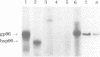
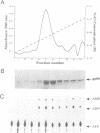
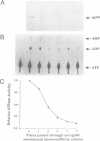
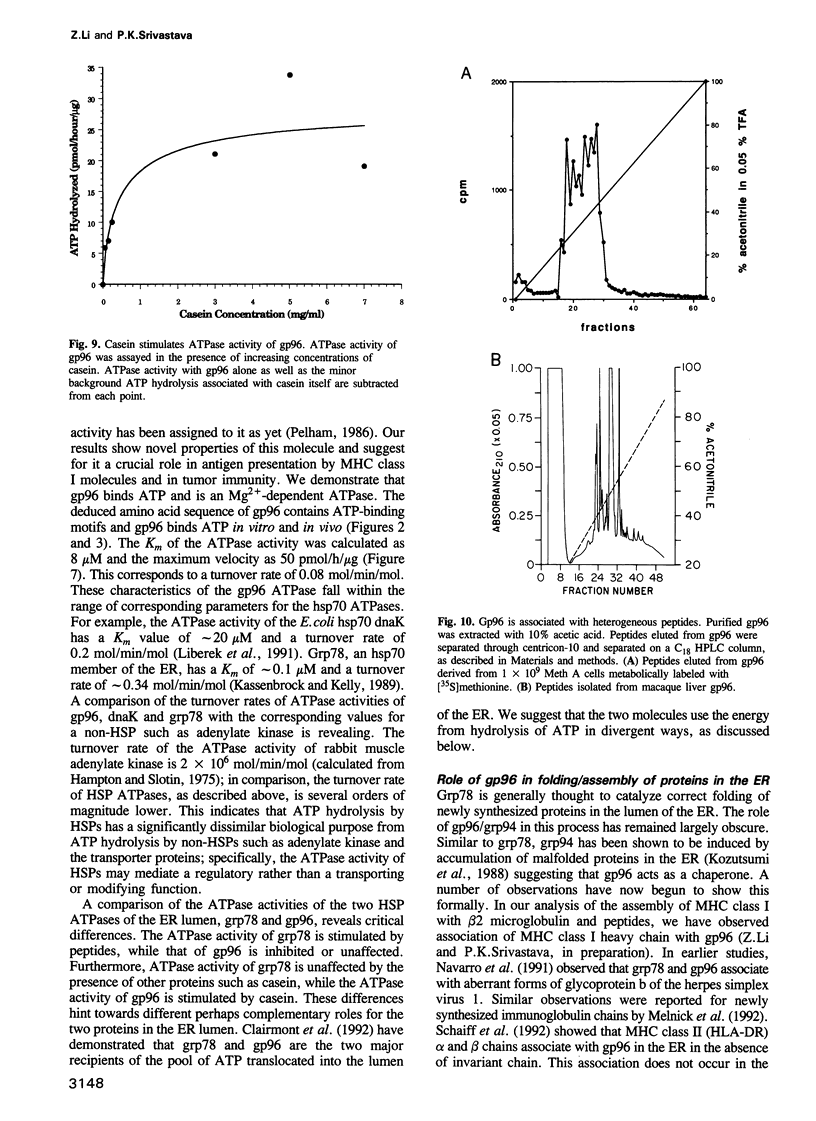
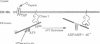Abstract
Immunization of mice with gp96/grp94 heat shock proteins (HSPs) elicits tumor-specific cellular immunity to the tumors from which gp96 is isolated. However, the cDNA sequence of gp96 is identical among tumors and normal tissues. This raises the question regarding the structural basis of the specific immunogenicity of gp96. As HSPs bind a wide array of molecules including peptides, we have proposed that gp96 may not be immunogenic per se, but may chaperone antigenic peptides. Furthermore, gp96 is localized predominantly in the lumen of the endoplasmic reticulum (ER) suggesting that it may act as a peptide acceptor and as accessory to peptide loading of MHC class I molecules. We demonstrate here that gp96 molecules contain ATP-binding cassettes, bind ATP and possess an Mg(2+)-dependent ATPase activity. Gp96 preparations are also observed to contain tightly bound peptides, which can be eluted by acid extraction. These properties of gp96 are consistent with its proposed roles in chaperoning antigenic peptides and in facilitating MHC class I--peptide assembly in the ER lumen. We present a model to explain how interaction of gp96 with MHC class I may result in transfer of peptides to the latter.
Full text
PDF
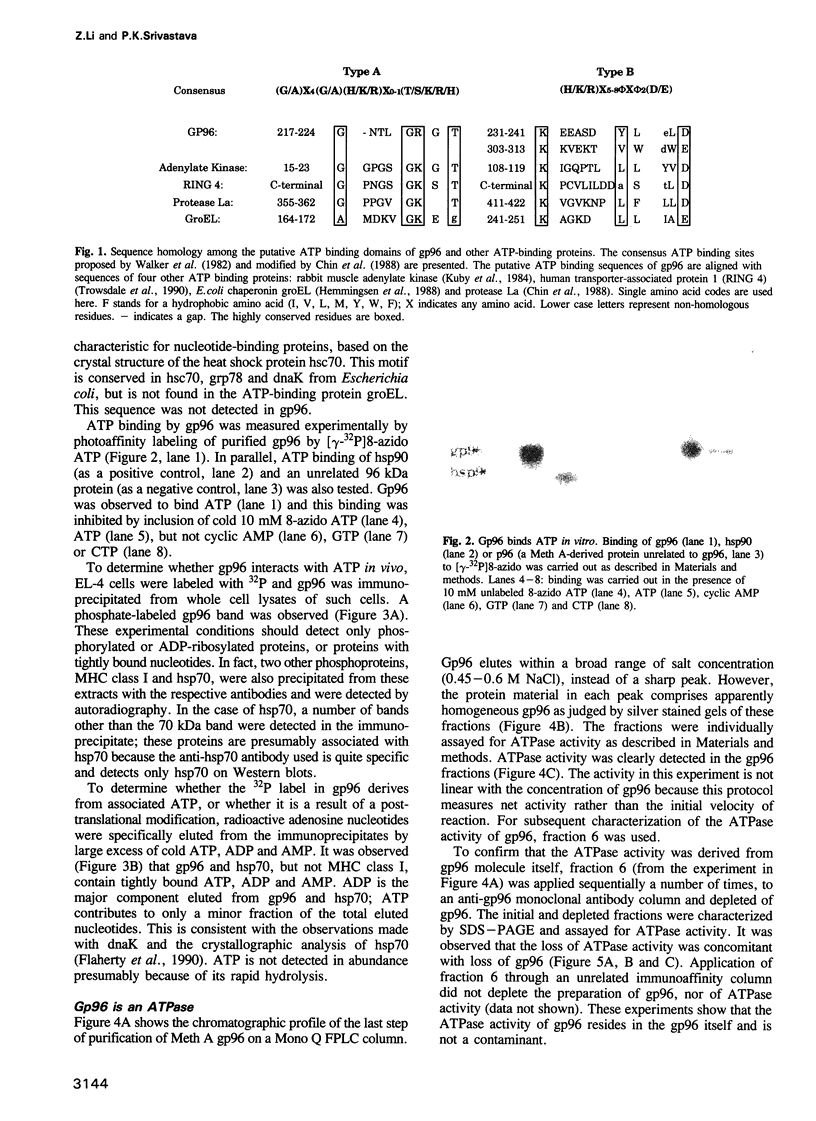
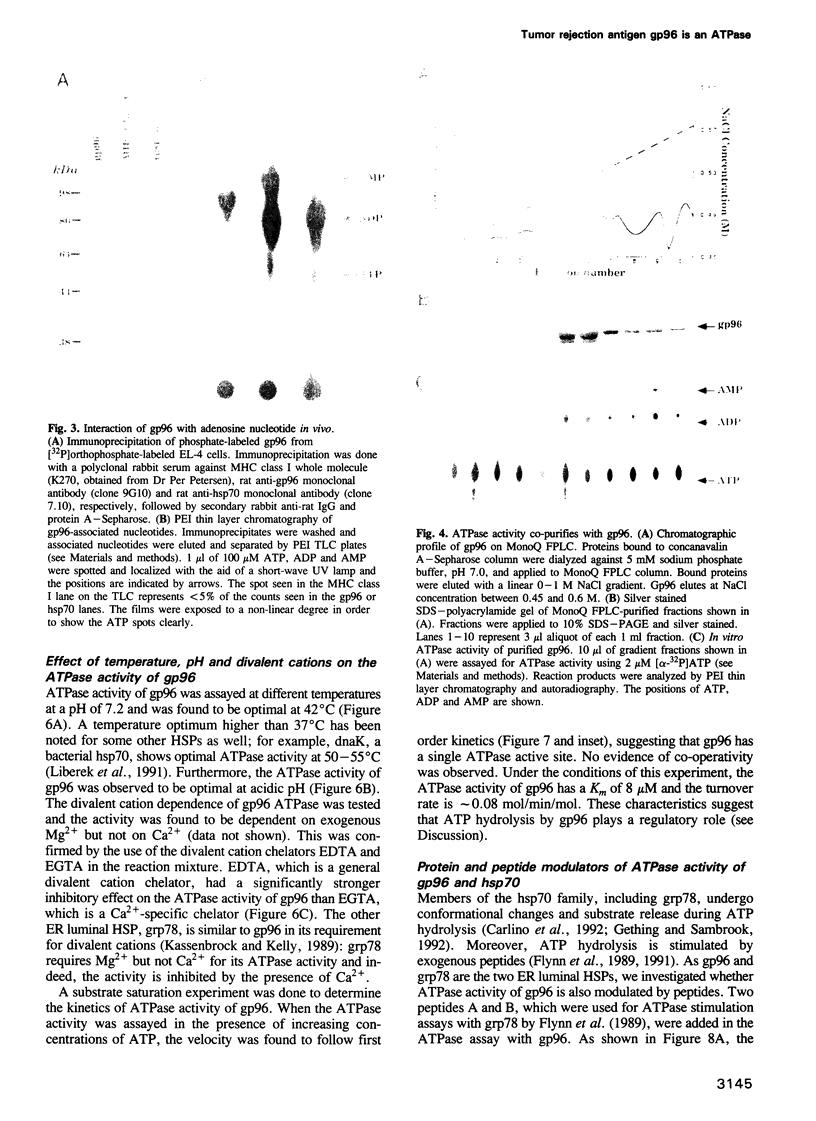
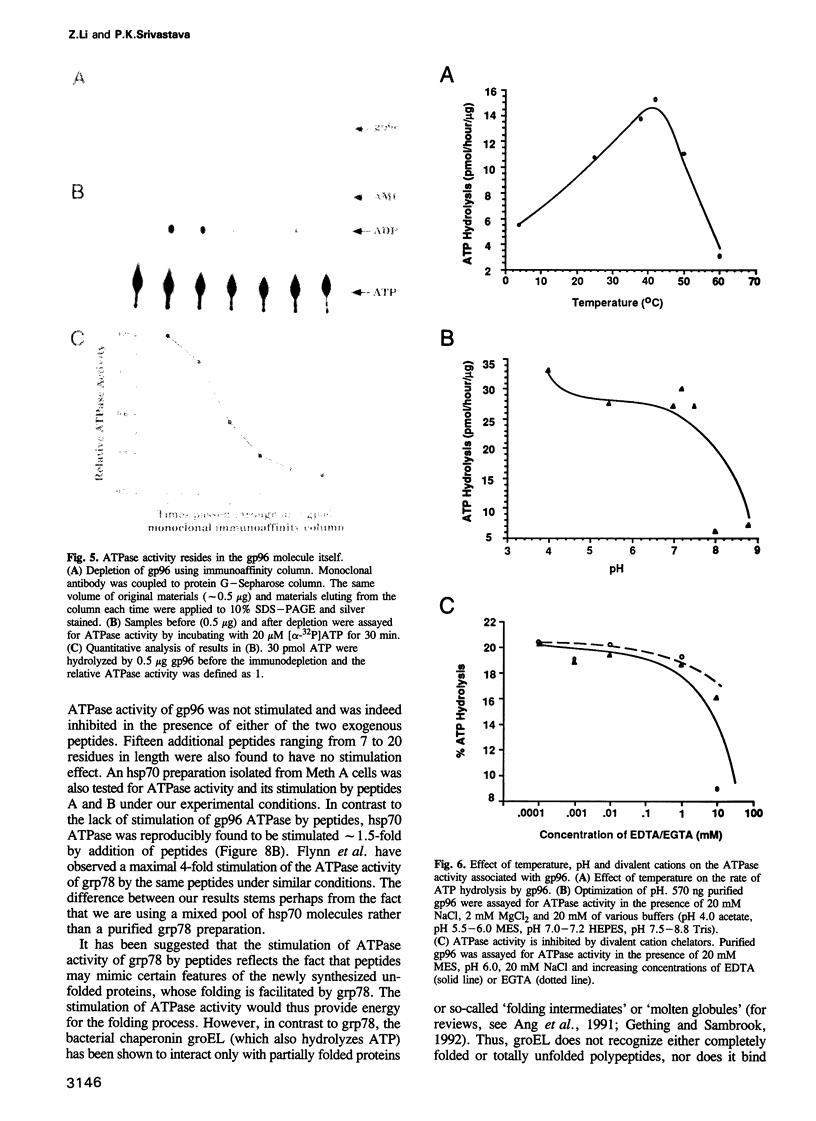
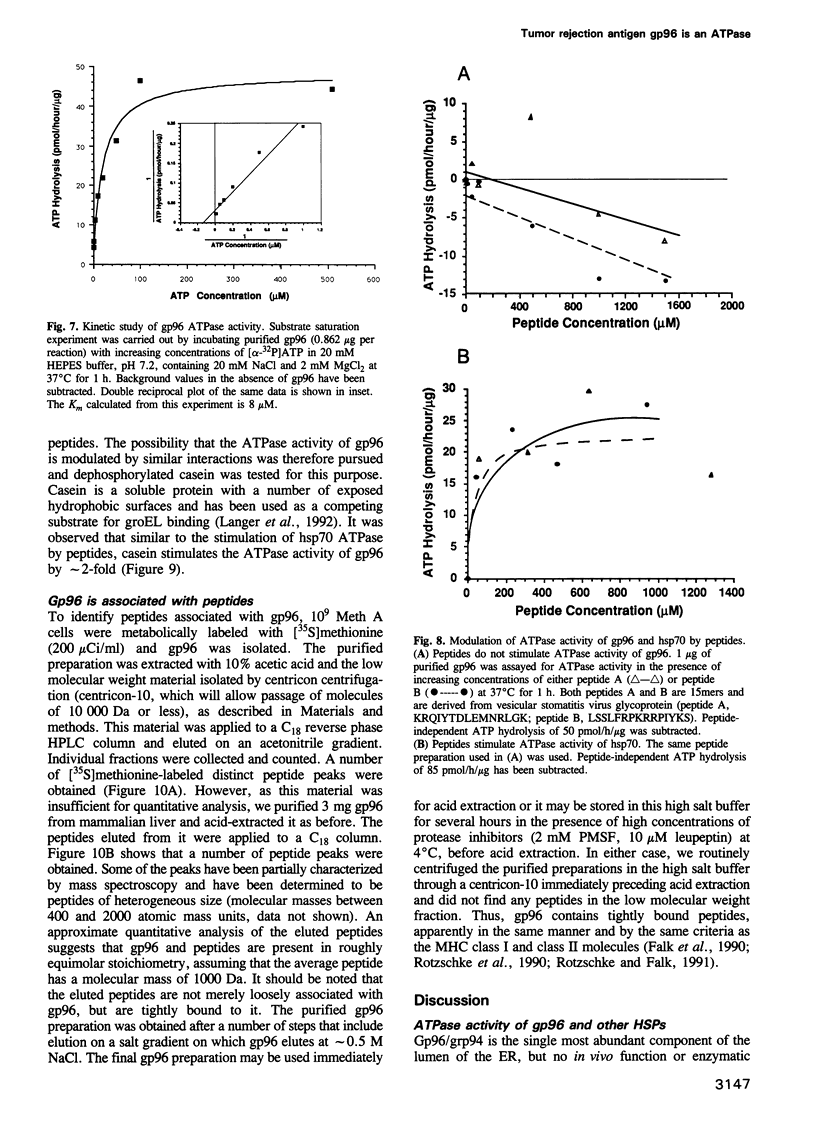



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J., Payne J. A., Murray R., Frelinger J. A., Cresswell P. Differential transport requirements of HLA and H-2 class I glycoproteins. Immunogenetics. 1989;29(6):380–388. doi: 10.1007/BF00375866. [DOI] [PubMed] [Google Scholar]
- Ang D., Liberek K., Skowyra D., Zylicz M., Georgopoulos C. Biological role and regulation of the universally conserved heat shock proteins. J Biol Chem. 1991 Dec 25;266(36):24233–24236. [PubMed] [Google Scholar]
- Boon T. Toward a genetic analysis of tumor rejection antigens. Adv Cancer Res. 1992;58:177–210. doi: 10.1016/s0065-230x(08)60295-x. [DOI] [PubMed] [Google Scholar]
- Booth C., Koch G. L. Perturbation of cellular calcium induces secretion of luminal ER proteins. Cell. 1989 Nov 17;59(4):729–737. doi: 10.1016/0092-8674(89)90019-6. [DOI] [PubMed] [Google Scholar]
- Carlino A., Toledo H., Skaleris D., DeLisio R., Weissbach H., Brot N. Interactions of liver Grp78 and Escherichia coli recombinant Grp78 with ATP: multiple species and disaggregation. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2081–2085. doi: 10.1073/pnas.89.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. T., Goff S. A., Webster T., Smith T., Goldberg A. L. Sequence of the lon gene in Escherichia coli. A heat-shock gene which encodes the ATP-dependent protease La. J Biol Chem. 1988 Aug 25;263(24):11718–11728. [PubMed] [Google Scholar]
- Clairmont C. A., De Maio A., Hirschberg C. B. Translocation of ATP into the lumen of rough endoplasmic reticulum-derived vesicles and its binding to luminal proteins including BiP (GRP 78) and GRP 94. J Biol Chem. 1992 Feb 25;267(6):3983–3990. [PubMed] [Google Scholar]
- Degen E., Williams D. B. Participation of a novel 88-kD protein in the biogenesis of murine class I histocompatibility molecules. J Cell Biol. 1991 Mar;112(6):1099–1115. doi: 10.1083/jcb.112.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- FOLEY E. J. Antigenic properties of methylcholanthrene-induced tumors in mice of the strain of origin. Cancer Res. 1953 Dec;13(12):835–837. [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Rammensee H. G. Cellular peptide composition governed by major histocompatibility complex class I molecules. Nature. 1990 Nov 15;348(6298):248–251. doi: 10.1038/348248a0. [DOI] [PubMed] [Google Scholar]
- Flaherty K. M., DeLuca-Flaherty C., McKay D. B. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990 Aug 16;346(6285):623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- Flaherty K. M., McKay D. B., Kabsch W., Holmes K. C. Similarity of the three-dimensional structures of actin and the ATPase fragment of a 70-kDa heat shock cognate protein. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5041–5045. doi: 10.1073/pnas.88.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn G. C., Chappell T. G., Rothman J. E. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 1989 Jul 28;245(4916):385–390. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- Flynn G. C., Pohl J., Flocco M. T., Rothman J. E. Peptide-binding specificity of the molecular chaperone BiP. Nature. 1991 Oct 24;353(6346):726–730. doi: 10.1038/353726a0. [DOI] [PubMed] [Google Scholar]
- Galvin K., Krishna S., Ponchel F., Frohlich M., Cummings D. E., Carlson R., Wands J. R., Isselbacher K. J., Pillai S., Ozturk M. The major histocompatibility complex class I antigen-binding protein p88 is the product of the calnexin gene. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8452–8456. doi: 10.1073/pnas.89.18.8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Hampton A., Slotin L. A. Inactivation of rabbit, pig, and carp adenylate kinases by N6-o- and p-fluorobenzoyladenosine 5'-triphosphates. Biochemistry. 1975 Dec 16;14(25):5438–5444. doi: 10.1021/bi00696a009. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Hobson A. C., Weatherwax R., Ames G. F. ATP-binding sites in the membrane components of histidine permease, a periplasmic transport system. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7333–7337. doi: 10.1073/pnas.81.23.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstenbach F., David V., Watkins S., Brenner M. B. Endoplasmic reticulum resident protein of 90 kilodaltons associates with the T- and B-cell antigen receptors and major histocompatibility complex antigens during their assembly. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4734–4738. doi: 10.1073/pnas.89.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN G., SJOGREN H. O., KLEIN E., HELLSTROM K. E. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res. 1960 Dec;20:1561–1572. [PubMed] [Google Scholar]
- Kassenbrock C. K., Kelly R. B. Interaction of heavy chain binding protein (BiP/GRP78) with adenine nucleotides. EMBO J. 1989 May;8(5):1461–1467. doi: 10.1002/j.1460-2075.1989.tb03529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozutsumi Y., Segal M., Normington K., Gething M. J., Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988 Mar 31;332(6163):462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- Kuby S. A., Palmieri R. H., Frischat A., Fischer A. H., Wu L. H., Maland L., Manship M. Studies on adenosine triphosphate transphosphorylases. Amino acid sequence of rabbit muscle ATP-AMP transphosphorylase. Biochemistry. 1984 May 22;23(11):2393–2399. doi: 10.1021/bi00306a012. [DOI] [PubMed] [Google Scholar]
- Langer T., Lu C., Echols H., Flanagan J., Hayer M. K., Hartl F. U. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992 Apr 23;356(6371):683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- Lee A. S., Bell J., Ting J. Biochemical characterization of the 94- and 78-kilodalton glucose-regulated proteins in hamster fibroblasts. J Biol Chem. 1984 Apr 10;259(7):4616–4621. [PubMed] [Google Scholar]
- Liberek K., Marszalek J., Ang D., Georgopoulos C., Zylicz M. Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luescher I. F., Lóez J. A., Malissen B., Cerottini J. C. Interaction of antigenic peptides with MHC class I molecules on living cells studied by photoaffinity labeling. J Immunol. 1992 Feb 15;148(4):1003–1011. [PubMed] [Google Scholar]
- Lévy F., Gabathuler R., Larsson R., Kvist S. ATP is required for in vitro assembly of MHC class I antigens but not for transfer of peptides across the ER membrane. Cell. 1991 Oct 18;67(2):265–274. doi: 10.1016/0092-8674(91)90178-2. [DOI] [PubMed] [Google Scholar]
- Maki R. G., Old L. J., Srivastava P. K. Human homologue of murine tumor rejection antigen gp96: 5'-regulatory and coding regions and relationship to stress-induced proteins. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5658–5662. doi: 10.1073/pnas.87.15.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzarella R. A., Green M. ERp99, an abundant, conserved glycoprotein of the endoplasmic reticulum, is homologous to the 90-kDa heat shock protein (hsp90) and the 94-kDa glucose regulated protein (GRP94). J Biol Chem. 1987 Jun 25;262(18):8875–8883. [PubMed] [Google Scholar]
- Melnick J., Aviel S., Argon Y. The endoplasmic reticulum stress protein GRP94, in addition to BiP, associates with unassembled immunoglobulin chains. J Biol Chem. 1992 Oct 25;267(30):21303–21306. [PubMed] [Google Scholar]
- Navarro D., Qadri I., Pereira L. A mutation in the ectodomain of herpes simplex virus 1 glycoprotein B causes defective processing and retention in the endoplasmic reticulum. Virology. 1991 Sep;184(1):253–264. doi: 10.1016/0042-6822(91)90842-y. [DOI] [PubMed] [Google Scholar]
- PREHN R. T., MAIN J. M. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957 Jun;18(6):769–778. [PubMed] [Google Scholar]
- Palladino M. A., Jr, Srivastava P. K., Oettgen H. F., DeLeo A. B. Expression of a shared tumor-specific antigen by two chemically induced BALB/c sarcomas. Cancer Res. 1987 Oct 1;47(19):5074–5079. [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Deres K., Schild H., Norda M., Metzger J., Jung G., Rammensee H. G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990 Nov 15;348(6298):252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K. Naturally-occurring peptide antigens derived from the MHC class-I-restricted processing pathway. Immunol Today. 1991 Dec;12(12):447–455. doi: 10.1016/0167-5699(91)90018-O. [DOI] [PubMed] [Google Scholar]
- Schaiff W. T., Hruska K. A., Jr, McCourt D. W., Green M., Schwartz B. D. HLA-DR associates with specific stress proteins and is retained in the endoplasmic reticulum in invariant chain negative cells. J Exp Med. 1992 Sep 1;176(3):657–666. doi: 10.1084/jem.176.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaknovich R., Shue G., Kohtz D. S. Conformational activation of a basic helix-loop-helix protein (MyoD1) by the C-terminal region of murine HSP90 (HSP84). Mol Cell Biol. 1992 Nov;12(11):5059–5068. doi: 10.1128/mcb.12.11.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille C., Chomez P., Wildmann C., Van Pel A., De Plaen E., Maryanski J. L., de Bergeyck V., Boon T. Structure of the gene of tum- transplantation antigen P198: a point mutation generates a new antigenic peptide. J Exp Med. 1990 Jul 1;172(1):35–45. doi: 10.1084/jem.172.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. K., Chen Y. T., Old L. J. 5'-structural analysis of genes encoding polymorphic antigens of chemically induced tumors. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3807–3811. doi: 10.1073/pnas.84.11.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. K., Das M. R. The serologically unique cell surface antigen of Zajdela ascitic hepatoma is also its tumor-associated transplantation antigen. Int J Cancer. 1984 Mar 15;33(3):417–422. doi: 10.1002/ijc.2910330321. [DOI] [PubMed] [Google Scholar]
- Srivastava P. K., DeLeo A. B., Old L. J. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc Natl Acad Sci U S A. 1986 May;83(10):3407–3411. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. K., Heike M. Tumor-specific immunogenicity of stress-induced proteins: convergence of two evolutionary pathways of antigen presentation? Semin Immunol. 1991 Jan;3(1):57–64. [PubMed] [Google Scholar]
- Srivastava P. K., Maki R. G. Stress-induced proteins in immune response to cancer. Curr Top Microbiol Immunol. 1991;167:109–123. doi: 10.1007/978-3-642-75875-1_7. [DOI] [PubMed] [Google Scholar]
- Srivastava P. K. Protein tumor antigens. Curr Opin Immunol. 1991 Oct;3(5):654–658. doi: 10.1016/0952-7915(91)90092-f. [DOI] [PubMed] [Google Scholar]
- Szikora J. P., Van Pel A., Brichard V., André M., Van Baren N., Henry P., De Plaen E., Boon T. Structure of the gene of tum- transplantation antigen P35B: presence of a point mutation in the antigenic allele. EMBO J. 1990 Apr;9(4):1041–1050. doi: 10.1002/j.1460-2075.1990.tb08208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A., Elliott T., Cerundolo V., Foster L., Barber B., Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990 Jul 27;62(2):285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Hanson I., Mockridge I., Beck S., Townsend A., Kelly A. Sequences encoded in the class II region of the MHC related to the 'ABC' superfamily of transporters. Nature. 1990 Dec 20;348(6303):741–744. doi: 10.1038/348741a0. [DOI] [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Van den Eynde B., Lethé B., Van Pel A., De Plaen E., Boon T. The gene coding for a major tumor rejection antigen of tumor P815 is identical to the normal gene of syngeneic DBA/2 mice. J Exp Med. 1991 Jun 1;173(6):1373–1384. doi: 10.1084/jem.173.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada I., Rindress D., Cameron P. H., Ou W. J., Doherty J. J., 2nd, Louvard D., Bell A. W., Dignard D., Thomas D. Y., Bergeron J. J. SSR alpha and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J Biol Chem. 1991 Oct 15;266(29):19599–19610. [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech H., Buchner J., Zimmermann R., Jakob U. Hsp90 chaperones protein folding in vitro. Nature. 1992 Jul 9;358(6382):169–170. doi: 10.1038/358169a0. [DOI] [PubMed] [Google Scholar]