Abstract
Polymyxins, which are increasingly being used to treat infections caused by multidrug-resistant bacteria, perform poorly against Serratia marcescens. To investigate the underlying mechanisms, Tn5 mutagenesis was performed and two mutants exhibiting increased polymyxin B (PB) susceptibility were isolated. The mutants were found to have Tn5 inserted into the arnB and arnC genes. In other bacteria, arnB and arnC belong to the seven-gene arn operon, which is involved in lipopolysaccharide (LPS) modification. LPSs of arn mutants had greater PB-binding abilities than that of wild-type LPS. Further, we identified PhoP, a bacterial two-component response regulator, as a regulator of PB susceptibility in S. marcescens. By the reporter assay, we found PB- and low-Mg2+-induced expression of phoP and arn in the wild-type strain but not in the phoP mutant. Complementation of the phoP mutant with the full-length phoP gene restored the PB MIC and induction by PB and low Mg2+ levels, as in the wild type. An electrophoretic mobility shift assay (EMSA) further demonstrated that PhoP bound directly to the arn promoter. The PB challenge test confirmed that pretreatment with PB and low Mg2+ levels protected S. marcescens from a PB challenge in the wild-type strain but not in the phoP mutant. Real-time reverse transcriptase-PCR also indicated that PB serves as a signal to regulate expression of ugd, a gene required for LPS modification, in S. marcescens through a PhoP-dependent pathway. Finally, we found that PB-resistant clinical isolates displayed greater expression of arnA upon exposure to PB than did susceptible isolates. This is the first report to describe the role of S. marcescens arn in PB resistance and its modulation by PB and Mg2+ through the PhoP protein.
INTRODUCTION
Cationic antimicrobial polypeptides (CAPs) play an important role in host defenses against microbial infections by affecting membrane integrity and are key effectors of the host innate immune response (1). CAPs, having antimicrobial and immunomodulatory activities, are being developed as a promising new class of therapeutic drugs (2). Microbial pathogens have evolved distinct mechanisms to resist killing by CAPs (3, 4), including expelling CAPs through pumps and cleaving CAPs with proteases. One of the important mechanisms of resistance to CAPs in Gram-negative bacteria involves remodeling of the composition of the outer membrane through modification of lipopolysaccharide (LPS) with positively charged substituents, which leads to the repulsion of CAPs (4). In Escherichia coli and Salmonella enterica serovar Typhimurium, a seven-gene polycistronic unit (arnBCADTEF or pmrHFIJKLM, referred to as the arn or pmr operon, respectively) is involved in LPS modification; all genes of the arn operon except arnF are necessary for the biosynthesis and addition of 4-aminoarabinose (Ara4N) to the 4′ phosphate of lipid A, a modification that contributes to reduction of the net negative charge of LPS and consequently decreases the attraction and binding of CAPs to the outer membrane (5, 6).
In a large number of bacterial species, the genes conferring resistance to CAPs are regulated by bacterial two-component systems (7–12). In Salmonella, transcriptional activation of the pmr operon requires the PmrA-PmrB two-component regulatory system, where PmrB is the sensor kinase and PmrA is the cognate response regulator that controls pmr operon expression directly (7). Decreases in Mg2+ concentrations promote PmrA-dependent upregulation of the pmr operon. This process also requires the PhoP (regulator)-PhoQ (sensor kinase) two-component system, a master regulator of S. enterica virulence functions (8, 13). PhoP positively controls the pmr operon at the transcriptional level by increasing production of PmrD (8), which then activates the PmrA protein posttranslationally, resulting in modification of LPS. More recently, studies have shown that the transcription of PhoP-activated genes is upregulated by sublethal concentrations of CAPs (14) and that CAPs can bind to and activate the PhoQ sensor directly (15).
Serratia marcescens, which was first described in 1819, was thought for years to be a nonpathogen; S. marcescens is now an accepted clinical pathogen, causing nosocomial infections and a full spectrum of clinical diseases, including urinary tract infections and pneumonia (16). The significance of S. marcescens was further highlighted by the emergence of multidrug-resistant (MDR) isolates. Recently, polymyxins, a type of CAPs (17, 18), were being increasingly used for the treatment of infections caused by MDR bacteria (18, 19). Although polymyxin B (PB) demonstrated good activity against most Enterobacteriaceae species, the drug showed poor activity against Proteus and Serratia spp. (19, 20). Previously, we reported that a Proteus mirabilis two-component response regulator, RppA, is involved in PB resistance through regulation of the expression of its downstream genes, ugd and pmrI, which are responsible for the synthesis and/or modification of LPS (17, 21, 22). UDP-glucose dehydrogenase (Ugd) is an enzyme that converts UDP-glucose into UDP-glucuronic acid. Both UDP-glucose and UDP-glucuronic acid are precursors for the synthesis of extracellular polysaccharides and LPS in many pathogenic bacteria. pmrI, one gene of the pmrHFIJKLM operon that encodes UDP-glucuronic acid decarboxylase, is involved in LPS modification. Mutation of rppA, ugd, or pmrI in P. mirabilis leads to increased PB sensitivity (17, 21, 22).
In this study, by using a Tn5 transposon mutagenesis approach, we identified two genes, arnB and arnC, that are involved in PB resistance in S. marcescens. We also found that expression of the S. marcescens arn operon is under the control of the two-component regulator PhoP. To our knowledge, this is the first report describing the role of the arn operon and its regulation by the PhoP response regulator in S. marcescens.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacteria were routinely cultured at 37°C in Luria-Bertani (LB) medium (17). Clinical isolates of S. marcescens were collected at the National Taiwan University Hospital. These isolates were obtained from sputum (R1, R2, S1, and S2), wounds (R3, S3, and S4), and urine (R4, R5, R6, S5, and S6). All of the isolates were identified by conventional biochemical identification methods, and results were confirmed by the Vitek automated system (bioMérieux). Random amplified polymorphic DNA (RAPD) analysis (Operon Technologies) was used to exclude repeat isolates.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain, mutation, or plasmid | Genotype or relevant phenotypea | Reference or source |
|---|---|---|
| Serratia marcescens strains | ||
| 3927 | Wild-type; Tetr Cmr Ampr Sms Kms | Clinical isolate |
| arnB(t) | 3927 derivative; arnB Tn5-mutagenized mutant; PBs Kmr | This study |
| arnC(t) | 3927 derivative; arnC Tn5-mutagenized mutant; PBs Kmr | This study |
| phoP | 3927 derivative; phoP-knockout mutant; Kmr | This study |
| phoP(c) | phoP mutant containing pBAD33 (Smr)-phoP; phoP-complemented strain; Smr | This study |
| arnB | 3927 derivative; arnB-knockout mutant; Kmr | This study |
| arnB(c) | arnB mutant containing pBAD33 (Smr)-arnB; arnB-complemented strain; Smr | This study |
| arnC(tc) | arnC(t) mutant containing pBAD33 (Smr)-arnC; arnC-complemented strain; Smr | This study |
| R1–R6 | PBr | Clinical isolate |
| S1–S6 | PBs | Clinical isolate |
| E. coli | ||
| DH5α | fhuA2 lac(del)U169 phoA glnV44 φ80′ lacZ(del)M15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17 | Invitrogen |
| S17-1 λ pir | λ pir lysogen of S17-1 (thi pro hsdR− hsdM+ recA RP4 2-Tc::Mu-Km::Tn7 [Tpr Smr]); permissive host able to transfer suicide plasmids requiring Pir protein by conjugation to recipient cells | 21 |
| BL21(DE3) | F− ompT hsdSB (rB− mB−) gal dcm (DE3) | Invitrogen |
| Plasmids | ||
| pGEM-T Easy | High-copy-number TA cloning vector; Ampr | Promega |
| pGEM-4Z | High-copy-number cloning vector; Ampr | Promega |
| yT&A::xylE | High-copy-number TA cloning vector containing xylE coding region; Ampr | Provided by Yang |
| pET32a(+) | Expression vector containing T7 promoter; Ampr | Novagen |
| pET32a(+)-phoP | pET32a(+) containing intact phoP sequence; Ampr | This study |
| pUT/mini-Tn5-Km | Suicide plasmid requiring Pir protein for replication and containing mini-Tn5 cassette containing Kmr gene | 22 |
| pBAD33 | Low-copy-number cloning vector, P15A replicon; Cmr | Addgene |
| pACYC184-Sm | Low-copy-number cloning vector, P15A replicon; Smr Tetr | This study |
| pACYC184-Sm-phoP-xylE | pACYC184-Sm containing intact phoP promoter sequence and xylE ORF; Smr Tetr | This study |
| pACYC184-Sm-arn-xylE | pACYC184-Sm containing intact arn promoter sequence and xylE ORF; Smr Tetr | This study |
Tet, tetracycline; Amp, ampicillin; Km, kanamycin; Cm, chloramphenicol; Sm, streptomycin; Tp, trimethoprim.
Transposon mutagenesis and identification of the mutated gene.
S. marcescens mini-Tn5 kanamycin (Km) mutagenized mutants were isolated as described previously (17). PB-susceptible clones were identified by replica plating on LB plates containing 400 μg/ml PB. Chromosomal DNA was extracted from the mutants and partially digested with EcoRV, and fragments over 4 kb were cloned into SmaI-digested pGEM-4Z (Promega). Following transformation of Escherichia coli TOP10 cells, Km-resistant Tn5 Km-containing clones were selected. Using I-out and O-out primers, the nucleotide sequences flanking the Tn5 cassette were obtained and subjected to BLAST analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome). We then searched the sequence in the released genome sequence of S. marcescens (https://www.sanger.ac.uk/cgi-bin/blast/submitblast/s_marcescens) and cloned the full gene by PCR TA cloning using primers from highly conserved sequences around the Tn5-interrupted genes (arnB and arnC) in the released genome sequences of S. marcescens. The remaining genes of the arn operon were also cloned by PCR TA cloning, using primers from highly conserved sequences. The nucleotide sequence was determined using a 373A DNA sequencer (Applied Biosystems). Alignment of protein amino acids with counterpart proteins was performed using DNAman software (version 4.15).
Gene knockout by homologous recombination.
For construction of the arnB and phoP mutants, the primer pairs arnBko-F/arnBko-R-XbaI and phoPko-F/phoPko-R-XbaI (see Table S1 in the supplemental material) were used to amplify the central regions of arnB and phoP, respectively. PCR products were cloned into pGEM-T Easy (Promega) and ligated with a Km cassette by XbaI digestion. The phoP- and arnB-containing plasmids were excised as SalI/SphI and NotI fragments, respectively, and ligated into pUT vectors digested with SalI/SphI and NotI, respectively, to form pUT-phoP and pUT-arnB. For gene inactivation by homologous recombination, pUT-phoP or pUT-arnB was transferred to S. marcescens 3927 by conjugation. Transconjugants were spread on LB plates containing kanamycin (100 μg/ml). Mutant candidates were screened by colony PCR, and Southern blot hybridization was performed to confirm the mutant genotypes (data not shown). Results confirmed that a single crossover event had occurred (23) (data not shown).
Construction of phoP-, arnB-, and arnC-complemented strains.
Full-length phoP, arnB, and arnC were amplified by PCR (primers are listed in Table S1 in the supplemental material) and cloned into pGEM-T Easy (Promega). The DNA fragments containing full-length phoP, arnB, and arnC were excised with XhoI/SacI and ligated into SalI/SacI-digested low-copy-number plasmid pBAD33 (also carrying a streptomycin resistance cassette for selection, since S. marcescens 3927 is resistant to chloramphenicol) to generate the phoP, arnB, and arnC complementation plasmids, respectively. Arabinose (0.02%)-induced expression of these genes was driven by the PBAD promoter in the pBAD33 plasmid. The phoP, arnB, and arnC complementation plasmids were then transformed into the mutants to generate the respective complemented strains.
MIC assay.
In vitro determination of MICs for PB was performed by the broth microdilution method, according to the guidelines proposed by the Clinical and Laboratory Standards Institute (24).
Preparation and analysis of LPS.
The extraction and analysis of LPS were performed as described previously (17).
PB binding of LPS.
The experiments to determine binding of PB by LPS were performed as described previously (17).
Binding of wild-type S. marcescens and arnB mutant with fluorescent polymyxin B.
Overnight cultures of bacterial strains were washed with phosphate-buffered saline (PBS), followed by dilution in PBS to an optical density at 600 nm (OD600) of 0.05. Oregon Green 514-conjugated polymyxin B (P13236; Invitrogen) was added to 50 μl of diluted cells at select concentrations, and the cells were incubated at 37°C for 10 min. Cells were then washed with PBS, resuspended in 50 μl of PBS, and placed in 96-well plates for analysis. The fluorescence (excitation, 480 nm; emission, 535 nm) and OD600 values for each well were determined using a microplate reader (SpectraMax M5; Molecular Devices) (25).
Reporter assay.
The promoter region of the putative phoP or arn operon was amplified by SphI- and PstI-included primers (see Table S1 in the supplemental material) and cloned into pGEM-T Easy to create pGEMphoPp or pGEMarnp, respectively. pGEMphoPp and pGEMarnp were digested by SphI/PstI, and the promoter-containing fragments were ligated with xylE-containing pACYC184 digested by SphI/PstI to construct the phoP- and arn-xylE reporter plasmids. The reporter plasmids containing the wild-type, phoP mutant, and phoP-complemented strains were grown overnight in N minimal medium (NMM) [5 mM KCl, 7.5 mM (NH4)2SO4, 0.5 mM K2SO4, 1 mM KH2PO4, 38 mM glycerol, 0.1% Casamino Acids, 0.1 M Tris-HCl, pH 7.4] with 2 mM MgCl2 or LB broth with 100 μg/ml streptomycin, diluted 100-fold in the same medium, and grown to an OD600 value of 0.3. Then cells were incubated under conditions with or without 1 μg/ml PB (in LB broth) and with low or high Mg2+ levels (in NMM). The xylE activity was measured 1, 2, and 3 h after incubation (22).
Purification of His-tagged recombinant protein and electrophoretic mobility shift assay.
The full-length phoP gene was cloned into the plasmid pET32a(+) with BamHI and XhoI to generate plasmid pET32a(+)-PhoP. Overexpression and purification of His-tagged PhoP were performed as described previously (22). The purity of the PhoP preparation was confirmed by SDS-PAGE. The amplified promoter DNA (0.1 μg each) was incubated with 0, 0.5, 1, or 2 μM His-tagged PhoP protein in 10 μl of binding buffer (22). After incubation for 30 min at room temperature, the reaction mixtures were loaded onto 5% nondenaturing polyacrylamide gels, and the gels were subjected to electrophoresis at 100 V for 1.5 to 2 h before being stained with ethidium bromide (22).
PB challenge test for S. marcescens after pretreatment with Mg2+ or PB.
For Mg2+ pretreatment, cells were harvested from an overnight culture grown in NMM with 2 mM MgCl2, washed with NMM without MgCl2, and diluted 1:50 in NMM with 10 μM MgCl2 (low Mg). Bacteria were grown at 37°C, with aeration, to an optical density at 600 nm (OD600) of about 0.6 and were challenged with PB (1,000 μg/ml). Bacterial growth was monitored at 1-h intervals after incubation at 37°C with aeration. In addition, PB (1 μg/ml) was added to cultures at OD600 of 0.6 in NMM with 2 mM MgCl2, cells were then induced for 1 h, and the PB challenge test was performed in the same way as described above. For PB pretreatment, overnight bacterial cultures were diluted, grown to an OD600 of 0.6 at 37°C in LB broth (containing about 0.1 mM Mg2+) with or without MgCl2 (20 mM), and challenged with the MIC dose of PB for the wild-type, phoP mutant, and phoP-complemented strains (2,048, 2, and 2,048 μg/ml, respectively). After being challenged with the respective MIC dose of PB for 1 h, bacteria were diluted and plated on LB agar plates, and the numbers of CFU were determined after overnight incubation. For PB induction, 1 μg/ml PB was added to cultures with an OD600 of 0.3, cells were grown to an OD600 of 0.6, and then the PB challenge was conducted. The percent survival was calculated as (CFU of PB-challenged culture/CFU of no-challenge culture) × 100% (26).
Reverse transcription assays.
Total RNA was extracted from overnight LB cultures. Reverse transcriptase (RT)-PCR analyses were carried out by reverse transcription of total RNA using random primers or gene-specific primers and avian myeloblastosis virus reverse transcriptase (Roche). The resulting cDNA was amplified using the primer pairs of arnBCrt-F/arnBCrt-R and arnCArt-F/arnCArt-R (see Table S1 in the supplemental material) for the arnBC and arnCA intergenic regions, respectively (Fig. 1). For control reactions, RNA without RT or chromosomal DNA was used as a template. To study the effects of phoP mutation on the expression of ugd mRNA, overnight LB cultures of the wild-type, phoP knockout, and phoP-complemented strains were diluted 100-fold with LB broth and grown to an OD600 of 0.3, and the cultures were further grown at 37°C to an OD600 of 0.6, without or with 1 μg/ml PB. Then, total RNA was extracted and real-time RT-PCR was performed as described previously (17), to monitor the expression of ugd mRNA. arnA expression in clinical S. marcescens isolates (Table 2) was measured in the same way. The mRNA levels were normalized against 16S rRNA levels.
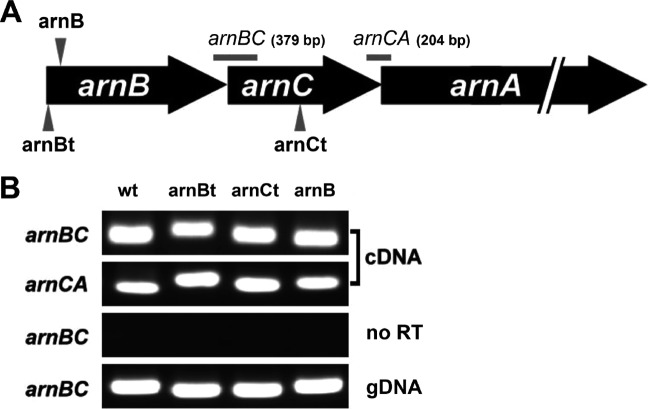
FIG 1.
(A) Organization of S. marcescens arnBCA. Only arnBCA of the arn operon is shown. Arrow, knockout or Tn5-interrupted site for the arnB, arnB(t), or arnC(t) mutant; horizontal line, region amplified by PCR. The product sizes are indicated in parentheses. (B) RT-PCR analysis of arn transcription in wild-type S. marcescens and the arnB(t), arnC(t), and arnB mutants. RT-PCR using the primer pair annealing to arnB/arnC or arnC/arnA (the region shown in panel A) was performed with RNA isolated from overnight cultures of the wild-type and mutant strains. For control reactions, RNA without reverse transcriptase (no RT) or genomic DNA (gDNA) was used as a template. wt, wild-type strain; arnB, arnB-knockout mutant; arnBt, arnB Tn5-mutagenized mutant; arnCt, arnC Tn5-mutagenized mutant.
TABLE 2.
PB MICs, arnA expression, and specimen types for wild-type S. marcescens and clinical isolates
| Parameter | Wild-type | R1 | R2 | R3 | R4 | R5 | R6 | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml) | 2,048 | 4,096 | >4,096 | 4,096 | >4,096 | 4,096 | >4,096 | 2 | 4 | 2 | 2 | 4 | 2 |
| arnA expressiona | |||||||||||||
| Nil | 1 | 0.5 (0.2) | 2.9 (1.2) | 0.5 (0.2) | 2.0 (0.2) | 1.2 (0.4) | 0.6 (0.2) | 1.0 (0.4) | 2.5 (0.9) | 2.0 (1.0) | 1.0 (0.4) | 0.8 (0.2) | 0.6 (0.1) |
| PB | 4.1 (0.7) | 3.7 (0.2) | 8.8 (0.3) | 2.0 (0.2) | 10 (0.7) | 5.0 (1.0) | 3.0 (0.6) | 0.9 (0.6) | 2.8 (0.4) | 0.8 (0.5) | 1.2 (0.3) | 0.9 (0.2) | 0.6 (0.1) |
| Fold changeb | 4.1 | 7.4 | 3.0 | 4.0 | 5.0 | 4.2 | 5.0 | 0.9 | 1.1 | 0.4 | 1.2 | 1.1 | 1.0 |
| Specimen type | Sputum | Sputum | Sputum | Wound | Urine | Urine | Urine | Sputum | Sputum | Wound | Wound | Urine | Urine |
The arnA mRNA level of S. marcescens in the presence of polymyxin B (PB) or not (nil) is shown. The value obtained for the wild-type strain in the absence of PB was set at 1, and other values (with standard deviations in parentheses) are relative to that value. R1 to R6, PB-resistant clinical isolates; S1 to S6, PB-susceptible clinical isolates.
Fold change is arnA mRNA level in the presence of PB divided by that in the absence of PB.
Nucleotide sequence accession numbers.
The nucleotide sequences of the S. marcescens phoPQ and arnBCADTEF genes have been deposited in GenBank under accession no. KJ534564 and KJ560442, respectively.
RESULTS
Isolation of PB-sensitive S. marcescens mutants.
In order to investigate the underlying mechanisms of PB resistance in S. marcescens, we used a mini-Tn5 transposon mutagenesis approach, as described previously (17), to isolate PB-sensitive mutants of S. marcescens. Through characterization of these mutants, we identified two mutants, the arnB(t) and arnC(t) strains (Tn5-mutagenized mutants), that were 1,024-fold more susceptible to PB than wild-type S. marcescens (MICs, 2 versus 2,048 μg/ml) (Table 2). The nucleotide sequence was obtained from the cloned DNA fragment containing the mini-Tn5 in the mutants. By searching the released genome sequence of S. marcescens using the sequence we obtained, we found that Tn5 was inserted into two genes, named arnB and arnC. The genes were cloned and sequenced. The nucleotide sequences of arnB and arnC were found to be 95% and 96% identical, respectively, to the corresponding sequence of the sequenced S. marcescens Db11.
arnB and arnC are within a putative seven-gene arn operon.
The sequences of arnB and arnC were similar to the first and second genes of the arn (or pmr) operon (arnBCADTEF) in Yersinia pestis (http://www.ncbi.nlm.nih.gov/nuccore/NC_004088.1?report=genbank&from=2110265&to=2117676), an enterobacterium phylogenetically more closely related to S. marcescens (27), E. coli, and Salmonella (5, 28). Analysis of the amino acid sequences indicated that arnB and arnC may encode bacterial UDP-4-amino-4-deoxy-l-arabinose-oxoglutarate aminotransferase and undecaprenyl phosphate-4-amino-4-deoxy-l-arabinose transferase, respectively. S. marcescens ArnB and ArnC proteins are homologous to those of S. enterica serovar Typhimurium (68 and 74% identity and 80 and 88% similarity, respectively), E. coli (70 and 76% identity and 81 and 87% similarity, respectively), and Yersinia pestis (76 and 86% identity and 84 and 92% similarity, respectively). Further analysis of the deduced amino acid sequences revealed that the Tn5-inserted S. marcescens locus may encode seven putative proteins, with 90%, 83%, 83%, 80%, and 82% similarities to Y. pestis ArnA, ArnD, ArnT, ArnE, and ArnF, respectively (GenBank protein accession numbers AAM85486 to AAM85490).
The S. marcescens arnB-knockout mutant exhibited increased susceptibility to PB.
To demonstrate the role of the arnB gene in regulating PB susceptibility, we constructed the arnB mutant (see Materials and Methods). The MIC of PB for the arnB mutant was 2 μg/ml, compared with the wild-type value of 2,048 μg/ml (Table 2). The finding that complementation of the arnB and arnC genes in the arnB and arnC(t) mutants, respectively, restored the wild-type PB MIC (2,048 μg/ml) further confirms that arnB and arnC are determinants for PB susceptibility in S. marcescens. In addition, by RT-PCR using primer pairs annealing to the arnB/arnC and arnC/arnA genes, we detected amplicons of the expected sizes, i.e., approximately 0.4 kb (arnBC) and 0.2 kb (arnCA), from RNA samples from the wild-type strain and mutants, including the arnB(t), arnC(t), and arnB strains (Fig. 1). This implies that the transcript containing arnB, arnC, and arnA genes was produced in the arnB(t), arnC(t), and arnB mutants. The transcriptional readthrough may explain the restoration of wild-type PB susceptibility in the arnB and arnC(t) mutants complemented with arnB and arnC alone, respectively.
LPSs of S. marcescens arnB and arnC mutants had greater PB-binding abilities than that of wild type.
LPS modifications play important roles in PB susceptibility in many Gram-negative bacteria, including Salmonella, Yersinia, Pseudomonas, E. coli, and P. mirabilis (9, 10, 22, 29). To investigate the underlying cause of PB sensitivity in the arn mutants, we compared the LPS profiles of the arn mutants with that of the wild-type strain. SDS-PAGE analysis of LPS extracted from equal amounts of wild-type or mutant cells revealed that the arn mutants had LPS profiles similar to that of the wild-type strain (data not shown). In addition, the arnB (arnB-knockout) and arnC(t) (Tn5-mutagenized) mutants synthesized similar amounts of LPS versus the wild-type strain (11.5 and 11.79 versus 12.95 mg/ml), using equal amounts of wild-type and mutant cells.
To explain why the arn mutants have increased PB susceptibility, we thus tested whether the LPS purified from the mutants and the wild-type strain had different binding activities with PB. Equal amounts of LPS from the mutants and the wild-type strain were incubated with PB, and the unbound fraction was subjected to the E. coli inhibition assay (17). As shown in Fig. 2A, LPS from the arnB or arnC(t) mutant bound larger amounts of PB than did LPS from the wild-type strain, and the arnB(c) and arnC(tc) strains displayed binding patterns similar to that of the wild-type strain. The whole-cell binding of the arnB mutant with fluorescent PB was also better than that of wild-type S. marcescens (Fig. 2B). Since identical LPS concentrations and cell densities were used in these binding assays, these data indicate that there was a qualitative change in the LPS of the arn mutants and that this change caused LPS from the arn mutants and arnB mutant cells to have higher binding activities with PB. The increased PB-binding activity of the arn mutants may have contributed to their sensitivity to PB.
FIG 2.
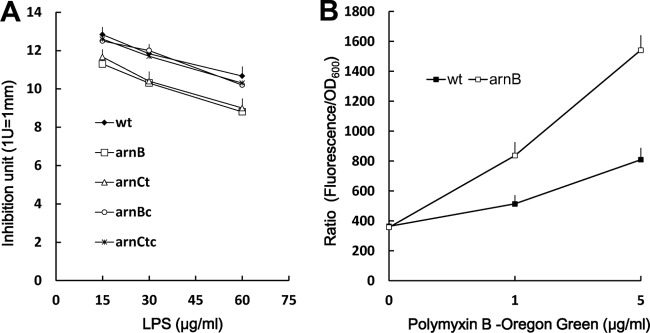
Polymyxin B-binding ability of wild-type S. marcescens and arn mutants. (A) PB-binding abilities of LPSs purified from the wild-type, arnB, arnC(t), arnB(c), and arnC(tc) strains. Various amounts of purified LPS were subjected to the PB-binding assay (see Materials and Methods). (B) Binding of wild-type and arnB mutant cells with fluorescent polymyxin B. The wild-type or arnB mutant cells were incubated for 10 min with Oregon Green 514-conjugated polymyxin B at the indicated concentrations and washed before being resuspended for analysis in 96-well plates. The fluorescence of each well was determined using a microplate reader. Data are reported as a ratio of fluorescence intensity to OD600. Each experiment was repeated in triplicate, and the data are averages and standard deviations. wt, wild-type strain; arnB, arnB-knockout mutant; arnCt, arnC Tn5-mutagenized mutant; arnBc, arnB-complemented strain; arnCtc, arnC-complemented strain.
Expression of the S. marcescens arn operon was induced by PB and low Mg2+ levels through the PhoP-dependent pathway.
Knowing that PB can regulate the PB susceptibility of P. mirabilis in an Arn (Pmr)-dependent manner through the RppAB two-component system (21, 22) and that the Salmonella PhoPQ two-component signal transduction system can sense PB to control pmr gene expression (i.e., arn operon) (14, 30, 31), we searched for phoPQ homologues in S. marcescens Db11 and found the PhoP and PhoQ counterparts of S. marcescens Db11, with high levels of identity (90% for PhoP and 80% for PhoQ) and similarity (94% for PhoP and 89% for PhoQ) to those of Y. pestis. In addition, expression of PhoPQ and RppAB, which are both involved in PB susceptibility, has been shown to be autoregulated and induced by PB and low Mg2+ levels in Salmonella (13, 32) and Proteus (17). Therefore, we constructed a Serratia phoP mutant and found its PB MIC to be 2 μg/ml. Then we investigated the expression of arnB and phoP in the wild-type strain and the phoP-knockout mutant in the presence of 1 μg/ml PB (in LB broth) or different concentrations of Mg2+ (in NMM medium) with the xylE reporter assay. RT-PCR using primer pairs annealing to the putative arnB and arnC genes and arnC and arnA genes, respectively, in wild-type S. marcescens demonstrated that the arnB, arnC, and arnA genes are cotranscribed (Fig. 1), indicating that they share the promoter upstream of the putative arnB gene. Therefore, the promoter region upstream of the putative arnB was used to construct the xylE transcriptional fusion (arnB promoter sequence fused with xylE gene with xylE ribosome binding site). We found that phoP and arnB promoter activities of the phoP-knockout mutant were low in comparison with the wild-type strain (Fig. 3A and 4A) during incubation in the absence of PB and that PB could induce the promoter activities of phoP and arnB in the wild-type strain but not in the phoP-knockout mutant after induction for 1, 2, and 3 h (Fig. 3A and 4A). Low Mg2+ levels induced expression of phoP and arnB in the wild-type strain but not in the phoP-knockout mutant (Fig. 3B and 4B). The results indicated that expression of the S. marcescens arn operon requires the phoP gene, which is positively autoregulated. The PB- or low-Mg2+-induced promoter activities of phoP and arnB were restored to about the wild-type level in the phoP-complemented strain (Fig. 3 and 4). In order to determine whether PhoP could regulate the expression of phoP and arnB directly by binding the putative phoP and arnB promoters in S. marcescens, an electrophoretic mobility shift assay (EMSA) was conducted. Figure 5 shows that PhoP proteins can specifically bind the putative phoP and arnB promoter fragments but not the irrelevant 16S ribosomal DNA fragments (compare Fig. 5, lanes 1 to 4 and 8 to 11 with lanes 5 to 7). Taken together, these findings suggest that PB and low Mg2+ levels can modulate the expression of phoP and arnB through the PhoP-dependent pathway.
FIG 3.
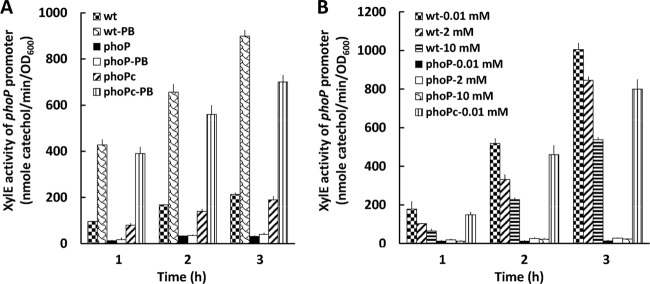
Activity of xylE in phoP-xylE reporter plasmid-transformed wild-type S. marcescens, phoP mutant, and phoP-complemented strains, in the presence or absence of 1 μg/ml polymyxin B (PB) (A) and with various concentrations of Mg2+ (0.01, 2, and 10 mM) (B), after induction for 1, 2, and 3 h. The data represent the averages of three independent experiments with standard deviations. wt, wild-type strain; phoP, phoP mutant; phoPc, phoP-complemented strain.
FIG 4.
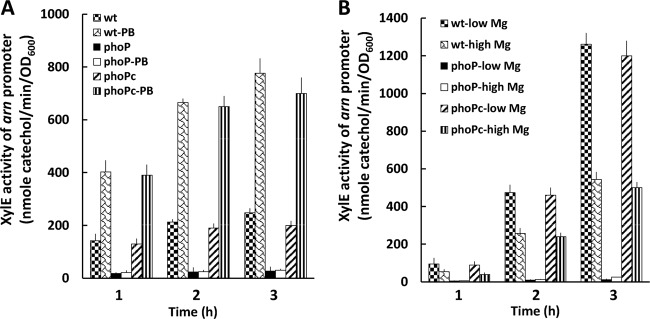
Activity of xylE in arn-xylE reporter plasmid-transformed wild-type S. marcescens, phoP mutant, and phoP-complemented strains, in the presence or absence of 1 μg/ml polymyxin B (PB) (A) and with low (0.01 mM) or high (10 mM) Mg2+ levels (B), after induction for 1, 2, and 3 h. The data represent the averages of three independent experiments with standard deviations. wt, wild-type strain; phoP, phoP mutant; phoPc, phoP-complemented strain.
FIG 5.
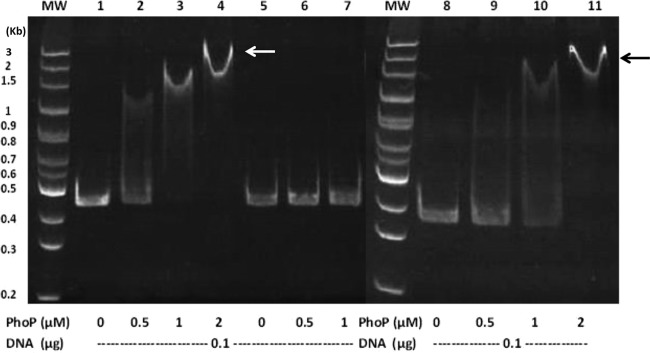
Electrophoretic mobility shift assay of purified S. marcescens PhoP with the phoP or arn promoter fragment. DNA fragments (0.1 μg) of the phoP promoter (431 bp), arn promoter (342 bp), or negative-control DNA (424 bp) obtained by PCR were incubated with the indicated concentrations (0, 0.5, 1, and 2 μM) of the PhoP protein. After protein-DNA complex formation, the fragments were resolved on a 5% nondenaturing polyacrylamide gel. Arrows, protein-DNA complex. Lane MW, size markers; lanes 1 to 4, phoP promoter with PhoP; lanes 8 to 11, arn promoter with PhoP; lanes 5 to 7, negative-control DNA with PhoP.
PB or Mg2+ pretreatment protected wild-type S. marcescens but not the phoP-knockout mutant from PB challenge.
Knowing that Serratia PhoP regulated the expression of the arn operon in response to PB and Mg2+ levels, we tested the effects of pretreatment with PB or Mg2+ on the growth and survival of wild-type S. marcescens, phoP mutant, and phoP-complemented [phoP(c)] strains after challenge with PB. The phoP mutant pretreated with low-dose PB (1 μg/ml) or low Mg2+ levels (0.1 mM) in NMM (see Materials and Methods) displayed decreased OD600 values during the 7-h period after being challenged with 1,000 μg/ml PB, but the wild-type and phoP(c) strains exhibited increased growth (Fig. 6A and B). In addition, we performed the PB survival assay by treating bacterial strains with either 1 μg/ml PB or high Mg2+ levels (20 mM) in LB broth and then challenging bacterial cells with the respective MIC dose of PB for the wild-type, phoP mutant, and phoP(c) strains. The survival rates of the wild-type and phoP(c) strains were decreased to around 20% of the no-challenge control by pretreatment with 20 mM Mg2+ and were increased 6-fold, relative to the no-challenge control, by PB pretreatment (Fig. 6C). In contrast, treatment with 1 μg/ml PB or high Mg2+ levels had almost no effect on the survival of the phoP mutant (Fig. 6C). Together, these data indicate that 1 μg/ml PB or Mg2+ may serve as the signal to protect S. marcescens from the PB challenge through a PhoP-dependent pathway, which activates expression of the LPS-modifying arn operon.
FIG 6.
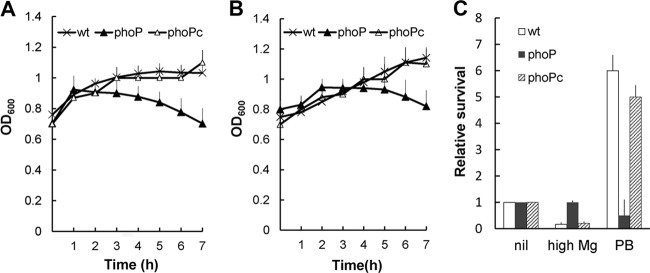
PB challenge test for S. marcescens after pretreatment with PB or Mg2+. (A) Cells of wild-type, phoP mutant, and phoP-complemented strains were grown in N minimal medium with 2 mM MgCl2 to an OD600 of 0.6, induced with 1 μg/ml PB for 1 h, and then challenged with PB (1,000 μg/ml). Bacterial growth (OD600) was monitored at 1-h intervals. (B) Cells of wild-type, phoP mutant, and phoP-complemented strains were grown in N minimal medium with 10 μM MgCl2 to an OD600 of 0.6 and then challenged with PB (1,000 μg/ml). Bacterial growth (OD600) was monitored at 1-h intervals. (C) Cells of wild-type, phoP mutant, and phoP-complemented strains were grown in LB broth, with or without the addition of 20 mM MgCl2 (high Mg2+), to OD600 of 0.6 and then challenged for 1 h with the respective MIC dose of PB for wild-type, phoP mutant, and phoP-complemented strains. For PB pretreatment, 1 μg/ml PB was added to the cultures with OD600 of 0.3, cells were grown to OD600 of 0.6, and then the PB challenge was conducted as described above. After the challenge with PB, CFU were determined and the percent survival was calculated. The relative survival values were obtained, with the values for wild-type, phoP mutant, and phoP-complemented strains in the absence of PB set at 1. All data represent the averages of three independent experiments with standard deviations. wt, wild-type strain; phoP, phoP mutant; phoPc, phoP-complemented strain.
Expression of S. marcescens ugd is regulated by PB through the PhoP-dependent pathway.
The enzymes encoded by the arn operon are responsible for LPS modification with Ara4N, and the ugd gene is required for biosynthesis of Ara4N (5). The Ugd protein of Salmonella, Yersinia, and Proteus is under the control of the two-component pathway PhoPQ or RppAB in response to PB and/or Mg2+ (21, 31, 33). In this regard, S. marcescens Ugd has 79% identity and 86% similarity to that of Y. pestis KM10+. Knowing that Serratia PhoP regulated the expression of the arn operon in response to PB, we examined the effects of PB on the expression of ugd in the wild-type S. marcescens, phoP mutant, and phoP(c) strains by real-time RT-PCR. The results indicated that ugd mRNA expression was induced by PB in the wild-type and complemented strains but not in the phoP mutant (Fig. 7). Our data indicated that both arn and ugd genes may respond to the PB signal through the PhoP pathway to modify the LPS and give rise to PB resistance in S. marcescens.
FIG 7.
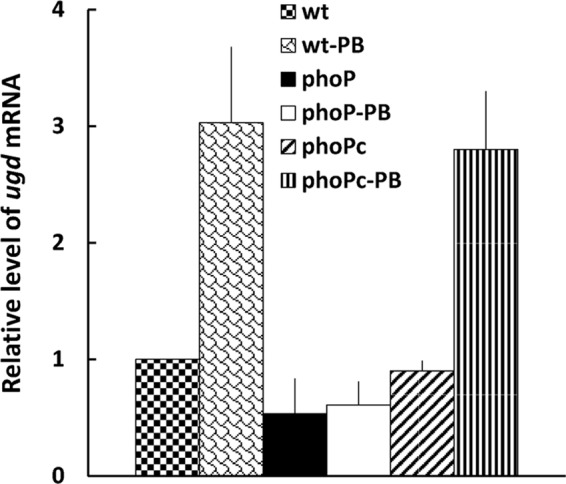
Effects of S. marcescens phoP mutation on the expression of ugd mRNA in the presence or absence of 1 μg/ml PB (see Materials and Methods). The ugd mRNA amounts in the wild-type, phoP-knockout mutant, and phoP-complemented strains were quantified by real-time RT-PCR. The value obtained for the wild-type cells in the absence of PB was set at 1. The data represent the averages of three independent experiments with standard deviations. wt, wild-type strain; phoP, phoP mutant; phoPc, phoP-complemented strain.
Expression of the arnA gene was induced by PB in PB-resistant clinical isolates of S. marcescens.
To further confirm the significance of the PhoP-dependent arn expression in PB resistance, we analyzed the expression of the arnA gene by real-time RT-PCR in clinical isolates of S. marcescens. We found that expression levels were comparable in the PB-resistant and -susceptible isolates in the absence of PB; however the expression of arnA was induced 3- to 7.4-fold in the presence of PB in the PB-resistant isolates but not in the susceptible ones (Table 2).
DISCUSSION
The ability of S. marcescens to survive the killing action of CAPs is clearly important in the pathogenesis of S. marcescens (27). Although some studies revealed that proteases and LPS modification appeared to be necessary for CAP resistance in other bacteria (3, 4), the molecular mechanisms underlying the CAP resistance of S. marcescens remain totally unknown. In this study, we isolated several mutants that were more sensitive to PB than the wild type. Two mutants were found to have Tn5 inserted in the arnB and arnC genes of what appears to be the counterpart of the pmrF operon in S. enterica and E. coli (5, 28). The arn operon in S. marcescens and Y. pestis is flanked by the btuD (encoding a transporter of vitamin B12) and nlpC (encoding a lipoprotein hydrolase) genes.
LPS modifications of Gram-negative organisms are regulated by two-component systems in response to environmental conditions (13, 33–36). In Salmonella, the LPS-modifying arn operon is induced by Fe3+, which is sensed by the PmrA cognate sensor PmrB, and by low Mg2+ levels, in a way that requires PmrAB, the Mg2+-responding PhoPQ, and PmrD protein (8, 31). E. coli induces PmrA-dependent PB resistance in response to Fe3+ but is blind to the low Mg2+ signal due to a highly divergent PmrD protein (37, 38). In P. mirabilis, we identified a two-component response regulator (RppA) as a regulator of PB susceptibility by directly controlling the expression of pmrI (arnA) in response to PB (22). Here we report that PB and Mg2+ can promote PhoP-dependent arn expression and PB resistance in S. marcescens through direct binding of PhoP in the arn promoter despite lacking a PmrD protein. Similarly, in PmrD-lacking Yersinia pestis, PhoP has been shown to regulate expression of the arn operon by directly binding the promoter region of the arn operon (33). In summary, bacterial species may use disparate regulatory pathways to control genes encoding conserved proteins. Moreover, the PhoP proteins of Salmonella and Yersinia trigger ugd expression by direct binding of the ugd promoter (31, 33). Accordingly, we found that Serratia ugd is under the control of PhoP in this study (Fig. 7).
Several lines of evidence indicate that the S. marcescens PhoPQ system, by sensing PB and Mg2+, can regulate the expression of the arn operon to modify LPS and lead to PB resistance. First, all of the phoP, arnB, and arnC(t) mutants displayed a PB-sensitive phenotype, and the phoP(c), arnB(c), and arnC(tc) strains exhibited the wild-type MIC. PB and low Mg2+ levels protected S. marcescens from the PB challenge in the wild-type strain and the phoP-complemented strain but not in the phoP mutant (Fig. 6). Second, the PB binding assay demonstrated that LPS of arnB and arnC(t) mutants had increased PB-binding ability, which implied that certain LPS changes exist in the mutants (Fig. 2). Third, phoP and arn operon promoter activities were increased by PB and low Mg2+ levels in the wild-type and PhoP-complemented strains but not in the phoP mutant (Fig. 3 and 4). Fourth, EMSA indicated that PhoP can bind the phoP and arn promoter directly (Fig. 5). Fifth, expression of the ugd gene, encoding the Ara4N precursor for LPS modification, was also increased in response to PB in the wild-type and phoP(c) strains but not in the phoP mutant (Fig. 7). The significance of arn-mediated PB resistance was also demonstrated by the higher arnA expression of PB-resistant clinical isolates of S. marcescens versus susceptible isolates in response to PB (Table 2). The presence of a conserved PhoP binding site in the arn promoter among different Serratia species (Fig. 8) highlights the significance of the PhoP-regulated arn pathway in PB resistance.
FIG 8.

Conservation of the PhoP box in the arn promoter among different Serratia species. Underlining, −10 region; shading, PhoP binding sites, separated by 5 bp.
S. marcescens PhoPQ has been demonstrated to control critical virulence phenotypes and be involved in the adaptation to growth with scarce Mg2+ and in the presence of PB, which constitute signals to activate the PhoPQ system (27). Here we found that S. marcescens PhoP can sense both Mg2+ and PB to activate the expression of ugd and arn genes, which are involved in LPS synthesis and modification. Given that LPS is involved in aspects of bacterial virulence such as adherence and subsequent invasion into host cells (39–41), we also found that S. marcescens arnB and arnC mutants exhibited significantly reduced ability for invasion into human epithelial cells (data not shown).
In this work, for the first time, we characterized an arn operon that is necessary for LPS modification and PB resistance, as well as its modulation by PB and Mg2+ through the PhoP protein, in S. marcescens. These data suggest that inhibition of the PhoP-regulated arn pathway can make S. marcescens, which is highly resistant to PB, become more vulnerable to PB treatment. It is tempting to suggest that the pathway is a potential target for drug development. In this regard, HQ17-2, a natural product from the lacquer tree, has been shown to inhibit the expression of rppA and pmrI in P. mirabilis (42).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Science Council and the National Taiwan University Hospital (Taipei, Taiwan).
We thank Yeong-Shiau Pu (National Taiwan University Hospital) for providing the NTUB1 cell line and Yang Tsuey-Ching (National Yang-Ming University) for giving us the yT&A::xylE plasmid.
Footnotes
Published ahead of print 23 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00013-14.
REFERENCES
- 1.Hancock RE, Scott MG. 2000. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. U. S. A. 97:8856–8861. 10.1073/pnas.97.16.8856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falagas ME, Kasiakou SK. 2005. Colistin: the revival of polymyxins for the management of multidrug-resistant Gram-negative bacterial infections. Clin. Infect. Dis. 40:1333–1341. 10.1086/429323 [DOI] [PubMed] [Google Scholar]
- 3.Fernandez L, Alvarez-Ortega C, Wiegand I, Olivares J, Kocincova D, Lam JS, Martinez JL, Hancock RE. 2013. Characterization of the polymyxin B resistome of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 57:110–119. 10.1128/AAC.01583-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peschel A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10:179–186. 10.1016/S0966-842X(02)02333-8 [DOI] [PubMed] [Google Scholar]
- 5.Gunn JS, Lim KB, Krueger J, Kim K, Guo L, Hackett M, Miller SI. 1998. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 27:1171–1182. 10.1046/j.1365-2958.1998.00757.x [DOI] [PubMed] [Google Scholar]
- 6.Gunn JS, Ryan SS, Van Velkinburgh JC, Ernst RK, Miller SI. 2000. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 68:6139–6146. 10.1128/IAI.68.11.6139-6146.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunn JS. 2008. The Salmonella PmrAB regulon: lipopolysaccharide modifications, antimicrobial peptide resistance and more. Trends Microbiol. 16:284–290. 10.1016/j.tim.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 8.Kox LF, Wosten MM, Groisman EA. 2000. A small protein that mediates the activation of a two-component system by another two-component system. EMBO J. 19:1861–1872. 10.1093/emboj/19.8.1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marceau M, Sebbane F, Ewann F, Collyn F, Lindner B, Campos MA, Bengoechea JA, Simonet M. 2004. The pmrF polymyxin-resistance operon of Yersinia pseudotuberculosis is upregulated by the PhoP-PhoQ two-component system but not by PmrA-PmrB, and is not required for virulence. Microbiology 150:3947–3957. 10.1099/mic.0.27426-0 [DOI] [PubMed] [Google Scholar]
- 10.Moskowitz SM, Ernst RK, Miller SI. 2004. PmrAB, a two-component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J. Bacteriol. 186:575–579. 10.1128/JB.186.2.575-579.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moss JE, Fisher PE, Vick B, Groisman EA, Zychlinsky A. 2000. The regulatory protein PhoP controls susceptibility to the host inflammatory response in Shigella flexneri. Cell Microbiol. 2:443–452. 10.1046/j.1462-5822.2000.00065.x [DOI] [PubMed] [Google Scholar]
- 12.Newcombe J, Jeynes JC, Mendoza E, Hinds J, Marsden GL, Stabler RA, Marti M, McFadden JJ. 2005. Phenotypic and transcriptional characterization of the meningococcal PhoPQ system, a magnesium-sensing two-component regulatory system that controls genes involved in remodeling the meningococcal cell surface. J. Bacteriol. 187:4967–4975. 10.1128/JB.187.14.4967-4975.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groisman EA. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 183:1835–1842. 10.1128/JB.183.6.1835-1842.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bader MW, Navarre WW, Shiau W, Nikaido H, Frye JG, McClelland M, Fang FC, Miller SI. 2003. Regulation of Salmonella Typhimurium virulence gene expression by cationic antimicrobial peptides. Mol. Microbiol. 50:219–230. 10.1046/j.1365-2958.2003.03675.x [DOI] [PubMed] [Google Scholar]
- 15.Bader MW, Sanowar S, Daley ME, Schneider AR, Cho U, Xu W, Klevit RE, Le Moual H, Miller SI. 2005. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 122:461–472. 10.1016/j.cell.2005.05.030 [DOI] [PubMed] [Google Scholar]
- 16.Mahlen SD. 2011. Serratia infections: from military experiments to current practice. Clin. Microbiol. Rev. 24:755–791. 10.1128/CMR.00017-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang WB, Chen IC, Jiang SS, Chen HR, Hsu CY, Hsueh PR, Hsu WB, Liaw SJ. 2008. Role of RppA in the regulation of polymyxin B susceptibility, swarming, and virulence factor expression in Proteus mirabilis. Infect. Immun. 76:2051–2062. 10.1128/IAI.01557-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans ME, Feola DJ, Rapp RP. 1999. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant Gram-negative bacteria. Ann. Pharmacother. 33:960–967. 10.1345/aph.18426 [DOI] [PubMed] [Google Scholar]
- 19.Gales AC, Jones RN, Sader HS. 2006. Global assessment of the antimicrobial activity of polymyxin B against 54 731 clinical isolates of Gram-negative bacilli: report from the SENTRY antimicrobial surveillance programme (2001–2004). Clin. Microbiol. Infect. 12:315–321. 10.1111/j.1469-0691.2005.01351.x [DOI] [PubMed] [Google Scholar]
- 20.Zavascki AP, Goldani LZ, Li J, Nation RL. 2007. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J. Antimicrob. Chemother. 60:1206–1215. 10.1093/jac/dkm357 [DOI] [PubMed] [Google Scholar]
- 21.Jiang SS, Lin TY, Wang WB, Liu MC, Hsueh PR, Liaw SJ. 2010. Characterization of UDP-glucose dehydrogenase and UDP-glucose pyrophosphorylase mutants of Proteus mirabilis: defectiveness in polymyxin B resistance, swarming, and virulence. Antimicrob. Agents Chemother. 54:2000–2009. 10.1128/AAC.01384-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang SS, Liu MC, Teng LJ, Wang WB, Hsueh PR, Liaw SJ. 2010. Proteus mirabilis pmrI, an RppA-regulated gene necessary for polymyxin B resistance, biofilm formation, and urothelial cell invasion. Antimicrob. Agents Chemother. 54:1564–1571. 10.1128/AAC.01219-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soo PC, Wei JR, Horng YT, Hsieh SC, Ho SW, Lai HC. 2005. Characterization of the dapA-nlpB genetic locus involved in regulation of swarming motility, cell envelope architecture, hemolysin production, and cell attachment ability in Serratia marcescens. Infect. Immun. 73:6075–6084. 10.1128/IAI.73.9.6075-6084.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 9th ed. Approved standard M07-A9 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 25.Cullen TW, Giles DK, Wolf LN, Ecobichon C, Boneca IG, Trent MS. 2011. Helicobacter pylori versus the host: remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS Pathog. 7:e1002454. 10.1371/journal.ppat.1002454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia Vescovi E, Soncini FC, Groisman EA. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165–174. 10.1016/S0092-8674(00)81003-X [DOI] [PubMed] [Google Scholar]
- 27.Barchiesi J, Castelli ME, Di Venanzio G, Colombo MI, Garcia Vescovi E. 2012. The PhoP/PhoQ system and its role in Serratia marcescens pathogenesis. J. Bacteriol. 194:2949–2961. 10.1128/JB.06820-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan A, Guan Z, Raetz CR. 2007. An undecaprenyl phosphate-aminoarabinose flippase required for polymyxin resistance in Escherichia coli. J. Biol. Chem. 282:36077–36089. 10.1074/jbc.M706172200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran AX, Lester ME, Stead CM, Raetz CR, Maskell DJ, McGrath SC, Cotter RJ, Trent MS. 2005. Resistance to the antimicrobial peptide polymyxin requires myristoylation of Escherichia coli and Salmonella Typhimurium lipid A. J. Biol. Chem. 280:28186–28194. 10.1074/jbc.M505020200 [DOI] [PubMed] [Google Scholar]
- 30.Mouslim C, Groisman EA. 2003. Control of the Salmonella ugd gene by three two-component regulatory systems. Mol. Microbiol. 47:335–344. 10.1046/j.1365-2958.2003.03318.x [DOI] [PubMed] [Google Scholar]
- 31.Mouslim C, Latifi T, Groisman EA. 2003. Signal-dependent requirement for the co-activator protein RcsA in transcription of the RcsB-regulated ugd gene. J. Biol. Chem. 278:50588–50595. 10.1074/jbc.M309433200 [DOI] [PubMed] [Google Scholar]
- 32.Groisman EA, Mouslim C. 2006. Sensing by bacterial regulatory systems in host and non-host environments. Nat. Rev. Microbiol. 4:705–709. 10.1038/nrmicro1478 [DOI] [PubMed] [Google Scholar]
- 33.Winfield MD, Latifi T, Groisman EA. 2005. Transcriptional regulation of the 4-amino-4-deoxy-l-arabinose biosynthetic genes in Yersinia pestis. J. Biol. Chem. 280:14765–14772. 10.1074/jbc.M413900200 [DOI] [PubMed] [Google Scholar]
- 34.Geurtsen J, Dzieciatkowska M, Steeghs L, Hamstra HJ, Boleij J, Broen K, Akkerman G, El Hassan H, Li J, Richards JC, Tommassen J, van der Ley P. 2009. Identification of a novel lipopolysaccharide core biosynthesis gene cluster in Bordetella pertussis, and influence of core structure and lipid A glucosamine substitution on endotoxic activity. Infect. Immun. 77:2602–2611. 10.1128/IAI.00033-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunn JS. 2001. Bacterial modification of LPS and resistance to antimicrobial peptides. J. Endotoxin Res. 7:57–62. 10.1177/09680519010070011001 [DOI] [PubMed] [Google Scholar]
- 36.Raetz CR, Reynolds CM, Trent MS, Bishop RE. 2007. Lipid A modification systems in Gram-negative bacteria. Annu. Rev. Biochem. 76:295–329. 10.1146/annurev.biochem.76.010307.145803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winfield MD, Groisman EA. 2004. Phenotypic differences between Salmonella and Escherichia coli resulting from the disparate regulation of homologous genes. Proc. Natl. Acad. Sci. U. S. A. 101:17162–17167. 10.1073/pnas.0406038101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zwir I, Shin D, Kato A, Nishino K, Latifi T, Solomon F, Hare JM, Huang H, Groisman EA. 2005. Dissecting the PhoP regulatory network of Escherichia coli and Salmonella enterica. Proc. Natl. Acad. Sci. U. S. A. 102:2862–2867. 10.1073/pnas.0408238102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta SK, Masinick S, Garrett M, Hazlett LD. 1997. Pseudomonas aeruginosa lipopolysaccharide binds galectin-3 and other human corneal epithelial proteins. Infect. Immun. 65:2747–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.West NP, Sansonetti P, Mounier J, Exley RM, Parsot C, Guadagnini S, Prevost MC, Prochnicka-Chalufour A, Delepierre M, Tanguy M, Tang CM. 2005. Optimization of virulence functions through glucosylation of Shigella LPS. Science 307:1313–1317. 10.1126/science.1108472 [DOI] [PubMed] [Google Scholar]
- 41.Zaidi TS, Fleiszig SM, Preston MJ, Goldberg JB, Pier GB. 1996. Lipopolysaccharide outer core is a ligand for corneal cell binding and ingestion of Pseudomonas aeruginosa. Invest. Ophthalmol. Vis. Sci. 37:976–986 [PubMed] [Google Scholar]
- 42.Liu MC, Lin SB, Chien HF, Wang WB, Yuan YH, Hsueh PR, Liaw SJ. 2012. 10′(Z),13′(E)-Heptadecadienylhydroquinone inhibits swarming and virulence factors and increases polymyxin B susceptibility in Proteus mirabilis. PLoS One 7:e45563. 10.1371/journal.pone.0045563 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.