Abstract
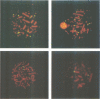
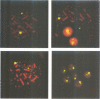
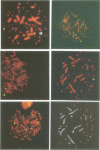
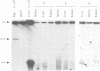
Marek's disease virus (MDV), a lymphotropic herpesvirus, induces T-cell lymphomas in chicken, its natural host. The lymphoma cells are latently infected with MDV but the viral contribution to the transformed phenotype is not understood. To investigate the virus-cell interaction, we focused on the status of MDV in the transformed cells. By the use of highly sensitive fluorescent in situ hybridization with metaphase chromosomes, we found (i) MDV DNA to be randomly integrated at multiple sites in the chromosomes of primary lymphoma cells from chicken tissues; (ii) extrachromosomal, circular MDV genomes were absent and linear virion DNA was usually not detectable in the latently infected lymphoma cells; (iii) the pattern of integration sites revealed the clonal origin of the tumour cells; which (iv) was retained in in vitro established cell lines derived from primary lymphomas; (v) activation of the lytic phase of MDV's life cycle occurred in vitro suggesting that MDV can escape from its integrated status by an unknown mechanism.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anvret M., Karlsson A., Bjursell G. Evidence for integrated EBV genomes in Raji cellular DNA. Nucleic Acids Res. 1984 Jan 25;12(2):1149–1161. doi: 10.1093/nar/12.2.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baichwal V. R., Sugden B. Latency comes of age for herpesviruses. Cell. 1988 Mar 25;52(6):787–789. doi: 10.1016/0092-8674(88)90419-9. [DOI] [PubMed] [Google Scholar]
- Bradley G., Hayashi M., Lancz G., Tanaka A., Nonoyama M. Structure of the Marek's disease virus BamHI-H gene family: genes of putative importance for tumor induction. J Virol. 1989 Jun;63(6):2534–2542. doi: 10.1128/jvi.63.6.2534-2542.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calnek B. W. Influence of age at exposure on the pathogenesis of Marek's disease. J Natl Cancer Inst. 1973 Sep;51(3):929–939. doi: 10.1093/jnci/51.3.929. [DOI] [PubMed] [Google Scholar]
- Calnek B. W. Marek's disease--a model for herpesvirus oncology. Crit Rev Microbiol. 1986;12(4):293–320. doi: 10.3109/10408418509104432. [DOI] [PubMed] [Google Scholar]
- Calnek B. W., Schat K. A., Ross L. J., Shek W. R., Chen C. L. Further characterization of Marek's disease virus-infected lymphocytes. I. In vivo infection. Int J Cancer. 1984 Mar 15;33(3):389–398. doi: 10.1002/ijc.2910330318. [DOI] [PubMed] [Google Scholar]
- Calnek B. W., Schat K. A., Shek W. R., Chen C. L. In vitro infection of lymphocytes with Marek's disease virus. J Natl Cancer Inst. 1982 Sep;69(3):709–713. [PubMed] [Google Scholar]
- Calnek B. W., Shek W. R., Schat K. A. Spontaneous and induced herpesvirus genome expression in Marek's disease tumor cell lines. Infect Immun. 1981 Nov;34(2):483–491. doi: 10.1128/iai.34.2.483-491.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. B., Sondermeijer P. J., Velicer L. F. Identification of a unique Marek's disease virus gene which encodes a 38-kilodalton phosphoprotein and is expressed in both lytically infected cells and latently infected lymphoblastoid tumor cells. J Virol. 1992 Jan;66(1):85–94. doi: 10.1128/jvi.66.1.85-94.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. B., Velicer L. F. Multiple bidirectional initiations and terminations of transcription in the Marek's disease virus long repeat regions. J Virol. 1991 May;65(5):2445–2451. doi: 10.1128/jvi.65.5.2445-2451.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman J., Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delecluse H. J., Bartnizke S., Hammerschmidt W., Bullerdiek J., Bornkamm G. W. Episomal and integrated copies of Epstein-Barr virus coexist in Burkitt lymphoma cell lines. J Virol. 1993 Mar;67(3):1292–1299. doi: 10.1128/jvi.67.3.1292-1299.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delecluse H. J., Hammerschmidt W. Status of Marek's disease virus in established lymphoma cell lines: herpesvirus integration is common. J Virol. 1993 Jan;67(1):82–92. doi: 10.1128/jvi.67.1.82-92.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K., Nazerian K. Induction of Marek's disease virus antigens by IdUrd in a chicken lymphoblastoid cell line. J Gen Virol. 1977 Mar;34(3):413–419. doi: 10.1099/0022-1317-34-3-413. [DOI] [PubMed] [Google Scholar]
- Gardella T., Medveczky P., Sairenji T., Mulder C. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J Virol. 1984 Apr;50(1):248–254. doi: 10.1128/jvi.50.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley E. A., Agger S., McNeil J. A., Lawrence J. B., Calendar A., Lenoir G., Thorley-Lawson D. A. When Epstein-Barr virus persistently infects B-cell lines, it frequently integrates. J Virol. 1991 Mar;65(3):1245–1254. doi: 10.1128/jvi.65.3.1245-1254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isfort R., Jones D., Kost R., Witter R., Kung H. J. Retrovirus insertion into herpesvirus in vitro and in vivo. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):991–995. doi: 10.1073/pnas.89.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D., Lee L., Liu J. L., Kung H. J., Tillotson J. K. Marek disease virus encodes a basic-leucine zipper gene resembling the fos/jun oncogenes that is highly expressed in lymphoblastoid tumors. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4042–4046. doi: 10.1073/pnas.89.9.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Falk L., Bjursell G., Adams A., Lindahl T. Human lymphoblastoid cell lines derived from individuals without lymphoproliferative disease contain the same latent forms of Epstein-Barr virus DNA as those found in tumor cells. Int J Cancer. 1977 Aug 15;20(2):173–180. doi: 10.1002/ijc.2910200203. [DOI] [PubMed] [Google Scholar]
- Kawamura M., Hayashi M., Furuichi T., Nonoyama M., Isogai E., Namioka S. The inhibitory effects of oligonucleotides, complementary to Marek's disease virus mRNA transcribed from the BamHI-H region, on the proliferation of transformed lymphoblastoid cells, MDCC-MSB1. J Gen Virol. 1991 May;72(Pt 5):1105–1111. doi: 10.1099/0022-1317-72-5-1105. [DOI] [PubMed] [Google Scholar]
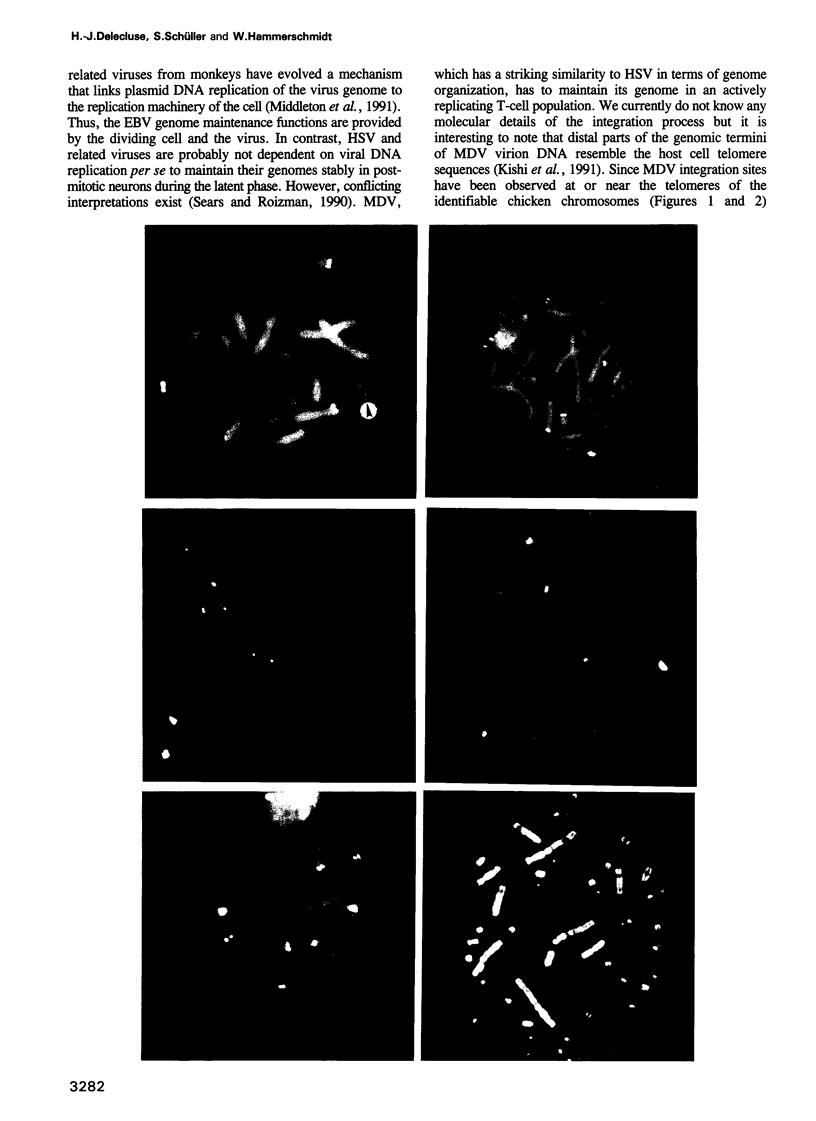
- Kishi M., Bradley G., Jessip J., Tanaka A., Nonoyama M. Inverted repeat regions of Marek's disease virus DNA possess a structure similar to that of the a sequence of herpes simplex virus DNA and contain host cell telomere sequences. J Virol. 1991 Jun;65(6):2791–2797. doi: 10.1128/jvi.65.6.2791-2797.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. B., Villnave C. A., Singer R. H. Sensitive, high-resolution chromatin and chromosome mapping in situ: presence and orientation of two closely integrated copies of EBV in a lymphoma line. Cell. 1988 Jan 15;52(1):51–61. doi: 10.1016/0092-8674(88)90530-2. [DOI] [PubMed] [Google Scholar]
- Li Q. X., Young L. S., Niedobitek G., Dawson C. W., Birkenbach M., Wang F., Rickinson A. B. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature. 1992 Mar 26;356(6367):347–350. doi: 10.1038/356347a0. [DOI] [PubMed] [Google Scholar]
- Lillycrop K. A., Dent C. L., Wheatley S. C., Beech M. N., Ninkina N. N., Wood J. N., Latchman D. S. The octamer-binding protein Oct-2 represses HSV immediate-early genes in cell lines derived from latently infectable sensory neurons. Neuron. 1991 Sep;7(3):381–390. doi: 10.1016/0896-6273(91)90290-g. [DOI] [PubMed] [Google Scholar]
- Matsuo T., Heller M., Petti L., O'Shiro E., Kieff E. Persistence of the entire Epstein-Barr virus genome integrated into human lymphocyte DNA. Science. 1984 Dec 14;226(4680):1322–1325. doi: 10.1126/science.6095452. [DOI] [PubMed] [Google Scholar]
- Middleton T., Gahn T. A., Martin J. M., Sugden B. Immortalizing genes of Epstein-Barr virus. Adv Virus Res. 1991;40:19–55. doi: 10.1016/s0065-3527(08)60276-6. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D. L., Fraser N. W. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature. 1983 Apr 7;302(5908):523–525. doi: 10.1038/302523a0. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Fraser N. W. Latent herpes simplex virus type 1 DNA contains two copies of the virion DNA joint region. J Virol. 1985 Sep;55(3):849–852. doi: 10.1128/jvi.55.3.849-852.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears A. E., Roizman B. Amplification by host cell factors of a sequence contained within the herpes simplex virus 1 genome. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9441–9444. doi: 10.1073/pnas.87.23.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shek W. R., Calnek B. W., Schat K. A., Chen C. H. Characterization of Marek's disease virus-infected lymphocytes: discrimination between cytolytically and latently infected cells. J Natl Cancer Inst. 1983 Mar;70(3):485–491. [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Sugaya K., Bradley G., Nonoyama M., Tanaka A. Latent transcripts of Marek's disease virus are clustered in the short and long repeat regions. J Virol. 1990 Dec;64(12):5773–5782. doi: 10.1128/jvi.64.12.5773-5782.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K., Shimizu N., Sakuma S., Ono Y. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J Virol. 1986 Mar;57(3):1016–1022. doi: 10.1128/jvi.57.3.1016-1022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zur Hausen H., Schulte-Holthausen H. Presence of EB virus nucleic acid homology in a "virus-free" line of Burkitt tumour cells. Nature. 1970 Jul 18;227(5255):245–248. doi: 10.1038/227245a0. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]