Abstract
Sterile alpha motif and histidine-aspartic domain-containing protein 1 (SAMHD1) is a deoxynucleoside triphosphate (dNTP) triphosphohydrolase recently recognized as an antiviral factor that acts by depleting dNTP availability for viral reverse transcriptase (RT). SAMHD1 restriction is counteracted by the human immunodeficiency virus type 2 (HIV-2) accessory protein Vpx, which targets SAMHD1 for proteosomal degradation, resulting in an increased availability of dNTPs and consequently enhanced viral replication. Nucleoside reverse transcriptase inhibitors (NRTI), one of the most common agents used in antiretroviral therapy, compete with intracellular dNTPs as the substrate for viral RT. Consequently, SAMHD1 activity may be influencing NRTI efficacy in inhibiting viral replication. Here, a panel of different RT inhibitors was analyzed for their different antiviral efficacy depending on SAMHD1. Antiviral potency was measured for all the inhibitors in transformed cell lines and primary monocyte-derived macrophages and CD4+ T cells infected with HIV-1 with or without Vpx. No changes in sensitivity to non-NRTI or the integrase inhibitor raltegravir were observed, but for NRTI, sensitivity significantly changed only in the case of the thymidine analogs (AZT and d4T). The addition of exogenous thymidine mimicked the change in viral sensitivity observed after Vpx-mediated SAMHD1 degradation, pointing toward a differential effect of SAMHD1 activity on thymidine. Accordingly, sensitivity to AZT was also reduced in CD4+ T cells infected with HIV-2 compared to infection with the HIV-2ΔVpx strain. In conclusion, reduction of SAMHD1 levels significantly decreases HIV sensitivity to thymidine but not other nucleotide RT analog inhibitors in both macrophages and lymphocytes.
INTRODUCTION
Sterile alpha motif and histidine-aspartic domain-containing protein 1 (SAMHD1) is a recently identified human immunodeficiency virus type 1 (HIV-1) host restriction factor that limits retroviral replication at the reverse transcription stage of the viral life cycle (1–5). SAMHD1 functions as a deoxynucleoside triphosphate (dNTP) triphosphohydrolase that regulates the intracellular pool of dNTPs (6). It restricts HIV-1 infection in immune cells of myeloid lineage and in quiescent CD4-positive T lymphocytes (1, 2, 4). SAMHD1 reduces cellular dNTP levels to concentrations below the threshold required for reverse transcription of the viral RNA genome into DNA (4, 5). SAMHD1 is counteracted by the retroviral Vpx protein that is encoded by simian immunodeficiency virus (SIV) and HIV-2, but this gene is lacking from the HIV-1 and feline immunodeficiency virus (FIV) genomes (7). However, and despite the lack of Vpx function, HIV-1 is still able to replicate in noncycling myeloid cells, albeit at low levels (7).
Most current standard three-drug antiretroviral regimens involve RT inhibitors combined with a protease inhibitor. Approved antiretroviral drugs targeting the DNA polymerase activity of HIV RT can be classified into two major groups: nucleoside analogue inhibitors (NRTI; zidovudine [AZT], lamivudine [3TC], stavudine [d4T], didanosine [ddI], zalcitabine [ddC], abacavir [ABC], emtricitabine [FTC], and the acyclic nucleotide phosphonate tenofovir [TFV]) and nonnucleoside analogue reverse transcriptase inhibitors (NNRTI; nevirapine [NVP], delavirdine, efavirenz [EFV], and etravirine). NRTI are phosphorylated to their triphosphate form to act as competitive inhibitors of HIV RT. In contrast, NNRTI bind at a hydrophobic pocket adjacent to the polymerase active site (reviewed in references 8 and 9). Consequently, NRTI but not NNRTI compete with intracellular dNTPs for incorporation into newly transcribed viral DNA during the reverse transcription step.
NRTI may compete with intracellular dNTPs; therefore, SAMHD1 may be influencing HIV-1 sensitivity to NRTI. Here, we show that SAMHD1 did not affect viral sensitivity to all NRTI but exclusively to thymidine analogs in both T cell lines and primary cells, suggesting that SAMHD1 may have a differential effect over the different dNTPs.
MATERIALS AND METHODS
Cells.
Peripheral blood mononuclear cells (PBMCs) were obtained from blood of healthy donors using Ficoll-Paque density gradient centrifugation and monocytes, and CD4+ T lymphocytes were purified using negative-selection antibody cocktails (StemCell Technologies). Monocytes were cultured in complete culture medium: RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco), penicillin, and streptomycin (Gibco) and differentiated to monocyte-derived macrophages (MDM) for 4 days in the presence of macrophage colony-stimulating factor (M-CSF; Peprotech) at 100 ng/ml. CD4+ T lymphocytes were activated in complete RPMI medium, with interleukin 2 (IL-2) (16 U/ml) and phytohemagglutinin (PHA; 4 μg/ml; Sigma-Aldrich), for 3 days. PBMCs from healthy donors were cultured in complete culture medium and stimulated with a CD3-CD8-bispecific antibody (NIH AIDS Reagents Program) in the presence of IL-2, as previously described, for 5 days (10).
The human cell lines Jurkat and MT-4 (AIDS Reagent Program, National Institutes of Health, Bethesda, MD) were grown in complete RPMI medium with 10% FBS, penicillin, and streptomycin. HEK293-T cells (AIDS Reagent Program, National Institutes of Health, Bethesda, MD) were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Madrid, Spain) supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/ml penicillin, and 100 μg/ml streptomycin.
Drugs.
3-Azido-3-deoxythymidine (zidovudine [AZT]) and AMD3100 were purchased from Sigma-Aldrich (Madrid, Spain). Stavudine (d4T), lamivudine (3TC), zalcitabine (ddC), didanosine (ddI), tenofovir disoproxil fumarate (TFV), efavirenz (EFV), and nevirapine (NVP) were obtained from the NIH AIDS Research and Reference Reagent Program. Abacavir (ABC) was purchased from Selleckchem (Munich, Germany). Raltegravir (RAL) was obtained from Merck.
Nucleotides (thymidine and 2′-deoxycytidine hydrochloride) were purchased from Sigma-Aldrich. Thymidine was dissolved in RPMI (pH 4.1) and 2′-deoxycytidine hydrochloride in RPMI (pH 7.4).
RNA interference.
Small interfering RNA (siRNA) were purchased from Dharmacon (siGENOME SMARTpool; Dharmacon, Thermo-Scientific). Monocytes were transfected with 50 pmol of the corresponding siRNA using the monocyte Amaxa nucleofection kit (Lonza) as previously described (11). Transfected monocytes were left untreated overnight and then differentiated to macrophages as described above.
SAMHD1 mRNA quantification.
For relative mRNA quantification, RNA was extracted using the NucleoSpin RNA II kit (Magerey-Nagel), as recommended by the manufacturer, including the DNase I treatment step. Reverse transcriptase was performed using the high-capacity cDNA reverse transcription kit (Life Technologies). mRNA relative levels of SAMHD1 were measured by two-step quantitative RT-PCR and normalized to GAPDH mRNA expression using the threshold cycle (ΔΔCT) method. Primers and DNA probes were purchased from Life Technologies (TaqMan gene expression assays).
Virus.
The envelope-deficient HIV-1 NL4-3 clone encoding internal ribosome entry site (IRES)-green fluorescent protein (GFP) (NL4-3-GFP) was pseudotyped with vesicular stomatitis virus G protein (VSV-G) by cotransfection of HEK293T cells using polyethylenimine (Polysciences) as previously described (11). NL4-3 virus modified to incorporate Vpx into HIV-1 virions (HIV-1*GFP) (1) was produced by transfection into HEK293T cells, together with pSIV3+ encoding Vpx (12), when appropriate, as previously described (1). A plasmid encoding ROD9 HIV-2 GFP-expressing virus and the corresponding ΔVpx mutant were used to transfect HEK293T cells to produce viral stocks as previously described (1). For the production of viruslike particles carrying Vpx (VLPVpx), HEK293T cells were cotransfected with pSIV3+ and a VSV-G-expressing plasmid. Three days after transfection, supernatants were harvested, filtered, and stored at −80°C. Viral stocks were concentrated using Lenti-X concentrator (Clontech). Viruses were titrated by infection of TZM cells followed by GFP quantification by flow cytometry.
Virus infections.
MT-4 cells, Jurkat cells, and MDM were pretreated with VLPVpx for 4 h before infection or left with fresh medium as a control. Cells were then infected with VSV-pseudotyped NL4-3-GFP, and antiviral drugs and/or nucleotides were added immediately before infection. Activated CD4+ T lymphocytes and CD3/CD8-stimulated PBMCs were spinoculated in the presence of the corresponding virus (HIV-1*GFP or HIV-2 for CD4+ T lymphocytes and PBMCs, respectively) for 90 min at 1,200 × g. Viral replication was measured in all cases 2 days later by flow cytometry. Measurement of cell cytotoxicity was performed by a methyl tetrazolium-based colorimetric assay (MTT method) as described before (13) or, in the case of lymphocytes, by relative quantification of the gate of live cells by flow cytometry.
The anti-HIV activity of the different compounds was determined by infection of cells in the presence of different concentrations of the drug, and 50% effective concentrations (EC50) were calculated, as previously described (14, 15).
Western blotting.
Cells were rinsed in ice-cold phosphate-buffered saline (PBS), and extracts were prepared in lysis buffer (50 mM Tris HCl [pH 7.5], 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 10 mM Na β-glycerophosphate, 50 mM NaF, 5 mM Na pyrophosphate, 270 mM sucrose, and 1% Triton X-100) supplemented with protease inhibitor (Roche) and 1 mM phenylmethylsulfonyl fluoride. Lysates were subjected to SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane (ImmunolonP; Thermo). The following antibodies were used for immunoblotting: anti-rabbit and anti-mouse horseradish peroxidase-conjugated secondary antibodies (1:5,000; Pierce), anti-human Hsp90 (BD Biosciences; catalog no. 610418), and anti-SAMHD1 (1:2,500, ab67820; Abcam).
Intracellular SAMHD1 staining by flow cytometry.
Cells were fixed for 20 min with 4% fluorescent antibody (FA) followed by permeabilization for 15 min with 0.5% Triton X-100 in PBS. After incubation for 1 h in PBS containing 2% bovine serum albumin (BSA), cells were stained with the rabbit polyclonal anti-SAMHD1 (1:100, catalog no. 12586-1-AP; Proteintech) for 1 h followed by incubation for 20 min with the goat anti-rabbit Alexa 633 antibody (1:1,000; Life Technologies), both diluted in the blocking medium. Flow cytometry was performed in a FACS LSRII flow cytometer (BD Biosciences). Data were analyzed using the FlowJo software (BD Biosciences).
Determination of dNTP intracellular levels.
MDM were rinsed and lysed with trichloroacetic acid (TCA; 0.5 M). Cellular proteins were cleared by centrifugation, and supernatant was neutralized with 0.5 M tri-n-octylamine in 1,1,2-trichlorotrifluoroethane, and after centrifugation, aqueous phase was recovered and dried in a SpeedVac. Pellets were resuspended in Tris-HCl buffer (40 mM, pH 7.4).
dNTP content was determined using a polymerase-based method (16) with minor modifications. Briefly, 20 μl of reaction mixture contained 5 μl of dNTP extract in 40 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 5 mM dithiothreitol, 0.25 μM oligoprimer, 0.75 μM [8-3H]dATP, 12 to 21 Ci/mmol (or [methyl-3H]dTTP for the dATP assay), and 1.7 units of Thermo Sequenase DNA polymerase (GE Healthcare). Reaction mixtures with aqueous dNTP standards were processed in parallel. After incubation at 48°C for 60 min, 18 μl of the mix was spotted on a Whatman DE81 paper and left to dry. The filters were washed 3 times for 10 min with 5% Na2HPO4, once with water, and once with absolute ethanol and left to dry again. The retained radioactivity was determined by scintillation counting, and dNTP amounts were calculated from interpolation on the calibration curves. To ensure the reliability of the results, triplicates of 2 different dilutions of each dNTP extract (usually undiluted and 1:3 water-diluted) were processed in each independent experiment.
Statistical methods.
Data were analyzed with the PRISM statistical package. If not stated otherwise, all data were normally distributed and expressed as means ± standard deviation (SD). P values were calculated using an unpaired, two-tailed, Student t test.
RESULTS
SAMHD1 expression is required for maintaining AZT antiviral potency in T cell lines.
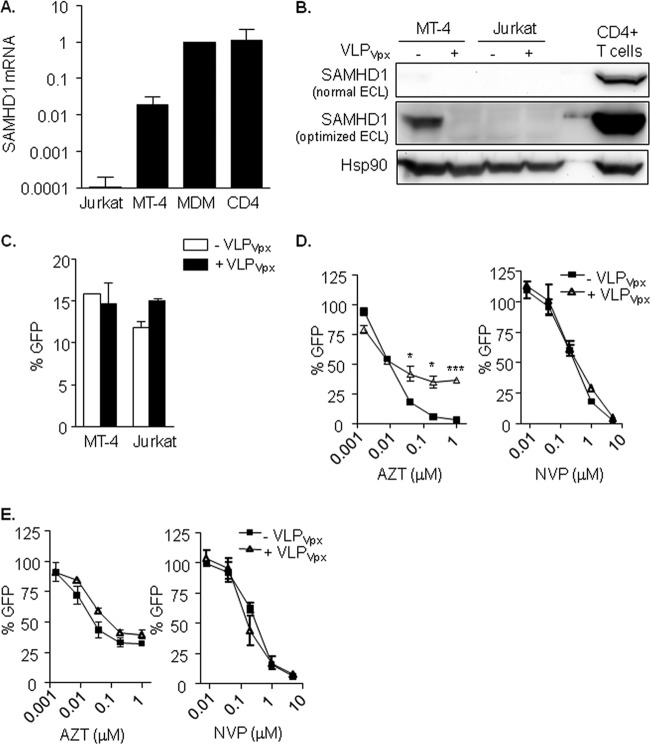
SAMHD1 expression is variable in distinct human cell lines and tissue types (6, 17), although high levels of expression have been reported for HIV-1 target cells, such as macrophages and CD4+ T lymphocytes. mRNA and protein expression of SAMHD1 was assessed in two HIV-1 highly susceptible T cell lines (MT-4 and Jurkat) and primary HIV-1 target cells (MDM and activated CD4+ T cells), with the aim to identify cell lines with different degrees of SAMHD1 expression (Fig. 1A and B). Jurkat T cells expressed very low levels of SAMHD1 mRNA and protein expression was undetectable, as previously reported (17). On the contrary, MDM or activated CD4+ T cells expressed high levels of both SAMHD1 mRNA and protein, whereas expression levels were intermediate in the MT-4 cell line.
FIG 1.
(A) SAMHD1 mRNA expression in T cell lines and primary cells. Relative mRNA expression of the SAMHD1 gene showing the diverse expression levels depending on cell type. (B) SAMHD1 protein expression and degradation by VLPVpx in T cell lines. Western blot of SAMHD1 expression in MT-4 and Jurkat T cell lines treated or not with VLPVpx for 24 h. Activated CD4+ T cells are included as a reference to illustrate the different degree of expression. SAMHD1 protein was detected only in MT-4 cells and after optimizing band detection by enhanced chemiluminescence (ECL). (C) HIV-1 replication is not affected by Vpx in MT-4 and Jurkat T cell lines. Percentage of infected cells after treatment with VLPVpx. Cells were transduced with VLP carrying HIV-2 Vpx or left untreated for 4 h and then infected with a VSV-pseudotyped HIV-1 GFP-expressing virus. After 48 h, infection was assessed by flow cytometry. (D and E) Absence of SAMHD1 affects AZT antiviral potency in T cell lines. Dose response of AZT and NVP in MT-4 (D) and Jurkat (E) T cell lines treated or not with VLPVpx. In the absence of SAMHD1 expression, either by targeting its degradation with VLPVpx (D; MT-4) or due to lack of expression (E; Jurkat), AZT antiviral activity is decreased. Means ± standard deviations (SD) from at least 2 independent experiments performed in duplicate are shown. MDM, monocyte-derived macrophages. *, P < 0.05; ***, P < 0.0005.
Despite the observed differences in SAMHD1 expression, both MT-4 and Jurkat T cells were equally susceptible to HIV-1 infection (Fig. 1C, black bars), and infection was not enhanced by degradation of SAMHD1 with VLPVpx in MT-4 cells (Fig. 1B, lines 1 and 2, and C, white bars), confirming that SAMHD1 is not restricting viral replication in transformed cell lines, where availability of dNTPs is presumably high. However, when antiviral activity of the NRTI AZT and the NNRTI NVP were assessed, the activity of AZT was significantly decreased in the absence of SAMHD1, i.e., Jurkat cells (Fig. 1E, left) and MT-4 cells treated with VLPVpx (Fig. 1D, left). Conversely, the antiviral effect of NVP remained unaffected (Fig. 1D and E, right). These results suggest that the limitation of intracellular dNTPs resulting from SAMHD1 activity may be enhancing the efficacy of NRTI for incorporation into viral DNA. Moreover, it suggests that despite undetectable viral restriction by SAMHD1, it remains active at modulating dNTP availability.
SAMHD1 regulates viral sensitivity exclusively to NRTI thymidine analogs in MDM.
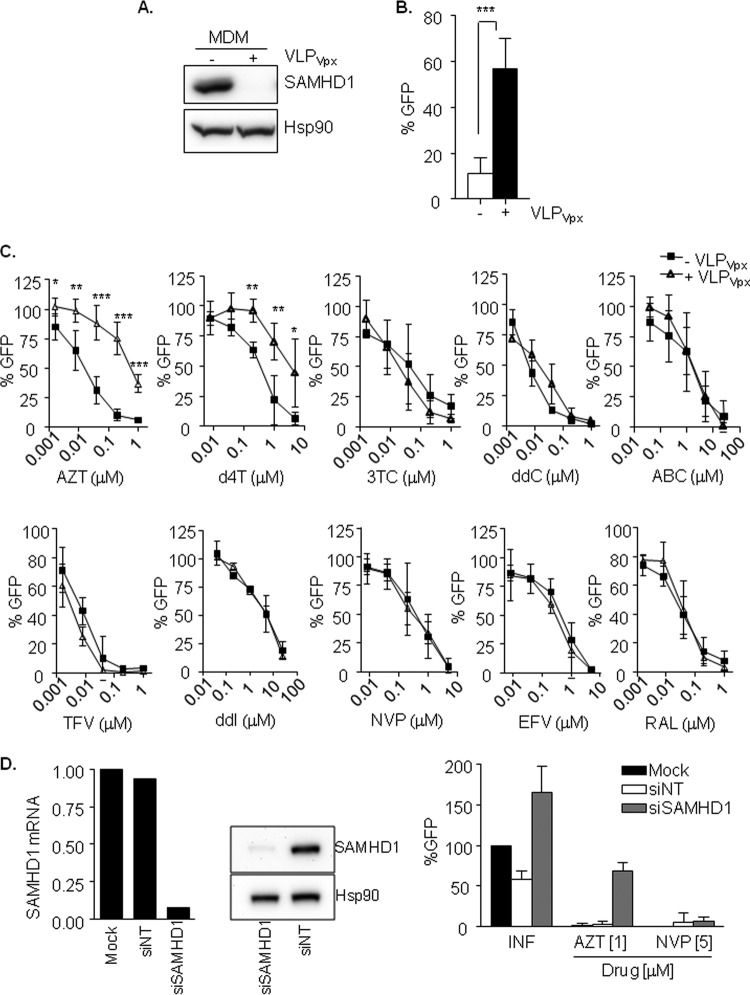
To further investigate SAMHD1-mediated regulation of NRTI viral sensitivity, antiviral activity of a panel of NRTI and NNRTI were evaluated in MDM in the presence or not of HIV-2 Vpx. VLPVpx induced the degradation of SAMHD1 (Fig. 2A) and a 5-fold and significant (P < 0.0001) enhancement of HIV-1 replication (Fig. 2B).
FIG 2.
(A) Degradation of SAMHD1 by VLPVpx in MDM. Western blot of SAMHD1 expression in MDM treated or not with VLPVpx. (B) Degradation of SAMHD1 by Vpx enhances HIV-1 replication in MDM. MDM previously treated or not with VLPVpx were infected with a VSV-pseudotyped HIV-1 GFP virus and replication was assessed 2 days later by measuring GFP expression. Fivefold change in HIV-1 replication was observed after Vpx-mediated SAMHD1 degradation. Means ± SD from at least six independent donors performed in duplicate are shown. (C) Decreased sensitivity of thymidine analogs NRTI after Vpx-mediated SAMHD1 degradation in MDM. Dose response of NRTI (AZT, d4T, 3TC, ddC, ABC, TFV, and ddI), NNRTI (NVP and EFV), and integrase inhibitor raltegravir as a control. Inhibition of HIV infection was measured as the percentage of GFP+ cells relative to the no-drug condition. Means ± SD from at least three independent donors performed in duplicate are shown. (D) siRNA-mediated knockdown of SAMHD1 in MDM affects AZT antiviral potency. Specific siRNA-mediated inhibition of SAMHD1 mRNA (left) or protein expression (middle) led to an increase in HIV-1 replication (right) compared to mock-transfected MDM or MDM transfected with a control siRNA (siNT). Absence of SAMHD1 correlated with a decreased sensitivity of AZT (1 μM), whereas no changes in NVP sensitivity (5 μM) were observed. Values from a representative donor performed in duplicate are shown.
After incubation with VLPVpx, macrophages were treated with NRTI (AZT, d4T, 3TC, ddC, ABC, ddI, and TFV), NNRTI (EFV and NVP), or the integrase inhibitor raltegravir as a control, at different concentrations prior to infection with VSV-pseudotyped HIV-GFP. As shown (Fig. 2C), macrophages transduced with VLPVpx showed a significantly reduced viral sensitivity to NRTI thymidine analogs (AZT and d4T) compared to that of untreated macrophages (P < 0.0001 and P = 0.03 at highest concentrations tested for AZT and d4T, respectively; Fig. 2C, firsts two panels). No differences were observed for the cytidine, guanosine, and adenosine analogs tested, NNRTI or raltegravir. Calculation of 50% effective concentrations (EC50) of macrophages expressing or not Vpx showed a fold change of 26 and 7 for AZT and d4T, respectively, whereas no change in EC50 was observed for any other drug (Table 1). Importantly, knockdown of SAMHD1 expression by RNA interference showed similar results, i.e., an increase in viral replication consequence of SAMHD1 absence and decreased sensitivity to AZT (Fig. 2D), discarding that the observed effect is due to the presence of Vpx.
TABLE 1.
Anti-HIV-1 activity of NRTI, NNRTI, or the integrase inhibitora
| Drug | Jurkat cells |
MT-4 cells |
MDM cells |
CD4+ T cells |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC50 (μM) |
FC | EC50 (μM) |
FC | EC50 (μM) |
FC | EC50 (μM) |
FC | |||||
| −Vpx | +Vpx | −Vpx | +Vpx | −Vpx | +Vpx | −Vpx | +Vpx | |||||
| AZT | 0.023 | 0.034 | 1 | 0.017 | 0.082 | 5 | 0.015 | 0.389 | 26 | 0.068 | 4.607 | 67 |
| d4T | 5.000 | 4.300 | 1 | 0.962 | 2.376 | 2 | 0.300 | 2.009 | 7 | 1.245 | 1/>5b | 1/>4b |
| 3TC | 0.164 | 0.156 | 1 | 0.816 | 0.758 | 1 | 0.081 | 0.022 | 1 | 0.524 | 0.372 | 1 |
| ddC | 0.052 | 0.059 | 1 | 0.292 | 0.308 | 1 | 0.113 | 0.042 | 1 | 0.115 | 0.081 | 1 |
| ABC | 0.053 | 0.059 | 1 | 0.001 | 0.001 | 1 | 0.003 | 0.003 | 1 | 0.015 | 0.009 | 1 |
| TFV | 11.598 | 10.858 | 1 | 2.877 | 2.162 | 1 | 6.114 | 5.599 | 1 | 1.949 | 6.822 | 3 |
| ddI | 2.045 | 2.572 | 1 | 1.470 | 2.692 | 2 | 1.805 | 2.251 | 1 | 0.402 | 0.481 | 1 |
| NVP | 0.090 | 0.095 | 1 | 0.237 | 0.295 | 1 | 0.274 | 0.361 | 1 | 1.017 | 1.256 | 1 |
| EFV | 0.038 | 0.072 | 2 | 0.047 | 0.055 | 1 | 0.071 | 0.071 | 1 | 0.068 | 0.091 | 1 |
| RAL | 0.003 | 0.007 | 3 | 0.005 | 0.004 | 1 | 0.022 | 0.031 | 1 | 0.017 | 0.015 | 1 |
NRTI are AZT, d4T, 3TC, ddC, TFV, ddI, and ABC; NNRTI are NVP and EFV; the integrase inhibitor is RAL. EC50 values were calculated in T cell lines negative or expressing low levels of SAMHD1 (Jurkat and MT4) and primary cells MDM and CD4+ T cells. EC50, effective concentration required to block HIV-1 replication by 50%; FC, fold change or ratio of the EC50 with Vpx (+) and the EC50 without Vpx (−). Results are the means from at least two independent experiments done in triplicates.
EC50 > 5 μM in 2/5 donors, which resulted in a fold change of <4.
To further study the effect of Vpx-induced degradation of SAMHD1 in viral sensitivity to thymidine analogs, the intracellular dNTP pool availability for reverse transcription was measured. As previously reported, treatment of macrophages with VLPVpx led to an increase of all intracellular dNTP (Fig. 3), suggesting that the reduced efficacy of thymidine analogs observed upon degradation of SAMHD1 may be the result of direct competition with intracellular dNTPs.
FIG 3.
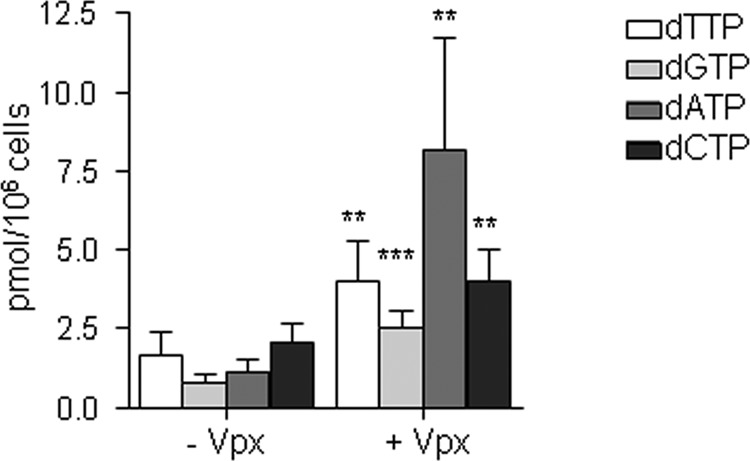
Intracellular dNTP levels in MDM. MDM were transduced with VLPVpx for 24 h and intracellular dNTPs were extracted, and dNTP content was determined using a polymerase-based method. Means ± SD from five independent donors are shown.**, P < 0.005; ***, P < 0.0005.
Imbalance of the dNTP intracellular pool mimics SAMHD1-mediated decrease in viral sensitivity to NRTI thymidine analogs.
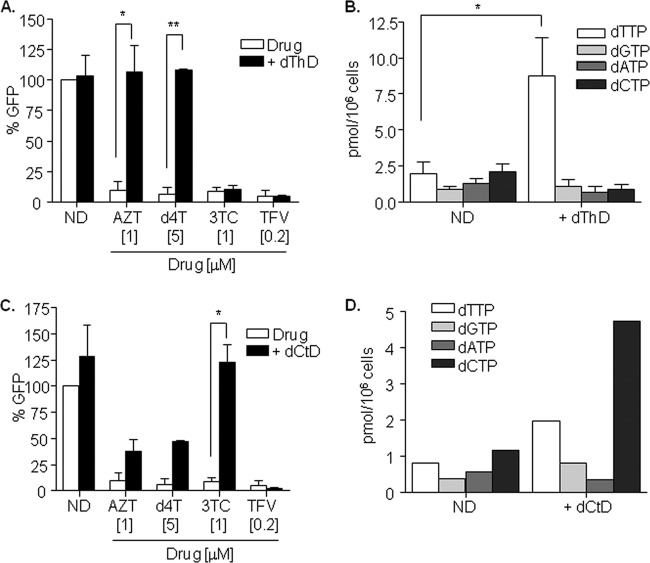
Competition with the intracellular pool of dNTPs is the most plausible hypothesis underlying the reduced efficacy of NRTI thymidine analogs. Therefore, the addition of exogenous thymidine may mimic the effect observed by SAMHD1 degradation. Thymidine (dThD; 1 mM) was added to macrophages together with NRTI thymidine analogs AZT and d4T, as well as to cytosine analog 3TC and adenosine analog TFV as controls, prior to HIV-1 infection (Fig. 4A). No differences in viral replication were observed as a result of adding dThD. However, AZT and d4T completely lost their antiviral activity, therefore mimicking the effect of SAMHD1, arguing in favor of the competition with intracellular dNTPs as the mechanism underlying the loss of antiviral sensitivity. No changes were observed in viral sensitivity to 3TC and TFV. As expected, dTTP intracellular levels were significantly increased compared to those of untreated macrophages (5-fold increase, P < 0.0001; Fig. 4B), whereas no differences were observed for any other dNTP. Similarly, exogenous addition of cytidine led to the loss of antiviral activity of the NRTI cytidine analog 3TC (Fig. 4C), a consequence of the increase in dCTP intracellular levels (Fig. 4D). The addition of exogenous cytidine also partially affects dTTP intracellular levels, which is also reflected in the infection outcome where AZT and d4T partly lost their antiviral potency (Fig. 4C and D). However, these effects were not observed after degradation of SAMHD1, although intracellular concentrations of both nucleotides were similarly affected after Vpx-mediated SAMHD1 degradation, further supporting a preferential effect of SAMHD1 activity on thymidines.
FIG 4.
(A) NRTI thymidine analog antiviral activity is inhibited by exogenous addition of thymidine in MDM. Thymidine (1 mM) was added to the culture medium together with the corresponding drug 4 h prior to infection with VSV-pseudotyped HIV-1 GFP virus, and replication was assessed 2 days later. Means ± SD from two independent donors performed in duplicate are shown. (B) Intracellular dNTP levels in MDM treated with exogenous addition of dThD. dThD (1 mM) was added to the culture medium for 24 h before dNTPs were extracted and measured. Means ± SD from three independent donors are shown. (C) NRTI cytidine analog antiviral activity is inhibited by exogenous addition of cytidine in MDM. Cytidine (1 mM) was added to the culture medium together with the corresponding drug 4 h prior to infection with VSV-pseudotyped HIV-1 GFP virus, and replication was assessed 2 days later. Means ± SD from two independent donors performed in duplicate are shown. (D) Intracellular dNTP levels in MDM treated with exogenous addition of dCtD. dCtD (1 mM) was added to the culture medium for 24 h before dNTPs were extracted and measured. A representative experiment is shown. dThD, deoxythymidine; dCtd, deoxycytidine; ND, no drug. *, P < 0.05; **, P < 0.005.
Effect of SAMHD1 degradation on HIV-1 sensitivity to NRTI thymidine analogs in activated CD4+ T cells.
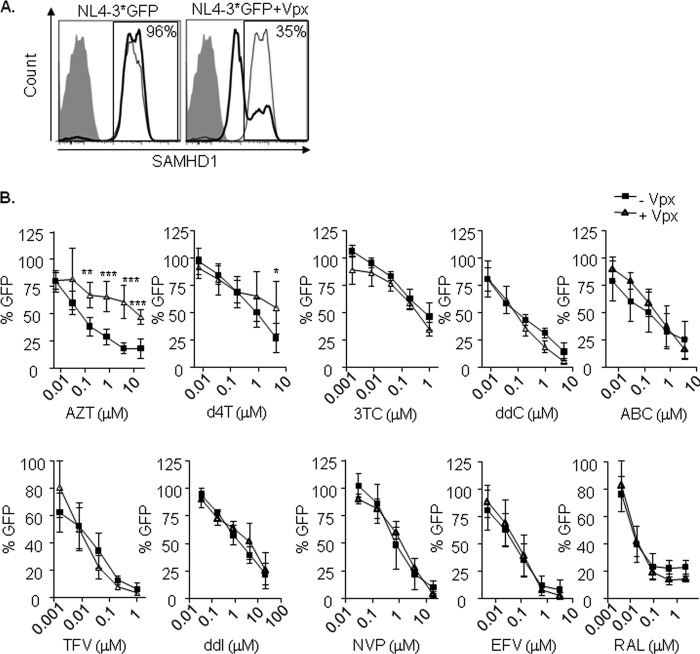
Activated primary CD4+ T lymphocytes were infected with or without Vpx, using a modified NL4-3 virus that incorporates HIV-2 Vpx (NL4-3*GFP and NL4-3*GFP+Vpx, respectively). Infection with HIV-1 expressing Vpx was able to induce SAMHD1 degradation (from 96% SAMHD1-expressing cells in NL4-3*GFP-infected cells to 35% after infection with NL4-3*GFP+Vpx; Fig. 5A), although HIV-1 infection did not differ as a result of SAMHD1 degradation, as previously reported for cycling cells (data not shown) (18).
FIG 5.
(A) Degradation of SAMHD1 by Vpx in lymphocytes. Flow cytometry histograms showing intracellular staining of SAMHD1 in lymphocytes infected with an NL4-3*GFP (left) or NL4-3*GFP with Vpx (right). Three days after infection, cells were fixed, permeabilized, and stained using a primary-specific SAMHD1 antibody followed by an allophycocyanin (APC)-conjugated secondary antibody (gray-line histogram, uninfected cells; black-line histogram, infected cells). The secondary antibody alone was used as a control (shaded histogram). Representative histograms of one experiment are shown. The experiment was performed in three independent donors. (B) Decreased sensitivity of thymidine NRTI in CD4+ T lymphocytes infected with HIV-1. Dose response of NRTI (AZT, d4T, 3TC, ddC, ABC, TFV, and ddI), NNRTI (NVP and EFV), and integrase inhibitor raltegravir as a control. PHA-activated CD4+ T lymphocytes were infected with or without Vpx using a GFP-expressing NL4-3 virus, modified to incorporate HIV-2 Vpx (NL4-3*GFP). Inhibition of HIV infection was measured as the percentage of GFP+ cells relative to the no-drug condition. Means ± SD from at least three independent donors performed in duplicate are shown. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
To determine the effect of SAMHD1 on NRTI efficacy, activated CD4+ T lymphocytes were treated with NRTI (AZT, d4T, 3TC, ddC, ABC, ddI, and TFV), NNRTI (EFV and NVP), or raltegravir as a control and then infected with NL4-3*GFP with and without Vpx. Similar to macrophages, degradation of SAMHD1 by infection with NL4-3*GFP+Vpx reduced viral sensitivity to AZT and d4T (Fig. 5B, first two panels). Results were more homogenous in the case of AZT than d4T, where a lack of antiviral activity was observed in 2 donors in contrast to the more limited effect shown in 3 others (see Fig. S1 in the supplemental material). This observation highlights the existence of important interdonor differences, putatively reflecting the lesser antiviral potency of d4T than of AZT (50- to 200-fold reduction in the EC50 depending on the cell type; Table 1), and that resulted in an apparently limited effect. No differences were observed for the cytidine, guanosine, and adenosine analogs tested, NNRTI or raltegravir. Vpx induced a 67-fold change in EC50 for AZT; no change in EC50 was observed for d4T, although a reduced sensitivity was observed at the highest concentration used (Table 1 and Fig. 5B, second panel). No change in EC50 was observed for any other drug (Table 1).
Decreased potency of AZT in HIV-2-infected PBMCs depends on SAMHD1.
Although data from HIV-2-infected patients treated with AZT and other NRTI are limited to small cohorts of patients, differences in antiviral activity of AZT on HIV-2 infection has been previously reported (19), but the underlying mechanisms are still under debate. In contrast to HIV-1, HIV-2 encodes Vpx protein and consequently it harbors the ability to overcome the restriction imposed by SAMHD1. Therefore, Vpx-mediated degradation of SAMHD1 may play a role on the decreased AZT viral sensitivity observed.
PBMCs from healthy donors were activated with a CD3-CD8-bispecific antibody for 5 days, prior to infection with wild-type HIV-2 or HIV-2 defective for Vpx (HIV-2ΔVpx strain). Infection with wild-type HIV-2 was able to partially induce SAMHD1 degradation (from 71% SAMHD1-expressing cells in the HIV-2ΔVpx strain-infected cells to 45% after infection with HIV-2; Fig. 6A). Viral replication was not significantly altered by Vpx (means of 4.6% and 3.1% GFP+ cells after infection with wild-type HIV-2 and the HIV-2ΔVpx strain, respectively; Fig. 6B). Importantly, and in accordance with previous results, a significant decrease of AZT antiviral potency against HIV-2 was observed compared to that against HIV-2ΔVpx infection (P < 0.0001; Fig. 6B). As expected, similar results regarding infection and limited AZT antiviral potency were obtained in parallel infections using HIV-1 virus modified to incorporate or not Vpx (Fig. 6C). In summary, these results point to SAMHD1 as a contributor to the limited antiviral activity of AZT in HIV-2 infections.
FIG 6.
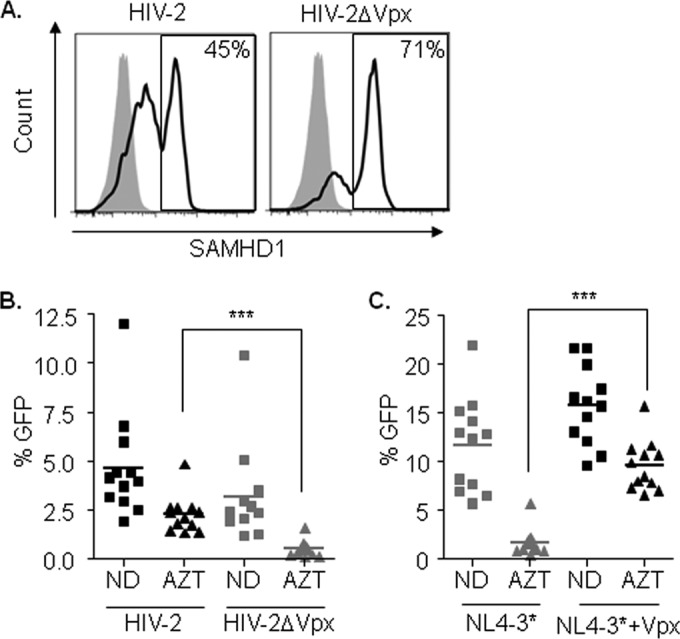
(A) Degradation of SAMHD1 by Vpx in PBMCs by HIV-2. Flow cytometry histograms showing intracellular staining of SAMHD1 in lymphocytes infected with wild-type HIV-2 (left) or HIV-2 defective for Vpx protein (right). Three days after infection, cells were fixed, permeabilized, and stained using a primary-specific SAMHD1 antibody followed by an APC-conjugated secondary antibody. The secondary antibody alone was used as a control (shaded histogram). Representative histograms of one experiment are shown. The experiment was performed in three independent donors. (B) AZT antiviral potency is decreased in HIV-2-infected PBMCs in vitro. CD3/CD8-activated PBMCs from donors (n = 12) were infected with wild-type GFP-expressing HIV-2 or HIV-2 defective for Vpx protein. Antiviral activity of AZT (1 μg/ml) was assessed. (C) AZT antiviral potency is decreased in HIV-1-infected PBMCs in vitro. CD3/CD8-activated PBMCs from the same donors as in panel B (n = 12) were infected with NL4-3*GFP carrying or not Vpx protein. Antiviral activity of AZT (1 mg/ml) was assessed. ND, no drug. ***, P < 0.0005.
DISCUSSION
While cell receptor and coreceptor recognition by retroviral envelope proteins is a primary determinant of cell tropism, viruses have evolved to overcome other cell type-specific metabolic blocks to viral replication. Limitation of intracellular dNTP levels, the substrates for DNA polymerization, represents one such metabolic bottleneck (20). The HIV RT requires cellular dNTPs, and thus the rate of proviral DNA synthesis is kinetically dependent on the cellular dNTP concentrations. The steady-state level of cellular dNTPs is tightly regulated primarily by the cell cycle and, indeed, dNTP levels in nondividing cells are low compared to those of dividing cells (21, 22). SAMHD1, a newly identified HIV-1 restriction factor, modulates the concentration of intracellular dNTPs, limiting its availability for the reverse transcription of incoming virus (1–5, 23). Although differences in expression between cell lines and primary tissues exist, SAMHD1 expression is similar between HIV-1-resistant and -susceptible cells (6, 17). Posttranscriptional control by phosphorylation has been proposed as a plausible hypothesis underlying differences in SAMHD1 function between resistant and susceptible cells, although it has not been experimentally proved yet (24–26). Here, we evaluated SAMHD1 activity in SAMHD1-negative or low-expressing transformed cell lines, as well as in primary cells, demonstrating that SAMHD1 activity is maintained in the absence of viral restriction and is specifically influencing thymidine NRTI analog antiviral efficacy.
Intracellular dNTP levels are significantly higher in rapidly dividing cancer cells and transformed cell lines than in resting cells (22, 27). In accordance, no differences in viral replication were observed in the MT-4 cell line after SAMHD1 degradation (Fig. 1C). Similarly, a lack of enhanced replication after SAMHD1 degradation was observed in stimulated CD4+ T cells and PBMCs compared to macrophages (Fig. 5A and data not shown), likely due to the kinetic parameters of RT (8). The dNTPs found in macrophages are below the Km and dissociation constant (Kd) of RT (20, 22), and therefore the increase in dNTP levels upon SAMHD1 degradation enhances viral replication (Fig. 2B and 3).
Taking into account SAMHD1-mediated regulation of dNTP intracellular levels, which compete with NRTI for incorporation into viral DNA, it is intuitive to think that SAMHD1 function may be influencing HIV-1 sensitivity to RT inhibitors. Similar to previous studies (18), SAMHD1 function did not affect viral sensitivity to NNRTI. NNRTI bind to an allosteric site of the HIV-1 RT and therefore, unlike NRTI, do not compete against cellular dNTPs. However, we identified a SAMHD1-dependent difference in viral sensitivity to thymidine but not other NRTI analogs in both macrophages and CD4+ T cells. Lack of antiviral potency for NRTI has already been suggested after SAMHD1 degradation (18). In line with our results, SAMHD1 has been associated to a differential efficacy of NRTI (28), although differences were found in all analogs tested in macrophages but not in activated T cells (18) or in the THP1 cell line (18, 28). In contrast, our work uses an extended panel of NRTI as well as relevant controls such as NNRTI and the integrase inhibitor raltegravir, which allow us to better demonstrate the specificity of the effect. Moreover, we show similar results in different cell types (MT-4, macrophages, and lymphocytes). The discordance between studies using primary cells might be explained by the method used for macrophage differentiation in cell culture (e.g., macrophage colony-stimulating factor [M-CSF] instead of granulocyte-macrophage colony-stimulating factor [GM-CSF]), which affects macrophage susceptibility to HIV-1 infection (29) and also dNTP intracellular levels, both parameters being higher in M-CSF-differentiated macrophages (see Fig. S2 in the supplemental material) (data not shown).
Considering that SAMHD1 is not able to efficiently hydrolyze NRTI (18, 28) and the observation that exogenous addition of nucleosides mimicked SAMHD1 activity, our results are in accordance with the idea that reduced efficacy of thymidine analogs observed upon degradation of SAMHD1 may be the result of direct competition with increased intracellular dNTPs (Fig. 2 and 3) (18) but not to the NRTI activation pathway (28).
Steady-state and pre-steady-state kinetic experiments characterizing dNTP binding and incorporation during reverse transcription have not identified significant differences between the different dNTPs, that is, similar binding Kd in the low μM range for all dNTPs (8). Conversely, SAMHD1 requires dGTP to assemble into catalytically active tetramers (3, 30, 31), and recent evidences suggest that it may have differential affinity for each of the four dNTPs (32, 33). In line with this data, our results suggest that SAMHD1 degradation or loss of function may preferentially affect dTTP availability and, therefore, significantly affect the antiviral potency of thymidine analogs.
NRTI must undergo anabolic phosphorylation to an active 5′-triphosphate moiety by enzymes in the target cells (reviewed in reference 34). AZT and d4T require the activity of the thymidine kinase 1 (TK1) that is specifically expressed during the S-phase of the cell cycle. The dependence of AZT and d4T on TK1 for activation results in markedly reduced anti-HIV activity in resting cells but in a high antiviral potency upon PBMC stimulation (35). Similarly, SAMHD1 antiretroviral activity is modulated by cell cycle-dependent posttranslational modifications. Phosphorylation of SAMHD1 by cyclin-dependent kinases (CDK), whose activation is tightly controlled depending on the moment of the cell cycle (36), have been demonstrated as the regulatory mechanism of SAMHD1-mediated viral restriction in cycling cells (24–26). Our results do not point to a SAMHD1-dependent regulation of TK1 function, as similar results were obtained in different cell types, i.e., cycling cells (immortalized cell lines and activated lymphocytes) or differentiated cells (macrophages), expressing different levels of SAMHD1 (Fig. 1A and B). Despite the cell type, we observed a similar reduced sensitivity to thymidine NRTI as a consequence of SAMHD1 absence, suggesting that cell cycle phase is not a strong influence and may indicate that TK1 activity is preserved independent of the specific cell cycle phase and SAMHD1 expression. However, the coordinated regulation of nucleotide metabolism throughout the cell cycle phases might be rendering thymidine more susceptible to changes in the intracellular nucleotide pool, and therefore a concerted action between multiple enzymes affecting dNTP levels and particularly thymidine availability may be strongly influencing the efficacy of thymidine analogs as antiviral agents.
Like HIV-1, HIV-2 causes AIDS, and therefore persons infected with HIV-2 may benefit from treatment with antiretroviral drugs, including RT and protease inhibitors. However, information on how HIV-2-infected persons should be best treated is limited because of the absence of randomized clinical trials and because most infections occur in developing countries where few patients are treated. Although data from HIV-2-infected patients treated with AZT and other NRTI are limited to small cohorts of patients, several observations suggest differences in the antiviral activity of AZT between HIV-2 and HIV-1 (19, 37, 38). Both HIV-1 and HIV-2 RTs have similar abilities to incorporate AZT, suggesting that the observed HIV-2 resistance to AZT is not mediated by an increased discrimination against this drug (19). The Vpx-mediated degradation of SAMHD1 may be contributing to the mechanism underlying AZT resistance by HIV-2.
Mutations in SAMHD1 are associated with the autoimmune disorder Aicardi-Goutières syndrome (39, 40) and recently to relapsed/refractory chronic lymphocytic leukemia (CLL) (41). Elevated cellular dNTP level is considered a biochemical marker of transformed/cancerous cells (42). Since nucleotide metabolism plays a role in transformation and tumor progression, inhibition of this pathway has long been considered a therapeutic strategy for cancer. Nucleoside analogs are a class of cytotoxic drugs that have played an important role in the treatment of hematological neoplasms, especially lymphoid and myeloid malignancies (43). Therefore, the limited sensitivity to thymidine analogs due to SAMHD1 loss of function described here might also be relevant for the treatment of certain tumors, where resistance to current therapies based on nucleoside analogs is observed.
Inhibition or degradation of SAMHD1 significantly decreases HIV sensitivity to thymidine but not other nucleotide RT analog inhibitors in both macrophages and lymphocytes. Our results indicate that sensitivity to AZT may serve as a marker of SAMHD1 function in proliferating cells where virus restriction cannot be detected due to high dNTP levels. They further support the regulation of dNTPs as the underlying mechanism of SAMHD1-mediated HIV-1 restriction.
Supplementary Material
ACKNOWLEDGMENTS
We thank the National Institutes of Health (AIDS Research and Reference Reagent Program) and the EU Programme EVA Centralised Facility for AIDS Reagents, NIBSC, United Kingdom, for reagents.
This work was supported by the Spanish MINECO projects BFU2012-31569 and PI13/01083. E.P. and A.R. are research fellows from MINECO, and J.T.-T. was funded by the United Mitochondrial Disease Foundation (postdoctoral grant 12-029).
Footnotes
Published ahead of print 9 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03145-14.
REFERENCES
- 1.Baldauf HM, Pan X, Erikson E, Schmidt S, Daddacha W, Burggraf M, Schenkova K, Ambiel I, Wabnitz G, Gramberg T, Panitz S, Flory E, Landau NR, Sertel S, Rutsch F, Lasitschka F, Kim B, Konig R, Fackler OT, Keppler OT. 2012. SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells. Nat. Med. 18:1682–1687. 10.1038/nm.2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Descours B, Cribier A, Chable-Bessia C, Ayinde D, Rice G, Crow Y, Yatim A, Schwartz O, Laguette N, Benkirane M. 2012. SAMHD1 restricts HIV-1 reverse transcription in quiescent CD4(+) T-cells. Retrovirology 9:87. 10.1186/1742-4690-9-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstone DC, Ennis-Adeniran V, Hedden JJ, Groom HC, Rice GI, Christodoulou E, Walker PA, Kelly G, Haire LF, Yap MW, de Carvalho LP, Stoye JP, Crow YJ, Taylor IA, Webb M. 2011. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 480:379–382. 10.1038/nature10623 [DOI] [PubMed] [Google Scholar]
- 4.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. 2011. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474:654–657. 10.1038/nature10117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L, Bloch N, Maudet C, Bertrand M, Gramberg T, Pancino G, Priet S, Canard B, Laguette N, Benkirane M, Transy C, Landau NR, Kim B, Margottin-Goguet F. 2012. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat. Immunol. 13:223–228. 10.1038/ni.2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N, Zhang W, Cao X. 2000. Identification of human homologue of mouse IFN-gamma induced protein from human dendritic cells. Immunol. Lett. 74:221–224. 10.1016/S0165-2478(00)00276-5 [DOI] [PubMed] [Google Scholar]
- 7.Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J. 2011. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474:658–661. 10.1038/nature10195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarafianos SG, Marchand B, Das K, Himmel DM, Parniak MA, Hughes SH, Arnold E. 2009. Structure and function of HIV-1 reverse transcriptase: molecular mechanisms of polymerization and inhibition. J. Mol. Biol. 385:693–713. 10.1016/j.jmb.2008.10.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menendez-Arias L. 2013. Molecular basis of human immunodeficiency virus type 1 drug resistance: overview and recent developments. Antiviral Res. 98:93–120. 10.1016/j.antiviral.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Li C, Huang J, Cung T, Seiss K, Beamon J, Carrington MF, Porter LC, Burke PS, Yang Y, Ryan BJ, Liu R, Weiss RH, Pereyra F, Cress WD, Brass AL, Rosenberg ES, Walker BD, Yu XG, Lichterfeld M. 2011. CD4+ T cells from elite controllers resist HIV-1 infection by selective upregulation of p21. J. Clin. Invest. 121:1549–1560. 10.1172/JCI44539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pauls E, Jimenez E, Ruiz A, Permanyer M, Ballana E, Costa H, Nascimiento R, Parkhouse RM, Pena R, Riveiro-Munoz E, Martinez MA, Clotet B, Este JA, Bofill M. 2013. Restriction of HIV-1 replication in primary macrophages by IL-12 and IL-18 through the upregulation of SAMHD1. J. Immunol. 190:4736–4741. 10.4049/jimmunol.1203226 [DOI] [PubMed] [Google Scholar]
- 12.Mangeot PE, Negre D, Dubois B, Winter AJ, Leissner P, Mehtali M, Kaiserlian D, Cosset FL, Darlix JL. 2000. Development of minimal lentivirus vectors derived from simian immunodeficiency virus (SIVmac251) and their use for gene transfer into human dendritic cells. J. Virol. 74:8307–8315. 10.1128/JVI.74.18.8307-8315.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballana E, Pauls E, Clotet B, Perron-Sierra F, Tucker GC, Este JA. 2011. Beta5 integrin is the major contributor to the alphaVintegrin-mediated blockade of HIV-1 replication. J. Immunol. 186:464–470. 10.4049/jimmunol.1002693 [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez E, Ballana E, Clotet B, Este JA. 2011. Development of resistance to VIR-353 with cross-resistance to the natural HIV-1 entry virus inhibitory peptide (VIRIP). AIDS 25:1557–1583. 10.1097/QAD.0b013e328348a733 [DOI] [PubMed] [Google Scholar]
- 15.Permanyer M, Ballana E, Ruiz A, Badia R, Riveira-Munoz E, Gonzalo E, Clotet B, Este JA. 2012. Antiretroviral agents effectively block HIV replication after cell-to-cell transfer. J. Virol. 86:8773–8780. 10.1128/JVI.01044-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferraro P, Franzolin E, Pontarin G, Reichard P, Bianchi V. 2010. Quantitation of cellular deoxynucleoside triphosphates. Nucleic Acids Res. 38:e85. 10.1093/nar/gkp1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Silva S, Hoy H, Hake TS, Wong HK, Porcu P, Wu L. 2013. Promoter methylation regulates SAMHD1 gene expression in human CD4+ T cells. J. Biol. Chem. 288:9284–9292. 10.1074/jbc.M112.447201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amie SM, Daly MB, Noble E, Schinazi RF, Bambara RA, Kim B. 2013. Anti-HIV host factor SAMHD1 regulates viral sensitivity to nucleoside reverse transcriptase inhibitors via modulation of cellular deoxyribonucleoside triphosphate (dNTP) levels. J. Biol. Chem. 288:20683–20691. 10.1074/jbc.M113.472159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid P, MacInnes H, Cong ME, Heneine W, Garcia-Lerma JG. 2005. Natural resistance of human immunodeficiency virus type 2 to zidovudine. Virology 336:251–264. 10.1016/j.virol.2005.03.030 [DOI] [PubMed] [Google Scholar]
- 20.Amie SM, Noble E, Kim B. 2013. Intracellular nucleotide levels and the control of retroviral infections. Virology 436:247–254. 10.1016/j.virol.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjursell G, Skoog L. 1980. Control of nucleotide pools in mammalian cells. Antibiot. Chemother. 28:78–85 [DOI] [PubMed] [Google Scholar]
- 22.Rampazzo C, Miazzi C, Franzolin E, Pontarin G, Ferraro P, Frangini M, Reichard P, Bianchi V. 2010. Regulation by degradation, a cellular defense against deoxyribonucleotide pool imbalances. Mutat. Res. 703:2–10. 10.1016/j.mrgentox.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 23.Pauls E, Ballana E, Este JA. 2013. Nucleotide embargo by SAMHD1: a strategy to block retroviral infection. Antiviral Res. 97:180–182. 10.1016/j.antiviral.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 24.Cribier A, Descours B, Valadao AL, Laguette N, Benkirane M. 2013. Phosphorylation of SAMHD1 by cyclin A2/CDK1 regulates its restriction activity toward HIV-1. Cell Rep. 3:1036–1043. 10.1016/j.celrep.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 25.White TE, Brandariz-Nunez A, Valle-Casuso JC, Amie S, Nguyen LA, Kim B, Tuzova M, Diaz-Griffero F. 2013. The retroviral restriction ability of SAMHD1, but not its deoxynucleotide triphosphohydrolase activity, is regulated by phosphorylation. Cell Host Microbe 13:441–451. 10.1016/j.chom.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.St. Gelais C, de Silva S, Hach JC, White TE, Diaz-Griffero F, Yount JS, Wu L. 2014. Identification of cellular proteins interacting with the retroviral restriction factor SAMHD1. J. Virol. 88:5834–5844. 10.1128/JVI.00155-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angus SP, Wheeler LJ, Ranmal SA, Zhang X, Markey MP, Mathews CK, Knudsen ES. 2002. Retinoblastoma tumor suppressor targets dNTP metabolism to regulate DNA replication. J. Biol. Chem. 277:44376–44384. 10.1074/jbc.M205911200 [DOI] [PubMed] [Google Scholar]
- 28.Huber AD, Michailidis E, Schultz ML, Ong YT, Bloch N, Puray-Chavez MN, Leslie MD, Ji J, Lucas AD, Kirby KA, Landau NR, Sarafianos SG. 27 May 2014. SAMHD1 has differential impact on the efficacies of HIV nucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 10.1128/AAC.02745-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diget EA, Zuwala K, Berg RK, Laursen RR, Soby S, Ostergaard L, Melchjorsen J, Mogensen TH. 2013. Characterization of HIV-1 infection and innate sensing in different types of primary human monocyte-derived macrophages. Mediators Inflamm. 2013:208412. 10.1155/2013/208412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji X, Wu Y, Yan J, Mehrens J, Yang H, DeLucia M, Hao C, Gronenborn AM, Skowronski J, Ahn J, Xiong Y. 2013. Mechanism of allosteric activation of SAMHD1 by dGTP. Nat. Struct. Mol. Biol. 20:1304–1309. 10.1038/nsmb.2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan J, Kaur S, DeLucia M, Hao C, Mehrens J, Wang C, Golczak M, Palczewski K, Gronenborn AM, Ahn J, Skowronski J. 2013. Tetramerization of SAMHD1 is required for biological activity and inhibition of HIV infection. J. Biol. Chem. 288:10406–10417. 10.1074/jbc.M112.443796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong Y. 2014. Kill HIV by starvation: SAMHD1 as a potent viral restriction factor and a master regulator of cellular dNTP metabolism, abstr 114. Top. Antivir. Med. 22(e-1):58–59 [Google Scholar]
- 33.Miazzi C, Ferraro P, Pontarin G, Rampazzo C, Reichard P, Bianchi V. 2014. Allosteric regulation of the human and mouse deoxyribonucleotide triphosphohydrolase sterile alpha-motif/histidine-aspartate domain containing protein 1 (SAMHD1). J. Biol. Chem. 10.1074/jbc.M114.571091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein DS, Moore KH. 2001. Phosphorylation of nucleoside analog antiretrovirals: a review for clinicians. Pharmacotherapy 21:11–34. 10.1592/phco.21.1.11.34439 [DOI] [PubMed] [Google Scholar]
- 35.Gao WY, Shirasaka T, Johns DG, Broder S, Mitsuya H. 1993. Differential phosphorylation of azidothymidine, dideoxycytidine, and dideoxyinosine in resting and activated peripheral blood mononuclear cells. J. Clin. Invest. 91:2326–2333. 10.1172/JCI116463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wells AD, Morawski PA. 2014. New roles for cyclin-dependent kinases in T cell biology: linking cell division and differentiation. Nat. Rev. Immunol. 14:261–270. 10.1038/nri3625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adje-Toure CA, Cheingsong R, Garcia-Lerma JG, Eholie S, Borget MY, Bouchez JM, Otten RA, Maurice C, Sassan-Morokro M, Ekpini RE, Nolan M, Chorba T, Heneine W, Nkengasong JN. 2003. Antiretroviral therapy in HIV-2-infected patients: changes in plasma viral load, CD4+ cell counts, and drug resistance profiles of patients treated in Abidjan, Cote d'Ivoire. AIDS 17(Suppl 3):S49–S54 [PubMed] [Google Scholar]
- 38.van der Ende ME, Guillon C, Boers PH, Ly TD, Gruters RA, Osterhaus AD, Schutten M. 2000. Antiviral resistance of biologic HIV-2 clones obtained from individuals on nucleoside reverse transcriptase inhibitor therapy. J. Acquir. Immune Defic. Syndr. 25:11–18. 10.1097/00126334-200009010-00002 [DOI] [PubMed] [Google Scholar]
- 39.Crow YJ, Rehwinkel J. 2009. Aicardi-Goutieres syndrome and related phenotypes: linking nucleic acid metabolism with autoimmunity. Hum. Mol. Genet. 18:R130–R136. 10.1093/hmg/ddp293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rice GI, Bond J, Asipu A, Brunette RL, Manfield IW, Carr IM, Fuller JC, Jackson RM, Lamb T, Briggs TA, Ali M, Gornall H, Couthard LR, Aeby A, Attard-Montalto SP, Bertini E, Bodemer C, Brockmann K, Brueton LA, Corry PC, Desguerre I, Fazzi E, Cazorla AG, Gener B, Hamel BC, Heiberg A, Hunter M, van der Knaap MS, Kumar R, Lagae L, Landrieu PG, Lourenco CM, Marom D, McDermott MF, van der Merwe W, Orcesi S, Prendiville JS, Rasmussen M, Shalev SA, Soler DM, Shinawi M, Spiegel R, Tan TY, Vanderver A, Wakeling EL, Wassmer E, Whittaker E, Lebon P, Stetson DB, Bonthron DT, Crow YJ. 2009. Mutations involved in Aicardi-Goutieres syndrome implicate SAMHD1 as regulator of the innate immune response. Nat. Genet. 41:829–832. 10.1038/ng.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clifford R, Louis T, Robbe P, Ackroyd S, Burns A, Timbs AT, Wright Colopy G, Dreau H, Sigaux F, Judde JG, Rotger M, Telenti A, Lin YL, Pasero P, Maelfait J, Titsias M, Cohen DR, Henderson SJ, Ross MT, Bentley D, Hillmen P, Pettitt A, Rehwinkel J, Knight SJ, Taylor JC, Crow YJ, Benkirane M, Schuh A. 2013. SAMHD1 is mutated recurrently in chronic lymphocytic leukemia and is involved in response to DNA damage. Blood 123:1021–1031. 10.1182/blood-2013-04-490847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aird KM, Zhang R. 29 January 2014. Nucleotide metabolism, oncogene-induced senescence and cancer. Cancer Lett. 10.1016/j.canlet.2014.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galmarini CM, Mackey JR, Dumontet C. 2002. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol. 3:415–424. 10.1016/S1470-2045(02)00788-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.