Abstract
Fungal filamentous growth depends on continuous membrane insertion at the tip, the delivery of membrane-bound positional markers, and the secretion of enzymes for cell wall biosynthesis. This is achieved through exocytosis. At the same time, polarized growth requires membrane and protein recycling through endocytosis. Endocytic vesicles are thought to enter the protein degradation pathway or recycle their content to the cell surface. In Saccharomyces cerevisiae, the Rcy1 F-box protein is involved in the recycling process of a v-SNARE protein. We identified a Rcy1 orthologue, RcyA, in the filamentous fungus Aspergillus nidulans as a protein interacting with the KipA kinesin-7 motor protein in a yeast two-hybrid screen. The interaction was confirmed through bimolecular fluorescence complementation. RcyA possesses an F-box domain at the N terminus and a prenylation (CaaX) motif at the C terminus. RcyA shows also similarity to Sec10, a component of the exocyst complex. The RcyA protein localized to the hyphal tip and forming septa, likely through transportation on secretory vesicles and partially on early endosomes, but independently of KipA. Deletion of rcyA did not cause severe morphological changes but caused partial defects in the recycling of the SynA v-SNARE protein and the positioning of the cell end markers TeaA and TeaR. In addition, deletion of rcyA led to increased concentrations of the KipA protein, whereas the transcript concentration was unaffected. These results suggest that RcyA probably labels KipA for degradation and thereby controls the protein amount of KipA.
INTRODUCTION
During cellular growth and reproduction, the concentration of many cellular components needs to be increased. However, certain cellular components are required only during certain stages or for special events and thus the turnover of such components needs to be strictly regulated (1). Specific labeling of proteins for degradation occurs through covalent labeling of small modifiers such as ubiquitin. Polyubiquitination depends on E3 ubiquitin ligases in the anaphase-promoting complex/cyclosome (APC/C) or the Skp/Cullin/F-box (SCF) complex. Polyubiquitination allows recognition and subsequent proteolysis of the substrate proteins by the 26S proteasome (1). The core of the SCF complex is formed by Skp1, Rbx1, and cullin (Cul1) as a scaffold protein. Rbx1 resides in the catalytic part of the complex and binds to the ubiquitin ligase, whereas Skp1 interacts with the F-box domain of different proteins. F-box proteins are specific adaptors to E3 ligases and determine the fate of different substrate proteins. The number of F-box proteins ranges from 20 in Saccharomyces cerevisiae to more than 70 in humans. A similar number of F-box proteins was reported in Aspergillus nidulans, 42 of which have already been studied to some extent and play a role in cellular differentiation and sexual development (2–4).
Another function of ubiquitination is related to the recycling of membranes. As an alternative to the cytoplasmic proteasome, monoubiquitination leads to protein degradation through the vacuole/lysosome pathway. Monoubiquitination can also be a signal for membrane protein trafficking, sorting of transmembrane proteins, regulation of transcription factors, and posttranslational histone modifications (5). A protein involved in the recycling of membrane proteins in S. cerevisiae is the F-box protein Rcy1 (6). Rcy1 regulates the endosome-Golgi transport by ubiquitination of recycling proteins, specifically, the recycling of the v-SNARE protein synaptobrevin (Snc1) to a nondegradative (Golgi) compartment. Snc1 is recycled from the Golgi compartment to the plasma membrane via secretory vesicles. Deletion of rcy1 leads to an accumulation of Snc1 at early endosomes and causes a cold-sensitive growth defect. Rcy1 is an effector of the Rab GTPase Ypt31/32 and is also necessary for the recycling of the subtilisin-like protease Kex2 and the phopholipid translocase Cdc50/Drs2 (7, 8). In contrast, the Rcy1 orthologue in Schizosaccharomyces pombe, Pof6, is involved in cell separation and is essential for viability (9). The role of Pof6 and Skp1 in cell separation is unclear, but it was speculated that it could involve the exocyst complex. Moreover, it was proposed that Rcy1/Pof6 forms a non-SCF complex with Skp1 during v-SNARE recycling, but more recent studies have shown reduced ubiquitination of Snc1 in rcy1Δ mutants and an accumulation of (nonubiquitinable) Snc1-K63R in early endosomes, supporting the idea that Rcy1/Skp1 is part of a functional ubiquitin-ligase complex (10).
Growth in filamentous fungi depends on the continuous flow of vesicles, which deliver enzymes for cell wall biosynthesis to the growing tip (11). In addition, subapical endocytosis is required for recycling of excess membrane and membrane-bound proteins (12–15). The direction of vesicle flow is strictly regulated through the orientation and polarization of the microtubule and the actin cytoskeletons. Vesicles can be transported along either cytoskeleton for specific purposes. Whereas microtubules are necessary for long-distance transportation of excocytic vesicles and endosomes, actin serves as track for short-distance transportation of secretory vesicles prior to fusion with the membrane. In Aspergillus nidulans, close to the growing tip, almost all microtubules are oriented with their plus ends toward the tip, whereas actin filaments have their origin at the tip membrane. This organization is achieved through interplay between the two cytoskeletons through the activity of a class of proteins named cell end markers (16, 17). They were discovered in S. pombe and were characterized afterward in A. nidulans (17–20). Cell end markers are transported through microtubules to the tip, are tethered to the membrane through a prenyl anchor, and form a protein complex. One of the protein complex proteins in A. nidulans is the formin SepA. Why the cell end markers remain at the very tip, although continuous membrane insertion should cause their diffusion along the membrane, is still an open issue.
In this work, we report on the characterization of RcyA, the A. nidulans orthologue of Rcy1 and Pof6. In the filamentous fungus, RcyA appears to be involved in membrane recycling through recycling of v-SNARE, which is necessary for the positioning of cell end markers. In addition, RcyA controls the concentration of the kinesin-7 motor protein KipA, suggesting KipA as a novel target of RcyA.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
Supplemented minimal media (MM) for A. nidulans were prepared as described, and standard strain construction procedures were performed as previously described (21). A list of A. nidulans strains used in this study is given in Table 1. Standard laboratory Escherichia coli strains (XL-1 blue and Top 10 F′) were used. Plasmids are listed in Table 2.
TABLE 1.
A. nidulans strains used in this studya
| Strain | Genotype | Reference or source |
|---|---|---|
| TN02A3 | pyrG89 argB2 ΔnkuA::argB pyroA4 | 22 |
| RMS011 | pabaA1 yA2 ΔargB::trpCΔB trpC801 veA1 | 23 |
| SJW02 | wA3 pyroA4 alcA(p)-GFP-tubA ΔargB::trpCΔB | J. Warmbold, Marburg, Germany |
| SSK44 | pabaA1 wA3 ΔargB::trpCΔB ΔkipA::pyr4 veA1 | 24 |
| SSK92 | wA3 pyroA4 alcA(p)-GFP-kipA | 24 |
| SSK114 | pyrG89 ΔargB::trpCΔB pyroA4 veA1 alcA(p)-GFP-kipArigor::pyr-4 | 24 |
| LO1535 | fwA1 nicA2 pyrG89 pyroA4 ΔnkuA::argB synA(p)-GFP-synA | 25 |
| SNT56 | pabaA1 teaA(p)-mRFP1-teaA teaR(p)-GFP-teaR | 19 |
| SNZ14 | alcA(p)-GFP-uncArigor pyroA4 | 26 |
| SSH01 | TN02A3 transformed with pSH13 [alcA(p)-GFP-rcyA] | This study |
| SSH08 | SSK44 crossed with SSH01 [ΔkipA alcA(p)-GFP-rcyA] | This study |
| SSH14 | TN02A3 transformed with pSH30 [alcA(p)-mRFP1-rcyA pyrG89] | This study |
| SSH27 | wA3 yA2 ΔargB::trpCΔB trpC801 [alcA(p)-GFP-kipA] | 27 |
| SSH36 | ΔrcyA::pyrGAf in TN02A3 pyroA4 | This study |
| SSH37 | SSK92 crossed with SSH14 [alcA(p)-GFP-kipA alcA(p)-mRFP1-rcyA] | This study |
| SSH43 | SSH27 crossed with SSH36 [alcA(p)-GFP-kipA ΔrcyA] | This study |
| SSH44 | SNT56 crossed with SSH36 [ΔrcyA teaA(p)-mRFP1-teaA teaR(p)GFP-teaR] | This study |
| SSH55 | SNZ14 crossed with SSH14 [alcA(p)-gfp-uncArigor alcA(p)-mRFP1-rcyA] | This study |
| SSH56 | LO1535 crossed with SSH14 [synA(p)-GFP-synA alcA(p)-mRFP1-rcyA] | This study |
| SSH66 | SSK114 crossed with SSH14 [alcA(p)-GFP-kipArigor alcA(p)-mRFP1-rcyA] | This study |
| SSH67 | TN02A3 transformed with pSH46 [alcA(p)-mRFP1-rcyAΔF-box pyrG89] | This study |
| SSH69 | SSH67 crossed with SSH27 [alcA(p)-mRFP1-rcyAΔF-box alcA(p)-GFP-kipA] | This study |
| SSH73 | TN02A3 transformed with pSH47 [rcy(p)-GFP-rcyA pyroA4] | This study |
| SSH89 | SSH36 crossed with LO1535 [ΔrcyA synA(p)-GFP-synA] | This study |
| SSH91 | SSK114 crossed with SSH14 [alcA(p)-GFP-kinArigor alcA(p)-mRFP1-rcyA] | This study |
| SCos135 | TN02A3 transformed with p1789 [alcA(p)-GFP-rabA pyrG89] | C. Seidel, Karlsruhe, Germany |
| SNG67 | SCos135 crossed with RMS011 [alcA(p)-GFP-rabA pabaA1] | This study |
| SSH93 | SNG67 crossed with SSH14 [alcA(p)-GFP-rabA alcA(p)-mRFP1-rcyA] | This study |
All strains harbored the veA1 mutation in addition.
TABLE 2.
List of plasmids used in this study
| Plasmid | Description | Reference or source |
|---|---|---|
| pCMB17apx | alcA(p)-GFP, for N-terminal tagging of GFP to proteins of interest; contains N. crassa pyr-4 | 28 |
| pDM8 | GFP replaced mRFP1 in pCMB17apx | 29 |
| P1789 | alcA(p)-GFP-rabA::argB | 30 |
| pSH13 | alcA(p)-GFP-rcyA::pyr-4 | This study |
| pSH30 | alcA(p)-mRFP1-rcyA::pyroAAf | This study |
| pSH46 | alcA(p)-mRFP1-rcyAΔF-box::pyroAAf | This study |
| pSH47 | rcyA(p)-GFP-rcyA::pyr4 | This study |
Molecular techniques.
Standard DNA transformation procedures were used for A. nidulans (31) and E. coli (32). For PCR experiments, standard protocols were applied using a Biometra Personal cycler (Biometra, Göttingen) for the reaction cycles. DNA sequencing was done commercially (eurofins-MWG-operon, Ebersberg, Germany). Genomic DNA was extracted from A. nidulans with an innuPREP Plant DNA kit (analytikjena, Jena, Germany). DNA analyses (Southern hybridizations) were performed as described in reference 32.
Deletion of rcyA.
rcyA flanking regions were amplified by PCR using genomic DNA and primers rcyA-p3 (5′-AGGGTGAGCGCACAGCGAA-3′) and rcyA-p1 (5′-GAAGAGCATTGTTTGAGGCAATGTCTTTTCAAGGATTG-3′) for the upstream region of rcyA and rcyA-p5 (5′-ATCAGTGCCTCCTCTCAGACAGTGCAGCAACCGCTAATGTAT-3′) and rcyA-p8 (5′-CGTACAGAGTGCTTCCACTT-3′) for the downstream region. The pyrG gene from plasmid pFNO3 (S. Osmani) was amplified by PCR and used as the template together with rcyA-flanking regions for the fusion-PCR. The deletion cassette was amplified using the fusion-PCR method (33) and primers rcyA-p2 (5′-AGTCGAGAGTTCGAAGTCGT-3′) and rcyA-p7 (5′-AGTCTTTGGCATAGTCCGCA-3′). The resulting PCR product was transformed into pyrG89-auxotrophic A. nidulans strain TN02A3 (22), and transformants were selected and confirmed by Southern blotting (34).
Transformants were screened by PCR for the homologous integration event. Single integration of the construct was confirmed by Southern blotting. One rcyA-deletion strain was selected from the transformants and named SSH36.
Tagging of proteins with GFP.
For fluorescence microscopy, a 0.7-kb fragment of the rcyA gene was subcloned in the pCMB17apx plasmid (28), where N-terminal fusions of green fluorescent protein (GFP) to proteins of interest are expressed under the control of the alcA promoter, containing Neurospora crassa pyr-4 as a selection marker, yielding pSH13. GFP and pyr-4 were replaced with mRFP1 and pyroA, yielding pSH30. The resulting plasmids are listed in Table 2. The primer set used for rcyA was AN10061-AscI (5′-GGCGCGCCTATGTCAAAAGCGAGGAATGGC-3′) and AN10061-PacI (5′-TTAATTAACGTAGTTCTTCATCGACCATC-3′) (cloning sites are underlined). The ΔF box-deletion mutant was generated with the primers Delta-Fbox (5′-GGCGCGCCTAGGATAGGTTGCTGGGATGAAG-3′) and AN10061-PacI, and the PCR fragment was cloned into AscI-PacI-digested pSH30. All of these plasmids were transformed into the uracil-auxotrophic TN02A3 (ΔnkuA) strain (Table 1). The integration events were confirmed by PCR and Southern blotting.
Light/fluorescence microscopy.
For live-cell imaging of germlings and young hyphae, cells were grown on coverslips in 0.4 ml MM plus 2% glycerol (derepression of the alcA promoter), MM plus 2% glucose (repression of the alcA promoter), or MM plus 2% threonine (induction of the alcA promoter) (35). Cells were incubated at room temperature overnight. Images were captured at room temperature using an Axiophot microscope (Zeiss, Jena, Germany). Images were collected and analyzed with the AxioVision system (Zeiss).
For FM4-64 staining, germlings were grown in MM plus 2% glucose medium overnight and stained with 0.3 ml medium containing 10 μM FM4-64 (from a stock solution in dimethyl sulfoxide [DMSO]), kept for 15 min, washed in 2.5 ml of medium, and transferred to 2.5 ml of fresh medium (36).
Real-time PCR (RT-PCR).
For RNA isolation, mycelium was collected, shock-frozen in liquid nitrogen, and lyophilized. RNA was extracted with a Fungal RNA kit from Omega Bio-Tek following the manufacturer's protocol. RNA samples were obtained from TN02A3 (wild-type strain) and SSH36 (ΔrcyA strain). For DNA digestion, an Ambion Turbo DNA Free kit was used. For real-time PCR, a Bioline SensiFast SYBR kit and a Fluorescein One Step kit were used according to the manufacturer's protocol. Three technical replicates were performed. Histone H2B was taken as the housekeeping gene. kipA was amplified with primers RT-kipA-For (5′-GAGTGGATAGTGGATGCTCGTC-3′) and RT-kip-A-Rev (5′-CCATCACCCTCCTTACCAAACG-3′). For normalization of the kipA transcript levels, histone 2B primers H2B-RT fwd (5′-CTGCCGAGAAGAAGCCTAGCAC-3′) and H2B-RT rev (5′-GAAGAGTAGGTCTCCTTCCTGGTC-3′) were used.
Western blotting.
A. nidulans strains SSH27 (GFP-KipA) and SSH43 (GFP-KipA, ΔrcyA) were cultured in MM plus 2% glucose and strain SSH69 (GFP-KipA, mRFP1-RcyAΔF-box) in MM plus 2% threonine and 0.2% glucose for 24 h. The mycelium was ground in liquid nitrogen, resuspended in protein extraction buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM EDTA), supplemented with a cocktail of protease inhibitor (Sigma), and centrifuged at 10,000 × g for 15 min. The supernatant (clarified cell lysate) and a MiniProtean system (Bio-Rad) were used for Western blotting following the manufacturer's instructions and a polyclonal anti-GFP antibody (Sigma) and a monoclonal anti gamma-tubulin antibody (Sigma) as internal controls for the basal protein amount. The signal intensities of the Western blotting bands were measured with a Chemi-Smart-5100 geldoc device (Peqlab) and quantified with the ChemiCap program (peqlab). The wild-type value was set to 100%. The experiment was repeated twice with three different amounts of total protein.
RESULTS
Identification of the RcyA F-box protein in a yeast two-hybrid screening with KipA as bait.
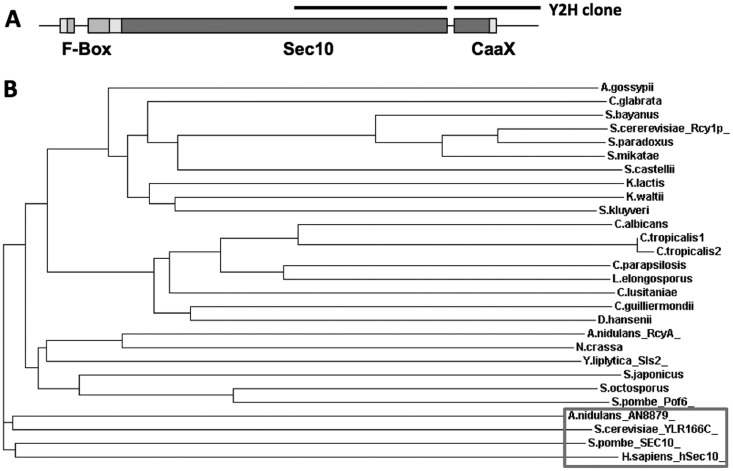
In a yeast two-hybrid screening with the KipA kinesin-7 motor protein of A. nidulans, several interacting proteins were identified (27). One of the candidates characterized here was AN10061. The yeast two-hybrid clone spans 1.4 kb of a cDNA corresponding to a region close to the 3′ end of the gene (Fig. 1A). The complete cDNA comprises 2.6 kb and encodes a polypeptide of 880 amino acids, which shares 35% amino acid identity with Sls2 of Yarrowia lipolytica (37), 29% identity with Rcy1 of S. cerevisiae (6, 38), and 32.5% identity with Pof6 of S. pombe (9). In accordance with the budding yeast's name, we used the name rcyA for the A. nidulans gene. The A. nidulans RcyA polypeptide of between 100 to 850 amino acids also shares 25% identity and 40% similarity with exocyst complex component Sec10. In addition, RcyA contains an F-box domain at the N terminus. Sec10 is conserved in all eukaryotes, and the A. nidulans orthologue is AN8879 (39, 40). This A. nidulans Sec10 orthologue shares 24% identity and 39% similarity with the human Sec10 protein. At the extreme C terminus of RcyA, a putative prenylation motif (CaaX) was detected using the PrePS program (http://mendel.imp.ac.at/sat/PrePS/index.html). Proteins with these three features (similarity to Sec10, F-box proteins at the N terminus, and a CaaX motif at the C terminus) were found only in fungi, while Sec10 orthologues are present in all eukaryotes (Fig. 1B).
FIG 1.
(A) Scheme of the RcyA protein. The F-box domain, the region with similarity to Sec10, and the CAAX motif are labeled. The original yeast two-hybrid (Y2H) clone is indicated above the scheme. (B) Relatedness analysis of RcyA. Fungal orthologues of RcyA and of Sec10 were aligned by tCoffee, and the tree was plotted at the EBI website (http://www.ebi.ac.uk/Tools/msa/tcoffee/). For the alignment, we used the Rcy1 orthologues of the following organisms: Saccharomyces cerevisiae (Rcy1p) (YJL204C), Saccharomyces paradoxus (spar343-g75.1), Saccharomyces mikatae (smik835-g3.1), Saccharomyces bayanus (sbayc610-g6.1), Candida glabrata (CAGL0F02497g), Saccharomyces castellii (Scas531.3), Kluyveromyces waltii (Kwal33.13585), Kluyveromyces lactis (KLLA0C03300g), Saccharomyces kluyveri (SAKL0C04004g), Ashbya gossypii (AFR644C), Candida lusitaniae (CLUG04919), Debaryomyces hansenii (DEHA2E19052g), Candida guilliermondii (PGUG03406.1), Candida tropicalis1 (CTRG05296.3), Candida tropicalis2 (CTRG06241.3), Candida albicans (orf19.3203), Candida parapsilosis (CPAG03322), Lodderomyces elongisporus (LELG03751), Yarrowia lipolytica (Sls2) (YALI0B19074g), Aspergillus nidulans (RcyA) (AN10061), Neurospora crassa (NCU03658), Schizosaccharomyces japonicus (SJAG02753), Schizosaccharomyces octosporus (SOCG02810), and Schizosaccharomyces pombe (Pof6) (SPCC18.04). In addition, we included the Se10 orthologues of the following organisms (boxed): Saccharomyces cerevisiae (YLR166C), Aspergillus nidulans (AN8879), Schizosaccharomyces pombe (SEC10) (SPAC13F5.06c), and Homo sapiens (hSec10) gi|24418661|sp|O00471.1|.
Localization of GFP-RcyA.
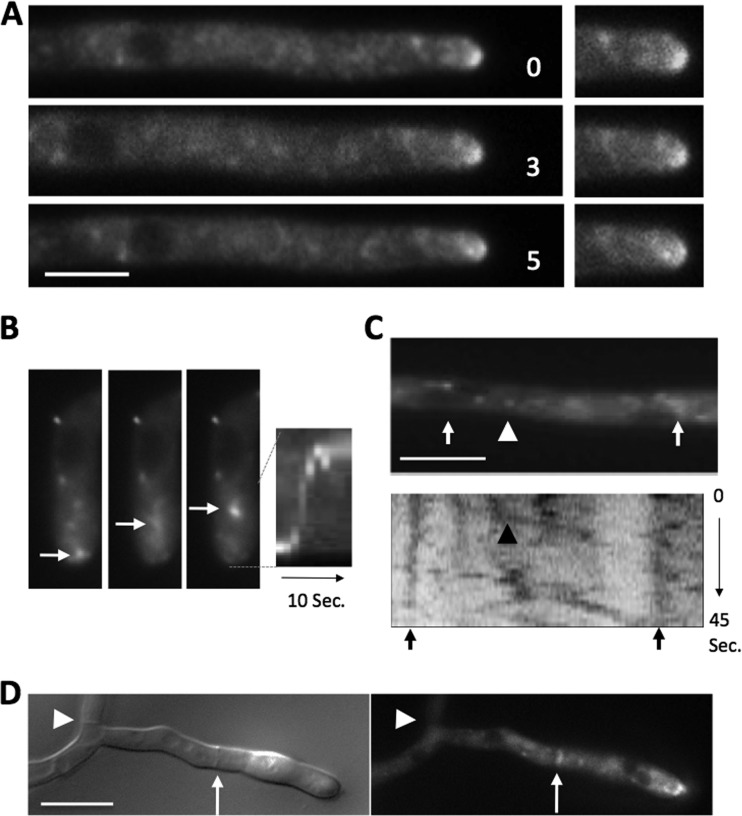
In order to unravel the molecular function of RcyA, we studied the subcellular distribution of the protein. RcyA was fused to GFP at the N terminus and expressed from the endogenous promoter or the inducible promoter of the alcohol dehydrogenase (alcA) (35). With glucose as the carbon source, the promoter is repressed; with glycerol as the carbon source, it is derepressed and expressed at a low level; and with threonine or ethanol as the carbon source, high expression levels can be obtained. The localization pattern of GFP-RcyA expressed under the control of the endogenous promoter was similar to that of GFP-RcyA expressed under the control of the alcA promoter derepressed condition with glycerol as the carbon source. GFP-RcyA accumulated at the tip in a dynamic manner (Fig. 2A; see also Movie S1 in the supplemental material). The fluorescence signal sometimes moved back from the tip to a subapical region (Fig. 2A and B, arrows). Along hyphae, especially in regions further back, GFP-RcyA moved as small spots bidirectionally (Fig. 2C, arrowheads) and localized in larger accumulations close to the nuclei (Fig. 2B and C, arrows). The small spots moved at 2.0 μm/s on average (1.8 μm/s ± 0.5 μm/s standard deviation [SD]; n = 10) and at a maximum of 3.0 μm/s (Fig. 2C). GFP-RcyA also localized at forming septa (Fig. 2D, arrow) but not at mature septa (Fig. 2D, arrowhead).
FIG 2.
Localization of RcyA in A. nidulans. (A) GFP-RcyA was expressed under the control of the native promoter (SSH73). The elapsed time is given in seconds. (B) Time-lapse fluorescence microscopy images and the corresponding kymograph of the strain (SSH01). The three images correspond to three consecutive frames (300 ms each). The complete sequence spans 10 s. (C) A single picture of a time-lapse sequence of GFP-RcyA in strain SSH01 with the corresponding kymograph. The arrowheads indicate rapid moving RcyA spots. (D) Localization of mFRP1-RcyA at a forming septum (arrow) in the SSH54 strain. Strains with the RcyA construct under the control of the alcA promoter were grown with MM plus 2% glycerol in the medium. Scale bars represent 5 μm.
RcyA transport.
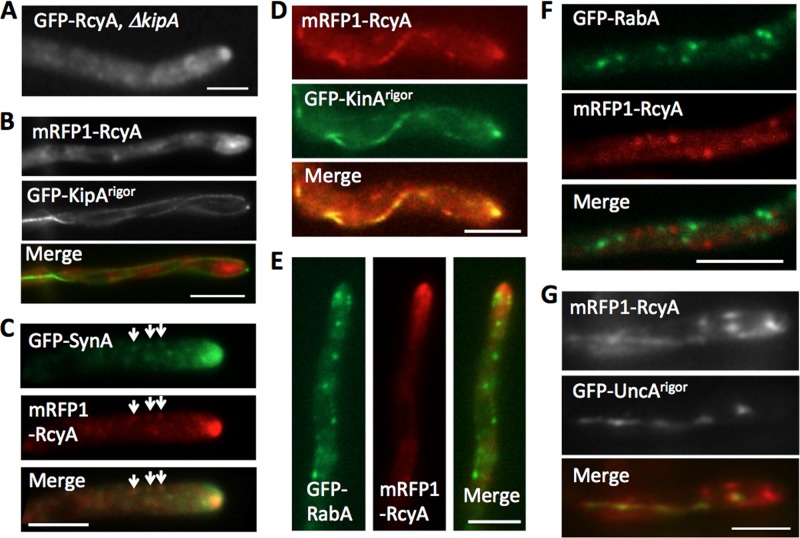
Because RcyA was identified as a kinesin-7-interacting protein by yeast two-hybrid screening (27), we anticipated that the dynamics of RcyA would depend on KipA. To test this hypothesis, we studied the localization of RcyA in the absence of KipA. However, no obvious difference from the wild type was observed. GFP-RcyA still accumulated at hyphal tips in the kipA-deletion strain and moved bidirectionally on small spots at a similar speed (Fig. 3A and data not shown). In addition, the signal of GFP-KipA at microtubule plus ends did not colocalize with that of mRFP1-RcyA (data not shown). In the case of KipA, a rigor version of the motor was used. Such rigor variants of kinesin bind irreversibly to microtubules (26, 41, 42). In a strain expressing GFP-KipArigor, mRFP-RcyA did not colocalize along the microtubules decorated with GFP-KipArigor (Fig. 3B). These results indicate that KipA is not required for RcyA movement and distribution in the hyphae.
FIG 3.
Transport of RcyA. (A) Fluorescence microscopy image of GFP-RcyA in kipA-deletion strain SSH08. (B) mRFP1-RcyA did not colocalize along the microtubule decorated with GFP-KipArigor in strain SSH66. (C) Colocalization of GFP-SynA (secretory vesicles) and mRFP1-RcyA in strain SSH56. (D) mRFP1-RcyA colocalized along the microtubule decorated with GFP-KinArigor in strain SSH91. (E and F) mRFP1-RcyA did not colocalize with GFP-RabA (early endosomes) around the hyphal tip (E) and backward region (F) in strain SSH93. (G) mRFP1-RcyA partially colocalized along the microtubule decorated with GFP-UncArigor in strain SSH55. Scale bars represent 5 μm.
The dynamic behavior of GFP-RcyA around the hyphal tip resembled the movement of secretory vesicles. Therefore, we compared the localization of mRFP1-RcyA with that of GFP-SynA (v-SNARE) as a marker for such vesicles (Fig. 3C; see also Movie S2 in the supplemental material) (25). Since the fluorescent signal of secretory vesicles appeared to be very weak and since the vesicles moved very quickly, it was hard to document exact colocalization in movies, but their dynamic behaviors looked very similar. Besides that, SynA and RcyA colocalized at small dots at subapical regions, which might represent the late Golgi or trans-Golgi network (Fig. 3C, arrows) (43). Because conventional kinesin (kinesin-1) is involved in protein secretion, we hypothesized that KinA could be involved in RcyA movement (44–46). Indeed, mRFP-RcyA colocalized with GFP-KinArigor (Fig. 3D) (47). These results further suggest that RcyA is transported to the hyphal tip on secretory vesicles.
Because the bidirectional movement of RcyA at backward regions resembled the movement of early endosomes (26, 30), we compared the localization of mRFP1-RcyA with that of GFP-RabA (Rab GTPase) as a marker for early endosomes (Fig. 3E and F; see also Movie S2 in the supplemental material). However, colocalization of RabA and RcyA was hardly observed. Likewise, mRFP-RcyA colocalized only partially along microtubules decorated with GFP-UncArigor (Fig. 3G). UncA is involved in early endosome movement (15, 26). These results suggest that only a fraction of RcyA is transported along microtubules on early endosomes.
Deletion of rcyA.
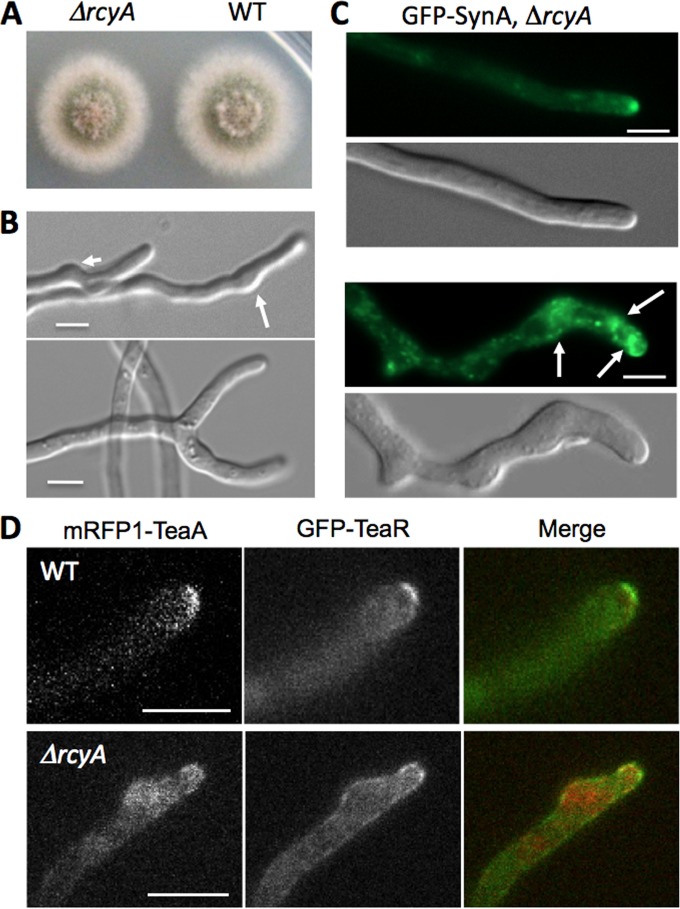
In order to determine the function of RcyA in A. nidulans, we created an rcyA-deletion strain. The rcyA-deletion strain grew as well as the wild type on agar plates, and no effect on conidiation or conidium density was observed (Fig. 4A). However, hyphae of the rcyA-deletion strain sometimes showed abnormal swellings, branch formation close to the tip, and split tips (Fig. 4B). The abnormal swellings around the tip were observed in 5% of the hyphae, and the tip split phenotype was observed in 1% of the hyphal tips (n = 400), but this phenotype was not observed in the wild type.
FIG 4.
Deletion of rcyA. (A) Colonies of an rcyA-deletion strain (SSH36) and the wild-type (WT) strain (TN02A3) grown on MM with 2% glucose. Plates were incubated at 37°C for 48 h. (B) Swellings around the tips (arrows, upper) and the tip split phenotype (lower panel) in the rcyA-deletion strain. (C) Normal localization of GFP-SynA at most hyphal tips (upper panel) and accumulation of GFP-SynA at the subapical region of abnormally swollen tips in strain SSH89 (ΔrcyA) (lower panel). (D) Localization of the cell end markers mRFP1-TeaA and GFP-TeaR in the SNT56 (WT, upper panel) and SSH44 (ΔrcyA, lower panel) control strains. Scale bars represent 5 μm.
Because Rcy1 in S. cerevisiae is thought to be involved in membrane recycling but not in endocytosis, we tested whether the absence of RcyA in A. nidulans would affect membrane recycling. To this end, the membrane was stained with the fluorescent dye FM4-64 (36). Hyphae were incubated 15 min in the presence of the dye and then, after washing with media, immediately analyzed in the microscope at room temperature. The rcyA-deletion strain did not show obvious differences from the wild type in the internalization of FM4-64 (data not shown). When we treated the hyphae with the dye on ice for 15 min before microscopic analysis, the internalization of FM4-64 was partially delayed in the rcyA-deletion strain (data not shown). However, this could have been an indirect effect, given that the rcy1 deletion caused a cold-sensitive phenotype in S. cerevisiae (10). Next, we tried to analyze membrane recycling, which means the transport of internalized dye from early endosomes back to the plasma membrane. It was hard to clearly visualize membrane recycling using this method, and no clear difference was observed in the numbers and sizes of early endosomes between the wild-type strain and the rcyA-deletion strain (data not shown).
Because S. cerevisiae Rcy1 is involved in v-SNARE Snc1 recycling, the localization of GFP-SynA (v-SNARE) was investigated in the rcyA-deletion strain (Fig. 4C). GFP-SynA localized at most hyphal tips without any obvious differences from the wild type (Fig. 4C, upper panel, and Fig. 3C), but accumulation of GFP-SynA was observed at subapical regions in abnormally swollen tips (Fig. 4C, lower panel).
Since the hyphae of the rcyA-deletion strain sometimes showed abnormal swellings or branch formation close to the tip, we investigated the localization of the two cell end markers TeaA and TeaR. Whereas both proteins were restricted to an area along the cytoplasmic membrane at the tip in the wild type, both proteins appeared less concentrated at the plasma membrane and localized at subapical swellings and also in the cytoplasm in the rcyA-deletion strain (Fig. 4D).
Overexpression of rcyA.
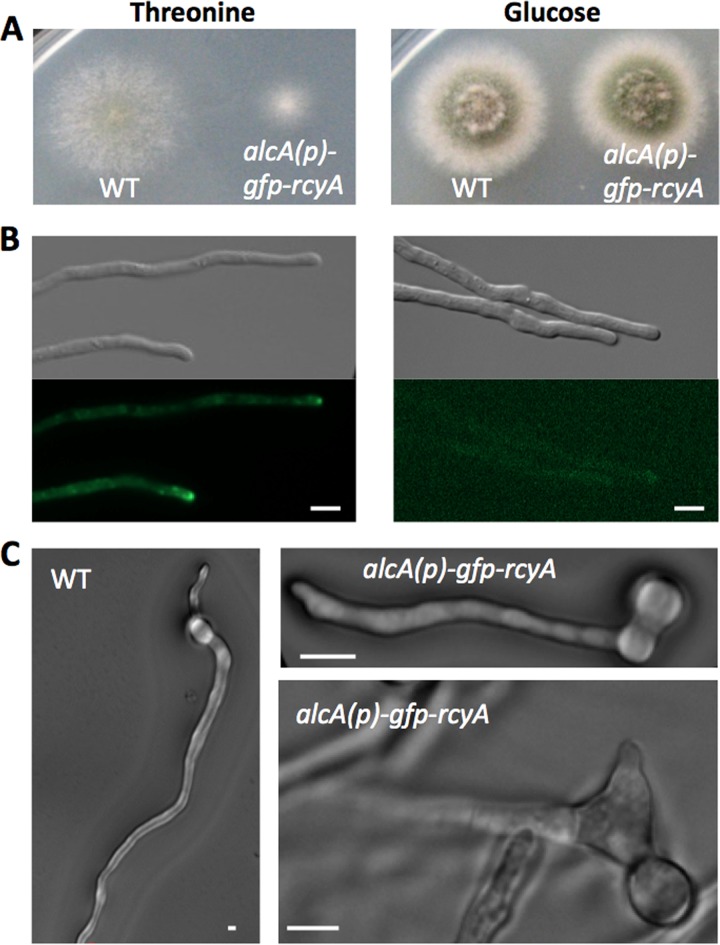
If RcyA is involved in membrane recycling, we anticipated not only that downregulation of rcyA would disturb hyphal morphology but also that an increase of the concentration could affect hyphal growth and morphology. To test this hypothesis, the alcA(p)-GFP-rcyA construct was induced with threonine as the carbon source. The first obvious phenotypic changes were the much slower colony growth compared to the wild type and the lack of conidia (Fig. 5A). Under repressed conditions (with glucose as the carbon source), colonies grew as fast as the wild type. The expression level of GFP-RcyA under induced and repressed conditions was visible as the intensity of the GFP signal (Fig. 5B). Another phenotype concerned spore germination. A. nidulans conidia normally form a second germ tube at the side opposite the first one (Fig. 5C, left) (48). However, conidia of the rcyA-overexpression strain sometimes did not form second germ tubes (35%; n = 100) or formed a second germ tube at a random position (10%; n = 100). If there was a second germ tube, occasionally it appeared swollen (Fig. 5C, right).
FIG 5.
Overexpression of rcyA. (A) Colonies of the wild-type and SSH14 strains grown on MM plus 2% threonine (left) or MM plus 2% glucose (right). Plates were incubated at 37°C for 48 h. (B) Differential interference contrast (DIC) and GFP signal of SSH14 grown on MM plus 2% threonine (left) or MM plus 2% glucose (right). (C) DIC images of the wild-type and SSH14 germ tubes grown in MM plus 2% threonine. Scale bars represent 5 μm.
RcyA controls the concentration of KipA.
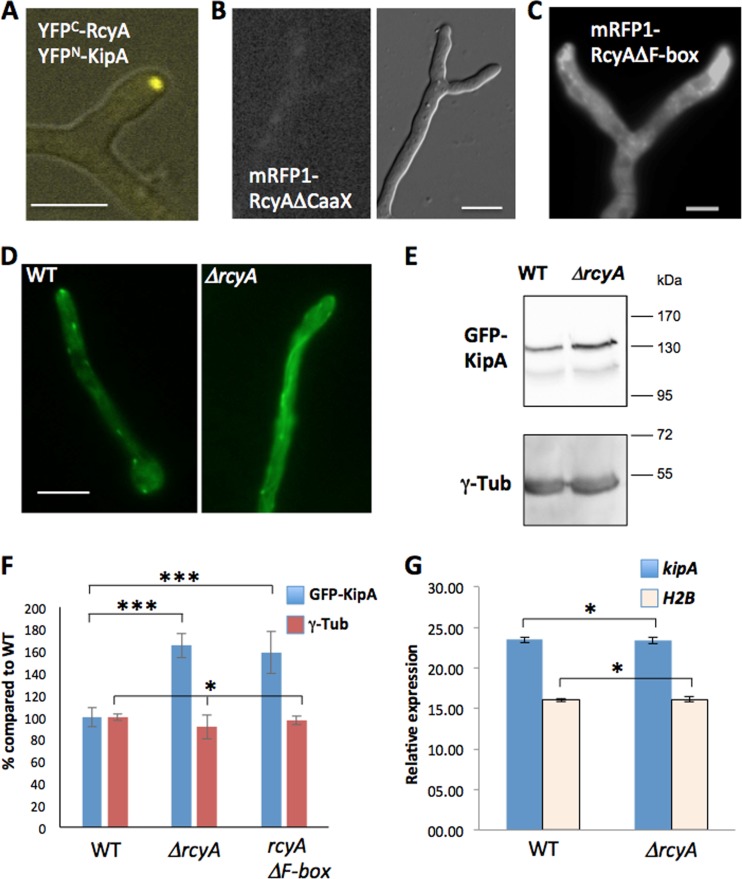
As described above, RcyA localization was independent of KipA. On the other hand, RcyA was isolated as a KipA-interacting protein. Therefore, we studied the role of this interaction. First, we confirmed the interaction using the bimolecular fluorescence complementation (BiFC) method. A bright signal close to the hyphal tip indicated that this interaction test result was positive (Fig. 6A).
FIG 6.
Effect of rcyA deletion on the KipA concentration. (A) Bimolecular fluorescence complementation of RcyA and KipA. (B and C) Localization of mRFP1-RcyA-deleted CaaX motif (B) and mRFP1-RcyA-deleted F-box (C). (D) Localization of GFP-KipA in the wild-type (SSH27) and SSH43 (ΔrcyA) strains. The microscopy medium was MM plus 2% glycerol. (E) Western blotting of cell lysate, SSH27 (GFP-KipA), SSH43 (GFP-KipA, ΔrcyA), and SSH69 (GFP-KipA, mRFP1-RcyAΔF-box) (strain SSH69 is not shown here), using a polyclonal anti-GFP antibody and a monoclonal anti-gamma-tubulin (γ-Tub) antibody as an internal control for the basal protein amount. (F) Relative levels of GFP-KipA and gamma-tubulin in the Western blotting. The wild-type value was set to 100% (n = 6). *, P > 0.05 (no significant difference); ***, P > 0.001 (highly significant difference). (G) Expression of the kipA gene. The graph represents the relative levels of expression of the kipA gene as measured by RT-PCR. H2B was used as a control. All experiments were done with three biological and two technical replicates. Scale bars represent 5 μm.
In order to characterize the role of the two important motifs of RcyA, the CaaX motif and the F-box domain, we created strains in which the corresponding regions were deleted. When the CaaX motif was missing, no mRFP1 signal was observed at hyphal tips and forming seta but very weak staining of the cytoplasm was observed (Fig. 6B, data not shown). In contrast, deletion of the F-box domain of RcyA showed signal accumulation around the tips of hyphae (Fig. 6C). Other morphological features resembled the ones observed for the rcyA deletion.
S. cerevisiae Rcy1 was shown to control the turnover of several proteins (9). We hypothesized that A. nidulans RcyA could be involved in the turnover of KipA and thus that kinesin-7 could be a novel target for the F-box protein. In order to test this hypothesis, we compared the KipA concentration, as a GFP-KipA fusion protein, in an rcyA-deletion strain with the concentration in the wild type. GFP-KipA associates with the microtubule plus ends and appears as comet-like moving structures in the wild type (Fig. 6D, left) (24). In contrast, in the absence of RcyA, microtubules were evenly decorated (Fig. 6D, right). Such a decoration of the entire microtubule was also observed when KipA was overexpressed (24). This result suggested that the KipA protein concentration was higher in the rcyA-deletion strain than in the wild type. To further prove this hypothesis, the protein amount of GFP-KipA was determined by Western blot analysis (Fig. 6E). As a control, gamma-tubulin was chosen. Indeed, the KipA concentration was increased by about 50% in the rcyA-deletion strain in comparison to the wild type (Fig. 6E and F). Likewise, the KipA concentration was increased when only the F-box domain of RcyA was deleted (Fig. 6F). Because the GFP-kipA construct was expressed from the alcA promoter in both the wild-type strain and the ΔrcyA strain, an effect of RcyA on the transcription of kipA was unlikely. Indeed, the mRNA levels of kipA revealed no difference in the two strains (Fig. 6G). These results suggest that the RcyA interaction is necessary for the control of the KipA turnover and that RcyA is the specific adaptor for KipA in the ubiquitination and subsequent proteasome-degradation pathway.
DISCUSSION
The growth form of filamentous fungi requires massive membrane flow for the continuous extension of the plasma membrane at hyphal tips and the delivery of enzymes required for cell wall biosynthesis. Both are achieved through the fusion of secretory vesicles with the membrane at the growing tip. However, there is excellent evidence that endocytosis is also important for polar growth (12, 49). On the one hand, excess membrane can be removed through endocytosis. Likewise, a slaB mutant in which membrane internalization is inhibited shows massive invaginations of the membrane (50). SlaB is a key regulator of F-actin and the endocytic internalization machinery. Interestingly, deletion of the gene is lethal, showing the importance of endocytosis for polar growth. However, deletion of rcyA did not show any obvious severe morphological phenotypes or defects in the uptake of FM4-64. This is in agreement with the findings in S. cerevisiae, where only membrane recycling but not membrane internalization is disturbed in the rcy1-deletion strain (10).
On the other hand, endocytosis actively occurs at a subapical ring of the hyphae and could contribute to the maintenance of polarity by recycling necessary components, such as cell end marker proteins (50). We found that the distribution of cell end marker proteins such as TeaR and TeaA in A. nidulans is impaired in the absence of RcyA. Since cell end marker proteins define the growth direction and are involved in branch formation, the misdistribution could be the reason for the observed changes in polar growth and branching in some minor fraction of the hyphae. Rcy1 in S. cerevisiae is thought to be involved in membrane recycling through the recycling of the v-SNARE Snc1 (8, 10). The v-SNARE SynA in A. nidulans occasionally accumulated at subapical regions in abnormally swollen tips (Fig. 4C, lower panel). This result supports the idea of a conserved function of RcyA with respect to SynA recycling; however, the defect of SynA recycling in the rcyA-deletion strain was only partial and appeared weaker than that of S. cerevisiae.
In comparison to the described role of S. cerevisiae Rcy1, Pof6 in S. pombe plays a critical role in cell separation, and gene deletion is lethal (9). We did not find any evidence for such a role in A. nidulans, although RcyA was found at septa. Of course, A. nidulans does not require cell separation and thus one would not expect a phenotype during vegetative growth. Since conidia are formed in a budding-like process, one would also not expect a phenotype corresponding to sporulation. However, one would expect a role of RcyA in filamentous fungi with a dimorphic switch between the filamentous form and a fission-yeast form, such as Penicillium marneffei.
Additionally, we identified KipA as a novel putative target for RcyA. The cellular concentration of KipA protein was increased upon deletion of the gene, while the gene expression level was comparable. We propose that RcyA is the E3 ubiquitin ligase adaptor responsible for the specific degradation of KipA in a SCF complex and that RcyA is necessary for the control of the KipA turnover. Here we cannot exclude the possibility that the increase of KipA protein levels in the rcyA-deletion strain is due to translational control. To exclude this possibility, the half-life time of KipA needs to be investigated. The overexpression of rcyA did not reduce the KipA concentration significantly and did not phenocopy the kipA-deletion phenotype (data not shown), suggesting that the residual KipA amount is sufficient. However, there is some similarity between the kipA-deletion phenotype and the rcyA-deletion phenotype, because both deletions disturb cell end marker organization. Nevertheless, TeaR was scattered along the plasma membrane in the rcyA-deletion strain, whereas it was organized in a compact structure in the kipA-deletion strain. This difference might be explained if we assume that membrane recycling is unaffected in the kipA-deletion strain but disturbed in the absence of RcyA. Taken together, our results are further evidence for a role of the endocytic ring in polarity maintenance and the importance of endocytosis in polar growth.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the German Science Foundation (DFG) Research Unit (FOR1334). N.T. was a Humboldt Fellow.
Footnotes
Published ahead of print 20 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00042-14.
REFERENCES
- 1.Peters JM. 1998. SCF and APC: the yin and yang of cell cycle regulated proteolysis. Curr. Opin. Cell Biol. 10:759–768. 10.1016/S0955-0674(98)80119-1 [DOI] [PubMed] [Google Scholar]
- 2.Krappmann S, Jung N, Medic B, Busch S, Prade RA, Braus GH. 2006. The Aspergillus nidulans F-box protein GrrA links SCF activity to meiosis. Mol. Microbiol. 61:76–88. 10.1111/j.1365-2958.2006.05215.x [DOI] [PubMed] [Google Scholar]
- 3.von Zeska Kress MR, Harting R, Bayram Ö, Christmann M, Irmer H, Valerius O, Schinke J, Goldman GH, Braus GH. 2012. The COP9 signalosome counteracts the accumulation of cullin SCF ubiquitin E3 RING ligases during fungal development. Mol. Microbiol. 83:1162–1177. 10.1111/j.1365-2958.2012.07999.x [DOI] [PubMed] [Google Scholar]
- 4.Colabardini AC, Humanes AC, Gouvea PF, Savoldi M, Goldman MH, Kress MR, Bayram Ö, Oliveira JV, Gomes MD, Braus GH, Goldman GH. 2012. Molecular characterization of the Aspergillus nidulans fbxA encoding an F-box protein involved in xylanase induction. Fungal Genet. Biol. 49:130–140. 10.1016/j.fgb.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 5.Schnell JD, Hicke L. 2003. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J. Biol. Chem. 278:35857–35860. 10.1074/jbc.R300018200 [DOI] [PubMed] [Google Scholar]
- 6.Wiederkehr A, Avaro S, Prescianotto-Baschong C, Haguenauer-Tsapis R, Riezman H. 2000. The F-box protein Rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in Saccharomyces cerevisiae. J. Cell Biol. 149:397–410. 10.1083/jcb.149.2.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuta N, Fujimura-Kamada K, Saito K, Yamamoto T, Tanaka K. 2007. Endocytic recycling in yeast is regulated by putative phospholipid translocases and the Ypt31/32p-Rcy1p pathway. Mol. Biol. Cell 18:295–312. 10.1091/mbc.E06-05-0461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen SH, Chen S, Tokarev AA, Liu F, Jedd G, Segev N. 2005. Ypt31/32 GTPases and their novel F-box effector protein Rcy1 regulate protein recycling. Mol. Biol. Cell 16:178–192. 10.1091/mbc.E04-03-0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermand D, Bamps S, Tafforeaus L, Vandenhaute J, Mäkelä TP. 2003. Skp1 and the F-box protein Pof6 are essential for cell separation in fission yeast. J. Biol. Chem. 278:9671–9677. 10.1074/jbc.M211358200 [DOI] [PubMed] [Google Scholar]
- 10.Chen SH, Shah AH, Segev N. 2011. Ypt31/32 GTPases and their F-box effector Rcy1 regulated ubiquitination of recycling proteins. Cell Logist. 1:21–31. 10.4161/cl.1.1.14695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riquelme M, Yarden O, Bartnicki-Garcia S, Bowman B, Castro-Longoria E, Free SJ, Fleissner A, Freitag M, Lew RR, Mouriño-Pérez R, Plamann M, Rasmussen C, Richthammer C, Roberson R-W, Sanchez-Leon E, Seiler S, Watters MK. 2011. Architecture and development of the Neurospora crassa hypha - a model cell for polarized growth. Fungal Biol. 115:446–474. 10.1016/j.funbio.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 12.Steinberg G. 14 May 2014. Endocytosis and early endosome motility in filamentous fungi. Curr. Opin. Microbiol. 10.1016/j.mib.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peñalva MA. 2010. Endocytosis in filamentous fungi: Cinderella gets her reward. Curr. Opin. Microbiol. 13:684–692. 10.1016/j.mib.2010.09.005 [DOI] [PubMed] [Google Scholar]
- 14.Peñalva MA, Galindo A, Abenza JF, Pinar M, Cacagno-Pizarelli AM, Arst HN, Pantazopoulou A. 2012. Searching for gold beyond mitosis: mining intracellular membrane traffic in Aspergillus nidulans. Cell Logist. 2:2–14. 10.4161/cl.19304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seidel C, Moreno-Velásquez SD, Riquelme M, Fischer R. 2013. Neurospora crassa NKIN2, a kinesin-3 motor, transports early endosomes and is required for polarized growth. Eukaryot. Cell 12:1020–1032. 10.1128/EC.00081-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer R, Zekert N, Takeshita N. 2008. Polarized growth in fungi - interplay between the cytoskeleton, positional markers and membrane domains. Mol. Microbiol. 68:813–826. 10.1111/j.1365-2958.2008.06193.x [DOI] [PubMed] [Google Scholar]
- 17.Takeshita N, Manck R, Grün N, de Vega S, Fischer R. 27 May 2014. Interdependence of the actin and the microtubule cytoskeleton during fungal growth. Curr. Opin. Microbiol. 10.1016/j.mib.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 18.Mata J, Nurse P. 1997. tea1 and the microtubular cytoskeleton are important for generating global spatial order within the fission yeast cell. Cell 89:939–949. 10.1016/S0092-8674(00)80279-2 [DOI] [PubMed] [Google Scholar]
- 19.Takeshita N, Higashitsuji Y, Konzack S, Fischer R. 2008. Apical sterol-rich membranes are essential for localizing cell end markers that determine growth directionality in the filamentous fungus Aspergillus nidulans. Mol. Biol. Cell 19:339–351. 10.1091/mbc.E07-06-0523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeshita N, Mania D, Herrero S, Ishitsuka Y, Nienhaus GU, Podolski M, Howard J, Fischer R. 2013. The cell-end marker TeaA and the microtubule polymerase AlpA contribute to microtubule guidance at the hyphal tip cortex of Aspergillus nidulans to provide polarity maintenance. J. Cell Sci. 126:5400–5411. 10.1242/jcs.129841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill TW, Käfer E. 2001. Improved protocols for Aspergillus minimal medium: trace element and minimal medium salt stock solutions. Fungal Genet. Newsl. 48:20–21 [Google Scholar]
- 22.Nayak T, Szewczyk E, Oakley CE, Osmani A, Ukil L, Murray SL, Hynes MJ, Osmani SA, Oakley BR. 2006. A versatile and efficient gene targeting system for Aspergillus nidulans. Genetics 172:1557–1566. 10.1534/genetics.105.052563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stringer MA, Dean RA, Sewall TC, Timberlake WE. 1991. Rodletless, a new Aspergillus developmental mutant induced by directed gene inactivation. Genes Dev. 5:1161–1171. 10.1101/gad.5.7.1161 [DOI] [PubMed] [Google Scholar]
- 24.Konzack S, Rischitor P, Enke C, Fischer R. 2005. The role of the kinesin motor KipA in microtubule organization and polarized growth of Aspergillus nidulans. Mol. Biol. Cell 16:497–506. 10.1091/mbc.E04-02-0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taheri-Talesh N, Horio T, Araujo-Bazan L, Dou X, Espeso EA, Penalva MA, Osmani A, Oakley BR. 2008. The tip growth apparatus of Aspergillus nidulans. Mol. Biol. Cell 19:1439–1449. 10.1091/mbc.E07-05-0464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zekert N, Fischer R. 2009. The Aspergillus nidulans kinesin-3 UncA motor moves vesicles along a subpopulation of microtubules. Mol. Biol. Cell 20:673–684. 10.1091/mbc.E08-07-0685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrero S, Takeshita N, Fischer R. 2011. The Aspergillus nidulans CENP-E kinesin motor KipA interacts with the fungal homologue of the centromere-associated protein CENP-H at the kinetochore. Mol. Microbiol. 80:981–994. 10.1111/j.1365-2958.2011.07624.x [DOI] [PubMed] [Google Scholar]
- 28.Efimov V, Zhang J, Xiang X. 2006. CLIP-170 homologue and NUDE play overlapping roles in NUDF localization in Aspergillus nidulans. Mol. Biol. Cell 17:2021–2034. 10.1091/mbc.E05-11-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veith D, Scherr N, Efimov VP, Fischer R. 2005. Role of the spindle-pole body protein ApsB and the cortex protein ApsA in microtubule organization and nuclear migration in Aspergillus nidulans. J. Cell Sci. 118:3705–3716. 10.1242/jcs.02501 [DOI] [PubMed] [Google Scholar]
- 30.Abenza JF, Pantazopoulou A, Rodríguez JM, Galindo A, Peñalva MA. 2009. Long-distance movement of Aspergillus nidulans early endosomes on microtubule tracks. Traffic 10:57–75. 10.1111/j.1600-0854.2008.00848.x [DOI] [PubMed] [Google Scholar]
- 31.Yelton MM, Hamer JE, Timberlake WE. 1984. Transformation of Aspergillus nidulans by using a trpC plasmid. Proc. Natl. Acad. Sci. U. S. A. 81:1470–1474. 10.1073/pnas.81.5.1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Russel DW. 1999. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 33.Szewczyk E, Nayak T, Oakley CE, Edgerton H, Xiong Y, Taheri-Talesh N, Osmani SA, Oakley BR. 2006. Fusion PCR and gene targeting in Aspergillus nidulans. Nat. Protoc. 1:3111–3120. 10.1038/nprot.2006.405 [DOI] [PubMed] [Google Scholar]
- 34.Osmani A, Oakley BR, Osmani SA. 2006. Identification and analysis of essential Aspergillus nidulans genes using the heterokaryon rescue technique. Nat. Protoc. 1:2517–2526. 10.1038/nprot.2006.406 [DOI] [PubMed] [Google Scholar]
- 35.Waring RB, May GS, Morris NR. 1989. Characterization of an inducible expression system in Aspergillus nidulans using alcA and tubulin coding genes. Gene 79:119–130. 10.1016/0378-1119(89)90097-8 [DOI] [PubMed] [Google Scholar]
- 36.Peñalva MA. 2005. Tracing the endocytic pathway of Aspergillus nidulans with FM4-64. Fungal Genet. Biol. 42:963–975. 10.1016/j.fgb.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 37.Boisramé A, Beckerich JM, Gaillardin C. 1999. A mutation in the secretion pathway of the yeast Yarrowia lipolytica that displays synthetic lethality in combination with a mutation affecting the signal recognition particle. Mol. Gen. Genet. 261:601–609. 10.1007/s004380050002 [DOI] [PubMed] [Google Scholar]
- 38.Galan JM, Wiederkehr A, Seol JH, Haguenauer-Tsapis R, Deshaies RJ, Riezman H, Peter M. 2001. Skp1p and the F-box protein Rcy1p form a non-SCF complex involved in recycling of the SNARE Snc1p in yeast. Mol. Cell. Biol. 21:3105–3117. 10.1128/MCB.21.9.3105-3117.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo W, Roth D, Gatt E, Novick P. 1997. Identification and characterization of homologues of the exocyst component Sec10p. FEBS Lett. 404:135–139. 10.1016/S0014-5793(97)00109-9 [DOI] [PubMed] [Google Scholar]
- 40.Elias M, Drdova E, Ziak D, Bavlnka B, Hala M, Cvrckova F, Soukupova H, Zarsky V. 2003. The exocyst complex in plants. Cell Biol. Int. 27:199–201. 10.1016/S1065-6995(02)00349-9 [DOI] [PubMed] [Google Scholar]
- 41.Meluh PB, Rose MD. 1990. KAR3, a kinesin-related gene required for yeast nuclear fusion. Cell 60:1029–1041. 10.1016/0092-8674(90)90351-E [DOI] [PubMed] [Google Scholar]
- 42.Nakata T, Hirokawa N. 1995. Point mutation of adenosine triphosphate-binding motif generated rigor kinesin that selectively blocks anterograde lysosome membrane transport. J. Cell Biol. 131:1039–1053. 10.1083/jcb.131.4.1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinar M, Pantazopoulou A, Arst HN, Peñalva MA. 2013. Acute inactivation of the Aspergillus nidulans Golgi membrane fusion machinery: correlation of apical extension arrest and tip swelling with cisternal disorganization. Mol. Microbiol. 89:228–248. 10.1111/mmi.12280 [DOI] [PubMed] [Google Scholar]
- 44.Seiler S, Nargang FE, Steinberg G, Schliwa M. 1997. Kinesin is essential for cell morphogenesis and polarized secretion in Neurospora crassa. EMBO J. 16:3025–3034. 10.1093/emboj/16.11.3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuster M, Treitschke S, Kilaru S, Molloy J, Harmer NJ, Steinberg G. 2012. Myosin-5, kinesin-1 and myosin-17 cooperate in secretion of fungal chitin synthase. EMBO J. 31:214–227. 10.1038/emboj.2011.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Requena N, Alberti-Segui C, Winzenburg E, Horn C, Schliwa M, Philippsen P, Liese R, Fischer R. 2001. Genetic evidence for a microtubule-destabilizing effect of conventional kinesin and analysis of its consequences for the control of nuclear distribution in Aspergillus nidulans. Mol. Microbiol. 42:121–132. 10.1046/j.1365-2958.2001.02609.x [DOI] [PubMed] [Google Scholar]
- 47.Seidel C, Zekert N, Fischer R. 2012. The Aspergillus nidulans kinesin-3 tail is necessary and sufficient to recognize modified microtubules. PLoS One 7:e30976. 10.1371/journal.pone.0030976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeshita N, Fischer R. 2011. On the role of microtubules, cell end markers, and septal microtubule organizing centers on site selection for polar growth in Aspergillus nidulans. Fungal Biol. 115:506–517. 10.1016/j.funbio.2011.02.009 [DOI] [PubMed] [Google Scholar]
- 49.Valdez-Taubas J, Pelham HR. 2003. Slow diffusion of protein in the yeast plasma membrane allows polarity to be maintained by endocytic cycling. Curr. Biol. 13:1636–1640. 10.1016/j.cub.2003.09.001 [DOI] [PubMed] [Google Scholar]
- 50.Araujo-Bazán L, Peñalva MA, Espeso EA. 2008. Preferential localization of the endocytic internalization machinery to hyphal tips underlies polarization of the actin cytoskeleton in Aspergillus nidulans. Mol. Microbiol. 67:891–905. 10.1111/j.1365-2958.2007.06102.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.