Abstract
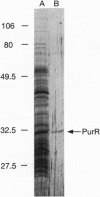
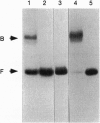
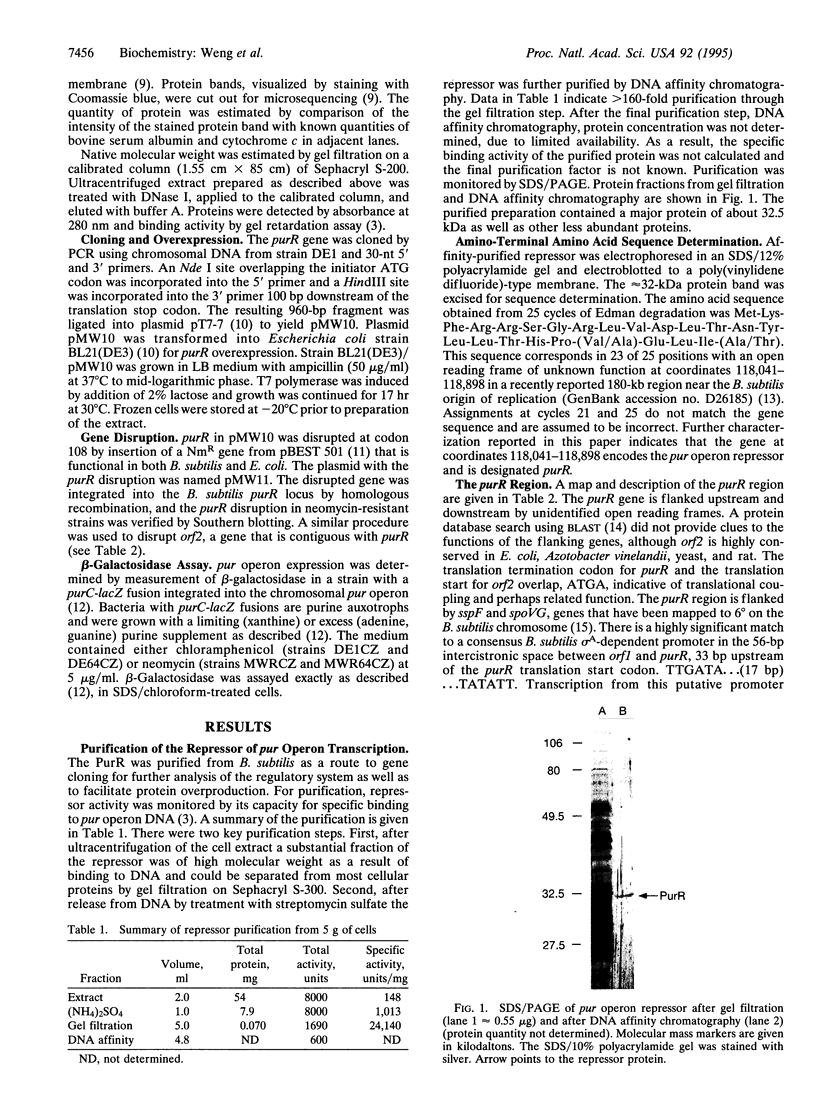
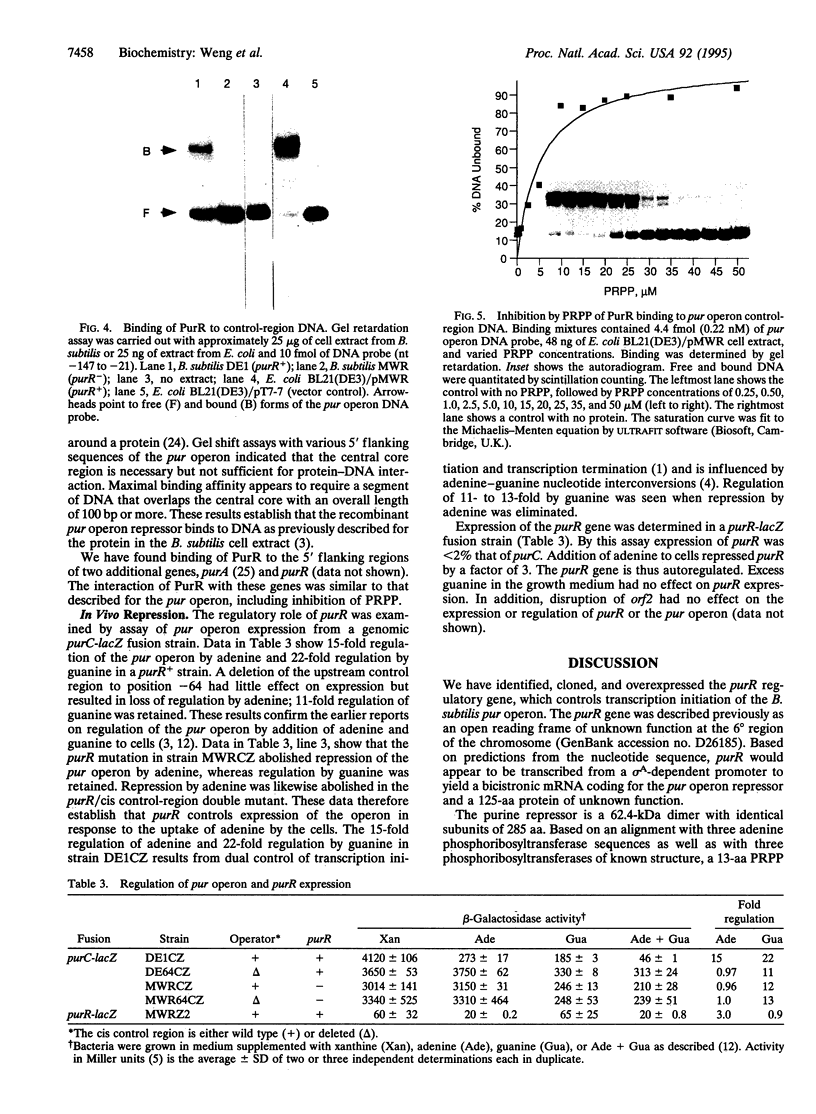
Transcription of the Bacillus subtilis pur operon is repressed in response to a signal of excess adenine. We have purified the repressor protein and have identified, cloned, and overexpressed the purR regulatory gene that controls transcription initiation of the operon. B. subtilis purR encodes a 62-kDa homodimer that binds to the pur operon control region. The PurR binding site which overlaps the promoter encompasses approximately 110 bp. The protein-DNA interaction is inhibited by 5-phosphoribosyl 1-pyrophosphate. A mutation that deletes the repressor binding site or one that disrupts purR abolishes binding activity in vitro and repression of transcription in vivo in response to the excess adenine signal. These results lead to a model in which an excess-adenine signal is transmitted to PurR via the 5-phosphoribosyl 1-pyrophosphate pool. In addition, purR is autoregulated. There is no structural or mechanistic similarity between the B. subtilis and Escherichia coli purine repressors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arnvig K., Hove-Jensen B., Switzer R. L. Purification and properties of phosphoribosyl-diphosphate synthetase from Bacillus subtilis. Eur J Biochem. 1990 Aug 28;192(1):195–200. doi: 10.1111/j.1432-1033.1990.tb19214.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Choi K. Y., Zalkin H. Structural characterization and corepressor binding of the Escherichia coli purine repressor. J Bacteriol. 1992 Oct;174(19):6207–6214. doi: 10.1128/jb.174.19.6207-6214.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eads J. C., Scapin G., Xu Y., Grubmeyer C., Sacchettini J. C. The crystal structure of human hypoxanthine-guanine phosphoribosyltransferase with bound GMP. Cell. 1994 Jul 29;78(2):325–334. doi: 10.1016/0092-8674(94)90301-8. [DOI] [PubMed] [Google Scholar]
- Ebbole D. J., Zalkin H. Bacillus subtilis pur operon expression and regulation. J Bacteriol. 1989 Apr;171(4):2136–2141. doi: 10.1128/jb.171.4.2136-2141.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbole D. J., Zalkin H. Cloning and characterization of a 12-gene cluster from Bacillus subtilis encoding nine enzymes for de novo purine nucleotide synthesis. J Biol Chem. 1987 Jun 15;262(17):8274–8287. [PubMed] [Google Scholar]
- Ebbole D. J., Zalkin H. Detection of pur operon-attenuated mRNA and accumulated degradation intermediates in Bacillus subtilis. J Biol Chem. 1988 Aug 5;263(22):10894–10902. [PubMed] [Google Scholar]
- Ebbole D. J., Zalkin H. Interaction of a putative repressor protein with an extended control region of the Bacillus subtilis pur operon. J Biol Chem. 1989 Feb 25;264(6):3553–3561. [PubMed] [Google Scholar]
- Hershey H. V., Gutstein R., Taylor M. W. Cloning and restriction map of the E. coli apt gene. Gene. 1982 Jul-Aug;19(1):89–92. doi: 10.1016/0378-1119(82)90192-5. [DOI] [PubMed] [Google Scholar]
- Hidaka Y., Tarlé S. A., O'Toole T. E., Kelley W. N., Palella T. D. Nucleotide sequence of the human APRT gene. Nucleic Acids Res. 1987 Nov 11;15(21):9086–9086. doi: 10.1093/nar/15.21.9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove-Jensen B., Harlow K. W., King C. J., Switzer R. L. Phosphoribosylpyrophosphate synthetase of Escherichia coli. Properties of the purified enzyme and primary structure of the prs gene. J Biol Chem. 1986 May 25;261(15):6765–6771. [PubMed] [Google Scholar]
- Itaya M., Kondo K., Tanaka T. A neomycin resistance gene cassette selectable in a single copy state in the Bacillus subtilis chromosome. Nucleic Acids Res. 1989 Jun 12;17(11):4410–4410. doi: 10.1093/nar/17.11.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T. Purification of sequence-specific binding proteins by DNA affinity chromatography. Methods Enzymol. 1991;208:10–23. doi: 10.1016/0076-6879(91)08004-2. [DOI] [PubMed] [Google Scholar]
- Mäntsälä P., Zalkin H. Cloning and sequence of Bacillus subtilis purA and guaA, involved in the conversion of IMP to AMP and GMP. J Bacteriol. 1992 Mar;174(6):1883–1890. doi: 10.1128/jb.174.6.1883-1890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson D., Hove-Jensen B., Arnvig K. Primary structure of the tms and prs genes of Bacillus subtilis. Mol Gen Genet. 1989 Sep;218(3):565–571. doi: 10.1007/BF00332425. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Nakai S., Yoshikawa H. Systematic sequencing of the 180 kilobase region of the Bacillus subtilis chromosome containing the replication origin. DNA Res. 1994;1(1):1–14. doi: 10.1093/dnares/1.1.1. [DOI] [PubMed] [Google Scholar]
- Rolfes R. J., Zalkin H. Escherichia coli gene purR encoding a repressor protein for purine nucleotide synthesis. Cloning, nucleotide sequence, and interaction with the purF operator. J Biol Chem. 1988 Dec 25;263(36):19653–19661. [PubMed] [Google Scholar]
- Scapin G., Grubmeyer C., Sacchettini J. C. Crystal structure of orotate phosphoribosyltransferase. Biochemistry. 1994 Feb 15;33(6):1287–1294. doi: 10.1021/bi00172a001. [DOI] [PubMed] [Google Scholar]
- Schumacher M. A., Choi K. Y., Zalkin H., Brennan R. G. Crystal structure of LacI member, PurR, bound to DNA: minor groove binding by alpha helices. Science. 1994 Nov 4;266(5186):763–770. doi: 10.1126/science.7973627. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Zaluzec E. J., Wery J. P., Niu L., Switzer R. L., Zalkin H., Satow Y. Structure of the allosteric regulatory enzyme of purine biosynthesis. Science. 1994 Jun 3;264(5164):1427–1433. doi: 10.1126/science.8197456. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle E., Kornberg A. The partition locus of plasmid pSC101 is a specific binding site for DNA gyrase. EMBO J. 1988 Jun;7(6):1889–1895. doi: 10.1002/j.1460-2075.1988.tb03022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuryev A., Corden J. L. A Saccharomyces cerevisiae gene encoding a potential adenine phosphoribosyltransferase. Yeast. 1994 May;10(5):659–662. doi: 10.1002/yea.320100510. [DOI] [PubMed] [Google Scholar]