Abstract
The FhuA receptor protein is involved in energy-coupled transport of Fe3+ via ferrichrome through the outer membrane of Escherichia coli. Since no energy source is known in the outer membrane it is assumed that energy is provided through the action of the TonB, ExbB and ExbD proteins, which are anchored to the cytoplasmic membrane. By deleting 34 amino acid residues of a putative cell surface exposed loop, FhuA was converted from a ligand specific transport protein into a TonB independent and nonspecific diffusion channel. The FhuA deletion derivative FhuA delta 322-355 formed stable channels in black lipid membranes, in contrast to wild-type FhuA which did not increase membrane conductance. The single-channel conductance of the FhuA mutant channels was at least three times larger than that of the general diffusion porins of E. coli outer membrane. It is proposed that the basic structure of FhuA in the outer membrane is a channel formed by beta-barrels. Since the loop extending from residue 316 to 356 is part of the active site of FhuA, it probably controls the permeability of the channel. The transport-active conformation of FhuA is mediated by a TonB-induced conformational change in response to the energized cytoplasmic membrane. The ferrichrome transport rate into cells expressing FhuA delta 322-355 increased linearly with increasing substrate concentration (from 0.5 to 20 microM), in contrast to FhuA wild-type cells, which displayed saturation at 5 microM. This implies that in wild-type cells ferrichrome transport through the outer membrane is the rate-limiting step and that TonB, ExbB and ExbD are only required for outer membrane transport.
Full text
PDF


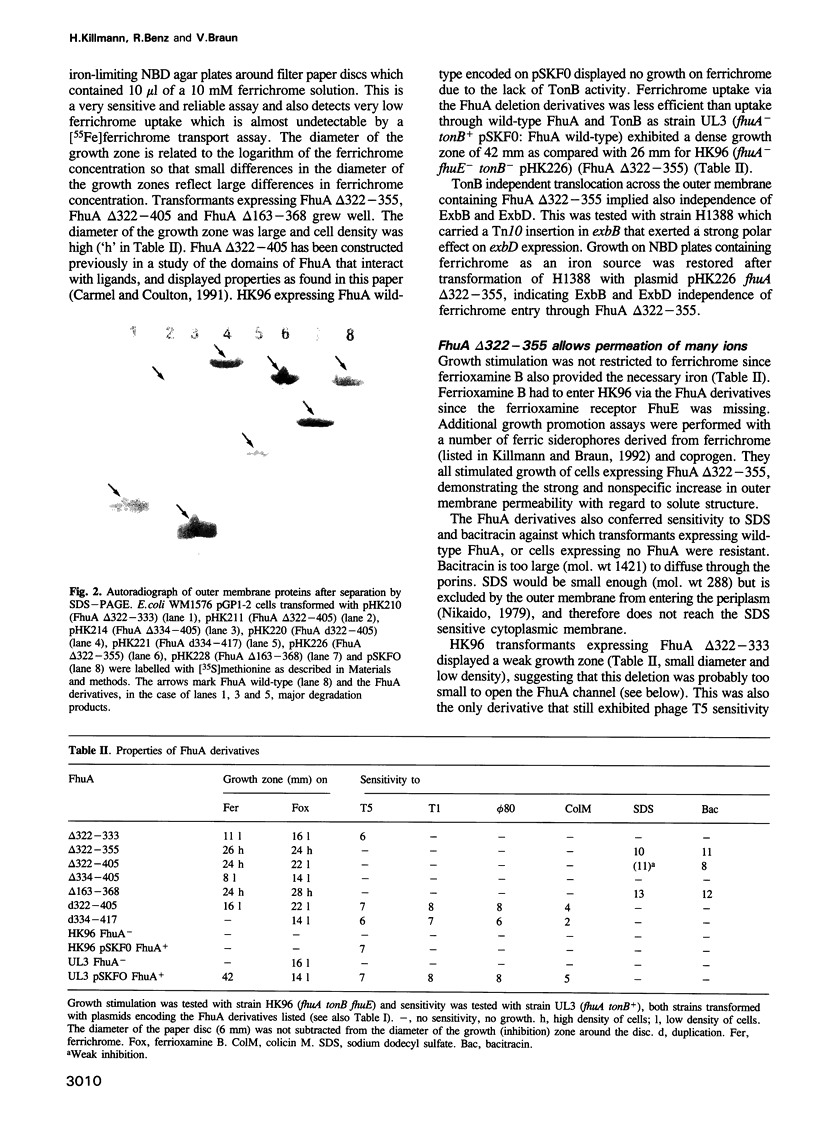
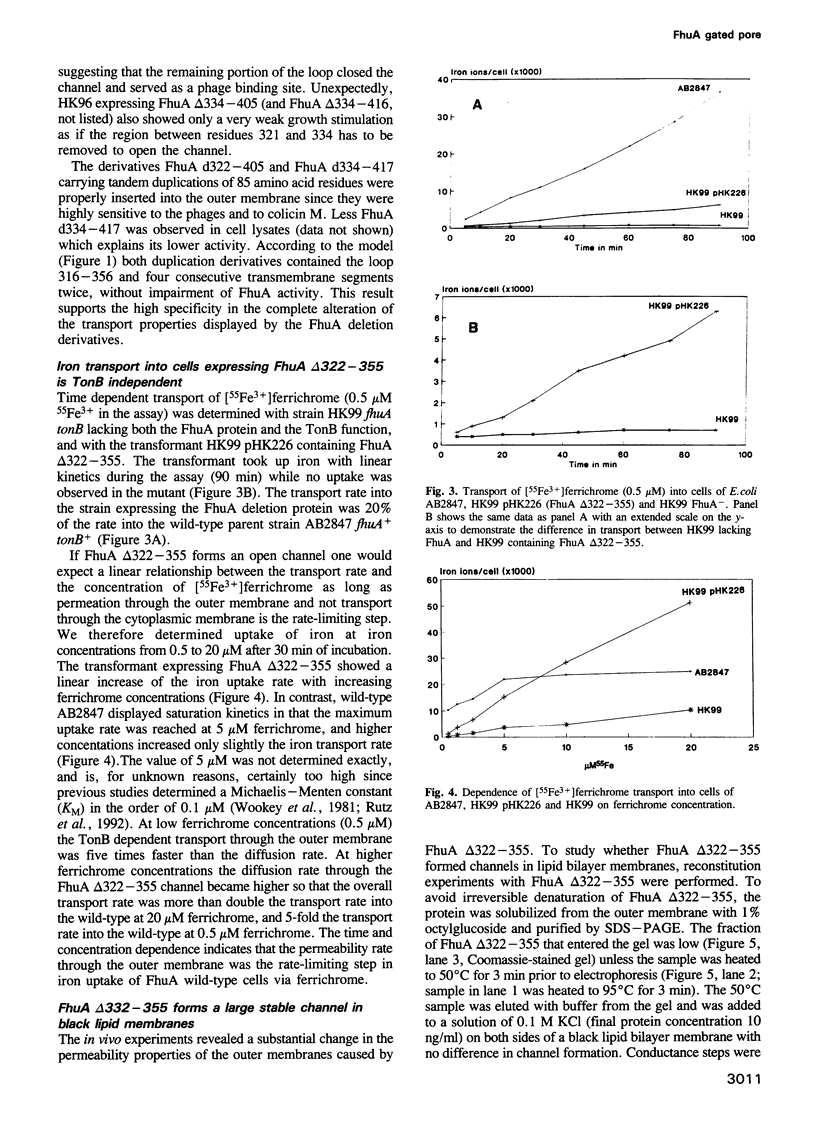
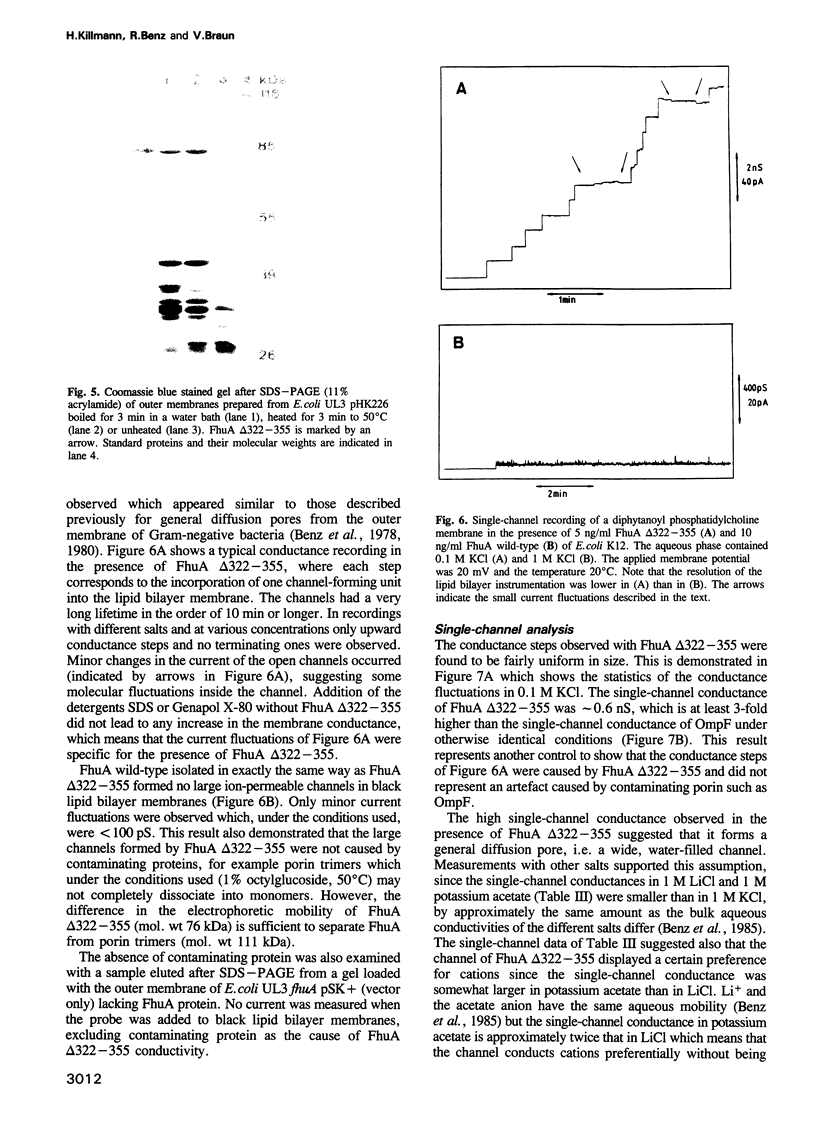



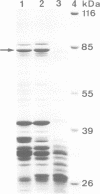
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Ishii J., Nakae T. Determination of ion permeability through the channels made of porins from the outer membrane of Salmonella typhimurium in lipid bilayer membranes. J Membr Biol. 1980 Aug 21;56(1):19–29. doi: 10.1007/BF01869348. [DOI] [PubMed] [Google Scholar]
- Benz R., Janko K., Boos W., Läuger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1978 Aug 17;511(3):305–319. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- Benz R., Schmid A., Hancock R. E. Ion selectivity of gram-negative bacterial porins. J Bacteriol. 1985 May;162(2):722–727. doi: 10.1128/jb.162.2.722-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Schmid A., Nakae T., Vos-Scheperkeuter G. H. Pore formation by LamB of Escherichia coli in lipid bilayer membranes. J Bacteriol. 1986 Mar;165(3):978–986. doi: 10.1128/jb.165.3.978-986.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R. Structure and function of porins from gram-negative bacteria. Annu Rev Microbiol. 1988;42:359–393. doi: 10.1146/annurev.mi.42.100188.002043. [DOI] [PubMed] [Google Scholar]
- Braun V., Frenz J., Hantke K., Schaller K. Penetration of colicin M into cells of Escherichia coli. J Bacteriol. 1980 Apr;142(1):162–168. doi: 10.1128/jb.142.1.162-168.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V. The structurally related exbB and tolQ genes are interchangeable in conferring tonB-dependent colicin, bacteriophage, and albomycin sensitivity. J Bacteriol. 1989 Nov;171(11):6387–6390. doi: 10.1128/jb.171.11.6387-6390.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer S., Tolley M., Trayer I. P., Barr G. C., Dorman C. J., Hannavy K., Higgins C. F., Evans J. S., Levine B. A., Wormald M. R. Structure and function of X-Pro dipeptide repeats in the TonB proteins of Salmonella typhimurium and Escherichia coli. J Mol Biol. 1990 Dec 20;216(4):883–895. doi: 10.1016/S0022-2836(99)80008-4. [DOI] [PubMed] [Google Scholar]
- Bäumler A. J., Hantke K. A lipoprotein of Yersinia enterocolitica facilitates ferrioxamine uptake in Escherichia coli. J Bacteriol. 1992 Feb;174(3):1029–1035. doi: 10.1128/jb.174.3.1029-1035.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel G., Coulton J. W. Internal deletions in the FhuA receptor of Escherichia coli K-12 define domains of ligand interactions. J Bacteriol. 1991 Jul;173(14):4394–4403. doi: 10.1128/jb.173.14.4394-4403.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Mason P., Cameron D. R., Carmel G., Jean R., Rode H. N. Protein fusions of beta-galactosidase to the ferrichrome-iron receptor of Escherichia coli K-12. J Bacteriol. 1986 Jan;165(1):181–192. doi: 10.1128/jb.165.1.181-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan S. W., Schirmer T., Rummel G., Steiert M., Ghosh R., Pauptit R. A., Jansonius J. N., Rosenbusch J. P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992 Aug 27;358(6389):727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- Eick-Helmerich K., Braun V. Import of biopolymers into Escherichia coli: nucleotide sequences of the exbB and exbD genes are homologous to those of the tolQ and tolR genes, respectively. J Bacteriol. 1989 Sep;171(9):5117–5126. doi: 10.1128/jb.171.9.5117-5126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E., Günter K., Braun V. Involvement of ExbB and TonB in transport across the outer membrane of Escherichia coli: phenotypic complementation of exb mutants by overexpressed tonB and physical stabilization of TonB by ExbB. J Bacteriol. 1989 Sep;171(9):5127–5134. doi: 10.1128/jb.171.9.5127-5134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günter K., Braun V. In vivo evidence for FhuA outer membrane receptor interaction with the TonB inner membrane protein of Escherichia coli. FEBS Lett. 1990 Nov 12;274(1-2):85–88. doi: 10.1016/0014-5793(90)81335-l. [DOI] [PubMed] [Google Scholar]
- Hancock R. W., Braun V. Nature of the energy requirement for the irreversible adsorption of bacteriophages T1 and phi80 to Escherichia coli. J Bacteriol. 1976 Feb;125(2):409–415. doi: 10.1128/jb.125.2.409-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannavy K., Barr G. C., Dorman C. J., Adamson J., Mazengera L. R., Gallagher M. P., Evans J. S., Levine B. A., Trayer I. P., Higgins C. F. TonB protein of Salmonella typhimurium. A model for signal transduction between membranes. J Mol Biol. 1990 Dec 20;216(4):897–910. doi: 10.1016/S0022-2836(99)80009-6. [DOI] [PubMed] [Google Scholar]
- Hantke K., Braun V. Functional interaction of the tonA/tonB receptor system in Escherichia coli. J Bacteriol. 1978 Jul;135(1):190–197. doi: 10.1128/jb.135.1.190-197.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann H., Fischer E., Kraut H., Braun V. Preparation of the FhuA (TonA) receptor protein from cell envelopes of an overproducing strain of Escherichia coli K-12. J Bacteriol. 1986 May;166(2):404–411. doi: 10.1128/jb.166.2.404-411.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J. Vitamin B12 transport in Escherichia coli: energy coupling between membranes. Mol Microbiol. 1990 Dec;4(12):2027–2033. doi: 10.1111/j.1365-2958.1990.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Kampfenkel K., Braun V. Membrane topology of the Escherichia coli ExbD protein. J Bacteriol. 1992 Aug;174(16):5485–5487. doi: 10.1128/jb.174.16.5485-5487.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampfenkel K., Braun V. Topology of the ExbB protein in the cytoplasmic membrane of Escherichia coli. J Biol Chem. 1993 Mar 15;268(8):6050–6057. [PubMed] [Google Scholar]
- Killmann H., Braun V. An aspartate deletion mutation defines a binding site of the multifunctional FhuA outer membrane receptor of Escherichia coli K-12. J Bacteriol. 1992 Jun;174(11):3479–3486. doi: 10.1128/jb.174.11.3479-3486.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebnik R., Braun V. Insertion derivatives containing segments of up to 16 amino acids identify surface- and periplasm-exposed regions of the FhuA outer membrane receptor of Escherichia coli K-12. J Bacteriol. 1993 Feb;175(3):826–839. doi: 10.1128/jb.175.3.826-839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster W., Braun V. Iron (III) hydroxamate transport into Escherichia coli. Substrate binding to the periplasmic FhuD protein. J Biol Chem. 1990 Dec 15;265(35):21407–21410. [PubMed] [Google Scholar]
- Nikaido H. Permeability of the outer membrane of bacteria. Angew Chem Int Ed Engl. 1979 May;18(5):337–350. doi: 10.1002/anie.197903373. [DOI] [PubMed] [Google Scholar]
- Olschläger T., Schramm E., Braun V. Cloning and expression of the activity and immunity genes of colicins B and M on ColBM plasmids. Mol Gen Genet. 1984;196(3):482–487. doi: 10.1007/BF00436196. [DOI] [PubMed] [Google Scholar]
- Postle K., Skare J. T. Escherichia coli TonB protein is exported from the cytoplasm without proteolytic cleavage of its amino terminus. J Biol Chem. 1988 Aug 5;263(22):11000–11007. [PubMed] [Google Scholar]
- Rutz J. M., Liu J., Lyons J. A., Goranson J., Armstrong S. K., McIntosh M. A., Feix J. B., Klebba P. E. Formation of a gated channel by a ligand-specific transport protein in the bacterial outer membrane. Science. 1992 Oct 16;258(5081):471–475. doi: 10.1126/science.1411544. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M., Hantke K., Braun V. Ferric-coprogen receptor FhuE of Escherichia coli: processing and sequence common to all TonB-dependent outer membrane receptor proteins. J Bacteriol. 1987 May;169(5):2044–2049. doi: 10.1128/jb.169.5.2044-2049.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffler H., Braun V. Transport across the outer membrane of Escherichia coli K12 via the FhuA receptor is regulated by the TonB protein of the cytoplasmic membrane. Mol Gen Genet. 1989 Jun;217(2-3):378–383. doi: 10.1007/BF02464907. [DOI] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare J. T., Postle K. Evidence for a TonB-dependent energy transduction complex in Escherichia coli. Mol Microbiol. 1991 Dec;5(12):2883–2890. doi: 10.1111/j.1365-2958.1991.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita S., Sato M., Toba M., Masahashi W., Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987;61(1):63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- Weiss M. S., Abele U., Weckesser J., Welte W., Schiltz E., Schulz G. E. Molecular architecture and electrostatic properties of a bacterial porin. Science. 1991 Dec 13;254(5038):1627–1630. doi: 10.1126/science.1721242. [DOI] [PubMed] [Google Scholar]
- Weiss M. S., Kreusch A., Schiltz E., Nestel U., Welte W., Weckesser J., Schulz G. E. The structure of porin from Rhodobacter capsulatus at 1.8 A resolution. FEBS Lett. 1991 Mar 25;280(2):379–382. doi: 10.1016/0014-5793(91)80336-2. [DOI] [PubMed] [Google Scholar]
- Weiss M. S., Schulz G. E. Structure of porin refined at 1.8 A resolution. J Mol Biol. 1992 Sep 20;227(2):493–509. doi: 10.1016/0022-2836(92)90903-w. [DOI] [PubMed] [Google Scholar]
- Wookey P. J., Hussein S., Braun V. Functions in outer and inner membranes of Escherichia coli for ferrichrome transport. J Bacteriol. 1981 Jun;146(3):1158–1161. doi: 10.1128/jb.146.3.1158-1161.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]