Abstract
The development of novel antimicrobial agents is urgently required to curb the widespread emergence of multidrug-resistant bacteria like colistin-resistant Pseudomonas aeruginosa. We previously synthesized a series of amphiphilic neamine derivatives active against bacterial membranes, among which 3′,6-di-O-[(2″-naphthyl)propyl]neamine (3′,6-di2NP), 3′,6-di-O-[(2″-naphthyl)butyl]neamine (3′,6-di2NB), and 3′,6-di-O-nonylneamine (3′,6-diNn) showed high levels of activity and low levels of cytotoxicity (L. Zimmermann et al., J. Med. Chem. 56:7691–7705, 2013). We have now further characterized the activity of these derivatives against colistin-resistant P. aeruginosa and studied their mode of action; specifically, we characterized their ability to interact with lipopolysaccharide (LPS) and to alter the bacterial outer membrane (OM). The three amphiphilic neamine derivatives were active against clinical colistin-resistant strains (MICs, about 2 to 8 μg/ml), The most active one (3′,6-diNn) was bactericidal at its MIC and inhibited biofilm formation at 2-fold its MIC. They cooperatively bound to LPSs, increasing the outer membrane permeability. Grafting long and linear alkyl chains (nonyl) optimized binding to LPS and outer membrane permeabilization. The effects of amphiphilic neamine derivatives on LPS micelles suggest changes in the cross-bridging of lipopolysaccharides and disordering in the hydrophobic core of the micelles. The molecular shape of the 3′,6-dialkyl neamine derivatives induced by the nature of the grafted hydrophobic moieties (naphthylalkyl instead of alkyl) and the flexibility of the hydrophobic moiety are critical for their fluidifying effect and their ability to displace cations bridging LPS. Results from this work could be exploited for the development of new amphiphilic neamine derivatives active against colistin-resistant P. aeruginosa.
INTRODUCTION
The emergence of multidrug-resistant bacteria (1, 2) and the formation of biofilms which evade the host immune response leading to more and more hospital-acquired infections are a major health concern (3–5). The lack of new antibiotics in the drug development pipeline, especially those that have new modes of action and that are active against Gram-negative bacteria, has worsened the situation. In this context, the search for new drugs acting on new targets is a critical challenge.
Cationic amphiphilic drugs are known to act on the bacterial membranes and/or lipopolysaccharide (LPS) of Pseudomonas aeruginosa, a Gram-negative bacterium frequently associated with severe infections in immunocompromised hosts or in patients suffering from cystic fibrosis. Among them, the cyclopeptide colistin (polymyxin E) (6–9) is currently used in the clinic for multiresistant infections, while other families, like cationic steroids (ceragenins [10, 11] or cationic antimicrobial peptides [e.g., magainin, dermaseptin, cecropin] [12–14]) and peptidomimetic compounds (e.g., MSI-78, MSI-594) (15), have also been identified to be antimicrobial agents with potent activity against P. aeruginosa. Due to their mode of action, which implies the formation of pores in the bacterial membranes (16, 17), in vitro resistance to these cationic amphiphiles is rarely observed. However, their use in the clinic is presently limited because of their toxicity, the cost of their synthesis, and, for some of them, their susceptibility to proteolysis (18).
In recent years, several studies have demonstrated the potential of exploiting aminoglycosides for the development of new cationic amphiphilic antimicrobial agents by converting part or all of their amine and hydroxyl functions into alkyl- or aryl-amide and ether groups, respectively (19–24).
The neamine core corresponds to the minimum scaffold necessary for aminoglycosides to bind to 16S rRNA (25, 26). Therefore, we selectively modified neamine at one or more of the hydroxyl functions in order to keep the four amine functions unchanged and potentially partially protonated at physiological pH, which may favor the binding of the molecule to rRNA but also to the negatively charged bacterial membranes.
We synthesized O-mono- and O-polyalkylated neamine derivatives, and among them, amphiphilic 3′,4′,6-tri-O-[(2″-naphthyl)methyl]neamine (3′,4′,6-tri2NM) (Fig. 1) has shown antibacterial activity against both susceptible and resistant Gram-positive and Gram-negative bacteria, such as methicillin-resistant Staphylococcus aureus, vancomycin-resistant Staphylococcus aureus, multiresistant P. aeruginosa, and Escherichia coli strains (27). In contrast to conventional aminoglycosides, this derivative was unable to bind to 16S rRNA in vitro and to inhibit P. aeruginosa protein synthesis (27) but, rather, was able to interact with LPS and induce P. aeruginosa membrane depolarization (28). Both effects suggest that this molecule possesses a novel mode of action related to its amphiphilic character, which allows it to bind to bacterial membranes, leading to their destabilization.
FIG 1.
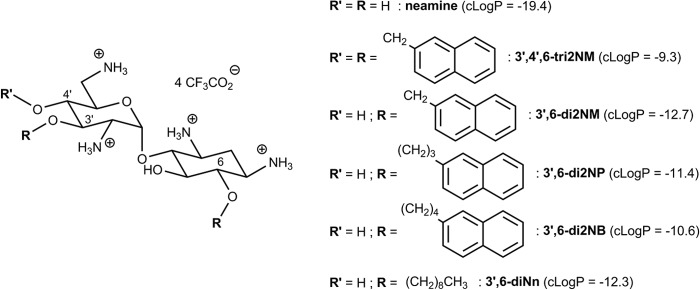
Structures and calculated partition coefficients (cLogP values) of neamine and amphiphilic derivatives.
Unfortunately, 3′,4′,6-tri2NM appeared to be cytotoxic, which was not the case for the less lipophilic dialkylated 3′,6-di-O-[(2″-naphthyl)methyl]neamine (3′,6-di2NM) active against susceptible and resistant S. aureus strains but not against Gram-negative bacteria (28). We therefore synthesized a series of lipophilic dialkylated neamine derivatives with the aim of reducing their affinity for eukaryotic cell membranes and enlarging the spectrum against Gram-negative bacteria (29). We varied the naphthyl neamine spacer from methyl to hexyl groups or shifted to linear alkyl chains as lipophilic groups. This tuning led to the identification of three lead compounds {3′,6-di-O-[(2″-naphthyl)propyl]neamine (3′,6-di2NP), 3′,6-di-O-[(2″-naphthyl)butyl]neamine (3′,6-di2NB), and 3′,6-di-O-nonylneamine (3′,6-diNn)} that are less toxic than 3′,4′,6-tri2NM and that have a broader spectrum of activity than 3′,6-di2NM (29).
The present study had three main objectives. The first one was to characterize the antimicrobial activity of these derivatives against colistin-sensitive and -resistant P. aeruginosa strains. To achieve this, we determined the activity of the new amphiphilic neamine derivatives against colistin-resistant strains of P. aeruginosa and compared it with the activities of gentamicin, a conventional aminoglycoside, and colistin. For the most active derivative, 3′,6-diNn, we also examined its bactericidal effect against planktonic bacteria as well as its effect on biofilm formation.
Our second objective was to better document the mode of action of these derivatives; more specifically, the objective was to determine their ability to interact with LPS and to alter bacterial outer membrane (OM) permeability. To achieve this, we characterized the ability of the 3′,6-dialkyl neamine derivatives to bind to LPS and to permeabilize the OM of P. aeruginosa, using as comparators the parent compound neamine and conventional aminoglycosides (neomycin B and gentamicin). To obtain more insight into the mechanism involved in OM permeabilization and the interactions with LPS, we explored the effect of amphiphilic neamine derivatives on the size, fluidity, and mean molecular area of LPS micelles.
The third objective was to obtain insight into the structure-activity relationships using compounds for which the naphthyl neamine spacer was increased from a methyl to a hexyl group or shifted to a linear alkyl chain.
Our results show that 3′,6-diNn is active against colistin-resistant strains of P. aeruginosa and impairs biofilm formation. Activity was associated with the self-promoted insertion of the molecule within the lipid A region of the OM outer leaflet in P. aeruginosa, leading to an increase in OM permeability. The naphthylalkyl derivatives (3′,6-di2NP and 3′,6-di2NB) were slightly less effective than the alkyl derivative (3′,6-diNn) in this respect. Studies on LPS micelles highlighted the formation of a fluidic cross-linked supramolecular network between LPS and amphiphilic neamine derivatives. This effect depended upon the structure of the selected amphiphilic neamine derivative (3′,6-di2NM < 3′,6-di2NP ∼ 3′,6-diNn < 3′,6-di2NB). Taking these data as a whole, we suggest that the interaction between these new amphiphilic antibacterials and LPS and the OM permeability that they induce are critical for their antibacterial activity against P. aeruginosa, including colistin-resistant strains.
MATERIALS AND METHODS
Materials.
P. aeruginosa ATCC 27853 was used as a reference strain, and colistin-resistant clinical strains were isolated from cystic fibrosis patients at the Hôpital Erasme (strains PA272, PA307, and PA313; O. Denis, Université Libre de Bruxelles, Brussels, Belgium) and the University Hospital of Besançon, Besançon, France (strain PA2938). LPS from P. aeruginosa (or from a Salmonella enterica serovar Minnesota R595 Re mutant for Langmuir compression isotherms) and N-phenyl-1-naphthylamine (NPN) were supplied from Sigma-Aldrich (St. Louis, MO). Laurdan (6-dodecanoyl-2-dimethyl-aminonaphthalene) and 5-]({4-[4,4-difluoro-5-(2-thienyl)-4-bora-3a,4a-diaza-S-indacene-3-yl]phenoxy}acetyl)amino]pentylamine hydrochloride salt (BODIPY-TR-cadaverine [BC]) were obtained from Molecular Probes (Invitrogen, Carlsbad, CA). Other reagents or antibiotics were purchased from E. Merck AG (Darmstadt, Germany), Sigma-Aldrich-Fluka (Lyon, France), or Acros Organics (Illkirch, France).
Amphiphilic neamine derivative synthesis.
Neamine was obtained as a hydrochloride salt by methanolysis of neomycin B in the presence of a concentrated aqueous solution of hydrochloric acid. The 3′,6-di-O-nonylneamine (3′,6-diNn), 3′,6-di-O-[(2″-naphthyl)methyl]neamine (3′,6-di2NM), 3′,6-di-O-[(2″-naphthyl)propyl]neamine (3′,6-di2NP), 3′,6-di-O-[(2″-naphthyl)butyl]neamine (3′,6-di2NB), and 3′,4′,6-tri-O-[(2″-naphthyl)methyl]neamine (3′,4′,6-tri2NM) derivatives (Fig. 1) were synthesized in three steps from neamine according to our previous reports (27, 29, 30) by (i) tritylation of the four amine functions, (ii) alkylation with 1-bromononane or the corresponding bromonaphthylalkane in dimethyl formamide (DMF) in the presence of NaH or under phase transfer conditions with toluene and a concentrated aqueous solution of sodium hydroxide using tetrabutylammonium iodide or fluoride as the phase transfer agent, and (iii) removal of the trityl protective groups in the presence of trifluoroacetic acid-anisole. All compounds were isolated as tetratrifluoroacetates.
Calculation of partition coefficients (clogP).
The lipophilicity of the selected amphiphilic neamine derivatives was calculated using MarvinSketch software (version 5.11.4, 2012; ChemAxon), as described previously (29).
MIC determination.
All strains were grown overnight at 35°C on Trypticase soy agar (TSA) petri dishes (BD Diagnostics, BD, Franklin Lakes, NJ). MICs were determined by microdilution using a fresh culture in cation-adjusted Mueller-Hinton broth (CA-MHB) and a starting inoculum of 106 cells, according to the recommendations of the Clinical and Laboratory Standards Institute (CLSI) for P. aeruginosa (31).
Bactericidal activity.
About 1 × 105 CFU in 100 μl phosphate-buffered saline (PBS) was incubated for 2 h at 37°C under agitation (130 rpm) with 3′,6-diNn at 1-, 2-, and 5-fold its MIC against P. aeruginosa ATCC 27853. Aliquots of 10 μl were diluted in CA-MHB and spread onto LB agar plates for colony counting.
Gene amplification and sequencing.
Bacterial chromosomal DNA was isolated using commercially available kits (Wizard genomic DNA purification kits; Promega Corporation). PCR amplifications of the genes pmrB, phoQ, parS, parR, cprS, cprR, and colS were performed using specific oligonucleotide primers designed by Primer3web (version 4.0.0) software (see Table S1 in the supplemental material). Amplicons were sequenced on both strands by using a BigDye Terminator kit (PE Applied Biosystems) in an ABI Prism 3100 DNA sequencer (PE Applied Biosystems). The resulting sequences were compared with those deposited in GenBank (www.ncbi.nih.gov/BLAST) and the Pseudomonas genome database (www.pseudomas.com).
Effect on formation of P. aeruginosa biofilm.
The effect of 3′,6-diNn on the formation of a P. aeruginosa ATCC 27853 biofilm was determined by quantifying the biomass using the crystal violet staining method (32), while the viability of bacterial cells was assessed by monitoring the intracellular hydrolysis of fluorescein diacetate (33). A few colonies from an overnight culture on TSA were suspended in CA-MHB to an optical density at 620 nm (OD620) of 0.5, diluted 50-fold with the same medium, and inoculated in 96-well plates physically treated to improve cell adhesion (catalog no. 655180; Greiner Bio-One). 3′,6-diNn diluted in CA-MHB was added to the bacterial suspension at increasing concentrations, and the plates were incubated 24 h at 37°C.
To quantify the biofilm mass, the plates were washed with PBS (200 μl). Biofilms were fixed by addition of 100 μl methanol and incubation for 15 min at room temperature. After removal of the methanol, 100 μl of a 10% crystal violet solution in PBS was added and the mixture was incubated for 20 min at room temperature. The wells were then thoroughly washed with pure water, and crystal violet was solubilized by addition of 200 μl of 66% aqueous acetic acid. After 1 h of incubation at room temperature, the absorbance at 590 nm was read on a SpectraMax M3 microplate reader (Molecular Devices, Sunnyvale, CA). The data were normalized using as references the absorbances of the control (no antibiotic added; 100%) and of the medium (no bacteria added; 0%). For assessment of bacterial viability, the wells were washed with 200 μl of MOPS (morpholinepropanesulfonic acid) buffer (0.14 M MOPS, 0.1 M NaCl, pH 7.4) and filled with 100 μl of the same buffer and 100 μl of a solution consisting of 0.2 mg/ml fluorescein diacetate in MOPS buffer. After 1 h of incubation at 37°C in the dark, the fluorescence was measured on a SpectraMax M3 microplate reader, with excitation and emission wavelengths set at 494 nm and 518 nm, respectively, with a monochromator band pass of 5 nm. Values were normalized as described above for the crystal violet assay.
BODIPY-TR-cadaverine displacement.
Binding to the lipid A region of LPS was determined using the BODIPY-TR-cadaverine displacement assay (34, 35), in which the probe bound to cell-free LPS is self-quenched but fluoresces when released in solution. Stock solutions of BODIPY-TR-cadaverine (500 μM) and LPS from P. aeruginosa (5 mg/ml) were prepared by dissolution in Tris buffer (50 mM, pH 7.4) (the Tris buffer was supplemented with 0.5% methanol for the former).
The assays were performed in 96-well black plates (OptiPlateTM-96F; PerkinElmer Ltd., Beaconsfield, United Kingdom). No interference with the emission of BODIPY-TR-cadaverine was detected in the presence of the 3′,6-dialkyl neamine derivatives. Stock solutions of BODIPY-TR-cadaverine and LPS were mixed in a final volume of 20 ml of Tris buffer to reach final concentrations of 5 μM and 10 μg/ml, respectively, and kept in the dark at room temperature for 4 h. Fifty microliters of antibacterials in Tris buffer and 50 μl of the LPS-probe mixture (final concentrations, 5 μg/ml of LPS, 2.5 μM BODIPY-TR-cadaverine) were added to the wells of the plates. The plate was kept for 1 h in the dark at room temperature until equilibration, and fluorescence was measured on a SpectraMax Gemini XS microplate spectrofluorimeter using excitation and emission wavelengths of 580 nm and 620 nm, respectively, with a monochromator band pass of 5 nm. A Hill equation was fitted to the data, as follows: , where Y is the probe displacement for a concentration C of the investigated molecule, Ymin is the minimal value of Y corresponding to no displacement of the probe, Ymax is the maximal value of Y corresponding to the maximal displacement of the probe, EC50 is the midpoint of the curve corresponding to half displacement of the probe for a symmetrical sigmoid, and n is the Hill coefficient, which gives the cooperativity of the process (36).
DLS.
The size and size distribution of LPS micelles incubated with the dialkyl neamine derivatives in the presence or absence of Ca2+ were evaluated by dynamic light scattering (DLS). Buffers and water were filtered over an Acrodisc syringe filter (pore size, 0.2 μm) before use. LPS from P. aeruginosa was diluted from a stock solution (5 mg/ml in water) in Tris buffer (10 mM, pH 7.4) to a final concentration of 5 μg/ml. The solution was vigorously stirred for a few minutes and kept overnight at 4°C. Compounds (final concentration, 10 μM) were added to LPS micelles, and after 5 min, DLS measurements were performed at 25°C in polystyrene cuvettes using a Zetasizer Nano SZ instrument (Malvern Instruments, Worcestershire, United Kingdom) equipped with an He-Ne laser and backscattering detection at 173°C. The size distribution was obtained by accumulation of four measurements consisting of 30 successive runs of 10 s each. To highlight the competition between Ca2+ and amphiphilic neamine derivatives and the cross-bridging role of multivalent cations, we determined the effect of Ca2+ on changes in the size distribution induced by EDTA and 3′,6-diNn. EDTA or 3′,6-diNn were added to LPS micelles, and the mixture was incubated with CaCl2 (final concentration, 1 mM) for 5 min. Normalized intensity autocorrelation functions were then analyzed by use of the the CONTIN algorithm of the Zetasizer Nano software supplied with the apparatus (37).
Langmuir studies.
Surface pressure-area (Π-A) compression isotherms were recorded on an automated Langmuir trough (Microtrough X; Kibron, Helsinki, Finland) equipped with a rectangular Teflon trough (width = 5.8 cm, length = 23.1 cm), two hydrophilic Delrin mobile barriers (symmetric compression), a platinum Wilhelmy wire, and a temperature probe. The system was enclosed in a Plexiglas box, and the temperature was maintained at 24.8 ± 0.7°C.
The cleanliness of the surface was checked by recording a compression isotherm of the pure subphase before each run. The platinum wire was cleaned by rinsing with isopropanol and heating to a red glow between two experiments. Tris HCl buffer at 10 mM and pH 7.4 in the presence or in the absence of 10 mM CaCl2 was used as the subphase. LPS from S. Minnesota, initially dissolved at a concentration of 0.5 mg/ml in CHCl3-methanol (2:1, vol/vol), was spread dropwise at the surface with a microsyringe (Hamilton). After an equilibration time of 15 min, the film was compressed at a rate of 5 mm min−1. In experiments with 3′,6-diNn, this compound was solubilized into the subphase at 0.1 μM before spreading of the LPS. Each compression isotherm was repeated at least two times, and the relative standard deviation (SD) in surface pressure and area was found to be ≤5%.
Laurdan generalized polarization.
The effect of 3′,6-dialkyl neamine derivatives on the packing of LPS micelles was further investigated by determining generalized polarization (GP) (38, 39). LPS from P. aeruginosa was diluted from a stock solution (5 mg/ml in water) in Tris buffer (10 mM, pH 7.4) to a final concentration of 5 μg/ml. Laurdan was added from a stock solution (50 μM in DMF) to a final concentration of 5 nM. The mixture was vigorously stirred, incubated at 37°C in the dark for 1 h, and kept at 4°C overnight. We checked by DLS that laurdan incorporation did not affect the size distribution of LPS micelles. One milliliter of sample was placed in a 1-ml quartz cuvette, and the fluorescence emission spectrum of laurdan between 350 and 570 nm (slit 10 nm) was recorded at 25°C on an LS55 spectrofluorimeter (PerkinElmer Ltd., Beaconsfield, United Kingdom) by 5 accumulated scans (500 nm per minute) with an excitation wavelength of 340 nm (slit width, 10 nm). An aliquot (10 μl) of concentrated test compound in Tris buffer was then added to obtain a final concentration of 10 μM, and the spectrum was collected after 10 min. The steady-state fluorescence parameter known as the excitation generalized polarization (GPex) quantitatively relates the spectral changes of laurdan in relation to its local environment and packing, by taking into account the relative fluorescence intensities of the blue and red edge regions of the emission spectra. GPex was calculated by the following equation: (I440 − I490)/(I440 + I490), where I440 and I490 are the fluorescence intensities measured from the spectra at 440 nm (ordered phase) and 490 nm (disordered phase), respectively.
Fluorescence of NPN.
The fluorescence of N-phenyl-1-naphthylamine (NPN) is highly dependent on the polarity of the solvent. When the barrier properties of the OM in Gram-negative bacteria are compromised, NPN is accumulated into the hydrophobic core of the OM and the fluorescence intensity is increased (40–42). P. aeruginosa ATCC 27853 was grown on TSA petri dishes overnight at 37°C. One colony was suspended in CA-MHB and incubated overnight at 37°C on a rotary shaker (130 rpm). The bacterial suspension was diluted 10-fold in CA-MHB and incubated (300 rpm, 37°C, 3 h) until it reached the end of the mid-logarithmic phase (OD620, ∼0.7 to 0.8). Bacteria were collected by centrifugation (3,000 × g, 20°C, 10 min), washed with HEPES buffer (5 mM, pH 7.4), centrifuged again, and resuspended in HEPES buffer to an OD600 of 0.50. The bacterial suspension can be stored for 3 to 4 h at room temperature without a change of the optical density. For each experiment, 10 μl of a 1 mM stock solution of NPN in acetone was added to 1 ml of bacterial suspension in a quartz cuvette, yielding an NPN concentration of 10 μM. Aliquots of concentrated solutions of test compound in pure water (1 to 5 mM) were added to reach a final concentration of 10 μM, and the fluorescence spectrum between 350 nm and 550 nm (slit, 2.5 nm) was recorded after 5 min at 37°C by 5 accumulated scans (500 nm per min) on an LS55 spectrofluorimeter (PerkinElmer Ltd., Beaconsfield, United Kingdom), using an excitation wavelength of 350 nm (slit width, 2.5 nm). The spectrum for a blank was collected and subtracted from the spectra collected after addition of test compounds. The fluorescence intensity of NPN in the presence of 10 μM colistin was used as a positive control for normalizing the results obtained with 3′,6-dialkyl neamine derivatives as a percentage of NPN uptake (43). We checked that the tested compounds did not modify the pH of the medium or the emission of NPN.
For investigating the role of metal cations bridging LPS molecules and their substitution by amphiphilic neamine derivatives, the fluorescence spectra of an NPN-containing bacterial suspension supplemented with 10 mM CaCl2 or MgCl2 (the concentration was determined to be that at which the cation effects were maximal) were recorded and compared to those obtained after addition of amphiphilic neamine derivatives in the same medium (only data obtained with CaCl2 are presented, as those obtained with MgCl2 were similar). We checked that addition of metal cations did not affect the spectral properties of NPN and the absence of NPN excretion during the time course of measurements by using 1 mM NaN3, known to limit cellular energy metabolism (42).
Statistical analysis.
All statistical analyses were performed with GraphPad InStat (version 3.06) software for Windows (GraphPad Prism Software, San Diego, CA). The significance of the differences between two sets of data was tested using two-way analysis of variance (ANOVA), followed by Bonferroni's posttest. Paired data were compared using repeated-measures ANOVA.
RESULTS
Antibacterial activity of 3′,6-dialkyl neamine derivatives against colistin-resistant P. aeruginosa strains.
Gentamicin and colistin showed MICs equal to 1 μg/ml against P. aeruginosa ATCC 27853, as previously described (44). The activity of the amphiphilic derivatives (3′,6-di2NP, 3′,6-di2NB, 3′,6-di2Nn, 3′,4′,6-tri2NM) appeared to be lower (range, 4 to 8 μg/ml). As previously reported (28), the neamine parent compound and 3′,6-di2NM were inactive (Table 1).
TABLE 1.
MICs of amphiphilic neamine derivatives against wild-type and colistin-resistant P. aeruginosa strains in comparison with those of gentamicin, neamine, and colistin
| Antibiotic | MIC (μg/ml) for the following P. aeruginosa strainsa: |
||||
|---|---|---|---|---|---|
| ATCC 27853 | Clinical strains resistant to colistin |
||||
| PA2938 | PA272 | PA307 | PA313 | ||
| Colistin | 1 | 64 | 64 | 64 | 32 |
| Gentamicin | 1 | 32 | 32 | 32 | 8 |
| Neamine | >128 | >128 | >128 | >128 | >128 |
| 3′,6-di2NM | 128 | 32 | 32 | 64 | 64 |
| 3′,6-di2NP | 8 | 4 | 8 | 8 | 8 |
| 3′,6-di2NB | 4 | 4 | 8 | 4 | 4 |
| 3′,4′,6-tri2NM | 8 | 8 | 32 | 16 | 4 |
| 3′,6-diNn | 4 | 2 | 4 | 2 | 2 |
CLSI (31) susceptibility breakpoints are >8 mg/liter for colistin resistance and >16 mg/liter for gentamicin resistance.
Focusing on colistin-resistant strains of P. aeruginosa, 3′,6-di2NB, 3′,6-di2NP, 3′,6-diNn, and 3′,4′,6-tri2NM displayed useful activity, with the MICs of most compounds ranging from 2 to 8 μg/ml and with 3′,6-diNn systematically showing the lowest MIC values. In contrast, gentamicin, neamine, and 3′,6-di2NM were inactive against these strains, except for gentamicin against PA313 (MIC, 8 μg/ml) (Table 1).
Acquired resistance to colistin in this species is associated with the overexpression of a large operon named arnCDATEF-pmrE, which is responsible for the addition of aminoarabinose molecules to lipid A. Its expression is regulated by at least four two-component regulatory systems (PmrAB, PhoPQ, ParRS, and CprRS) (45, 46). Since mutations in the genes phoQ, pmrB, parS, parR, colS, cprS, and cprR were previously identified to contribute to polymyxin resistance in P. aeruginosa clinical strains, we amplified and sequenced them in the four CF clinical strains selected (45–47). Sequences analysis (Table 2) revealed that all the strains harbored the amino acid substitution Tyr345His in the PmrB protein, which was also identified in many susceptible strains, such as strains PA14, PACS2 2192, and C3719. Additional mutations in the proteins PmrB (Asp47Asn, Val28Ala, Val28Ala, and Leu162Pro in strains PA2938, PA272, PA307, and PA313, respectively) and PhoQ (Ala23Val in all four strains) which explain the levels of resistance of the strains to colistin were identified. Of note, strain PA2938 also exhibited a mutation in the ParS protein (His398Arg), indicating that several mutations were accumulated in these strains.
TABLE 2.
Mutations identified in colistin-resistant strains
| Strain | Nucleotide (amino acid) mutation(s) |
||||||
|---|---|---|---|---|---|---|---|
| phoQ | pmrB | parS | parR | colS | cprS | cprR | |
| PA2938 | C68T (Ala23Val) | G139A (Asp47Asn), T1033C (Tyr345His) | G1245A (His398Arg) | − | − | − | − |
| PA272 | C68T (Ala23Val) | T83C (Val28Ala) T1033C (Tyr345His) | − | − | − | − | − |
| PA307 | C68T (Ala23Val) | T83C (Val28Ala) T1033C (Tyr345His) | − | − | − | − | − |
| PA313 | C68T (Ala23Val) | T485C (Leu162Pro) T1033C (Tyr345His) | − | − | − | − | − |
Bactericidal activity of 3′,6-dinonyl neamine in broth and effect on the formation of P. aeruginosa biofilm.
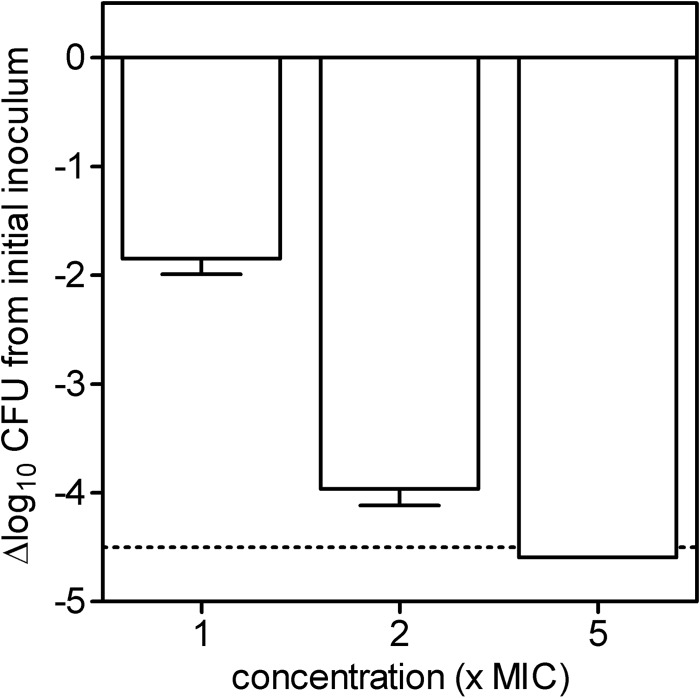
The capacity of increasing concentrations of the most active compound, 3′,6-diNn, to kill P. aeruginosa ATCC 27853 was then examined (Fig. 2). After 2 h, the decrease in the number of CFU (log scale) per milliliter reached 2, 4, and 5 at 1-, 2-, and 5-fold the MIC, respectively. At 2-fold the MIC, gentamicin also showed bactericidal activity.
FIG 2.
Bacterial killing of P. aeruginosa ATCC 27853 by 3′,6-diNn at different concentrations expressed as the multiple of the MIC. The ordinate shows the change in the number of CFU (log scale) per milliliter. The solid horizontal line corresponds to a bacteriostatic effect (no change from the initial inoculum), and the dotted horizontal line shows the limit of detection (4.5-log-CFU decrease). Values are means ± standard deviations (n = 3); when not visible, error bars are smaller than the symbols.
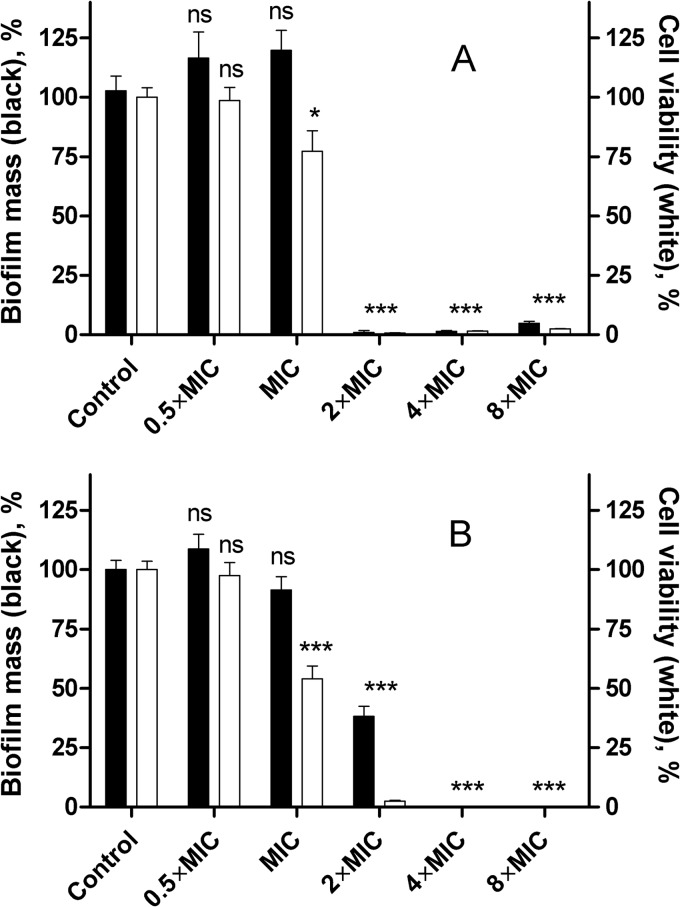
Because P. aeruginosa is also able to evade the host defense by growing as a biofilm, we examined whether 3′,6-diNn was able to prevent biofilm formation (Fig. 3A). The amphiphilic neamine derivative was able to totally prevent biofilm formation at 2-fold its MIC, as assessed by crystal violet staining, which could be explained by its drastic effect on viability and/or by the effect on the bacterial surface-associated functions of LPS. Similar effects were observed for gentamicin (Fig. 3B).
FIG 3.
Inhibition of biofilm formation of P. aeruginosa ATCC 27853 incubated with 3′,6-diNn (A) or gentamicin (B) at different concentrations expressed as a multiple of their corresponding MICs. biofilm mass (closed bars), assessed by the crystal violet method, and cell viability (open bars), assessed by the fluorescein diacetate method, were monitored. Data are means ± SDs of three independent experiments. ns, not significant; *, P < 0.05 compared to the control; ***, P < 0.001 compared to the control.
Binding of 3′,6-dialkyl neamine derivatives to LPS.
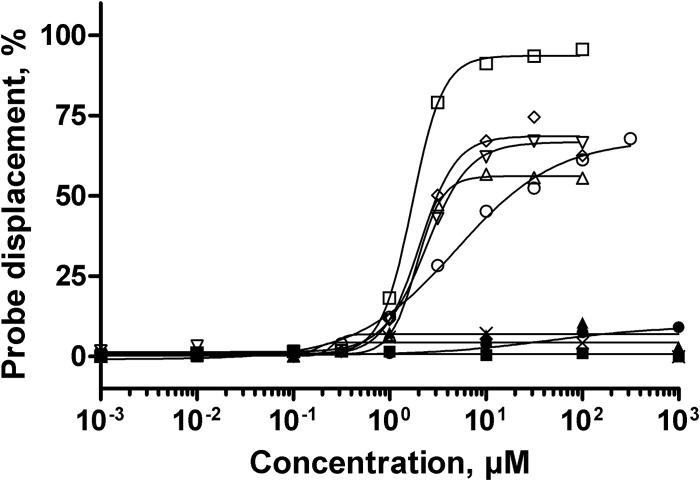
To go further into the mechanism involved in the activity of 3′,6-dialkyl neamine derivatives, we compared their ability to interact with LPS to that of 3′,4′,6-tri2NM previously described (28). Other comparators were selected: conventional aminoglycosides (gentamicin and neomycin B); the parent compound (neamine); and imipenem, a carbapenem displaying antibacterial activity by inhibition of peptidoglycan synthesis, which was used as a negative control (48) (Fig. 4). As expected, imipenem did not displace BODIPY-TR-cadaverine from its binding to LPS. Regardless of their concentrations, neamine, gentamicin, and neomycin B induced only slight probe displacement (lower than 5%), reflecting their low capacity to bind LPS in the lipid A region. In contrast, 3′,6-dialkyl neamine derivatives induced a dose-dependent displacement of the probe. This effect was more important for 3′,6-diNn (94% displacement) than for the derivatives 3′,6-di2NM, 3′,6-di2NP, and 3′,6-diNB (68%) and for the trisubstituted derivative (3′,4′,6-tri2NM) (56%). With respect to relative potencies, 3′,6-diNn appeared to be the most potent compound and 3′,6-di2NM was the least potent, with 3′,6-di2NP and 3′,6-diNB showing intermediate values close to the value observed for 3′,4′,6-tri2NM. Moreover, the Hill coefficient was greater than 1 for 3′,6-diNn, 3′,6-di2NB, 3′,6-di2NP, and 3′,4′,6-tri2NM and lower than 1 for 3′,6-di2NM (Table 3; Fig. 4).
FIG 4.
Relative affinity of 3′,6-dialkyl neamine derivatives for smooth LPS from P. aeruginosa. The dose-dependent displacement of the fluorescent probe BODIPY-TR-cadaverine from LPS induced by 3′,6-di2NM (○), 3′,6-di2NP (▽), 3′,6-di2NB (◇), and 3′,6-diNn (□) in comparison with that induced by 3′,4′,6-tri2NM (△), neamine (●), gentamicin (×), neomycin B (▲), and imipenem (◼) is shown. Fluorescence intensities were normalized as the percentages of probe displacement compared to the probe displacement induced by colistin, and a Hill equation was fitted to the data. Error bars are omitted for the sake of clarity (SDs, ∼5%).
TABLE 3.
Characteristic LPS-binding parametersa of amphiphilic neamine derivatives obtained from Hill regression of the data given in Fig. 4a
| Compound | EC50 (μM) | n (a.u.) | Ymax (%) |
|---|---|---|---|
| 3′,6-di2NM | 4.9 | 0.8 | 68 |
| 3′,6-di2NP | 2.4 | 1.9 | 67 |
| 3′,6-di2NB | 2.1 | 2.3 | 68 |
| 3′,6-diNn | 1.7 | 2.7 | 94 |
| 3′,4′,6-tri2NM | 1.9 | 3.2 | 56 |
EC50, concentration for half probe displacement; n, Hill coefficient; a.u., arbitrary units; Ymax, maximal probe displacement.
Effect of 3′,6-dialkyl neamine derivatives on the size and size distribution of LPS micelles.
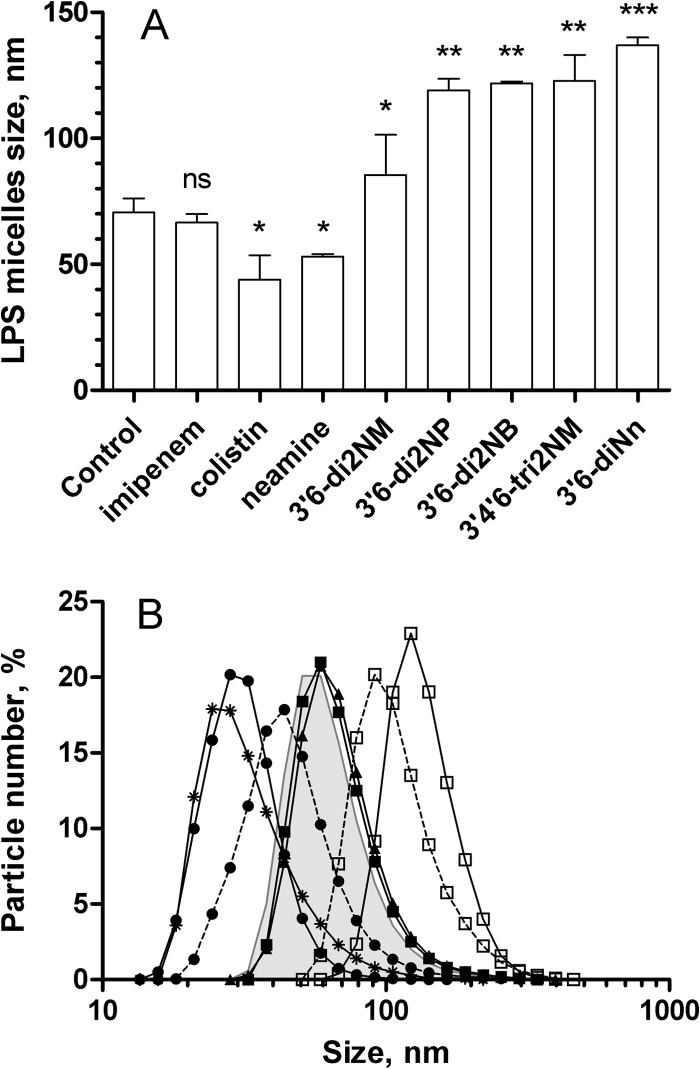
To give more insight into the consequences of the interactions between amphiphilic neamine derivatives and LPS, we determined by DLS the changes in the size of the LPS micelles that the compounds induced (Fig. 5A). Imipenem did not change the size distribution of LPS micelles (Fig. 5A). In contrast, neamine and colistin decreased the average diameter of LPS micelles, whereas the 3′,6-dialkyl neamine derivatives increased it (Fig. 5A). The effect observed with 3′,6-diNn was not significantly different from the effect induced by 3′,6-di2NP, 3′,6-di2NB, and 3′,4′,6-tri2NM. The effect induced by 3′,6-di2NM was slightly weaker than that observed with, e.g., 3′,6-diNn (P < 0.01).
FIG 5.
Effect of 3′,6-dialkyl neamine derivatives on the micelle size distribution of LPS from P. aeruginosa in solution (10 mM Tris HCl buffer, pH = 7.4). (A) Average diameter of LPS micelles in the presence of imipenem, colistin, neamine, and amphiphilic neamine derivatives. All compounds were added to a final concentration of 10 μM. ns, not significant; *, P < 0.05 compared to the control; **, P < 0.01 compared to the control; ***, P < 0.001 compared to the control. (B) Size distribution of LPS micelles (5 μg/ml) dispersed in buffer (gray surface) and after addition of 10 μM 3′,6-diNn (□, solid line), 1 mM EDTA (●, solid line), or 1 mM Ca2+ (▲, solid line). The combined effect of 1 mM Ca2+, 1 mM EDTA (●, dotted line) or Ca2+ 5 min later, and then 10 μM 3′,6-diNn (□, dotted line) 5 min after that is shown. Data are the means of three independent experiments. Error bars are omitted for clarity (SDs, ∼2%).
To test the hypothesis that an effect on the size of LPS micelles may result from reduced LPS-to-LPS interaction through possible displacement of divalent cations, we examined the effect of Ca2+ on the size distribution of LPS micelles in the absence and in the presence of 3′,6-dialkyl neamine derivatives (Fig. 5B). When EDTA (a calcium chelator) was added to LPS, a decrease in the mean diameter of LPS micelles was observed. When EDTA was added to micelles incubated with Ca2+, the effect of the micelle disrupter EDTA was partially countered by Ca2+, supporting the suggestion that EDTA reduces lateral cohesion between LPS molecules by disruption of Ca2+-mediated bridges. When 3′,6-diNn was added under the same conditions, Ca2+ partially prevented the increase in micelle diameter induced by the amphiphilic neamine derivative, supporting a competition between Ca2+ and 3′,6-diNn (Fig. 5B). Interestingly and regardless of the presence of Ca2+, the polydispersity index, which is an indication of the homogeneity of the size distribution, was slightly lower when amphiphilic neamine derivatives were added (data not shown).
Effect of 3′,6-dinonyl neamine on the molecular area of LPS and packing of LPS micelles.
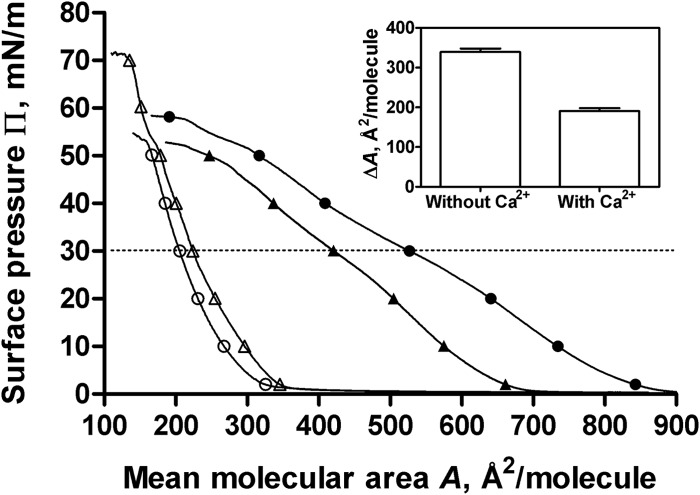
To go further into the characterization of the interaction of 3′,6-diNn with LPS micelles, we explored its effect on the packing of LPS micelles by determining the Langmuir compression isotherms and the generalized polarization (GP) using laurdan fluorescence. The compression isotherms of rough mutant LPS monolayers spread on the subphase containing 3′,6-diNn shifted to molecular areas higher than those for the pure buffer subphase, indicating an insertion of 3′,6-diNn into the LPS monolayer (Fig. 6). Moreover, the decrease in the compressibility modulus [C (s−1), where C is equal to −A(dΠ/dA)] when 3′,6-diNn was present (from 85.5 ± 4 to 39.7 ± 0.3) indicates that 3′,6-diNn neamine modifies the LPS film state into a more liquid-like one.
FIG 6.
Surface pressure (Π)-area (A) compression isotherms of rough mutant LPS Langmuir monolayers spread on buffer (10 mM Tris HCl, pH 7.4, 25°C) in the absence (open symbols) or in the presence (close symbols) of 0.1 μM 3′,6-diNn. Results obtained in the absence (○, ●) or in the presence (△, ▲) of 2 mM CaCl2 are also shown. Dotted line, Π equal to 30 mN/m. (Inset) Increase in the area by induced 3′,6-diNn at Π equal to 30 mN/m in the absence or in the presence of 2 mM CaCl2. Data are the means of three independent experiments.
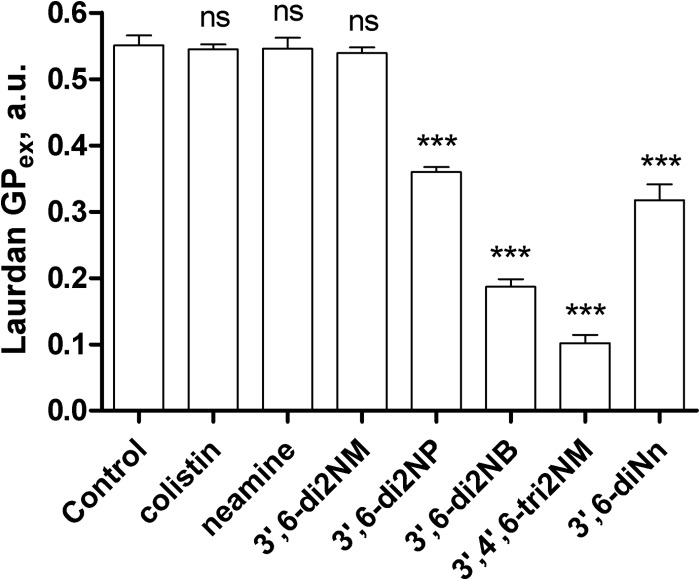
This effect is in agreement with data from laurdan fluorescence of LPS-laurdan mixed micelles. The GPex value decreased significantly (3′6-di2NP < 3′,6-di-2NB < 3′,4′,6-tri2NM), indicating an increasing liquid-like state in the lipid A region of LPS. The effect induced by 3′,6-diNn was not significantly different (P > 0.05) from that observed with 3′,6-di2NP. In contrast, colistin, neamine, and the 3′,6-di2NM derivative had no influence on the packing of lipid A in micelles, as indicated by unchanged GPex values compared to the value for the control (Fig. 7).
FIG 7.
Excitation generalized polarization (GPex) of laurdan fluorescence incorporated into LPS micelles (5 μg/ml 0.3%, wt/wt, laurdan) in the absence (Control) or in the presence of colistin, neamine, or amphiphilic derivatives. All compounds were added to a final concentration of 10 μM. Data are means ± SDs of three independent experiments. ns, not significant; ***, highly significant difference (P < 0.005) compared to the control. The result for 3′,6-di2NP was not significantly different from that for 3′,6-diNn. a.u., arbitrary units.
By determining Langmuir isotherm compression when CaCl2 was present in the subphase, we confirmed the role of Ca2+ on the effect induced by 3′,6-diNn. Addition of 3′,6-diNn in the presence of Ca2+ gave rise to an effect on the molecular area weaker than the effect obtained in the absence of Ca2+, suggesting that CaCl2 reduced the insertion of the 3′,6-diNn neamine derivative.
Effect of 3′,6-dialkyl neamine derivatives on outer membrane stability.
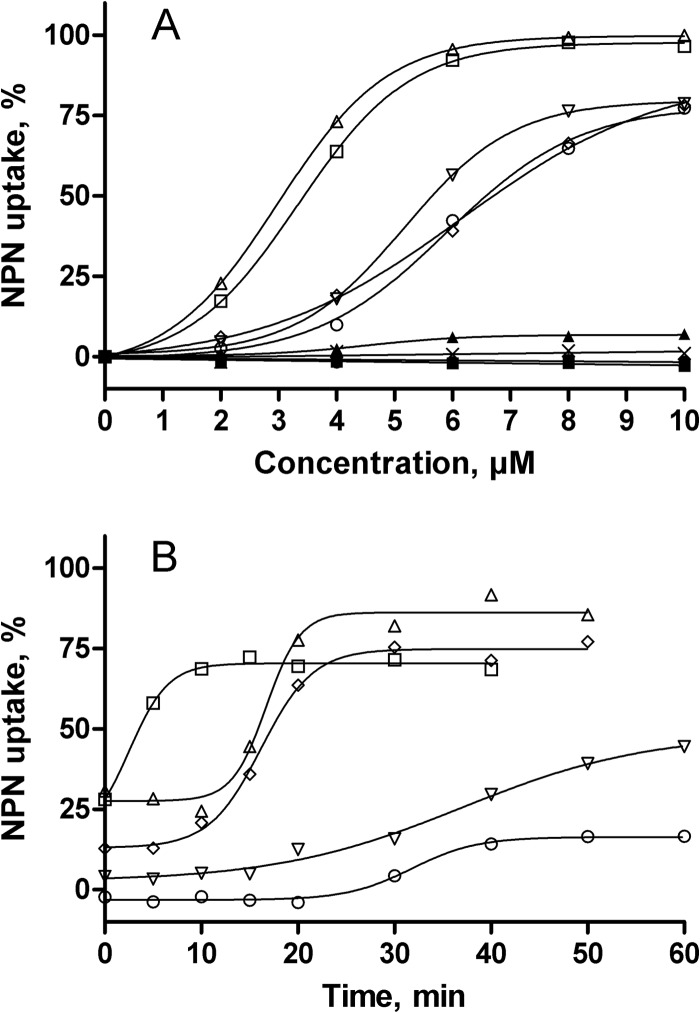
To assess the impact of the interactions between amphiphilic neamine derivatives and LPS on the OM barrier properties, we followed the uptake of NPN in the outer membrane of living P. aeruginosa cells. Again, imipenem was used as a negative control. Figure 8A shows the uptake of NPN in the membrane of bacteria exposed to increasing concentrations of the compounds. Gentamicin, neomycin B, and neamine induced slight or no changes in the OM barrier properties, as indicated by an NPN uptake that did not exceed 10%. The amphiphilic neamine derivatives induced a concentration-dependent NPN uptake within the OM. The 3′,6-diNn and 3′,4′,6-tri2NM neamine derivatives were the most efficient and the most potent, inducing NPN uptake of close to 100% at 5 μM, while 3′,6-di2NP, 3′,6-di2NB, and 3′,6-diNM caused only 70% uptake when concentrations reached at least 8 μM.
FIG 8.
Effect of 3′,6-dialkyl neamine derivatives on the OM permeability in living P. aeruginosa ATCC 27853 cells assessed by the increase in the fluorescence of NPN. (A) Dose-dependent NPN uptake within the bacterial OM expressed as a percentage of the maximal value recorded in the presence of 10 μM colistin, used as a positive control. Results obtained with 10 μM 3′,6-di2NM (○), 3′,6-di2NP (▽), 3′,6-di2NB (◇), and 3′,6-diNn (□) in comparison with those obtained with 3′,4′,6-tri2NM (△), neamine (●), gentamicin (×), neomycin B (▲), and imipenem (◼) are shown. Data are the means of three independent experiments. Error bars are omitted for clarity (SDs, ∼3%). (B) Time-dependent NPN uptake in the presence of Ca2+ (10 mM), expressed as a percentage of the values recorded in the absence of metal cations. Results were obtained with 10 μM 3′,6-di2NM (○), 3′,6-di2NP (▽), 3′,6-di2NB (◇), 3′,6-diNn (□), and 3′,4′,6-tri2NM (△).Data are the means of three different experiments. Error bars are omitted for clarity (SDs, ∼4%).
Based on the effect of Ca2+ on changes in the average diameter of LPS micelles and the Langmuir compression isotherms induced by 3′,6-diNn, we repeated these experiments in the presence of an excess of Ca2+ (Fig. 8B). As expected, the NPN uptake was reduced under these conditions and recovery was observed in a time-dependent fashion. Figure 8B shows the time course of NPN uptake in the presence of Ca2+ and amphiphilic neamine derivatives, expressed as the percentage of NPN uptake recovery compared to that observed under conditions where Ca2+ was omitted. In the presence of Ca2+, at time zero, the NPN uptake induced by derivatives was strongly reduced, reaching 25% (3′,6-diNn or 3′,4′,6-tri2NM), 10% (3′,6-di2NB), and almost 0% (3′,6-di2NP and 3′,6-di2NM) of the NPN uptake observed without Ca2+. The recovery was immediate and almost complete (75%) for 3′,6-diNn (lag time, 10 min), reached about 75 to 80% for 3′,4′,6-tri2NM or 3′,6-di2NB, and was very progressive and incomplete (<50%) for 3′,6-di2NP and 3′,6-di2NM, suggesting that the increase in OM permeability induced by amphiphilic neamine derivatives involves the substitution of divalent cations in the LPS layer of the OM.
DISCUSSION
LPS-LPS interactions, together with the bridging of the proximal negatively charged functional groups by metal ions like Ca2+ and Mg2+, are responsible for the barrier impermeability of bacteria. The outer membrane of Pseudomonas is considered particularly impermeable due to the unusual tight packing of acyl chains. In the search for new antibiotics active on bacterial membranes, we synthesized a library of more than 60 amphiphilic neamine derivatives (27, 29, 30). Three 3′,6-dialkyl neamine derivatives (3′,6-diNn, 3′,6-di2NP, and 3′,6-di2NB) were shown to be active against Gram-positive and Gram-negative strains and to have low cytotoxicity (29). The present study shows that these compounds are active against colistin-resistant P. aeruginosa strains and that their activity is most probably related to their capacity to interact with the LPS of the outer membrane of P. aeruginosa.
We demonstrated the importance of the electrostatic interactions with phosphate moieties of LPS (as evidenced by the effect of metal divalent cations on OM permeabilization and on the packing of LPS micelles) as well as the hydrophobic interactions between lipophilic groups of amphiphilic neamine derivatives and alkyl chains of lipid A of LPS (as shown by changes in the organization of LPS molecules and the increase in LPS micelle fluidity). The interactions of amphiphilic neamine derivatives with LPS could explain their antibacterial activity, especially against colistin-resistant strains of P. aeruginosa (Fig. 9). Indeed, the latter are characterized by a highly ordered state of LPS, the reduction of the net negative charge of lipid A (49), and the presence of an additional fatty acyl chain on lipid A (9). In contrast to the findings for colistin, the 3′,6-diNn would be able to insert within a more ordered network of LPS due to its high flexibility and its ability to increase the fluidity of LPS. However, LPS binding is probably not sufficient since 3′,6-di2NM interacts with LPS but does not show any antibacterial activity against sensitive and resistant P. aeruginosa strains. The fact that 3′,6-di2NM is characterized by a negative cooperative index regarding binding to LPS and would therefore be unable to self-promote its uptake route (50) could explain the absence of activity of 3′,6-di2NM.
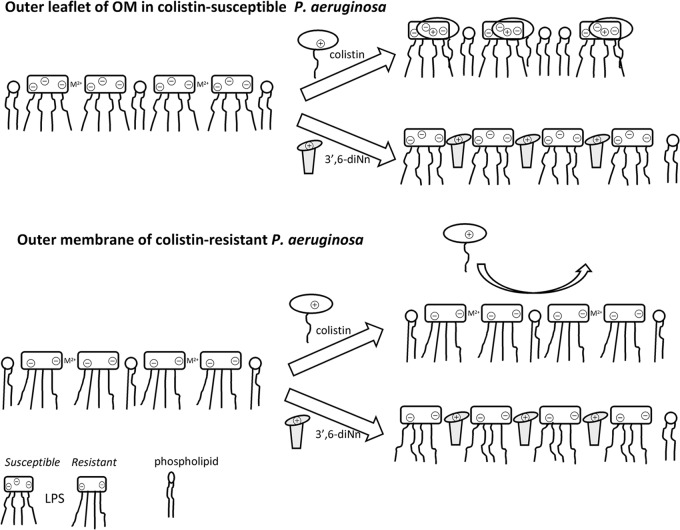
FIG 9.
Hypothesized mechanism of action of 3′,6-diNn against colistin-susceptible (top) and colistin-resistant (bottom) P. aeruginosa strains. (Top) The LPS layer was stabilized by cross-bridging through metal divalent cations (M2+). Insertion of colistin and 3′,6-diNn induces the formation of bimolecular complexes with LPS and disrupts the LPS cross-bridging. (Bottom) The highly ordered LPS layer acts as a barrier to colistin (no insertion), while the flexibility of 3′,6-diNn and its ability to fluidify the LPS network always permit primary interactions with self-promoted insertion.
From a relation-structure-activity point of view, our work afforded data on the structural characteristics of amphiphilic neamine derivatives needed to induce the loss of LPS-LPS interactions and alteration of OM permeability.
First, we evidenced the critical role of the length of the spacer between the hydrophobic tail and the polar core of the amphiphilic neamine core. When the spacer had from one to four carbon atoms (3′,6-diNM, 3′,6-diNP, and 3′,6-diNB), we showed that the effect on the GPex of laurdan inserted in LPS micelles develops in parallel with the length and the log P values of the corresponding derivatives (clogP values, −12.7, −11.4, and −10.6, respectively). The ascending time dependence of the Ca2+ effect in NPN uptake experiments observed from 3′,6-di2NM to 3′,6-di2NP and 3′,6-di2NB supports the suggestion that hydrophobic interactions, as a complement to electrostatic interactions, are required to bind LPS and cause OM destabilization.
Second, we demonstrated the importance of the number of naphthylalkyl groups (2 [3′,6-di2NM] versus 3 [3′,4′,6-tri2NM]) for the binding to LPS and the disordering effect of LPS micelles. Regardless, the amphiphilic dialkyl neamine derivatives studied are bulkier than Ca2+ or Mg2+ and therefore could act as a spacer in the plane of the bilayer, reducing the short-range attractive forces between LPS saccharide cores.
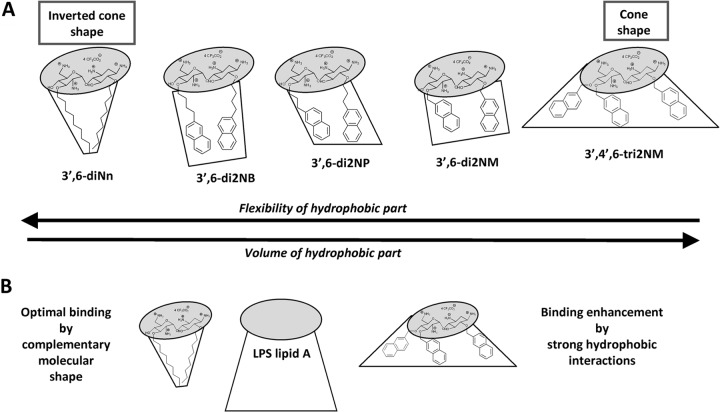
Third, focusing on the nature of the substituent (naphthylalkyl versus alkyl), we demonstrated that long alkyl chains (nonyl) grafted on neamine at positions 3′ and 6 induce the greatest change regarding the binding to LPS and the alteration of OM permeability. 3′,6-diNn is characterized by its flexibility and the narrowness of its alkyl chains (Fig. 10), optimizing insertion in the lipid A region of LPS. In this respect, the effect induced by 3′,6-di2NP was not significantly different from that obtained with 3′,6-diNn. The appearance of fluidic material extends previous data related to the formation of a supramolecular network between multicationic and multianionic substances (51, 52).
FIG 10.
Structure-activity relationship and main parameters (flexibility of hydrophobic moieties, molecular shape) involved in the interactions of amphiphilic neamine derivatives with LPS. Amphiphilic neamine derivatives and LPS are depicted with hydrophilic (gray) and hydrophobic (white) moieties. The complementary molecular shape between 3′,6-diNn and LPS is illustrated at the bottom of the scheme.
When all the data are taken together and when it is assumed that the volume of the hydrophobic part increases from linear dialkyl (3′,6-diNn) to the dinaphthylalkyl (3′,6-di2NM, 3′,6-di2NP, and 3′,6-di2NB) and the bulky trinaphthylalkyl (3′,4′,6-tri2NM) moieties, the maximal binding would be inversely proportional to the volume of the hydrophobic moiety. Both the lipophilicity and the molecular shape of the antibacterial would therefore be critical. More specifically, 3′,6-diNn, which is characterized by a pho/phi balance close to the optimal one (29), shows a molecular shape of an inverted cone with a large hydrophilic part and a small hydrophobic one. This could allow a close interaction between this derivative and the lipid A moiety of the LPS molecule, characterized by a conical complementary shape (with a small hydrophilic part and a large hydrophobic part), as illustrated in Fig. 10B.
Differences between the interactions of 3′,6-di2NP, 3′,6-di2NB, and 3′,6-diNn with LPS and the interaction of colistin with LPS could explain the activity of the amphiphilic neamine derivatives on colistin-resistant P. aeruginosa strains (43, 53). Large amphiphilic neamine derivatives could induce partial Ca2+ and Mg2+ substitutions with the aggregation of several smaller LPS primary particles into supramolecular LPS aggregates (54), as supported by the increase in the average diameter of LPS micelles. On the other hand, the addition of colistin to LPS micelles induces an important shift to a smaller average diameter of 35 ± 7 nm. This effect could be related to the ability to form bimolecular complexes with LPS, breaking supramolecular structures and leading to the dispersion of LPS in solution (55), as supported by the effect of EDTA on the mean diameter of LPS micelles. Similar effects have been reported for other peptides, like a lysine-rich synthetic hybrid magainin and mellitin variants originally designed and synthesized by Genaera Corporation (56) or parodaxin, a 33-residue excitatory fish defense peptide (57). One additional difference between 3′,6-dialkyl neamine derivatives and colistin is their ability to modify the fluidity of the LPS micelles. We did not observe any effect of colistin on fluidity. In contrast, amphiphilic neamine derivatives, which are more flexible than colistin, induced a large increase in membrane fluidity, leading to a facilitated insertion in the lipid bilayer. This extends the results published by others (58, 59) who suggested a critical role for the flexibility of the molecule and disordering, especially against colistin-resistant strains. These are characterized by changes in lipid A structures leading to modifications of hydrophobicity and molecular packing of LPS (60–63).
In conclusion, the observations described here support the proposal of the self-promoted insertion of amphiphilic neamine derivatives within the LPS layer of bacteria driven by electrostatic interactions with anionic LPS and stabilized by hydrophobic interactions. The ability of the amphiphilic neamine derivatives (3′,6-di2NP, 3′,6-di2NB, and 3′,6-diNn) to fluidify LPS could be critical in this respect as well as for their effect on OM permeabilization and their antibacterial activity against colistin-resistant P. aeruginosa. The molecular shape of the antibacterial and the flexibility of the hydrophobic moiety of 3′,6-dialkyl neamine derivatives are critical for their effects. Collectively, the findings from this work could be exploited for the development of new amphiphilic neamine derivatives active against colistin-resistant P. aeruginosa.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Belgian Funds for Scientific Research (Fonds National de la Recherche Scientifique no. 3.4.588.10 and 3.4.578.12), the Region Rhône-Alpes (ARC1, no. 12-00887201), and the Université Joseph Fourier (a fellowship to L.S.M.).
We gratefully acknowledge P. M. Tulkens (UCL/LDRI/FACM, Brussels, Belgium) and K. Lohner (University of Graz, Graz, Austria) for valuable discussions, O. Misir (UCL/LDRI/FACM, Brussels, Belgium), S. Guénard (CNR, Besançon, France), and A. Bollard (CNR, Besançon, France) for technical assistance, and E. Basseres (UCL/LDRI/FACM, Brussels, Belgium) for introducing us to biofilms. We also thank O. Denis (ULB, Brussels, Belgium) and P. Plésiat (Hôpital J. Minjoz, Besançon, France) for the kind gifts of the clinical strains.
Footnotes
Published ahead of print 27 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02536-13.
REFERENCES
- 1.van Duijn PJ, Dautzenberg MJ, Oostdijk EA. 2011. Recent trends in antibiotic resistance in European ICUs. Curr. Opin. Crit. Care 17:658–665. 10.1097/MCC.0b013e32834c9d87 [DOI] [PubMed] [Google Scholar]
- 2.Herzog T, Chromik AM, Uhl W. 2010. Treatment of complicated intra-abdominal infections in the era of multi-drug resistant bacteria. Eur. J. Med. Res. 15:525–532. 10.1186/2047-783X-15-12-525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Rojas A, Oliver A, Blazquez J. 2012. Intrinsic and environmental mutagenesis drive diversification and persistence of Pseudomonas aeruginosa in chronic lung infections. J. Infect. Dis. 205:121–127. 10.1093/infdis/jir690 [DOI] [PubMed] [Google Scholar]
- 4.Ranall MV, Butler MS, Blaskovich MA, Cooper MA. 2012. Resolving biofilm infections: current therapy and drug discovery strategies. Curr. Drug Targets 13:1375–1385. 10.2174/138945012803530251 [DOI] [PubMed] [Google Scholar]
- 5.Obritsch MD, Fish DN, MacLaren R, Jung R. 2005. Nosocomial infections due to multidrug-resistant Pseudomonas aeruginosa: epidemiology and treatment options. Pharmacotherapy 25:1353–1364. 10.1592/phco.2005.25.10.1353 [DOI] [PubMed] [Google Scholar]
- 6.Vaara M. 2013. Novel derivatives of polymyxins. J. Antimicrob. Chemother. 68:1213–1219. 10.1093/jac/dkt039 [DOI] [PubMed] [Google Scholar]
- 7.Bergen PJ, Landersdorfer CB, Lee HJ, Li J, Nation RL. 2012. ‘Old' antibiotics for emerging multidrug-resistant bacteria. Curr. Opin. Infect. Dis. 25:626–633. 10.1097/QCO.0b013e328358afe5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas S, Brunel JM, Dubus JC, Reynaud-Gaubert M, Rolain JM. 2012. Colistin: an update on the antibiotic of the 21st century. Expert Rev. Anti Infect. Ther. 10:917–934. 10.1586/eri.12.78 [DOI] [PubMed] [Google Scholar]
- 9.Velkov T, Roberts KD, Nation RL, Thompson PE, Li J. 2013. Pharmacology of polymyxins: new insights into an ‘old' class of antibiotics. Future Microbiol. 8:711–724. 10.2217/fmb.13.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epand RF, Pollard JE, Wright JO, Savage PB, Epand RM. 2010. Depolarization, bacterial membrane composition, and the antimicrobial action of ceragenins. Antimicrob. Agents Chemother. 54:3708–3713. 10.1128/AAC.00380-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epand RM, Epand RF, Savage PB. 2008. Ceragenins (cationic steroid compounds), a novel class of antimicrobial agents. Drug News Perspect. 21:307–311. 10.1358/dnp.2008.21.6.1246829 [DOI] [PubMed] [Google Scholar]
- 12.Joanne P, Falord M, Chesneau O, Lacombe C, Castano S, Desbat B, Auvynet C, Nicolas P, Msadek T, El Amri C. 2009. Comparative study of two plasticins: specificity, interfacial behavior, and bactericidal activity. Biochemistry 48:9372–9383. 10.1021/bi901222p [DOI] [PubMed] [Google Scholar]
- 13.Thwaite JE, Humphrey S, Fox MA, Savage VL, Laws TR, Ulaeto DO, Titball RW, Atkins HS. 2009. The cationic peptide magainin II is antimicrobial for Burkholderia cepacia-complex strains. J. Med. Microbiol. 58:923–929. 10.1099/jmm.0.008128-0 [DOI] [PubMed] [Google Scholar]
- 14.Fox MA, Thwaite JE, Ulaeto DO, Atkins TP, Atkins HS. 2012. Design and characterization of novel hybrid antimicrobial peptides based on cecropin A, LL-37 and magainin II. Peptides 33:197–205. 10.1016/j.peptides.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 15.Porcelli F, Buck-Koehntop BA, Thennarasu S, Ramamoorthy A, Veglia G. 2006. Structures of the dimeric and monomeric variants of magainin antimicrobial peptides (MSI-78 and MSI-594) in micelles and bilayers, determined by NMR spectroscopy. Biochemistry 45:5793–5799. 10.1021/bi0601813 [DOI] [PubMed] [Google Scholar]
- 16.Mihajlovic M, Lazaridis T. 2010. Antimicrobial peptides in toroidal and cylindrical pores. Biochim. Biophys. Acta 1798:1485–1493. 10.1016/j.bbamem.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazaridis T, He Y, Prieto L. 2013. Membrane interactions and pore formation by the antimicrobial peptide protegrin. Biophys. J. 104:633–642. 10.1016/j.bpj.2012.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradshaw J. 2003. Cationic antimicrobial peptides: issues for potential clinical use. BioDrugs 17:233–240. 10.2165/00063030-200317040-00002 [DOI] [PubMed] [Google Scholar]
- 19.Bera S, Zhanel GG, Schweizer F. 2008. Design, synthesis, and antibacterial activities of neomycin-lipid conjugates: polycationic lipids with potent gram-positive activity. J. Med. Chem. 51:6160–6164. 10.1021/jm800345u [DOI] [PubMed] [Google Scholar]
- 20.Bera S, Zhanel GG, Schweizer F. 2010. Antibacterial activities of aminoglycoside antibiotics-derived cationic amphiphiles. Polyol-modified neomycin B-, kanamycin A-, amikacin-, and neamine-based amphiphiles with potent broad spectrum antibacterial activity. J. Med. Chem. 53:3626–3631. 10.1021/jm1000437 [DOI] [PubMed] [Google Scholar]
- 21.Bera S, Zhanel GG, Schweizer F. 2010. Antibacterial activity of guanidinylated neomycin B- and kanamycin A-derived amphiphilic lipid conjugates. J. Antimicrob. Chemother. 65:1224–1227. 10.1093/jac/dkq083 [DOI] [PubMed] [Google Scholar]
- 22.Bera S, Dhondikubeer R, Findlay B, Zhanel GG, Schweizer F. 2012. Synthesis and antibacterial activities of amphiphilic neomycin B-based bilipid conjugates and fluorinated neomycin B-based lipids. Molecules 17:9129–9141. 10.3390/molecules17089129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herzog IM, Green KD, Berkov-Zrihen Y, Feldman M, Vidavski RR, Eldar-Boock A, Satchi-Fainaro R, Eldar A, Garneau-Tsodikova S, Fridman M. 2012. 6″-Thioether tobramycin analogues: towards selective targeting of bacterial membranes. Angew. Chem. Int. Ed. Engl. 51:5652–5656. 10.1002/anie.201200761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Keller K, Takemoto JY, Bensaci M, Litke A, Czyryca PG, Chang CW. 2009. Synthesis and combinational antibacterial study of 5″-modified neomycin. J. Antibiot. (Tokyo) 62:539–544. 10.1038/ja.2009.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fourmy D, Recht MI, Puglisi JD. 1998. Binding of neomycin-class aminoglycoside antibiotics to the A-site of 16 S rRNA. J. Mol. Biol. 277:347–362. 10.1006/jmbi.1997.1552 [DOI] [PubMed] [Google Scholar]
- 26.Francois B, Russell RJ, Murray JB, Aboul-ela F, Masquida B, Vicens Q, Westhof E. 2005. Crystal structures of complexes between aminoglycosides and decoding A site oligonucleotides: role of the number of rings and positive charges in the specific binding leading to miscoding. Nucleic Acids Res. 33:5677–5690. 10.1093/nar/gki862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baussanne I, Bussiere A, Halder S, Ganem-Elbaz C, Ouberai M, Riou M, Paris JM, Ennifar E, Mingeot-Leclercq MP, Decout JL. 2010. Synthesis and antimicrobial evaluation of amphiphilic neamine derivatives. J. Med. Chem. 53:119–127. 10.1021/jm900615h [DOI] [PubMed] [Google Scholar]
- 28.Ouberai M, El Garch F, Bussiere A, Riou M, Alsteens D, Lins L, Baussanne I, Dufrene YF, Brasseur R, Decout JL, Mingeot-Leclercq MP. 2011. The Pseudomonas aeruginosa membranes: a target for a new amphiphilic aminoglycoside derivative? Biochim. Biophys. Acta 1808:1716–1727. 10.1016/j.bbamem.2011.01.014 [DOI] [PubMed] [Google Scholar]
- 29.Zimmermann L, Bussiere A, Ouberai M, Baussanne I, Jolivalt C, Mingeot-Leclercq MP, Decout JL. 2013. Tuning the antibacterial activity of amphiphilic neamine derivatives and comparison to paromamine homologues. J. Med. Chem. 56:7691–7705. 10.1021/jm401148j [DOI] [PubMed] [Google Scholar]
- 30.Jackowski O, Bussière A, Vanhaverbeke C, Baussanne I, Peyrin E, Mingeot-Leclercq MP, Décout JL. 2012. Major increases of the reactivity and selectivity in aminoglycoside O-alkylation due to the presence of fluoride ions. Tetrahedron 68:737–746. 10.1016/j.tet.2011.10.102 [DOI] [Google Scholar]
- 31.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing: 23rd informational supplement. Document M100–S23. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 32.Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 40:175–179. 10.1016/S0167-7012(00)00122-6 [DOI] [PubMed] [Google Scholar]
- 33.Peeters E, Nelis HJ, Coenye T. 2008. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 72:157–165. 10.1016/j.mimet.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 34.Wood SJ, Miller KA, David SA. 2004. Anti-endotoxin agents. 1. Development of a fluorescent probe displacement method optimized for the rapid identification of lipopolysaccharide-binding agents. Comb. Chem. High Throughput Screen. 7:239–249. 10.2174/1386207043328832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood SJ, Miller KA, David SA. 2004. Anti-endotoxin agents. 2. Pilot high-throughput screening for novel lipopolysaccharide-recognizing motifs in small molecules. Comb. Chem. High Throughput Screen. 7:733–747. 10.2174/1386207043328229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunter CA, Anderson HL. 2009. What is cooperativity? Angew. Chem. Int. Ed. Engl. 48:7488–7499. 10.1002/anie.200902490 [DOI] [PubMed] [Google Scholar]
- 37.Provencher SW. 1982. A constrained regularization method for inverting data represented by linear algebraic or integral equations. Comput. Phys. Commun. 27:229–242. 10.1016/0010-4655(82)90174-6 [DOI] [Google Scholar]
- 38.Parasassi T, De Stasio G, Ravagnan G, Rusch RM, Gratton E. 1991. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of laurdan fluorescence. Biophys. J. 60:179–189. 10.1016/S0006-3495(91)82041-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parasassi T, Gratton E. 1995. Membrane lipid domains and dynamics as detected by laurdan fluorescence. J. Fluoresc. 5:59–69. 10.1007/BF00718783 [DOI] [PubMed] [Google Scholar]
- 40.Loh B, Grant C, Hancock RE. 1984. Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 26:546–551. 10.1128/AAC.26.4.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu M, Hancock RE. 1999. Interaction of the cyclic antimicrobial cationic peptide bactenecin with the outer and cytoplasmic membrane. J. Biol. Chem. 274:29–35. 10.1074/jbc.274.1.29 [DOI] [PubMed] [Google Scholar]
- 42.Helander IM, Mattila-Sandholm T. 2000. Fluorometric assessment of gram-negative bacterial permeabilization. J. Appl. Microbiol. 88:213–219. 10.1046/j.1365-2672.2000.00971.x [DOI] [PubMed] [Google Scholar]
- 43.Hancock RE, Wong PG. 1984. Compounds which increase the permeability of the Pseudomonas aeruginosa outer membrane. Antimicrob. Agents Chemother. 26:48–52. 10.1128/AAC.26.1.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buyck JM, Tulkens PM, Van Bambeke F. 2013. Pharmacodynamic evaluation of the intracellular activity of antibiotics towards Pseudomonas aeruginosa PAO1 in a model of THP-1 human monocytes. Antimicrob. Agents Chemother. 57:2310–2318. 10.1128/AAC.02609-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moskowitz SM, Brannon MK, Dasgupta N, Pier M, Sgambati N, Miller AK, Selgrade SE, Miller SI, Denton M, Conway SP, Johansen HK, Hoiby N. 2012. PmrB mutations promote polymyxin resistance of Pseudomonas aeruginosa isolated from colistin-treated cystic fibrosis patients. Antimicrob. Agents Chemother. 56:1019–1030. 10.1128/AAC.05829-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller AK, Brannon MK, Stevens L, Johansen HK, Selgrade SE, Miller SI, Hoiby N, Moskowitz SM. 2011. PhoQ mutations promote lipid A modification and polymyxin resistance of Pseudomonas aeruginosa found in colistin-treated cystic fibrosis patients. Antimicrob. Agents Chemother. 55:5761–5769. 10.1128/AAC.05391-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gutu AD, Sgambati N, Strasbourger P, Brannon MK, Jacobs MA, Haugen E, Kaul RK, Johansen HK, Hoiby N, Moskowitz SM. 2013. Polymyxin resistance of Pseudomonas aeruginosa phoQ mutants is dependent on additional two-component regulatory systems. Antimicrob. Agents Chemother. 57:2204–2215. 10.1128/AAC.02353-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. 2011. Carbapenems: past, present, and future. Antimicrob. Agents Chemother. 55:4943–4960. 10.1128/AAC.00296-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaara M, Vaara T. 1981. Outer membrane permeability barrier disruption by polymyxin in polymyxin-susceptible and -resistant Salmonella typhimurium. Antimicrob. Agents Chemother. 19:578–583. 10.1128/AAC.19.4.578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hancock RE, Bell A. 1988. Antibiotic uptake into gram-negative bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 7:713–720. 10.1007/BF01975036 [DOI] [PubMed] [Google Scholar]
- 51.Velkov T, Thompson PE, Nation RL, Li J. 2010. Structure-activity relationships of polymyxin antibiotics. J. Med. Chem. 53:1898–1916. 10.1021/jm900999h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pristovsek P, Kidric J. 1999. Solution structure of polymyxins B and E and effect of binding to lipopolysaccharide: an NMR and molecular modeling study. J. Med. Chem. 42:4604–4613. 10.1021/jm991031b [DOI] [PubMed] [Google Scholar]
- 53.Hancock RE, Chapple DS. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santos NC, Silva AC, Castanho MA, Martins-Silva J, Saldanha C. 2003. Evaluation of lipopolysaccharide aggregation by light scattering spectroscopy. Chembiochem 4:96–100. 10.1002/cbic.200390020 [DOI] [PubMed] [Google Scholar]
- 55.Giuliani A, Pirri G, Rinaldi AC. 2010. Antimicrobial peptides: the LPS connection. Methods Mol. Biol. 618:137–154. 10.1007/978-1-60761-594-1_10 [DOI] [PubMed] [Google Scholar]
- 56.Domadia PN, Bhunia A, Ramamoorthy A, Bhattacharjya S. 2010. Structure, interactions, and antibacterial activities of MSI-594 derived mutant peptide MSI-594F5A in lipopolysaccharide micelles: role of the helical hairpin conformation in outer-membrane permeabilization. J. Am. Chem. Soc. 132:18417–18428. 10.1021/ja1083255 [DOI] [PubMed] [Google Scholar]
- 57.Bhunia A, Domadia PN, Torres J, Hallock KJ, Ramamoorthy A, Bhattacharjya S. 2010. NMR structure of pardaxin, a pore-forming antimicrobial peptide, in lipopolysaccharide micelles: mechanism of outer membrane permeabilization. J. Biol. Chem. 285:3883–3895. 10.1074/jbc.M109.065672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lins RD, Straatsma TP. 2001. Computer simulation of the rough lipopolysaccharide membrane of Pseudomonas aeruginosa. Biophys. J. 81:1037–1046. 10.1016/S0006-3495(01)75761-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ravi HK, Stach M, Soares TA, Darbre T, Reymond JL, Cascella M. 2013. Electrostatics and flexibility drive membrane recognition and early penetration by the antimicrobial peptide dendrimer bH1. Chem. Commun. (Camb.) 49:8821–8823. 10.1039/c3cc44912b [DOI] [PubMed] [Google Scholar]
- 60.Murata T, Tseng W, Guina T, Miller SI, Nikaido H. 2007. PhoPQ-mediated regulation produces a more robust permeability barrier in the outer membrane of Salmonella enterica serovar Typhimurium. J. Bacteriol. 189:7213–7222. 10.1128/JB.00973-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soon RL, Li J, Boyce JD, Harper M, Adler B, Larson I, Nation RL. 2012. Cell surface hydrophobicity of colistin-susceptible vs resistant Acinetobacter baumannii determined by contact angles: methodological considerations and implications. J. Appl. Microbiol. 113:940–951. 10.1111/j.1365-2672.2012.05337.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pelletier MR, Casella LG, Jones JW, Adams MD, Zurawski DV, Hazlett KR, Doi Y, Ernst RK. 2013. Unique structural modifications are present in the lipopolysaccharide from colistin-resistant strains of Acinetobacter baumannii. Antimicrob. Agents Chemother. 57:4831–4840. 10.1128/AAC.00865-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prokhorenko IR, Zubova SV, Ivanov AY, Grachev SV. 2009. Interaction of Gram-negative bacteria with cationic proteins: dependence on the surface characteristics of the bacterial cell. Int. J. Gen. Med. 2:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.