Abstract
Pseudomonas aeruginosa is a ubiquitous versatile environmental microorganism with a remarkable ability to grow under diverse environmental conditions. Moreover, P. aeruginosa is responsible for life-threatening infections in immunocompromised and cystic fibrosis patients, as the extraordinary capacity of this pathogen to develop antimicrobial resistance dramatically limits our therapeutic arsenal. Its large genome carries an outstanding number of genes belonging to regulatory systems, including multiple two-component sensor-regulator systems that modulate the response to the different environmental stimuli. Here, we show that one of two systems, designated CreBC (carbon source responsive) and BlrAB (β-lactam resistance), might be of particular relevance. We first identified the stimuli triggering the activation of the CreBC system, which specifically responds to penicillin-binding protein 4 (PBP4) inhibition by certain β-lactam antibiotics. Second, through an analysis of a large comprehensive collection of mutants, we demonstrate an intricate interconnection between the CreBC system, the peptidoglycan recycling pathway, and the expression of the concerning chromosomal β-lactamase AmpC. Third, we show that the CreBC system, and particularly its effector inner membrane protein CreD, plays a major role in bacterial fitness and biofilm development, especially in the presence of subinhibitory concentrations of β-lactams. Finally, global transcriptomics reveals broad regulatory functions of CreBC in basic physiological aspects, particularly anaerobic respiration, in both the presence and absence of antibiotics. Therefore, the CreBC system is envisaged as a potentially interesting target for improving the efficacy of β-lactams against P. aeruginosa infections.
INTRODUCTION
Pseudomonas aeruginosa is a ubiquitous versatile environmental microorganism with a remarkable ability to grow under a variety of environmental conditions, including in soil and water and in human, animal, and plant hosts (1). Moreover, it is responsible for severe nosocomial infections in immunocompromised patients, as well as chronic lung infections in patients with cystic fibrosis (2). The genome of P. aeruginosa is relatively large (6.3 Mb) and carries a large number of genes belonging to regulatory systems (3, 4) and genes involved in the utilization of various carbon sources, anaerobic energy metabolisms through arginine catabolism (5), pyruvate fermentation (6), and denitrification in the presence of nitrogen oxides (7, 8), which might contribute to the environmental adaptability of this bacterium (8, 9). A well-established environment for P. aeruginosa growth under anoxic conditions is the biofilm developed within the mucus of the cystic fibrosis (CF) lung (8, 10). The nitrate and nitrite levels in CF mucus, generated in part by the host inflammatory response to infection, are sufficient to support the anaerobic metabolism of P. aeruginosa (8, 11).
P. aeruginosa is also a paradigmatic example of antimicrobial resistance development. Given its extraordinary capacity to develop resistance to nearly all of the antibiotics currently used in therapy, it is frequently associated with multidrug resistance (MDR) phenotypes (12, 13). Particularly noteworthy is its β-lactam resistance, caused by the selection of a complex repertoire of chromosomal mutations (12–14), leading to the hyperproduction of the chromosomal cephalosporinase AmpC (12, 15), which causes resistance to penicillins, cephalosporins, and monobactams. It is also well established that the expression of this concerning β-lactamase is linked to the peptidoglycan recycling pathways and therefore that blocking these processes is a promising strategy for overcoming β-lactam resistance (16).
Previous studies showed that P. aeruginosa has three muropeptide amidase genes (ampD, ampDh2, and ampDh3) and that their sequential inactivation leads to a stepwise upregulation of ampC expression, reaching full derepression with very high-level basal ampC expression in the triple mutant (17). Recent work showed, however, that one-step high-level resistance in clinical strains of P. aeruginosa frequently results from the inactivation of dacB, which encodes the nonessential penicillin-binding protein 4 (PBP4) (15). The inactivation of PBP4 was shown to give rise to a complex β-lactam resistance response, triggering a transcriptional regulator AmpR-dependent overproduction of the chromosomal β-lactamase AmpC and specific activation of the CreBC (carbon source responsive) (BlrAB [β-lactam resistance]) two-component regulator, evidenced through the induction of the expression of the inner membrane protein CreD (12, 15). A high similarity was previously noticed between CreBC from Escherichia coli and its BlrAB counterpart of Aeromonas spp., which plays an important role in the regulation of β-lactamases in these species lacking AmpR (18–20). BlrA acts as a regulator connected to a sensor kinase, BlrB. Although the mechanism by which this system drives the induction of expression in Aeromonas species is still unknown, a clear association has been established between BlrAB and the expression of different β-lactamases (18–20).
On the other hand, CreBC from E. coli is a transcriptional regulator that modulates the expression of up to 8 different genes in response to changes in the composition of nutrients in the medium (20, 21). Proteins from the CreBC system present a homology of 70% with those from BlrAB. Moreover, it has been demonstrated that the transcriptional regulator CreBC of E. coli is capable of modulating the expression of Aeromonas β-lactamases (18).
Although it is clear that a PBP4 mutation specifically activates the CreBC transcriptional regulator (15, 22) and that this is key to high-level resistance to β-lactam antibiotics, the underlying mechanism is still uncertain. Two-component sensor-regulator systems modify the expression of multiple genes in response to environmental stimuli (such as antibiotics) and are capable of interacting with other related metabolic pathways. In this sense, the CreBC regulon in P. aeruginosa might play an important role in ampC regulation/induction but also in other broader physiological responses to the presence of β-lactams and their effects on the cell wall. Indeed, CreB (BlrA) might perceive changes in the peptidoglycan and act as a transducer signal for these responses, including the expression of β-lactamases (18, 23, 24). In order to gain insights into these potential major regulatory roles, we performed a comprehensive analysis of the creBC and creD mutants of P. aeruginosa strain PAO1 to elucidate the role of the CreBC system of P. aeruginosa in the response to β-lactams, bacterial fitness, biofilm growth, and global regulation.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The complete list and description of the laboratory strains and plasmids used in this study are shown in Table 1. P. aeruginosa single or multiple knockout mutants involving ampD, ampR, ampC, ampG, creBC, creD, dacB, or nagZ were constructed according to well-established procedures (15, 17, 25) based on the Cre-lox system for gene deletion and antibiotic resistance marker recycling in P. aeruginosa (26). The previously constructed plasmids were used as the donor for the generation of knockout mutants by conjugational transfer to the corresponding P. aeruginosa strains (15, 25). For competition experiments, the P. aeruginosa PAO1, PAΔCreBC, and PAΔCreD strains were tagged at the att intergenic neutral chromosomal locus with two mini-Tn7 constructs harboring fluorescent and antibiotic resistance markers (green fluorescent protein [GFP]-gentamicin [Gm] and yellow fluorescent protein [YFP]-streptomycin [Str]) (Table 1), as previously described (27, 28).
TABLE 1.
Strains and plasmids used or constructed in this work
| Strain or plasmid | Genotype/relevant characteristic(s)a | Reference or source |
|---|---|---|
| P. aeruginosa strains | ||
| PAO1 | Reference strain completely sequenced | 9 |
| PAΔD | PAO1 ΔampD::lox; AmpD is an N-acetyl-anhydromuramyl–l-alanine amidase involved in peptidoglycan recycling; negative regulator of AmpC expression | 17 |
| PAΔdacB | PAO1 ΔdacB::lox; dacB encodes the nonessential penicillin-binding protein 4 | 15 |
| PAdacBΔD | PAdacB ΔampD::lox | 15 |
| PAΔDDh2Dh3 | PAO1 ΔampD::lox ΔampDh2::lox ΔampDh3::lox; AmpDh2 and AmpDh3 are the two additional AmpD homologues of P. aeruginosa | 17 |
| PAΔR | PAO1 ΔampR::lox; AmpR is a LysR-type transcriptional regulator required for ampC induction | 15 |
| PAΔC | PAO1 ΔampC::lox; ampC encodes the chromosomal β-lactamase AmpC | 15 |
| PAΔDΔC | PAO1 ΔampD::lox ΔampC::lox | 15 |
| PAdacBΔC | PAdacB ΔampC::lox | 15 |
| PAΔDDh2Dh3ΔC | PAO1 ΔampD::lox ΔampDh2::lox ΔampDh3::lox ΔampC::lox | 30 |
| PAΔnZ | PAO1 ΔnagZ::lox; nagZ is a β-N-acetylglucosaminidase that processes GlcNAc-1,6-anhydromuropeptides to generate 1,6 anhydromuropeptides | 34 |
| PAΔDnZ | PAO1 ΔampD::lox ΔnagZ::lox | 34 |
| PAΔdBnZ | PAO1 ΔdacB::lox ΔnagZ::lox | 34 |
| PAdacBΔDnZ | PAdacB ΔampD::lox ΔnagZ::lox | 34 |
| PAΔDDh2Dh3ΔnZ | PAO1 ΔampD::lox ΔampDh2::lox ΔampDh3::lox ΔnagZ::lox | 34 |
| PAΔG | PAO1 ΔampG::lox; AmpG encodes an inner membrane permease for GlcNAc-1,6-anhydromuropeptides | 25 |
| PAΔDG | PAO1 ΔampD::lox ΔampG::lox | 25 |
| PAΔdBG | PAO1 ΔdaB::lox ΔampG::lox | 25 |
| PAdacBADG | PAdacB ΔampD::lox ΔmpG::lox | 25 |
| PAΔcreBC | PAO1 ΔcreBC::lox; CreBC is a two-component response regulator | 15 |
| PAΔcreD | PAO1 ΔcreD::lox; inner membrane protein of unknown function known to be regulated by the CreBC system | 15 |
| PAOGFP | PAO1 tagged with eGFP-Gm in a mini-Tn7 construct | This work |
| PAOEYFP | PAO1 tagged with eYFP-Str in a mini-Tn7 construct | This work |
| PAΔcreBCGFP | PAΔcreBC tagged with eGFP-Gm in a mini-Tn7 construct | This work |
| PAΔcreBCEYFP | PAΔcreBC tagged with eYFP-Str in a mini-Tn7 construct | This work |
| PAΔcreDGFP | PAΔcreD tagged with eGFP-Gm in a mini-Tn7 construct | This work |
| PAΔcreDEYFP | PAΔcreD tagged with eYFP-Str in a mini-Tn7 construct | This work |
| Plasmids | ||
| Mini-Tn7-gfp2 | Plasmid based on pUCP19 (Ampr) with eGFP mini-Tn7, Gmr | 27 |
| Mini-Tn7PA1/04/03-eyfp-a | Plasmid based on pUCP19 (Ampr) with eYFP mini-Tn7, Strr | 28 |
| Mini-Tn7PA1/04/03-ecfp-a | Plasmid based on pUCP19 (Ampr) with eCFP mini-Tn7, Gmr | 28 |
eGFP, enhanced green fluorescent protein; eYFP, enhanced yellow fluorescent protein; eCFP, enhanced cyan fluorescent protein; Ampr, ampicillin resistant; Gmr, gentamicin resistant; Stmr, streptomycin resistant.
Quantification of basal and induced gene expression.
The relative mRNA levels of ampC and creD (blrD) were determined by real-time reverse transcription-PCR (RT-PCR), according to previously described protocols (15, 17). For induction experiments, the strains were incubated in LB broth for 3 h in the presence of 1/4 (0.25 μg/ml) and 1/2 (0.5 μg/ml) MICs of ceftazidime and imipenem, and in 50 μg/ml of cefoxitin as a control. Total RNA was obtained from these cultures with an RNeasy minikit (Qiagen, Hilden, Germany). Fifty nanograms of purified RNA was then used for one-step reverse transcription and real-time PCR using a QuantiTect SYBR green reverse transcription-PCR kit (Qiagen, Hilden, Germany) in a SmartCycler II apparatus (Cepheid, Sunnyvale, CA), according to previously described conditions and primers (see Table S1 in the supplemental material) (15, 17, 29). The rpsL housekeeping gene was used to normalize the expression levels, and the results were always referred to the basal level of expression for PAO1. All RT-PCRs were performed in duplicate, and the mean values of mRNA expression resulting from three independent experiments were considered in all cases.
In vitro competition experiments.
Competition experiments were performed between strains P. aeruginosa PAΔcreBCStr and PAΔcreDStr versus PAO1Gm and PAΔcreBCGm and PAΔcreDGm versus PAO1Str to override any effect that the markers might have on fitness. Exponentially growing cells in the broth of the corresponding mutant and wild-type strains were mixed in a 1:1 ratio and diluted in 0.9% saline solution. Approximately 103 cells from each of the mixtures were inoculated into eight (four for each combination of markers) 10-ml LB broth flasks containing no antibiotics or 1/4, 1/2, and 3/4 MICs of ceftazidime (0.25, 0.5, and 0.75 μg/ml, respectively) imipenem (0.25, 0.5, and 0.75 μg/ml, respectively) or cefoxitin (250, 500, and 750 μg/ml, respectively). Ciprofloxacin at 1/2 MIC (0.03 μg/ml) was used as a non-β-lactam control in the competition assays. The cultures were grown at 37°C and 180 rpm for 20 h, corresponding to approximately 20 generations. Serial dilutions were then plated in duplicate onto LB agar (LBA) with 10 μg/ml of gentamicin and LBA with 100 μg/ml of streptomycin in order to determine the number of mutant and wild-type CFU after overnight incubation at 37°C. The competition index (CI) was defined as the CFU mutant-to-CFU PAO1 ratio (30). The CI values were calculated for each of the eight independent competition experiments, and the median values were recorded. Statistical analysis of the distribution of the CI values was performed using the Mann-Whitney U test. P values of <0.05 were considered to be statistically significant. To assess the growth rates under noncompetitive conditions, the doubling times of exponentially growing cells in LB broth at 37°C and 180 rpm were determined by plating serial 10-fold dilutions on LBA at 1-h intervals. Three independent experiments were performed for each of the mutants, and the results were compared with those for PAO1 using Student's t test.
Biofilm growth.
Biofilm assays were performed according to previously described procedures (31). Briefly, 105 cells in stationary phase were inoculated into each of four wells per strain of a microtiter plate containing 100 μl of LB medium supplemented or not with 50 μg/ml of cefoxitin. After incubation for 24 h or 48 h at 37°C, the microtiter plates were gently rinsed with tap water. After removing all planktonic cells, the plates were air-dried and stained with 125 μl of a 0.1% crystal violet solution per well for 10 min. Next, the plates were gently rinsed with water and air-dried again. The dye was solubilized with 200 μl of 30% acetic acid for 25 min at room temperature. Once mixed by pipetting, 125 μl from each well was individually transferred to a clear flat-bottom 96-well plate. Finally, the absorbance was measured at 590 nm. All experiments were repeated on three independent occasions, and the final results were expressed as the mean values (± standard deviation) of 12 determinations (from three quadruplicate experiments).
Analysis of whole-genome gene expression.
Three independent replicates of PAO1 and PAΔcreBC were grown at 37°C to an optical density at 600 nm (OD600) of 1 in vigorously shaken 50-ml baffled flasks containing 10 ml of LB broth without antibiotics or supplemented with 50 μg/ml cefoxitin or 0.5 μg/ml ceftazidime. The cells were collected by centrifugation (8,000 × g for 5 min at 4°C), and total RNA was isolated using the RNeasy minikit (Qiagen), according to the manufacturer's instructions. RNA was dissolved in water and treated with 2 U of Turbo DNase (Ambion) for 30 min at 37°C to remove contaminating DNA. The reaction was stopped by adding 5 μl of DNase inactivation reagent. Ten micrograms of the total RNA was checked by running on an agarose gel prior to cDNA synthesis. cDNA synthesis, fragmentation, labeling, and hybridization were performed according to the Affymetrix GeneChip P. aeruginosa genome array expression analysis protocol. An expression analysis was performed as described previously (15, 32, 33). Only transcripts showing ≥2-fold increases or decreases were considered to be differentially expressed. In all cases, the posterior probability for differential expression (PPDE) was between 0.999 and 1. The expression of 3 selected representative genes was further analyzed using real-time RT-PCR according to the protocols described above using the primers listed in Table S1 in the supplemental material to confirm the microarray results.
Data analysis.
The GraphPad Prism 5 software was used for graphical representation and statistical analysis. Quantitative variables were compared using the Mann-Whitney U test or Student's t test, as appropriate. Categorical variables were compared using the χ2 test. A P value of <0.05 was considered statistically significant.
Microarray data accession number.
The microarray data for this study can be found under GEO accession no. GSE58758.
RESULTS AND DISCUSSION
The CreBC (BlrAB) system specifically responds to PBP4 inhibition by AmpC inducers.
In a previous work, we showed that the CreBC system is responsive to cefoxitin (a strong AmpC inducer and PBP4 inhibitor), as evidenced by a marked induction of creD expression in the presence of this cephalosporin (15). Moreover, we demonstrated that the inactivation of dacB, which encodes PBP4, leads to a constitutive upregulation of creD. These observations led us to generate the hypothesis that CreBC may specifically respond to PBP4 inhibition. To test this hypothesis, we determined ampC and creD induction in parallel in the presence of the cephalosporin ceftazidime (a weak AmpC inducer/weak PBP4 inhibitor) and the carbapenem imipenem (a strong AmpC inducer/strong PBP4 inhibitor), and cefoxitin was additionally used as a control. As shown in Table 2, in contrast to cefoxitin, ceftazidime had only a marginal effect on ampC and creD expression, whereas imipenem was a very potent ampC and creD inducer. Therefore, these results strongly suggest that the capacity of β-lactams to activate the CreBC system does not depend on structural aspects (cefoxitin and ceftazidime are both cephalosporins, in contrast to imipenem) but rather on cell target aspects, since imipenem and cefoxitin, in contrast to ceftazidime, share the single property of being potent PBP4 inhibitors. Thus, ampC and creBC regulatory pathways seem to essentially sense the same signals, which are directly or indirectly driven by the inhibition of PBP4 by certain β-lactams (AmpC inducers).
TABLE 2.
Level of expression of ampC and creD in the presence of cefoxitin, ceftazidime, and imipenem subinhibitory concentrations in P. aeruginosa strain PAO1
| β-Lactama | Concentration (μg/ml) | Relative mRNA level (mean ± SD)b |
|
|---|---|---|---|
| ampC | creD | ||
| CAZ | 0.25 (1/4 MIC) | 1.29 ± 0.1 | 1.7 ± 0.7 |
| 0.5 (1/2 MIC) | 1.26 ± 0.3 | 2.5 ± 1.4 | |
| IMP | 0.25 (1/4 MIC) | 193 ± 20.2 | 34 ± 10 |
| 0.5 (1/2 MIC) | 269 ± 73.1 | 70 ± 8 | |
| FOX | 50 | 78 ± 34 | 24 ± 9.1 |
CAZ, ceftazidime; IMP, imipenem; FOX, cefoxitin.
Relative amount of ampC and creD mRNA compared to PAO1 basal levels (set at 1).
Interplay between the CreBC (BlrAB) system and the AmpC regulatory pathway.
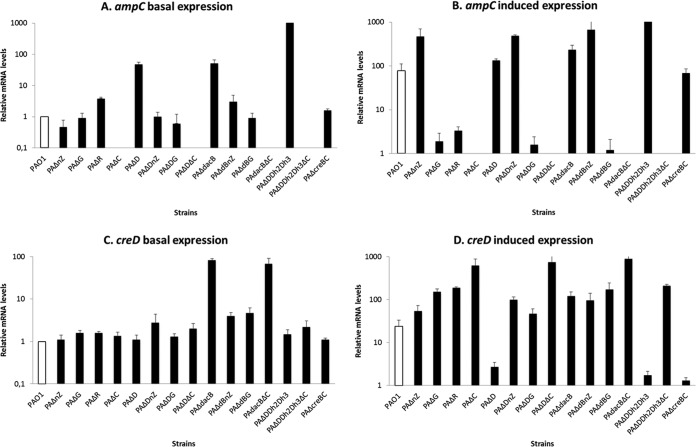
In order to gain insights into the role of the CreBC system in β-lactam resistance and its interplay with ampC regulation, a comprehensive analysis of ampC and creD expression under basal and cefoxitin-induced conditions was performed with a large collection of single and multiple mutants, including all the genes known to be involved in the AmpC regulatory pathway (described in Table 1), and the results are presented in Fig. 1A to D. As indicated in Fig. 1, the presented results include all available expression data, including those generated for the present work and those reported in our previous studies (15, 25, 34) for comparative purposes. The previous studies showed that inactivation of dacB leads to the specific activation of the CreBC system, as evidenced by the constitutive overexpression of creD. Moreover, we demonstrated that the CreBC system plays a major role in the high-level β-lactam resistance phenotype of the dacB mutant; while the ceftazidime MIC increased from 1 to 32 μg/ml in PAO1 after dacB inactivation, the dacB-creBC double mutant showed an MIC of only 4 μg/ml despite the fact that ampC expression was not modified (i.e., still overexpressed). Furthermore, the inactivation of the CreBC system in PAO1 dramatically decreased the spontaneous emergence of ceftazidime-resistant DacB mutants (15). On the other hand, inactivation of the CreBC system or CreD in wild-type PAO1 did not have any direct impact on β-lactam MICs (15). In the present work, through a complete analysis of the collection of PAO1 mutants involved in the AmpC regulatory pathway, we demonstrate that of all the single mutants analyzed, only dacB leads to a significant constitutive upregulation of creD (Fig. 1C). Interestingly, we also show in the present work that the inactivation of either nagZ or ampG in the dacB mutant determines a major attenuation of creD basal expression levels (Fig. 1C).
FIG 1.
ampC and creD basal (A and C) and induced (B and D) expression in the collection of mutants in the different components of AmpC regulatory pathways. The ampC and creD expression data for PAΔR, PAΔD, PAΔcreBC, PAΔDDh2Dh3 (15), and ampC expression data for nagZ (34) and ampG (25) mutants were obtained in previous works and included for comparative purposes. Data shown are means plus standard deviations.
As shown in Fig. 1D, a major impact of the ampC regulatory components on creD inducibility in the presence of cefoxitin was also evidenced. First, the inactivation in PAO1 of any of the components that need to be functional for ampC induction or constitutive overexpression, including ampG, nagZ, ampR, and ampC itself, leads to significantly enhanced creD inducibility. Second, as previously described (15), the inactivation of ampD blocked creD inducibility, but, interestingly, we show here that the inactivation of nagZ, ampG, or ampC fully restored creD inducibility in this mutant (Fig. 1D). Finally, the results of the present work show that the inactivation of ampC but not of nagZ or ampG in the dacB mutant also further increased creD inducibility (Fig. 1D).
Although the results presented here certainly highlight the complexity of the interplay between the CreBC and AmpC regulatory pathways, several conclusions can be reached. A first obvious conclusion is that ampC expression itself greatly affects creD inducibility. The explanation for this finding also appears to be obvious; the higher the expression of ampC, the greater the hydrolysis of cefoxitin, which avoids creD induction. Indeed, AmpC hydrolysis of cefoxitin as a feedback mechanism to tune down creD expression might explain many of the findings, including the increased creD inducibility in PAO1 ampG, nagZ, ampR, and ampC mutants and the restoration of creD inducibility in the ampD mutant after nagZ, ampG, or ampC inactivation. On the other hand, as previously suggested (15), the inhibition (by cefoxitin or other PBP4 inhibitors, such as imipenem) or inactivation of the peptidoglycan carboxypeptidase DacB directly triggers the activation of the CreBC system, leading to creD upregulation. Moreover, we show that through a still uncertain feedback mechanism, blocking the intracellular recycling of the produced cell wall metabolites through the inactivation of ampG or nagZ attenuates creD expression in the dacB mutant.
Role of the CreBC (BlrAB) system in bacterial fitness.
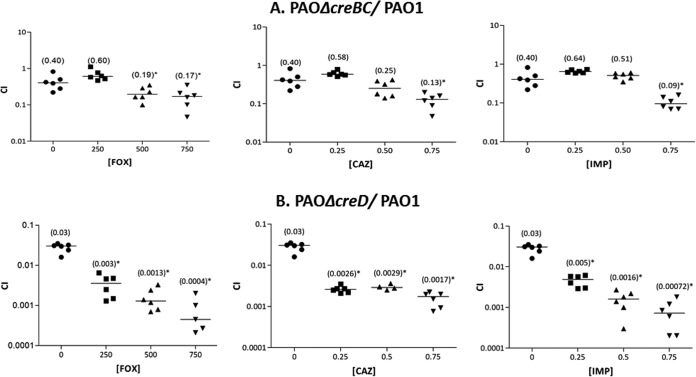
To assess the effect on bacterial fitness, in vitro competition experiments were performed between wild-type PAO1 and the corresponding mutants in the CreBC two-component regulator and its regulated inner membrane protein, CreD. As shown in Fig. 2, the inactivation of creBC produced a modest (CI, 0.4) but significant (P < 0.001) fitness cost. Moreover, a much higher fitness cost (CI, 0.03) was evidenced after the direct inactivation of creD. Interestingly, subinhibitory concentrations of all three β-lactams tested (therefore regardless of structure or whether they were potent AmpC inducers or not) significantly increased further the fitness cost of the mutants (Fig. 2). Nevertheless, the effect again was much more accentuated for the creD mutant than for the creBC mutant. Moreover, the effect was shown to be specific for β-lactams, since the CIs in the presence of 1/2 MICs of ciprofloxacin were not different from those without antibiotic for the CreBC versus PAO1 (0.49 ± 0.29 versus 0.57 ± 0.19) or CreD versus PAO1 (0.012 ± 0.008 versus 0.011 ± 0.013) competition experiments. All together, these results show that the CreBC system, particularly its regulated inner membrane protein CreD, plays an important role in bacterial fitness, especially in the presence of subinhibitory concentrations of β-lactams. The absence of major fitness differences depending on whether the specific β-lactam induces (imipenem or cefoxitin) or does not induce (ceftazidime) creD may suggest that the basal expression of creD plays a greater role in fitness than the effect derived from the further induction or not of its expression.
FIG 2.
Results of in vitro competition experiments of PAOΔcreBC versus PAO1 (A) and PAOΔcreD versus PAO1 (B) in the presence of β-lactams. In vitro competitions were performed using LB broth flasks containing 1/4, 1/2, and 3/4 MICs of ceftazidime (CAZ) (0.25, 0.5, and 0.75 μg/ml, respectively), imipenem (IMP) (0.25, 0.5, and 0.75 μg/ml, respectively), or cefoxitin (FOX) (250, 500, and 750 μg/ml, respectively). The CI values obtained for each of the eight independent experiments are plotted, and the median values are shown in parentheses. P values of <0.05 for the comparison of the CIs in the presence and absence of antibiotics are indicated with an asterisk.
Similar results were obtained when fitness was measured in terms of the growth rates in individual cultures (Table 3). While the duplication times were not sensitive enough to show the small fitness defect associated with CreBC inactivation in the competition experiments, they clearly evidenced the major cost associated with CreD inactivation and how it is significantly enhanced in the presence of subinhibitory concentrations of β-lactams (Table 3).
TABLE 3.
Duplication times of PAO1 and the corresponding CreBC and CreD mutants in the presence or absence of 1/2 MICs of ceftazidime or cefoxitin
| Strain | Duplication time (mean ± SD) (min) under antibiotic conditions of: |
||
|---|---|---|---|
| No antibiotic | 0.5 μg/ml ceftazidime | 500 μg/ml cefoxitin | |
| PAO1 | 29.8 ± 4.2 | 34 ± 1.6 | 30.4 ± 2.8 |
| PAΔcreBC | 29 ± 3.2 | 30.1 ± 4.0 | 33 ± 2.5 |
| PAΔcreD | 35 ± 1.2a | 42.5 ± 5.6a | 35.7 ± 2.9a |
Statistically significant differences (Student's t test) compared to PAO1 values.
Role of the CreBC (BlrAB) system in global regulation and response to β-lactams.
Using DNA microarrays, we compared the global expression profiles of PAO1 and PAOΔcreBC, in the absence of antibiotics, in the presence of subinhibitory (1/2 MIC [0.5 μg/ml]) concentrations of the weak AmpC inducer antipseudomonal cephalosporin ceftazidime, and in the presence of the strong AmpC inducer cephalosporin cefoxitin, which lacks antipseudomonal activity (i.e., the concentration used for AmpC induction, 50 μg/ml, is >20-fold lower than the MIC). This approach was therefore followed to evaluate the role of the CreBC system in (i) global regulation in the absence of antibiotics, (ii) the AmpC induction response, and (iii) β-lactam stress. The results are presented in Table 4 (genes showing modified expression in PAOΔcreBC compared to in PAO1 under the three evaluated conditions [no antibiotics, cefoxitin, and ceftazidime]), Table 5 (genes showing modified expression for each strain [PAO1 or PAOΔcreBC] in the presence or absence of cefoxitin or ceftazidime), and Fig. 3 (Venn diagram of differentially regulated genes).
TABLE 4.
Gene loci showing modified expression (≥2-fold change) in the creBC mutant compared to the parental wild-type PAO1 strain under basal conditions and after cefoxitin or ceftazidime incubation as determined using Affymetrix GeneChips
| Locus tag | Gene | Product | Fold change/condition under antibiotic conditionsa: |
||
|---|---|---|---|---|---|
| Basal | FOX | CAZ | |||
| PA0515 | Probable transcriptional regulator | 2.18 | −1.02 | −1.09 | |
| PA0517 | nirC | Probable c-type cytochrome precursor | 3.26 | 1.07 | 1.08 |
| PA0518 | nirM | Cytochrome c-551 precursor | 3.43 | −1.05 | 1.04 |
| PA0519 | nirS | Nitrite reductase precursor | 3.35 | −1.02 | −1.22 |
| PA0523 | norC | Nitric oxide reductase subunit C | 2.85 | 1.17 | −1.03 |
| PA0524 | norB | Nitric oxide reductase subunit B | 3.79 | 1.25 | 1.05 |
| PA0525 | Probable denitrification protein NorD | 2.26 | 1.08 | 1.26 | |
| PA1431 | rsaL | Regulatory protein RsaL | 2.15 | 1.68 | 1.91 |
| PA1596 | htpG | Heat shock protein HtpG | 1.07 | 1.93 | 2.16 |
| PA1901 | phzC2 | Phenazine biosynthesis protein PhzC | 1.71 | 2.03 | 1.8 |
| PA1903 | phzE2 | Phenazine biosynthesis protein PhzE | 1.89 | 2.06 | 1.51 |
| PA1905 | phzG2 | Probable pyridoxamine 5′-phosphate oxidase | 2.02 | 2.42 | 2.06 |
| PA2247 | bkdA1 | 2-Oxoisovalerate dehydrogenase (alpha-subunit) | 1.12 | 1.25 | 2.03 |
| PA2248 | bkdA2 | 2-Oxoisovalerate dehydrogenase (beta-subunit) | 1.13 | 1.58 | 2.58 |
| PA2300 | chicC | Chitinase | 1.5 | 2.18 | 1.49 |
| PA3126 | ibpA | Heat shock protein IbpA | 1.04 | 1.91 | 2.08 |
| PA3328 | Probable FAD-dependent monooxygenaseb | 1.77 | 2.42 | 2.1 | |
| PA3329 | Hypothetical protein | 1.57 | 2.14 | 1.94 | |
| PA3330 | Probable short-chain dehydrogenase | 1.86 | 2.61 | 2.27 | |
| PA3331 | Cytochrome P450 | 2.11 | 2.65 | 2.2 | |
| PA3332 | Conserved hypothetical protein | 1.83 | 2.46 | 1.88 | |
| PA3333 | fabH2 | 3-Oxoacyl-(acyl-carrier-protein) synthase III | 1.57 | 2.21 | 1.47 |
| PA3334 | Probable acyl carrier protein | 1.72 | 2.33 | 1.75 | |
| PA3874 | narH | Respiratory nitrate reductase beta chain | 2.93 | 1.78 | −1.05 |
| PA3875 | narG | Respiratory nitrate reductase alpha chain | 3.97 | 2.55 | −1.06 |
| PA3876 | narK2 | Nitrite extrusion protein 2 | 4.49 | 2.56 | 1.04 |
| PA3877 | narK1 | Nitrite extrusion protein 1 | 5.35 | 2.95 | 1.12 |
| PA3392 | nosZ | Nitrous oxide reductase precursor | 2.04 | −1.68 | −1.32 |
| PA3872 | narI | Respiratory nitrate reductase gamma chain | 2.14 | 1.43 | 1.01 |
| PA3873 | narJ | Respiratory nitrate reductase delta chain | 2.48 | 1.46 | 1.04 |
| PA3914 | moeA1 | Molybdenum cofactor biosynthetic protein A1 | 2.19 | 1.17 | 1.09 |
| PA3915 | moaB1 | Molybdenum cofactor biosynthetic protein B1 | 3.52 | 1.65 | 1.1 |
| PA4141 | Hypothetical protein | 2.09 | 2.36 | 2.37 | |
| PA4211 | phzB1 | Probable phenazine biosynthesis protein | 2.12 | 2.9 | 2.23 |
| PA4217 | phzS | Flavin-containing monooxygenase | 2.43 | 3.23 | 1.94 |
| PA4386 | groES | GroES protein | 2.21 | 1.63 | 1.4 |
| PA4762 | grpE | Heat shock protein GrpE | 1.13 | 1.98 | 2.66 |
| PA5053 | hslV | Heat shock protein HslV | 1.14 | 2.02 | 3 |
| PA5054 | hslU | Heat shock protein HslU | −1.05 | 1.67 | 2.24 |
| PA0465 | creD | Inner membrane protein CreD | 1.18 | −3.2 | 1.15 |
| PA0466 | Hypothetical protein | 1.18 | −2.02 | 1 | |
| PA0887 | acsA | Acetyl-coenzyme A synthetase | −1.55 | 1.23 | −2.34 |
| PA1123 | Hypothetical protein | −1.32 | −1.44 | −2.01 | |
| PA1183 | dctA | C4-dicarboxylate transport protein | −1.58 | −1.62 | −2.3 |
| PA1557 | ccoN2 | Cytochrome c oxidase, cbb3-type, CcoN subunit | −2.04 | −1.6 | −1.68 |
| PA3013 | foaB | Fatty acid oxidation complex beta-subunit | −1.56 | −1.72 | −2.16 |
| PA3040 | Conserved hypothetical protein | −1.16 | −1.31 | −2.07 | |
| PA3049 | Rmf | Ribosome modulation factor | −1.1 | −1.78 | −2.66 |
| PA3284 | Hypothetical protein | −1.35 | −1.81 | −2.12 | |
| PA3790 | oprC | Putative copper transport outer membrane porin OprC precursor | −1.62 | −1.72 | −2.23 |
| PA3841 | exoS | Exoenzyme S | −1.29 | −1.24 | −2.05 |
| PA4525 | pilA | Type 4 fimbrial precursor PilA | −1.71 | −2.18 | −2.83 |
| PA4587 | ccpR | Cytochrome c551 peroxidase precursor | −1.37 | −1.8 | −2.93 |
Bold type indicates increased gene expression (≥2-fold), and italics indicate decreased gene expression (≥2-fold).
FAD, flavin adenine dinucleotide.
TABLE 5.
Gene loci showing modified expression (≥2-fold change) in each of the strains tested under cefoxitin or ceftazidime incubation compared to the same strain under basal conditions as determined using Affymetrix GeneChips
| Locus tag | Gene | Product | Fold change/condition under antibiotic conditionsa: |
|||
|---|---|---|---|---|---|---|
| PAO1 FOX | creBC FOX | PAO1 CAZ | creBC CAZ | |||
| PA0465 | creD | Inner membrane protein CreD | 3.62 | −3.2 | 1.14 | 1.11 |
| PA0466 | 2.3 | −2.02 | 1.14 | −1.03 | ||
| PA0509 | nirN | Probable c-type cytochrome | 2.1 | −1.3 | −1.16 | −1.74 |
| PA0510 | Hypothetical protein | 2.18 | −1.39 | −1.44 | −2.18 | |
| PA0511 | nirJ | 2.17 | −1.2 | −1.34 | −2.24 | |
| PA0512 | Conserved hypothetical protein | 2.43 | −1.22 | −1.19 | −2.08 | |
| PA0513 | Probable transcriptional regulator | 2.42 | −1.12 | −1.59 | −2.91 | |
| PA0514 | nirL | 2.25 | −1.13 | −1.28 | −2.38 | |
| PA0515 | Probable transcriptional regulator | 2.46 | −1.01 | −1.17 | −2.77 | |
| PA0516 | nirF | 2.74 | 1.07 | −1.33 | −2.66 | |
| PA0517 | nirC | 3.56 | −1.01 | −1.22 | −3.66 | |
| PA0518 | nirM | 3.8 | −1.05 | −1.28 | −4.57 | |
| PA0519 | nirS | 3.86 | −1.02 | −1.21 | −4.94 | |
| PA0523 | norC | 1.85 | −1.18 | −3.45 | ||
| PA0524 | norB | 2.43 | −1.25 | −1.08 | −3.91 | |
| PA0525 | Probable denitrification protein NorD | 1.62 | −1.29 | −1.15 | −2.07 | |
| PA0918 | Cytochrome b561 | 2.41 | −1 | −1.14 | −1.78 | |
| PA1557 | ccoN2 | Cytochrome c oxidase, cbb3-type, CcoN subunit | 1.76 | 2.25 | 2.08 | 2.54 |
| PA1582 | sdhD | Succinate dehydrogenase (D subunit) | 1.38 | 1.54 | 2.02 | 1.63 |
| PA1588 | sucC | Succinyl-CoA synthetase beta chainb | 1.55 | 1.36 | 2.16 | 1.46 |
| PA2742 | rpmI | 50S ribosomal protein L35 | 1.48 | 1.61 | 2.03 | 1.74 |
| PA3205 | Hypothetical protein | 1.25 | 1.55 | −2.49 | −1.13 | |
| PA3392 | nosZ | Nitrous oxide reductase precursor | 3.79 | −1.68 | −1.53 | −4.1 |
| PA3393 | nosD | NosD protein | 2.11 | −1.39 | −1.28 | −1.87 |
| PA3394 | nosF | NosF protein | 2.2 | −1.27 | −1.24 | −1.80 |
| PA3395 | nosY | NosY protein | 2.21 | −1.28 | −1.01 | −1.26 |
| PA3872 | narI | Respiratory nitrate reductase gamma chain | −1.00 | −1.49 | −1.24 | −2.61 |
| PA3873 | narJ | Respiratory nitrate reductase delta chain | 1.26 | −1.34 | −1.14 | −2.7 |
| PA3874 | narH | Respiratory nitrate reductase beta chain | 1.15 | −1.43 | −1.1 | −3.37 |
| PA3875 | narG | Respiratory nitrate reductase alpha chain | 1.19 | −1.31 | −1.13 | −4.73 |
| PA3876 | narK2 | Nitrite extrusion protein 2 | 1.09 | −1.62 | −1.17 | −5.08 |
| PA3877 | narK1 | Nitrite extrusion protein 1 | 1.18 | −1.53 | −1.11 | −5.34 |
| PA3915 | moaB1 | Molybdopterin biosynthetic protein B1 | −1.03 | −1.20 | −1.15 | −3.67 |
| PA4110 | ampC | β-Lactamase precursor | 5.16 | 4.17 | 1.10 | −1.09 |
| PA4238 | rpoA | DNA-directed RNA polymerase alpha chain | 1.55 | 1.48 | 2.01 | 1.72 |
| PA4242 | rpmJ | 50S ribosomal protein L36 | 1.41 | 1.23 | 2.03 | 1.41 |
| PA4246 | rpsE | 30S ribosomal protein S5 | 1.46 | 1.72 | 2.11 | 1.91 |
| PA4247 | rplR | 50S ribosomal protein L18 | 1.41 | 1.28 | 2.03 | 1.58 |
| PA4254 | rpsQ | 30S ribosomal protein S17 | 1.68 | 1.43 | 2.41 | 1.88 |
| PA4257 | rpsC | 30S ribosomal protein S3 | 1.78 | 1.51 | 2.27 | 1.92 |
| PA4258 | rplV | 50S ribosomal protein L22 | 1.58 | 1.27 | 2.01 | 1.6 |
| PA4260 | rplB | 50S ribosomal protein L2 | 1.49 | 1.24 | 2.06 | 1.44 |
| PA4262 | rplD | 50S ribosomal protein L4 | 1.56 | 1.62 | 2.21 | 1.71 |
| PA4266 | fusA1 | Elongation factor G | 1.56 | 1.59 | 2.1 | 1.85 |
| PA4268 | rpsL | 30S ribosomal protein S12 | 1.96 | 1.61 | 2.47 | 1.63 |
| PA4272 | rplJ | 50S ribosomal protein L10 | 1.37 | 1.36 | 2.08 | 1.98 |
| PA4944 | hfG | 1.88 | 2.15 | 1.79 | 1.97 | |
| PA5212 | Hypothetical protein | 2.01 | 1.55 | −1.00 | −1.09 | |
Bold type indicates increased gene expression (≥2-fold), and italics indicate decreased gene expression (≥2-fold).
CoA, coenzyme A.
FIG 3.
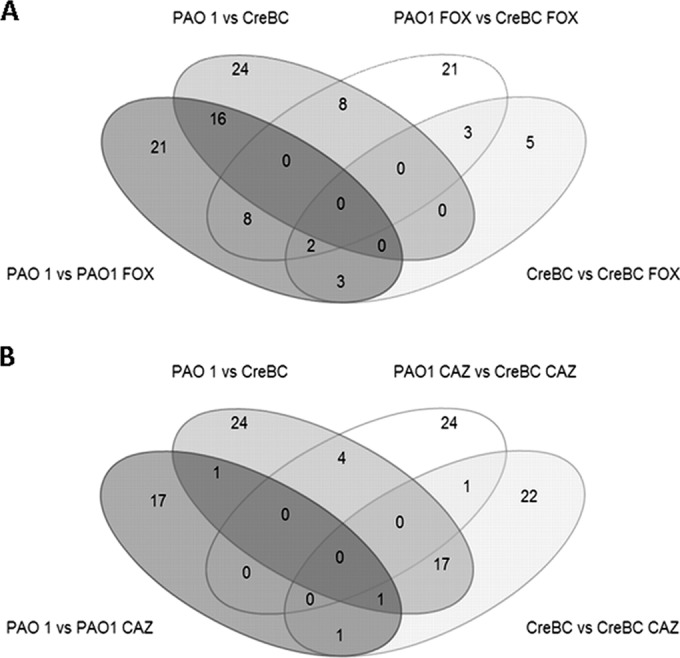
Venn diagrams of the differentially regulated genes. Shown are the distributions of significantly (P < 0.01) differentially regulated (≥2-fold) genes in PAO1 and PAOΔcreBC treated or not with 50 μg/ml cefoxitin (A) or 0.5 μg/ml ceftazidime (B).
As shown in Table 4, pairwise comparisons of the data sets of significantly differentially regulated genes (differential expression, ≥2-fold; P ≤ 0.01) identified a total of 53 genes showing modified expression in PAOΔcreBC with respect to that in PAO1 in either the absence of antibiotics (n = 24) or the presence of the AmpC inducer cefoxitin (n = 21) or the antipseudomonal cephalosporin ceftazidime (n = 24). Moreover, as shown in Table 5, the interstrain comparison identified a total of 23 genes differentially regulated in the presence of cefoxitin and 38 genes differentially regulated in the presence of ceftazidime. All together, these results clearly indicate that the CreBC system may act as a global regulator in P. aeruginosa, as was previously suggested for other Gram-negative bacteria (20).
To further address the interplay between creBC-dependent genes and antibiotic exposure, the differentially regulated genes were plotted in 4-way Venn diagrams (Fig. 3) and separated into cefoxitin (Fig. 3A) and ceftazidime (Fig. 3B) differentially regulated genes. Indeed, genes that were differentially regulated due to a lack of creBC, to β-lactam antibiotic exposure, or both showed an intricate interconnection.
As shown in Fig. 3A and Table 5, there were 21 genes upregulated in PAO1 after cefoxitin exposure and 24 genes showing modified expression (23 upregulated and 1 downregulated) in the creBC mutant compared to in PAO1 in the absence of antibiotics (Fig. 3A and Table 4). Interestingly, 16 of these genes were upregulated under the two conditions, indicating a clear interconnection between the CreBC system and global gene expression profiles resulting from cefoxitin exposure. Those genes include creD, as expected, and also the whole gene cluster for dissimilatory nitrite reductase (NIR) and the nitric oxide synthase (NOS) gene cluster for nitrous oxide reduction, both of which play a major role in denitrification during anaerobic metabolism. However, as previously noted (15), a remarkable exception to the role of the CreBC system in the response to the presence of the AmpC inducer cefoxitin is the expression of ampC itself, which is independent of the CreBC system (Tables 4 and 5).
On the other hand, the transcriptional response of PAO1 to subinhibitory concentrations of the antipseudomonal cephalosporin ceftazidime included the modification of the expression of 17 genes, all but 1 of which were upregulated (Fig. 3B and Table 5), including rpm, rpo, rps, and rpl ribosomal proteins. In contrast, of the 22 genes that showed modified expression in the creBC mutant, 21 were downregulated. Indeed, the responses of PAO1 and PAOΔcreBC to ceftazidime exposure had only one gene in common (Fig. 3B). Moreover, 17 of the 21 downregulated genes in the presence of ceftazidime were upregulated in the creBC mutant compared to in PAO1 in the absence of antibiotics (Tables 4 and 5 and Fig. 3B), confirming an important role of the CreBC system in the response to β-lactam challenge.
Moreover, as shown in Table 4, genes from the entire denitrification cascade were upregulated in the absence of antibiotics when creBC was inactivated, including genes of the nitrogen regulatory pathway (N2O reduction, [NOS], NO-2 reductase [NIR], and NO reductase [NOR], and nitrate/nitrite transport [NAR)]) and MoeA1 MoaB1 (molybdate metabolism) (35, 36). Genes from the first step, catalyzed by the membrane-bound nitrate reductase, NAR, are required for anaerobic nitrate respiration of P. aeruginosa in CF sputum (37). The reduction of nitrate by NAR is coupled to quinol oxidation and consumes two protons from the cytoplasm, thereby contributing to the creation of a proton gradient across the membrane (38, 39).
Nitrite reductase catalyzes the second step of denitrification, the reduction of nitrite to NO, and the nir gene cluster, which is located in the periplasm (40). The reduction of NO to N2O is catalyzed by NO reductase, which is encoded in the norCBD operon and is clustered with the nir genes for nitrite reductase in the genome of P. aeruginosa. NO reductase functions not only for anaerobic energy conservation as a respiratory enzyme but also for the detoxification of exogenous NO. The machinery for the detoxification of NO and its derivative reactive nitrogen species is necessary because the infected P. aeruginosa cells are subjected to nitrosative stress by attacks from the host immune system. The NO reductase-deficient mutant of P. aeruginosa shows a reduced survival rate in NO-producing macrophages (41). The final step of the denitrification pathway, the reduction of N2O to N2, is catalyzed by N2O reductase. In P. aeruginosa, N2O reductase is encoded in the nos operon. It has been also proposed that the MoeA-catalyzed product, an activated form of molybdate, regulates the nar operon (42).
Role of the CreBC (BlrAB) system in biofilm growth.
Considering the above results in light of previous findings revealing a role of the denitrification cascade in biofilm formation (37, 43), we evaluated the impact of the CreBC system in biofilm growth in the presence or absence of the AmpC inducer cefoxitin. As shown in Fig. 4, the inactivation of creD led to a significant reduction of biomass in both 24-h- and 48-h-old biofilms. Moreover, even at the low concentrations used (>20-fold below MICs), cefoxitin significantly reduced the biomass of 48-h biofilms from that in wild-type PAO1. Furthermore, the effect of cefoxitin on biofilm growth was dramatically enhanced in the creBC and creD mutants. Therefore, our results indicate a clear connection between the CreBC system, AmpC induction pathways, and biofilm formation.
FIG 4.
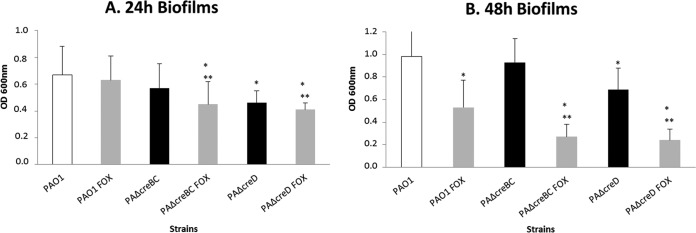
Biomass of 24-h (A) and 48-h (B) biofilms formed by PAO1, PAOΔcreBC, and PAOΔcreD strains exposed or not to 50 μg/ml cefoxitin (FOX). * and **, statistically significant differences for the comparisons with PAO1 and PAO1, respectively, exposed to cefoxitin. Data shown are means plus standard deviations.
Identification of the cre-tag.
In an attempt to identify if the genes that are differentially regulated by the CreBC system presented the CreBC-tag, a bioinformatics approach was applied to all CreBC-regulated genes from the microarray data for potential CreBC-binding sites. The criteria and consensus sequences described for E. coli (20, 21) and Aeromonas spp. (19, 20) from the CreBC-dependent genes were used. For a direct repeat to be classed as a cre-tag, it should be oriented in the same direction as a 3′-proximal gene and within 450 bp of the ATG initiation codon of that gene. Using this consensus sequence to search the upstream differentially regulated gene regions of the PAO1 genome led to the identification of the cre-tag in the upstream region of 10 of the 14 genes/operons analyzed (PA0515, nirS, rsaL, phzA2, PA3871, PA4141, nosZ, moeA1, phzA1, and groeS), with 50% of them being double direct repeats (Fig. 5). Moreover, an analysis of the upstream sequence of 100 randomly selected genes revealed that only 22 of them had the single tag, but no direct repeats were found, confirming the significant association between the presence of the cre-tag and experimental CreBC dependence.
FIG 5.
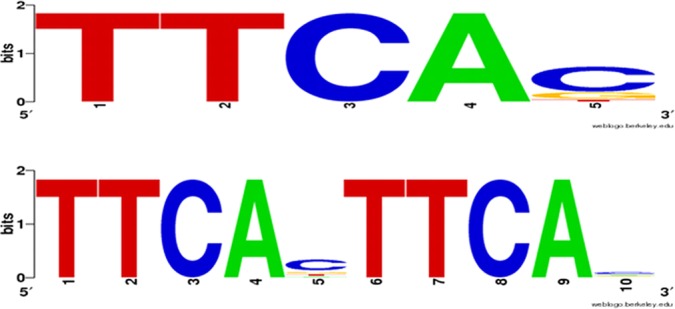
cre-tag analysis. The putative CreBC motif was found by analyzing the 500 bp upstream of differentially CreBC-regulated and CreBC-β-lactam-regulated genes/operons (P value, ≤0.01, ≥2-fold) (Tables 4 and 5) in PAOΔcreBC. The Pseudomonas Genome Database (35, 36) was used to identify the computationally predicted operons and the promoter-proximal TTCACnnnnnnTTCAC cre-tag motifs. The output is represented using WebLogo (53) for the creBC-dependent genes, including the presence of the single tag (top) and the direct double repeats (bottom).
In previous studies performed with E. coli, a direct analysis performed over the whole sequence led to the identification of eight genes that contained the cre-tag motif, ackA, talA, radC, malE, trgB, creD, yidS, and yieI (44). Nevertheless, our results reveal that none of those genes except for creD had cre-tag motifs or altered expression in P. aeruginosa. Zhou et al. (44) described in a systematical analysis of a CreBC mutant the differential expression of several open reading frames of unknown function, which were designated cbrA, cbrB, and cbrC (creB-regulated genes A, B, and C, respectively) (44). No phenotypic changes were observed for the multiple creABCD mutants analyzed, but significant differences were observed for the cbrA and cbrBC double mutants. Interestingly, subinhibitory concentrations of colicin M induce the transcription of genes involved in adaptive responses, including the two-component CreBC system associated with increased resistance to some colicins (45). Recent studies have demonstrated that CbrA, being a nonessential protein, confers high resistance to colicin M and renders cells resistant to osmotic shock in a CreBC-dependent manner (46). On the other hand, the overproduction of YieJ (formerly CbrC) enhances the tolerance of colicin E2 (a role previously assigned to CreD). One role of the Cre regulon might be to cause modification of the cell envelope, since previously identified colicin E2 and colicin M tolerance mechanisms are associated with a reduction in the ability of both bacteriocins to cross the envelope. Although P. aeruginosa lacks orthologs of these two genes, there are clear implications of this system in the response to cell wall stress in the presence of β-lactams. It has also been reported that all of the β-lactamase genes known for Aeromonas spp. have at least one copy of the cre/blr-tag sequence, TTCAC. Indeed, Aeromonas hydrophila genes ampH, cepH, and imiH are in fact regulated by the CreBC (blrAB) system as well as the blr regulon gene, blrD (creD) (20).
We previously reported that in P. aeruginosa, a PBP4 mutation leads to AmpR-dependent ampC overexpression and CreBC-dependent creD overexpression (15), thus revealing an efficient mechanism of one-step high level β-lactam resistance. A link between PBP4, β-lactamase expression, and the BlrAB system was detected in Aeromonas spp. as well (47). Indeed, the P. aeruginosa CreBC system is also more functionally similar to the BlrAB system (15). Previous works showed that the regulatory system of Aeromonas spp. (BlrAB) is highly homologous to the E. coli CreBC system (20, 21). Furthermore, different studies suggested that the CreBC system of E. coli can regulate the expression of Aeromonas spp. β-lactamase when cloned into E. coli (43, 48).
In summary, the results obtained in this work evidence (i) the specific response of the CreBC system to AmpC inducer/PBP4 inhibitor β-lactams, (ii) an interplay between the CreBC system, peptidoglycan recycling, and the AmpC regulatory pathways, (iii) the important impact of the CreBC system in bacterial fitness and biofilm formation in the presence and absence of β-lactams, and (iv) broad regulatory functions of the CreBC system in basic physiological aspects. Therefore, this work demonstrates a clear role of the CreBC system in β-lactam resistance that is typical of the BlrAB system and a physiological/metabolic role typical of the E. coli CreBC system. Therefore, the CreBC/BlrAB pathways studied so far in E. coli, Aeromonas spp., and P. aeruginosa appear to be a single system, which responds to signals generated in the cell wall, and these pathways regulate the expression of a set of genes in response to specific environments and metabolic signals, including the presence of β-lactams.
We demonstrate the presence of the putative cre-tag motif in a high percentage of the genes regulated by this system (single or direct repeat) that turn CreBC into a global regulator. On one hand, they play a major role in high-level β-lactam resistance (the blrAB resemblance). On the other hand, they act as a global transcription control regulator (the creBC role) that exerts its function over several keystone components, such as creD, which plays a major role in fitness maintenance, or larger subsets of genes, like the NAR operon, a key factor for the development and maintenance of biofilms (37), as well as the NIR and NOS gene clusters. Indeed, the nitrate sensor-response regulator extends beyond the regulation of nitrate metabolism and interferes in motility, biofilm formation, and virulence (49). Moreover, a recent work suggested that the activation of the nitrate respiratory chain in P. aeruginosa might be a response in order to compensate for the fitness cost associated with particular antibiotic resistance mechanisms (50). Therefore, our results complement previous studies performed with the AmpR mutant that point out a link between β-lactamase and global regulation in P. aeruginosa (3, 51, 52). Indeed, although the exact CreBC regulatory mechanisms are still unknown, we demonstrate the complexity and multiregulatory processes by which P. aeruginosa controls the expression of genes of diverse functions from β-lactam resistance to biofilm formation or anaerobic respiration. Therefore, the CreBC system is envisaged as a potentially interesting target for the development of new molecules for the treatment of P. aeruginosa infections.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Ministerio de Economía y Competitividad of Spain and the Instituto de Salud Carlos III, through the Spanish Network for the Research in Infectious Diseases (RD06/0008 and RD12/0015) and grants PS09/00033 and PI12/00103. The study is also supported by the Direcció General d′Universitats, Recerca i Transferència del Coneixement del Govern de les Illes Balears.
Footnotes
Published ahead of print 16 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02556-14.
REFERENCES
- 1.Silby MW, Winstanley C, Godfrey SA, Levy SB, Jackson RW. 2011. Pseudomonas genomes: diverse and adaptable. FEMS Microbiol. Rev. 35:652–680. 10.1111/j.1574-6976.2011.00269.x [DOI] [PubMed] [Google Scholar]
- 2.Lyczak JB, Cannon CL, Pier GB. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194–222. 10.1128/CMR.15.2.194-222.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balasubramanian D, Schneper L, Merighi M, Smith R, Narasimhan G, Lory S, Mathee K. 2012. The regulatory repertoire of Pseudomonas aeruginosa AmpC β-lactamase regulator AmpR includes virulence genes. PLoS One 7:e34067. 10.1371/journal.pone.0034067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mikkelsen H, Sivaneson M, Filloux A. 2011. Key two-component regulatory systems that control biofilm formation in Pseudomonas aeruginosa. Environ. Microbiol. 13:1666–1681. 10.1111/j.1462-2920.2011.02495.x [DOI] [PubMed] [Google Scholar]
- 5.Vander Wauven C, Piérard A, Kley-Raymann M, Haas D. 1984. Pseudomonas aeruginosa mutants affected in anaerobic growth on arginine: evidence for a four-gene cluster encoding the arginine deiminase pathway. J. Bacteriol. 160:928–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eschbach M, Schreiber K, Trunk K, Buer J, Jahn D, Schobert M. 2004. Long-term anaerobic survival of the opportunistic pathogen Pseudomonas aeruginosa via pyruvate fermentation. J. Bacteriol. 186:4596–4604. 10.1128/JB.186.14.4596-4604.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toyofuku M, Nomura N, Fujii T, Takaya N, Maseda H, Sawada I, Nakajima T, Uchiyama H. 2007. Quorum sensing regulates denitrification in Pseudomonas aeruginosa PAO1. J. Bacteriol. 189:4969–4972. 10.1128/JB.00289-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schobert M, Jahn D. 2010. Anaerobic physiology of Pseudomonas aeruginosa in the cystic fibrosis lung. Int. J. Med. Microbiol. 300:549–556. 10.1016/j.ijmm.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 9.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964. 10.1038/35023079 [DOI] [PubMed] [Google Scholar]
- 10.Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, Birrer P, Bellon G, Berger J, Weiss T, Botzenhart K, Yankaskas JR, Randell S, Boucher RC, Döring G. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 109:317–325. 10.1172/JCI13870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassett DJ, Cuppoletti J, Trapnell B, Lymar SV, Rowe JJ, Yoon SS, Hilliard GM, Parvatiyar K, Kamani MC, Wozniak DJ, Hwang SH, McDermott TR, Ochsner UA. 2002. Anaerobic metabolism and quorum sensing by Pseudomonas aeruginosa biofilms in chronically infected cystic fibrosis airways: rethinking antibiotic treatment strategies and drug targets. Adv. Drug Deliv. Rev. 54:1425–1443. 10.1016/S0169-409X(02)00152-7 [DOI] [PubMed] [Google Scholar]
- 12.Moyá B, Beceiro A, Cabot G, Juan C, Zamorano L, Albertí S, Oliver A. 2012. Pan-β-lactam resistance development in Pseudomonas aeruginosa clinical strains: molecular mechanisms, penicillin-binding proteins profiles and binding affinities. Antimicrob. Agents Chemother. 56:4771–4778. 10.1128/AAC.00680-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabot G, Ocampo-Sosa AA, Domínguez MA, Gago JF, Juan C, Tubau F, Rodriguez C, Moyà B, Peña C, Martínez-Martínez L, Oliver A, Spanish Network for Research in Infectious Disease (REIPI) 2012. Genetic markers of widespread extensively drug-resistant Pseudomonas aeruginosa high-risk clones. Antimicrob. Agents Chemother. 56:6349–6357. 10.1128/AAC.01388-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lister PD, Wolter DJ, Hanson ND. 2009. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 22:582–610. 10.1128/CMR.00040-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moya B, Dötsch A, Juan C, Blázquez J, Zamorano L, Haussler S, Oliver A. 2009. β-Lactam resistance response triggered by inactivation of a nonessential penicillin-binding protein. PLoS Pathog. 5:e1000353. 10.1371/journal.ppat.1000353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mark BL, Vocadlo DJ, Oliver A. 2011. Providing β-lactams a helping hand: targeting the AmpC β-lactamase induction pathway. Future Microbiol. 6:1415–1427. 10.2217/fmb.11.128 [DOI] [PubMed] [Google Scholar]
- 17.Juan C, Moyà B, Pérez JL, Oliver A. 2006. Stepwise upregulation of the Pseudomonas aeruginosa chromosomal cephalosporinase conferring high level beta-lactam resistance involves three AmpD homologues. Antimicrob. Agents Chemother. 50:1780–1787. 10.1128/AAC.50.5.1780-1787.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alksne LE, Rasmussen BA. 1997. Expression of the AsbA1, OXA-12, and AsbM1 beta-lactamases in Aeromonas jandaei AER 14 is coordinated by a two-component regulon. J. Bacteriol. 179:2006–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niumsup P, Simm AM, Nurmahomed K, Walsh TR, Bennett PM, Avison MB. 2003. Genetic linkage of the penicillinase gene, amp, and blrAB, encoding the regulator of β-lactamase expression in Aeromonas spp. J. Antimicrob. Chemother. 51:1351–1358. 10.1093/jac/dkg247 [DOI] [PubMed] [Google Scholar]
- 20.Avison MB, Niumpsup P, Nurmahomed K, Walsh TR, Bennett PM. 2004. Role of the ‘Cre/blr-tag’ DNA sequence in regulation of gene expression by the Aeromonas hydrophila beta-lactamase regulator, BlrA. J. Antimicrob. Chemother. 53:197–202. 10.1093/jac/dkh077 [DOI] [PubMed] [Google Scholar]
- 21.Cariss SJ, Tayler AE, Avison MB. 2008. Defining the growth conditions and promoter-proximal DNA sequences required for activation of gene expression by CreBC in Escherichia coli. J. Bacteriol. 190:3930–3939. 10.1128/JB.00108-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zamorano L, Moyà B, Juan C, Oliver A. 2010. Differential beta-lactam resistance response driven by ampD or dacB (PBP4) inactivation in genetically diverse Pseudomonas aeruginosa strains. J. Antimicrob. Chemother. 65:1540–1542. 10.1093/jac/dkq142 [DOI] [PubMed] [Google Scholar]
- 23.Boudreau MA, Fisher JF, Mobashery S. 2012. Messenger functions of the bacterial cell wall-derived muropeptides. Biochemistry 51:2974–2990. 10.1021/bi300174x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng X, Lin J. 2013. Beta-lactamase induction and cell wall metabolism in Gram-negative bacteria. Front. Microbiol. 4:128. 10.3389/fmicb.2013.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zamorano L, Reeve TM, Juan C, Moyà B, Cabot G, Vocadlo DJ, Mark BL, Oliver A. 2011. AmpG inactivation restores susceptibility of pan-β-lactam-resistant Pseudomonas aeruginosa clinical strains. Antimicrob. Agents Chemother. 55:1990–1996. 10.1128/AAC.01688-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quénée L, Lamotte D, Polack B. 2005. Combined sacB-based negative selection and Cre-lox antibiotic marker recycling for efficient gene deletion in Pseudomonas aeruginosa. Biotechniques 38:63–67. 10.2144/05381ST01 [DOI] [PubMed] [Google Scholar]
- 27.Kotch B, Jensen LE, Nybroe O. 2001. A panel of Tn7-based vectors for insertion of the gfp marker gene or for delivery of cloned DNA into Gram-negative bacteria at a neutral chromosomal site. J. Microbiol. Methods 45:187–195. 10.1016/S0167-7012(01)00246-9 [DOI] [PubMed] [Google Scholar]
- 28.Klausen M, Heydorn A, Ragas P, Lambertsen L, Aaes-Jørgensen A, Molin S, Tolker-Nielsen T. 2003. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 48:1511–1524. 10.1046/j.1365-2958.2003.03525.x [DOI] [PubMed] [Google Scholar]
- 29.Oh H, Stenhoff J, Jalal S, Wretlind B. 2003. Role of efflux pumps and mutations in genes for topoisomerases II and IV in fluoroquinolone-resistant Pseudomonas aeruginosa strains. Microb. Drug Resist. 9:323–328. 10.1089/107662903322762743 [DOI] [PubMed] [Google Scholar]
- 30.Moya B, Juan C, Albertí S, Pérez JL, Oliver A. 2008. Benefit of having multiple ampD genes for acquiring beta-lactam resistance without losing fitness and virulence in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 52:3694–3700. 10.1128/AAC.00172-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merritt JH, Kadouri DE, O'Toole GA. 2005. Growing and analyzing static biofilms. Curr. Protoc. Microbiol. Chapter 1:Unit 1B.1. 10.1002/9780471729259.mc01b01s00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blázquez J, Gómez-Gómez JM, Oliver A, Juan C, Kapur V, Martín S. 2006. PBP3 inhibition elicits adaptive responses in Pseudomonas aeruginosa. Mol. Microbiol. 62:84–99. 10.1111/j.1365-2958.2006.05366.x [DOI] [PubMed] [Google Scholar]
- 33.Mulet X, Maciá MD, Mena A, Juan C, Pérez JL, Oliver A. 2009. Azithromycin in Pseudomonas aeruginosa biofilms: bactericidal activity and selection of nfxB mutants. Antimicrob. Agents Chemother. 53:1552–1560. 10.1128/AAC.01264-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zamorano L, Reeve TM, Deng L, Juan C, Moyá B, Cabot G, Vocadlo DJ, Mark BL, Oliver A. 2010. NagZ inactivation prevents and reverts beta-lactam resistance, driven by AmpD and PBP 4 mutations, in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 54:3557–3563. 10.1128/AAC.00385-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winsor GL, Van Rossum T, Lo R, Khaira B, Whiteside MD, Hancock RE, Brinkman FS. 2009. Pseudomonas Genome Database: facilitating user-friendly, comprehensive comparisons of microbial genomes. Nucleic Acids Res. 37(Database issue):D483–D488. 10.1093/nar/gkn861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winsor GL, Lam DK, Fleming L, Lo R, Whiteside MD, Yu NY, Hancock RE, Brinkman FS. 2011. Pseudomonas Genome Database: improved comparative analysis and population genomics capability for Pseudomonas genomes. Nucleic Acids Res. 39(Database issue):D596–D600. 10.1093/nar/gkq869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer KL, Brown SA, Whiteley M. 2007. Membrane-bound nitrate reductase is required for anaerobic growth in cystic fibrosis sputum. J. Bacteriol. 189:4449–4455. 10.1128/JB.00162-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zumft WG, Körner H. 1997. Enzyme diversity and mosaic gene organization in denitrification. Antonie Van Leeuwenhoek 71:43–58. 10.1023/A:1000112008026 [DOI] [PubMed] [Google Scholar]
- 39.Zumft WG. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arai H. 2011. Regulation and function of versatile aerobic and anaerobic respiratory metabolism in Pseudomonas aeruginosa. Front. Microbiol. 2:103. 10.3389/fmicb.2011.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kakishima K, Shiratsuchi A, Taoka A, Nakanishi Y, Fukumori Y. 2007. Participation of nitric oxide reductase in survival of Pseudomonas aeruginosa in LPS-activated macrophages. Biochem. Biophys. Res. Commun. 355:587–591. 10.1016/j.bbrc.2007.02.017 [DOI] [PubMed] [Google Scholar]
- 42.Hasona A, Self WT, Ray RM, Shanmugam KT. 1998. Molybdate-dependent transcription of hyc and nar operons of Escherichia coli requires MoeA protein and ModE-molybdate. FEMS Microbiol. Lett. 169:111–116. 10.1111/j.1574-6968.1998.tb13306.x [DOI] [PubMed] [Google Scholar]
- 43.Avison MB, Horton RE, Walsh TR, Bennett PM. 2001. Escherichia coli CreBC is a global regulator of gene expression that responds to growth in minimal media. J. Biol. Chem. 276:26955–26961. 10.1074/jbc.M011186200 [DOI] [PubMed] [Google Scholar]
- 44.Zhou M, Tsumori N, Xu Q, Kushto GP, Andrews L. 2003. Reactions of B atoms and clusters with NO: experimental and theoretical characterization of novel molecules containing B, N, and O. J. Am. Chem. Soc. 125:11371–11378. 10.1021/ja0367187 [DOI] [PubMed] [Google Scholar]
- 45.Kamenšek S, Žgur-Bertok D. 2013. Global transcriptional responses to the bacteriocin colicin M in Escherichia coli. BMC Microbiol. 13:42. 10.1186/1471-2180-13-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Helbig S, Hantke K, Ammelburg M, Braun V. 2012. CbrA is a flavin adenine dinucleotide protein that modifies the Escherichia coli outer membrane and confers specific resistance to colicin M. J. Bacteriol. 194:4894–4903. 10.1128/JB.00782-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tayler AE, Ayala JA, Niumsup P, Westphal K, Baker JA, Zhang L, Walsh TR, Wiedemann B, Bennett PM, Avison MB. 2010. Induction of beta-lactamase production in Aeromonas hydrophila is responsive to beta-lactam-mediated changes in peptidoglycan composition. Microbiology 156:2327–2335. 10.1099/mic.0.035220-0 [DOI] [PubMed] [Google Scholar]
- 48.Rasmussen BA, Keeney D, Yang Y, Bush K. 1994. Cloning and expression of a cloxacillin-hydrolyzing enzyme and a cephalosporinase from Aeromonas sobria AER 14M in Escherichia coli: requirement for an E. coli chromosomal mutation for efficient expression of the class D enzyme. Antimicrob. Agents Chemother. 38:2078–2085. 10.1128/AAC.38.9.2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Alst NE, Picardo KF, Iglewski BH, Haidaris CG. 2007. Nitrate sensing and metabolism modulate motility, biofilm formation, and virulence in Pseudomonas aeruginosa. Infect. Immun. 75:3780–3790. 10.1128/IAI.00201-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olivares Pacheco J, Álvarez-Ortega C, Martinez JL. 2014. Metabolic compensation of fitness costs associated with overexpression of the multidrug efflux pump MexEF-OprN in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 58:3904–3913. 10.1128/AAC.00121-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balasubramanian D, Kumari H, Jaric M, Fernandez M, Turner KH, Dove SL, Narasimhan G, Lory S, Mathee K. 2014. Deep sequencing analyses expands [sic] the Pseudomonas aeruginosa AmpR regulon to include small RNA-mediated regulation of iron acquisition, heat shock and oxidative stress response. Nucleic Acids Res. 42:979–998. 10.1093/nar/gkt942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumari H, Murugapiran SK, Balasubramanian D, Schneper L, Merighi M, Sarracino D, Lory S, Mathee K. 2014. LTQ-XL mass spectrometry proteome analysis expands the Pseudomonas aeruginosa AmpR regulon to include cyclic di-GMP phosphodiesterases and phosphoproteins, and identifies novel open reading frames J. Proteomics 96: 328–42. 10.1016/j.jprot.2013.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crooks GE, Hon G, Chandonia JM, Brenner SE. 2004. WebLogo: a sequence logo generator. Genome Res. 14:1188–1190. 10.1101/gr.849004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.