7.3 ± 11.2 | 5.5 ± 9.4 | 5.4 ± 9.2 | 6.0 ± 11.4 | 3.2 ± 5.6 | 2.8 ± 5.0 | 2.8 ± 5.2 | 2.3 ± 4.8 |
| Smoking status (%) | 60.2 | 52.2 | 50.9 | 58.7 | 35.3 | 31.7 | 31.5 | 36.5 |
| Multivitamin use (%) | 38.3 | 37.1 | 34.6 | 32.3 | 42.9 | 44.3 | 42.6 | 38.3 |
| Menarche before age 12 y (%) | 24.4 | 23.2 | 21.7 | 21.5 | 25.9 | 24.3 | 21.8 | 20.7 |
| Oral contraceptive use (%) | 50.0 | 49.6 | 49.1 | 49.9 | 83.7 | 82.3 | 82.3 | 84.3 |
| Parous (%) | 92.2 | 92.5 | 93.3 | 92.2 | 68.4 | 69.7 | 72.9 | 70.7 |
| Breastfeeding ≥12 mo (%) | 18.3 | 19.4 | 18.0 | 13.1 | 23.8 | 28.4 | 28.4 | 21.0 |
| Postmenopausal (%) | 40.4 | 40.1 | 40.3 | 40.7 | 4.4 | 4.4 | 4.6 | 5.7 |
| Current hormone use (%) | 16.8 | 16.8 | 15.8 | 14.8 | 23.2 | 39.6 | 36.6 | 36.8 |
| Artificially sweetened soda (servings/d) | 0.6 ± 1.0 | 0.3 ± 0.7 | 0.2 ± 0.6 | 0.4 ± 0.8 | 1.1 ± 1.5 | 0.7 ± 1.1 | 0.5 ± 1.0 | 0.3 ± 1.0 |
| Total energy intake (kcal/d) | 1468.3 ± 474 | 1522.3 ± 479 | 1640.1 ± 497 | 1810.0 ± 535 | 1688.4 ± 516 | 1757.0 ± 527 | 1888.2 ± 546 | 2116.8 ± 567 |
Values (except for age) were standardized to the age distribution of the study population. AHEI, Alternate Healthy Eating Index; MET-h, metabolic equivalent task-hours; NHS, Nurses’ Health Study.
Mean ± SD (all such values).
The association between soda consumption and risk of RA is shown inTable 2. The incident rate of RA was 30 cases per 100,000 person-years in the NHS and 20 cases per 100,000 person-years in the NHS II. Higher soda consumption was significantly associated with increased risk of RA in the NHS but not in the NHS II. Compared with <1 serving/mo, the multivariable-adjusted HR was 1.58 (95% CI: 1.09, 2.27) for ≥1 servings soda intake/d in the NHS and 1.00 (95% CI: 0.62, 1.61) in the NHS II. The pooled multivariable-adjusted HR was 1.33 (95% CI: 1.00, 1.78; P = 0.067). For seropositive RA, it appeared that the association was stronger [HR (95% CI): 1.97 (1.27, 3.07) in the NHS and 1.20 (0.69, 2.10) in the NHS II]. The pooled results showed that participants who consumed >1 serving of soda/d had a 63% (HR: 1.63; 95% CI: 1.15, 2.30) increased risk of seropositive RA than did those who drank <1 serving/mo. No significant association was found for sugar-sweetened soda consumption and seronegative RA in either of the cohorts. The associations remained the same after further adjustment for weight change (data not shown).
TABLE 2.
HRs (95% CIs) for incident RA according to cumulative sugar-sweetened soda consumption in the NHS (1980–2008) and the NHS II (1991–2009)1
| Sugar-sweetened soda consumption |
|||||
| <1 serving/mo | 1–4 servings/mo | 2–6 servings/wk | ≥1 servings/d | P-trend2 | |
| All RA | |||||
| NHS | |||||
| No. of cases/person-years | 229/791,584 | 120/394,359 | 171/570,231 | 39/103,992 | |
| Age-adjusted model | 1 (Reference) | 1.05 (0.84, 1.31) | 1.07 (0.88, 1.31) | 1.46 (1.03, 2.06) | 0.04 |
| Multivariable-adjusted model3 | 1 (Reference) | 1.08 (0.86, 1.36) | 1.14 (0.92, 1.41) | 1.58 (1.09, 2.27) | 0.02 |
| NHS II | |||||
| No. of cases/person-years | 162/820,161 | 45/223,524 | 67/371,324 | 24/106,093 | |
| Age-adjusted model | 1 (Reference) | 1.01 (0.72, 1.41) | 0.93 (0.70, 1.24) | 1.22 (0.79, 1.88) | 0.43 |
| Multivariable-adjusted model3 | 1 (Reference) | 1.05 (0.75, 1.48) | 0.92 (0.68, 1.26) | 1.00 (0.62, 1.61) | 0.94 |
| NHS and NHS II pooled4 | |||||
| Age-adjusted model | 1 (Reference) | 1.04 (0.86, 1.25) | 1.02 (0.87, 1.21) | 1.36 (1.04, 1.78) | 0.03 |
| Multivariable-adjusted model3 | 1 (Reference) | 1.07 (0.89, 1.30) | 1.06 (0.89, 1.27) | 1.33 (1.00, 1.78) | 0.07 |
| Seropositive RA | |||||
| NHS | |||||
| No. of cases/person-years | 137/744,343 | 70/372,396 | 114/534,043 | 28/96,276 | |
| Age-adjusted model | 1 (Reference) | 1.01 (0.76, 1.36) | 1.20 (0.93, 1.54) | 1.76 (1.17, 2.67) | <0.01 |
| Multivariable-adjusted model3 | 1 (Reference) | 1.05 (0.78, 1.41) | 1.31 (1.00, 1.71) | 1.97 (1.27, 3.07) | <0.01 |
| NHS II | |||||
| No. of cases/person-years | 103/804,569 | 26/220,254 | 47/364,446 | 18/103,318 | |
| Age-adjusted model | 1 (Reference) | 0.88 (0.57, 1.36) | 1.03 (0.73, 1.45) | 1.48 (0.90, 2.45) | 0.11 |
| Multivariable-adjusted model3 | 1 (Reference) | 0.93 (0.60, 1.44) | 1.02 (0.70, 1.47) | 1.20 (0.69, 2.10) | 0.48 |
| NHS and NHS II pooled4 | |||||
| Age-adjusted model | 1 (Reference) | 0.97 (0.76, 1.24) | 1.14 (0.93, 1.39) | 1.64 (1.19, 2.26) | <0.01 |
| Multivariable-adjusted model3 | 1 (Reference) | 1.01 (0.79, 1.29) | 1.20 (0.97, 1.49) | 1.63 (1.15, 2.30) | <0.01 |
| Seronegative RA | |||||
| NHS | |||||
| No. of cases/person-years | 92/789,183 | 50/393,180 | 57/568,415 | 11/103,583 | |
| Age-adjusted model | 1 (Reference) | 1.12 (0.79, 1.58) | 0.89 (0.64, 1.25) | 1.03 (0.55, 1.95) | 0.85 |
| Multivariable-adjusted model3 | 1 (Reference) | 1.13 (0.79, 1.61) | 0.89 (0.63, 1.27) | 1.04 (0.53, 2.02) | 0.88 |
| NHS II | |||||
| No. of cases/person-years | 59/818,601 | 19/223,204 | 20/370,615 | 6/105,854 | |
| Age-adjusted model | 1 (Reference) | 1.25 (0.74, 2.11) | 0.78 (0.47, 1.29) | 0.82 (0.35, 1.90) | 0.4 |
| Multivariable-adjusted model3 | 1 (Reference) | 1.32 (0.77, 2.26) | 0.78 (0.45, 1.34) | 0.68 (0.28, 1.68) | 0.29 |
| NHS and NHS II pooled4 | |||||
| Age-adjusted model | 1 (Reference) | 1.15 (0.86, 1.54) | 0.86 (0.65, 1.13) | 0.95 (0.57, 1.58) | 0.54 |
| Multivariable-adjusted model3 | 1 (Reference) | 1.18 (0.88, 1.59) | 0.85 (0.64, 1.15) | 0.90 (0.52, 1.53) | 0.44 |
HRs were calculated by using time-varying Cox proportional hazards models. Sugar-sweetened soda included regular, caffeine-free cola, and other sugar-sweetened carbonated soda. NHS, Nurses’ Health Study; RA, rheumatoid arthritis.
P-trend values were derived from tests of linear trend across categories of soda consumption by treating the median value of each category as a continuous variable.
Adjusted for age, census tract median family income (quartiles), cigarette smoking status (never; past; current, 1–14 cigarettes/d; or current, ≥15 cigarettes/d), alcohol consumption (<5.0, 5.0–15.0, or >15 g/d), age at menarche (<12, 12, or >12 y), parity and breastfeeding (nulliparous, parous/no breastfeeding, parous/1–12 mo breastfeeding, or parous/>12 mo breastfeeding), hormone use (premenopausal, postmenopausal with never use, current use, or past use), physical activity (0–2.9, 3–8.9, 9–17.9, 18–26.9, or ≥27 metabolic equivalent task-hours/wk), BMI (in kg/m2; <20, 20–22.9, 23–24.9, 25–29.9, or ≥30), multivitamin use, Alternate Healthy Eating Index (quintiles), diabetes history, and total energy (kcal, quintiles).
The results from the 2 cohorts were pooled by using an inverse-variance–weighted, fixed-effects meta-analysis.
Because diet soda was a main substitution beverage for sugar-sweetened soda, we further examined whether diet soda consumption was also associated with the development of RA (Table 3). There was no significant association between diet soda consumption and risk of all RA, seropositive RA, and seronegative RA. These results were consistent between both cohorts.
TABLE 3.
HRs (95% CIs) for incident RA according to cumulative diet soda consumption in the NHS (1980–2008) and the NHS II (1991–2009)1
| Diet soda consumption |
|||||
| <1 serving/mo | 1–4 servings/mo | 2–6 servings/wk | ≥1 servings/d | P-trend2 | |
| All RA | |||||
| NHS | |||||
| No. of cases/person-years | 185/641,158 | 70/232,725 | 216/670,981 | 88/315,326 | |
| Age-adjusted model | 1 (Reference) | 1.05 (0.80, 1.39) | 1.10 (0.90, 1.35) | 0.98 (0.75, 1.26) | 0.78 |
| Multivariable-adjusted model3 | 1 (Reference) | 1.02 (0.77, 1.35) | 1.02 (0.83, 1.26) | 0.87 (0.66, 1.13) | 0.24 |
| NHS II | |||||
| No. of cases/person-years | 96/496,498 | 17/116,205 | 89/464,631 | 96/443,760 | |
| Age-adjusted model | 1 (Reference) | 0.72 (0.43, 1.21) | 0.92 (0.69, 1.23) | 1.09 (0.82, 1.44) | 0.27 |
| Multivariable-adjusted model3 | 1 (Reference) | 0.79 (0.46, 1.34) | 0.96 (0.70, 1.31) | 1.03 (0.75, 1.41) | 0.57 |
| NHS and NHS II pooled4 | |||||
| Age-adjusted model | 1 (Reference) | 0.97 (0.76, 1.23) | 1.04 (0.88, 1.23) | 1.02 (0.85, 1.24) | 0.52 |
| Multivariable-adjusted model3 | 1 (Reference) | 0.96 (0.75, 1.23) | 1.00 (0.84, 1.19) | 0.93 (0.76, 1.14) | 0.71 |
| Seropositive RA | |||||
| NHS | |||||
| No. of cases/person-years | 125/600,741 | 39/219,366 | 133/631,367 | 52/295,609 | |
| Age-adjusted model | 1 (Reference) | 0.87 (0.61, 1.25) | 1.00 (0.78, 1.28) | 0.85 (0.61, 1.18) | 0.42 |
| Multivariable adjusted model3 | 1 (Reference) | 0.84 (0.58, 1.21) | 0.93 (0.71, 1.20) | 0.75 (0.53, 1.05) | 0.14 |
| NHS II | |||||
| No. of cases/person-years | 62/486,459 | 10/114,690 | 63/457,377 | 59/434,053 | |
| Age-adjusted model | 1 (Reference) | 0.64 (0.33, 1.25) | 0.98 (0.68, 1.39) | 1.04 (0.73, 1.49) | 0.54 |
| Multivariable-adjusted model3 | 1 (Reference) | 0.70 (0.35, 1.38) | 1.03 (0.70, 1.51) | 0.99 (0.67, 1.46) | 0.87 |
| NHS and NHS II pooled4 | |||||
| Age-adjusted model | 1 (Reference) | 0.81 (0.59, 1.12) | 0.99 (0.81, 1.21) | 0.93 (0.73, 1.19) | 0.95 |
| Multivariable-adjusted model3 | 1 (Reference) | 0.81 (0.58, 1.11) | 0.96 (0.77, 1.19) | 0.84 (0.65, 1.09) | 0.38 |
| Seronegative RA | |||||
| NHS | |||||
| No. of cases/person-years | 60/639,122 | 31/232,061 | 83/668,876 | 36/314,328 | |
| Age-adjusted model | 1 (Reference) | 1.42 (0.92, 2.21) | 1.34 (0.96, 1.89) | 1.26 (0.83, 1.91) | 0.51 |
| Multivariable-adjusted model3 | 1 (Reference) | 1.39 (0.89, 2.17) | 1.23 (0.87, 1.75) | 1.11 (0.72, 1.71) | 0.99 |
| NHS II | |||||
| No. of cases/person-years | 34/495,617 | 7/116,014 | 26/463,798 | 37/442,836 | |
| Age-adjusted model | 1 (Reference) | 0.88 (0.39, 2.00) | 0.80 (0.48, 1.34) | 1.17 (0.73, 1.86) | 0.30 |
| Multivariable-adjusted model3 | 1 (Reference) | 0.97 (0.42, 2.25) | 0.84 (0.48, 1.46) | 1.12 (0.67, 1.88) | 0.45 |
| NHS and NHS II pooled4 | |||||
| Age-adjusted model | 1 (Reference) | 1.28 (0.87, 1.88) | 1.15 (0.87, 1.53) | 1.22 (0.89, 1.66) | 0.23 |
| Multivariable-adjusted model3 | 1 (Reference) | 1.29 (0.87, 1.90) | 1.10 (0.82, 1.48) | 1.11 (0.80, 1.55) | 0.58 |
1HRs were calculated by using time-varying Cox proportional hazards models. Diet soda included low-calorie cola, low-calorie caffeine-free cola, and other low-calorie carbonated beverages. NHS, Nurses’ Health Study; RA, rheumatoid arthritis.
2P values for trend were derived from tests of linear trend across categories of soda consumption by treating the median value of each category as a continuous variable.
3Adjusted for age, census tract median family income (quartiles), cigarette smoking status (never; past; current, 1–14 cigarettes/d; or current, ≥15 cigarettes/d), alcohol consumption (<5.0, 5.0–15.0, or >15 g/d), age at menarche (<12, 12, or >12 y), parity and breastfeeding (nulliparous, parous/no breastfeeding, parous/1–12 mo breastfeeding, or parous/>12 mo breastfeeding), hormone use (premenopausal, postmenopausal with never use, current use, or past use), physical activity (0–2.9, 3–8.9, 9–17.9, 18–26.9, or ≥27 metabolic equivalent task-hours/wk), BMI (in kg/m2; <20, 20–22.9, 23–24.9, 25–29.9, or ≥30), multivitamin use, Alternate Healthy Eating Index (quintiles), diabetes history, and total energy (kcal, quintiles).
4The results from 2 cohorts were pooled by using an inverse-variance–weighted, fixed-effects meta-analysis.
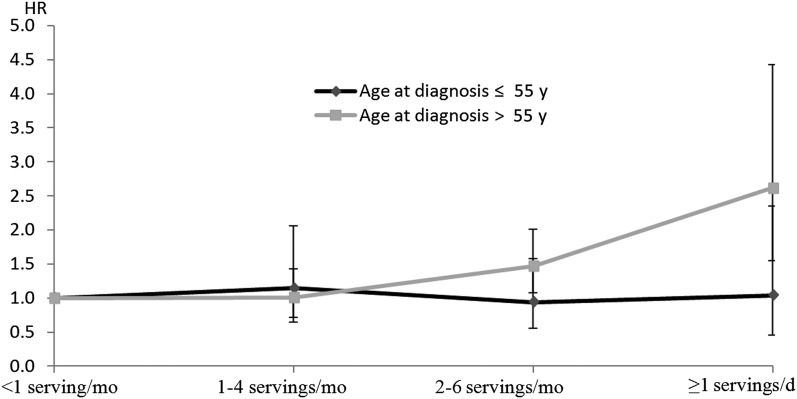
In the NHS, only 25% of the RA cases were diagnosed before age 55 y, whereas ˃80% of cases in the NHS II were diagnosed at <55 y of age. Consistent with the NHS II, no significant association was found when we restricted analyses to those with earlier seropositive RA onset (at or before age 55 y) in the NHS. For cases diagnosed after age 55 y, a stronger association was observed in the NHS (HR: 2.62; 95% CI: 1.55, 4.43 for ≥1 servings/d compared with <1 serving soda/mo) after adjustment for a series of lifestyle and dietary covariates (Figure 1). The P value for test of heterogeneity (Q statistics) was <0.001. The results for the NHS II were not shown because only 61 cases occurred after age 55, so the multivariable-adjusted model did not converge. The sensitivity analysis with the use of updated time-varying soda with lagged analysis showed an association similar to the main analysis, but the associations were attenuated in both cohorts. The pooled multivariable-adjusted HR for the comparison of the highest consumption category to the lowest consumption category for seropositive RA was 1.51 (95% CI: 1.03, 2.22; P-trend = 0.045) (Supplemental Table 1 under “Supplemental data” in the online issue). Similar results were also observed in the analysis that did not use the lagged design (which included the most recent exposure), and the pooled multivariable-adjusted HR was 1.48 (95% CI: 1.03, 2.13; P-trend = 0.02; data not shown). In the NHS, we found that the association of soda intake with RA risk was stronger among smokers with ≥10 pack-years compared with nonsmokers or light smokers with <10 pack-years (P-interaction = 0.02). For women with pack-years ≥10, those who consumed >1 serving of soda/d had a 2-fold increased risk (HR: 2.00; 95% CI: 1.22, 3.29) of developing RA than did those who consumed <1 serving/mo, whereas for those with <10 pack-years the corresponding HR was 1.23 (95% CI: 0.70, 2.14). However, we did not find any effect modification of smoking in the NHS II population. The P value for interaction did not change much in the analysis that included self-reported cancer. We also did not observe any significant interaction for alcohol consumption and BMI. After including fruit punch with sugar-sweetened soda for total sweetened beverage consumption, the observed association was attenuated to borderline statistical significance for seropositive RA. The pooled multivariable-adjusted HR for comparison of the highest consumption category to the lowest consumption category was 1.32 (95% CI: 0.96, 1.82; P-trend = 0.049; data not shown). We did not observe a significant association between fruit juice consumption and the risk of RA.
FIGURE 1.
HRs for incident seropositive rheumatoid arthritis according to sugar-sweetened soda consumption in the Nurses’ Health Study (1980–2008) stratified by age at diagnosis of rheumatoid arthritis. HRs were calculated by using time-varying Cox proportional hazards models. Results were adjusted for age, census tract median family income (quartiles), cigarette smoking status (never; past; current, 1–14 cigarettes/d; or current, ≥15 cigarettes/d), alcohol consumption (<5.0, 5.0–15.0, or ≥15 g/d), age at menarche (<12, 12, or >12 y), parity and breastfeeding (nulliparous, parous/no breastfeeding, parous/1–12 mo breastfeeding, or parous/>12 mo breastfeeding), hormone use (premenopausal, postmenopausal with never use, current use, or past use), physical activity (0–2.9, 3–8.9, 9–17.9, 18–26.9, or ≥27 metabolic equivalent task hours/wk), BMI (in kg/m2; <20, 20–22.9, 23–24.9, 25–29.9, or ≥30), multivitamin use, Alternate Healthy Eating Index (quintiles), diabetes history, and total energy (kcal, quintiles). Reference category, <1 serving/mo.
In the substitution analysis, substituting 1 serving of sugar-sweetened soda with 1 serving of skim milk was associated with a lower HR for seropositive RA of 20% (95% CI: 4%, 33%) (Supplemental Figure 1 under “Supplemental data” in the online issue). Substituting water, milk, tea, coffee, fruit juice, or diet soda was not associated with significantly lower HRs for seropositive RA.
DISCUSSION
In these 2 large prospective cohorts, each with >2 decades of follow-up, women who consumed more sugar-sweetened soda had an increased risk of seropositive RA but not seronegative RA. It appeared that the association was stronger for RA cases diagnosed at later ages. Diet soda consumption was not associated with the risk of RA in either of the cohorts. We did not observe significant associations between soda or diet soda consumption and the risk of seronegative RA.
Although numerous studies have shown associations between sugar-sweetened soda and the risk of T2D and CVD (13, 14, 23, 25), this is the first study, to our knowledge, to provide evidence that soda consumption may also play a role in the development of RA. The mechanism underlying this association is not completely understood. Sodas are composed of energy-containing sweeteners such as high-fructose corn syrup and sucrose and are thought to be associated with an increased risk of T2D and CVD through weight gain, insulin resistance, and inflammation (14). However, in our analysis, the association was not attenuated after adjustment for weight change from baseline and current BMI, suggesting that the deleterious effect of soda on RA development might not be through weight gain and obesity. A possible role of sugar in the etiology of RA may be through increased risk of infection. Published literature implicated refined dietary sugar, particularly sucrose, as a key causative agent in the development of periodontal infectious disease, which may share common pathologic mechanisms with RA (26, 27). There is also moderate evidence suggesting that infections increase the risk of RA (28). Exposure to infection may act as a trigger for RA, and a number of agents have been implicated; however, the epidemiologic data so far are inconclusive (29). The pathophysiology of RA involves several inflammatory cascades, which lead to the overproduction of many cytokines such as TNF and IL-6, and results in subsequent synovial inflammation and joint destruction. A recent study linking soda intake with coronary artery disease risk assessed cross-sectional associations between soda consumption and biomarkers and found that intake of soda was associated with significantly higher C-reactive protein, IL-6, and TNF receptor 2 concentrations (30). These results might provide a possible explanation for the potential mechanism underlying our findings, because it was reported that the advanced glycosylation products present in the caramel coloring of soda might increase insulin resistance in animal models (31, 32). Moreover, some studies found that insulin resistance was prevalent among RA patients, and the HOMA-IR was associated with inflammatory markers including IL-6, TNF receptor 2, and C-reactive protein (33, 34). In addition, soda intake was shown to be associated with high glycemic load by rapidly increasing blood glucose and insulin concentrations (35) and a high glycemic load was reported to induce insulin resistance and elevated concentrations of inflammatory biomarkers (36, 37). These lines of evidences may support the hypothesis that soda intake might increase the risk of RA by virtue of upregulating the inflammatory cytokines and decreasing insulin sensitivity.
Our results showed that diet soda was not associated with the risk of RA. Artificial sweeteners might have very different metabolic effects compared with added sugars. In a double-blinded randomized clinical trial, diet soda was shown to be a good alternative for sugar-sweetened soda in weight control (38). Evidence from large cohort studies suggested that diet soda consumption was not significantly associated with T2D and coronary artery disease (13, 14, 23). Also, short-term intervention studies found that consumption of artificial sweetener–containing food was associated with weight loss and reduced concentrations of inflammatory markers (39, 40). Because diet soda does not provide evidence to support a role in the development of inflammation, it is unlikely to contribute to the risk of RA.
An interesting finding in our study was that soda consumption was related only to increased risk of seropositive RA but not seronegative RA. Seropositive RA consists of the majority of RA cases, and ∼50–80% of patients with RA have positive RF, ACPA, or both (1). Compared with seronegative RA, seropositive RA has been more strongly associated with environmental risk factors such as cigarette smoking, which may induce the immunologic reaction against citrullinated peptides (41) and is also associated with a poorer prognosis (42). Soda may contribute to the genesis of autoantibodies that lead to the inflammatory cascades which might interact with other environmental risk factors such as cigarette smoking and diet, but the detailed biological mechanism remains unclear.
In the current analysis, we found that the associations were not quite consistent in the 2 cohorts. There were 2 main reasons that might account for this discrepancy. First, our analysis suggested that soda consumption was only associated with later onset of seropositive RA. Participants in the NHS II were, on average, ∼10 y younger than those in the NHS. Only 17% of the RA cases occurred at or after age 55 y in the NHS II, whereas 75% of cases were later onset in the NHS. Because the relation between dietary factors and disease usually exhibits long-term cumulative effects, it might be the additive detrimental effect of soda over years that results in chronic inflammation and finally leads to the development of RA. Second, there were significantly fewer cases in the NHS II than in the NHS, which might result in insufficient power to detect the potentially different risk of RA between higher soda consumption groups and lower soda consumption groups. Accordingly, it may be possible to find a stronger association between soda consumption and risk of seropositive RA during a longer period of follow-up in the NHS II when more RA cases occur among elderly women.
In sensitivity analyses that did not exclude the most recent dietary record, the association between soft drink consumption and seropositive RA was attenuated but remained significant, suggesting that preclinical symptoms of RA may lead to diet changes and reverse causation bias in analyses that include recent diet assessment. Analysis that used updated soda consumption (excluding the most recent dietary record) also showed attenuated associations, suggesting that long-term consumption of soda might be more relevant to the development of RA compared with short-term consumption. For the interaction analysis, we found systematically higher risks of developing RA across soda consumption groups among smokers with ≥10 pack-years in the NHS. This result might suggest a synergic effect between soda consumption and cigarette smoking on the development of inflammation, because cigarette smoking is associated with increased concentrations of inflammatory markers (43). In examining the relation between fruit juice and fruit punch and the risk of RA, we did not find any significant association in either of the cohorts. Unlike soda, which consists of large amounts of added high-fructose corn syrup and advanced glycosylation end products, fruit juices contain many beneficial components such as vitamins, minerals, soluble fiber, and phytochemicals that may impede systemic oxidation and inflammation (44). Our analysis showed that skim milk might be a good alternative for sugar-sweetened soda in lowering RA risk. A previous study found an inverse association between milk products and risk of RA perhaps through the immunomodulating effects of vitamin D (45). Although the potential biological mechanism is not known, it was reported that milk's many anti-inflammatory components such as lactadherin human milk oligosaccharides and IL-10 (46) might contribute to the decreased risk of RA.
Our study had several limitations. First, the study population included only women, so whether the association could also be present in men remains unknown. Because of the nature of observational study, it is not possible to establish causality between soda consumption and risk of RA, because we may not rule out the possibility of unmeasured confounding that might affect the observed association. However, we controlled for many known RA risk factors that were also plausibly associated with soda consumption, which minimized the impact of residual confounding.
In conclusion, our findings suggest that frequent intake of sugar-sweetened soda may be associated with increased risk of later-onset seropositive RA. More epidemiologic studies are required to confirm the association and to elucidate the potential biological mechanism.
Supplementary Material
Acknowledgments
We thank the participants in the NHS and NHS II cohorts for their dedication and continued participation in these longitudinal studies as well as NHS staffs in the Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, for their assistance with this project.
The authors’ responsibilities were as follows—YH and BL: designed the research, analyzed data, and wrote the manuscript; EWK, KHC, and MA-D: collected the data and interpreted the results; FBH, XG, JAS, and DHS: critically revised the manuscript. All of the authors were involved in drafting the article or revising it critically for important intellectual content and approved the final version to be published. None of the authors had a conflict of interest to declare.
Footnotes
Abbreviations used: ACPA, anticitrullinated peptide antibody; CVD, cardiovascular disease; FFQ, food-frequency questionnaire; NHS, Nurses’ Health Study; RA, rheumatoid arthritis; RF, rheumatoid factor; T2D, type 2 diabetes.
REFERENCES
- 1.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 2010;376:1094–108. [DOI] [PubMed] [Google Scholar]
- 2.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–19. [DOI] [PubMed] [Google Scholar]
- 3.Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev 2005;4:130–6. [DOI] [PubMed] [Google Scholar]
- 4.Lu B, Solomon DH, Costenbader KH, Keenan BT, Chibnik LB, Karlson EW. Alcohol consumption and markers of inflammation in women with preclinical rheumatoid arthritis. Arthritis Rheum 2010;62:3554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao KP, Alfredsson L, Karlson EW. Environmental influences on risk for rheumatoid arthritis. Curr Opin Rheumatol 2009;21:279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferraz Amaro I, Díaz González F, González Juanatey C, González Gay MA. Insulin resistance and rheumatoid arthritis. Rheumatol Clin 2011;7:124–9. [DOI] [PubMed] [Google Scholar]
- 7.Nurmohamed MT. Cardiovascular risk in rheumatoid arthritis. Autoimmun Rev 2009;8:663–7. [DOI] [PubMed] [Google Scholar]
- 8.Solomon DH, Love TJ, Canning C, Schneeweiss S. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis 2010;69:2114–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart JE, Laden F, Puett RC, Costenbader KH, Karlson EW. Exposure to traffic pollution and increased risk of rheumatoid arthritis. Environ Health Perspect 2009;117:1065–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benito-Garcia E, Feskanich D, Hu FB, Mandl LA, Karlson EW. Protein, iron, and meat consumption and risk for rheumatoid arthritis: a prospective cohort study. Arthritis Res Ther 2007;9:R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlson EW, Mandl LA, Aweh GN, Grodstein F. Coffee consumption and risk of rheumatoid arthritis. Arthritis Rheum 2003;48:3055–60. [DOI] [PubMed] [Google Scholar]
- 12.Malik VS, Popkin BM, Bray GA, Després J-P, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010;121:1356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr 2009;89:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34. [DOI] [PubMed] [Google Scholar]
- 15.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, Liang MH. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol 1995;5:297–302. [DOI] [PubMed] [Google Scholar]
- 16.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 17.Karlson EW, Chibnik LB, Tworoger SS, Lee I-M, Buring JE, Shadick NA, Manson JE, Costenbader KH. Biomarkers of inflammation and development of rheumatoid arthritis in women from two prospective cohort studies. Arthritis Rheum 2009;60:641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlson EW, Mandl LA, Hankinson SE, Grodstein F. Do breast-feeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the Nurses’ Health Study. Arthritis Rheum 2004;50:3458–67. [DOI] [PubMed] [Google Scholar]
- 19.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 20.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–40. [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Montoye HJ, Sallis JF, Paffenbarger RS. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25:71–80. [DOI] [PubMed] [Google Scholar]
- 22.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr 2011;93:1321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inskip H, Beral V, Fraser P, Haskey J. Methods for age-adjustment of rates. Stat Med 1983;2:455–66. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein AM, De Koning L, Flint AJ, Rexrode KM, Willett WC. Soda consumption and the risk of stroke in men and women. Am J Clin Nutr 2012;95:1190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genco RJ, Genco FD. Common risk factors in the management of periodontal and associated systemic diseases: the Dental Setting and Interprofessional Collaboration. J Evid Based Dent Pract 2014;14(suppl):4–16. [DOI] [PubMed] [Google Scholar]
- 27.Bartold PM, Marshall RI, Haynes DR. Periodontitis and rheumatoid arthritis: a review. J Periodontol 2005;76:2066–74. [DOI] [PubMed] [Google Scholar]
- 28.Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum 2002;46:2287–93. [DOI] [PubMed] [Google Scholar]
- 29.Silman AJ, Pearson JE. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res 2002;4(suppl 3):S265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation 2012;125:1735ndash41.. [DOI] [PMC free article] [PubMed]
- 31.Hofmann SM, Dong H-J, Li Z, Cai W, Altomonte J, Thung SN, Zeng F, Fisher EA, Vlassara H. Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes 2002;51:2082–9. [DOI] [PubMed] [Google Scholar]
- 32.Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, Peppa M, Rayfield EJ. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic. Proc Natl Acad Sci USA 2002;99:15596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung CP, Oeser A, Solus JF, Gebretsadik T, Shintani A, Avalos I, Sokka T, Raggi P, Pincus T, Stein CM. Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum 2008;58:2105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dessein PH, Joffe BI. Insulin resistance and impaired beta cell function in rheumatoid arthritis. Arthritis Rheum 2006;54:2765–75. [DOI] [PubMed] [Google Scholar]
- 35.Janssens JP, Shapira N, Debeuf P, Michiels L, Putman R, Bruckers L, Renard D, Molenberghs G. Effects of soft drink and table beer consumption on insulin response in normal teenagers and carbohydrate drink in youngsters. Eur J Cancer Prev 1999;8:289–95. [DOI] [PubMed] [Google Scholar]
- 36.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002;287:2414–23. [DOI] [PubMed] [Google Scholar]
- 37.Liu S, Manson JE, Buring JE, Stampfer MJ, Willett WC, Ridker PM. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr 2002;75:492–8. [DOI] [PubMed] [Google Scholar]
- 38.de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med 2012;367:1397–406. [DOI] [PubMed] [Google Scholar]
- 39.Sørensen LB, Raben A, Stender S, Astrup A. Effect of sucrose on inflammatory markers in overweight humans. Am J Clin Nutr 2005;82:421–7. [DOI] [PubMed] [Google Scholar]
- 40.Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr 2002;76:721–9. [DOI] [PubMed] [Google Scholar]
- 41.Szodoray P, Szabó Z, Kapitány A, Gyetvai Á, Lakos G, Szántó S, Szücs G, Szekanecz Z. Anti-citrullinated protein/peptide autoantibodies in association with genetic and environmental factors as indicators of disease outcome in rheumatoid arthritis. Autoimmun Rev 2010;9:140–3. [DOI] [PubMed] [Google Scholar]
- 42.van der Linden MPM, van der Woude D, Ioan-Facsinay A, Levarht EWN, Stoeken-Rijsbergen G, Huizinga TWJ, Toes REM, van der Helm-van Mill AHM. Value of anti–modified citrullinated vimentin and third-generation anti–cyclic citrullinated peptide compared with second-generation anti–cyclic citrullinated peptide and rheumatoid factor in predicting disease outcome in undifferentiated arthritis and rheumatoid arthritis . Arthritis Rheum 2009;60:2232–41. [DOI] [PubMed] [Google Scholar]
- 43.Levitzky YS, Guo C-Y, Rong J, Larson MG, Walter RE, Keaney JF, Sutherland PA, Vasan A, Lipinska I, Evans JC, et al. Relation of smoking status to a panel of inflammatory markers: the Framingham offs pring. Atherosclerosis 2008;201:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamprecht M, Obermayer G, Steinbauer K, Cvirn G, Hofmann L, Ledinski G, Greilberger JF, Hallstroem S. Supplementation with a juice powder concentrate and exercise decrease oxidation and inflammation, and improve the microcirculation in obese women: randomised controlled trial data. Br J Nutr 2013;110:1685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merlino LA, Curtis J, Mikuls T, Cerhan J, Criswell L, Saag K. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum 2004;50:72–7. [DOI] [PubMed] [Google Scholar]
- 46.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 2013;60:49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.