Abstract
Reduced Plasmodium falciparum sensitivity to short-course artemisinin (ART) monotherapy manifests as a long parasite clearance half-life. We recently defined three parasite founder populations with long half-lives in Pursat, western Cambodia, where reduced ART sensitivity is prevalent. Using the ring-stage survival assay, we show that these founder populations have reduced ART sensitivity in vitro at the early ring stage of parasite development and that a genetically admixed population contains subsets of parasites with normal or reduced ART sensitivity.
TEXT
Plasmodium falciparum resistance to frontline antimalarial drugs has repeatedly emerged in Southeast Asia and spread to Africa (1), prompting the World Health Organization in 2005 to recommend the worldwide use of artemisinin (ART)-based combination therapies (ACTs) for uncomplicated falciparum malaria (2). Parasites with reduced ART sensitivity have since become entrenched in western Cambodia (3–7), have emerged elsewhere in Southeast Asia (8–11), and are threatening the efficacy of all ACTs (12). Reduced ART sensitivity manifests as a long parasite clearance half-life in patients treated with an ART derivative or an ACT (13, 14). Based on a population structure analysis of genome-wide single-nucleotide polymorphism (SNP) data from 293 Cambodian parasites, we previously identified three highly differentiated founder populations (KH2, KH3, and KH4) of slow-clearing parasites in Pursat Province in western Cambodia and a subpopulation (KH1) of fast-clearing parasites in Ratanakiri Province in eastern Cambodia (15).
While the half-life distributions of KH2, KH3, and KH4 were similar (15), these data may be confounded by host factors (e.g., hemoglobin E [5], acquired immunity [16], and pharmacokinetics) or parasite stage at the time of ART treatment (17, 18), all of which may influence parasite clearance kinetics in vivo. To investigate the intrinsic ART sensitivity of founder populations in the absence of potential confounders, we sought to characterize them in vitro using the ring-stage survival assay (RSA0–3 h) (17). This assay measures the percentage of early (0- to 3-h) ring forms that survive a pharmacologically relevant dose (700 nM for 6 h) of dihydroartemisinin, the active metabolite of all ARTs. We selected 51 parasite isolates from Pursat and Ratanakiri (5, 15), adapted them to in vitro culture for several weeks, and genotyped them at 12 SNPs, as described previously (17). Four parasites that did not adapt to culture and three that differed genetically from the initial isolates were discarded.
The remaining 44 parasites (39 from Pursat, 5 from Ratanakiri) were genotyped from deep-sequencing read data at 681,546 high-quality exonic SNPs as part of the MalariaGEN Plasmodium falciparum community project (version 3.1 data release) (19). Based on an updated population structure analysis of genome-wide SNP data from 515 Cambodian parasites (O. Miotto et al., submitted for publication), we reassigned all 44 isolates to a core subpopulation (KH-C, n = 6), one of three western Cambodian founder populations (WKH-F01, n = 5; WKH-F02, n = 3; WKH-F04, n = 11), or an unclassified subpopulation (KH-U, n = 19). Using parasite clearance half-lives as phenotypes, KH-C was categorized as fast clearing, and WKH-F01, WKH-F02, and WKH-F04 were categorized as slow clearing (O. Miotto et al., submitted for publication). KH-U, which appears genetically admixed, shows a wide range of half-life values and cannot be reliably classified as fast clearing or slow clearing.
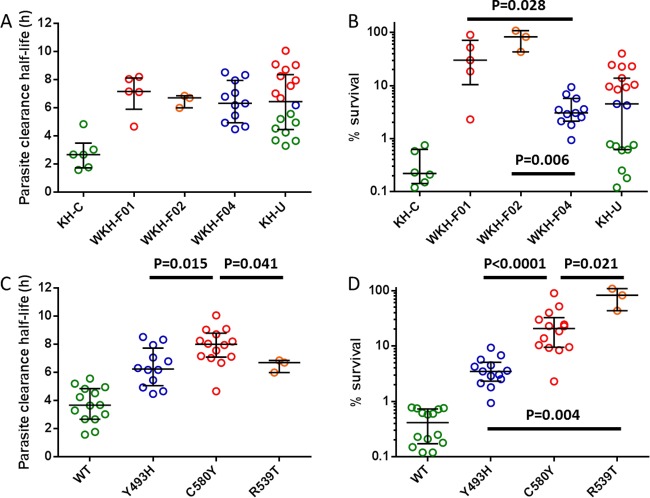
The median (range) half-life for parasites in KH-C was 2.68 h (1.58 to 4.84 h) and was significantly longer for parasites in WKH-F01 (7.18 h [4.67 to 8.21 h]; P = 0.009, Mann-Whitney test) (GraphPad Prism 6, GraphPad Software, La Jolla, CA), WKH-F02 (6.72 h [6.00 to 6.87 h]; P = 0.024), and WKH-F04 (6.32 h [4.49 to 8.54 h]; P = 0.0006) (Fig. 1A). Half-life values did not differ between the founder populations (P = 0.556, Kruskal-Wallis test). The distribution of half-lives in KH-U (median, 6.44 h [range, 3.31 to 10.1 h]) suggested that this group contains a mixture of fast-clearing and slow-clearing parasites (Fig. 1A). To investigate whether founder populations differ in their level of reduced ART sensitivity in vitro and whether KH-U parasites could be separated into parasites with normal or reduced ART sensitivity, we performed the RSA0–3 h on all of the isolates.
FIG 1.
Half-lives and percent-survival values of Cambodian Plasmodium falciparum isolates stratified by parasite subpopulation (A and B) or K13-propeller allele (C and D). The color codes for the WT, Y493H, C580Y, and R539T alleles used in panels A and B are indicated in panels C and D. All founder and mutant populations had significantly longer half-lives and higher percent-survival values than core and WT populations. P values were calculated using the Mann-Whitney test and are shown for significant differences among the founder or mutant populations.
The median (range) percent survival for parasites in KH-C was 0.22% (0.12% to 0.75%) and was significantly higher for parasites in WKH-F01 (30.0% [2.30% to 90.0%]; P = 0.004, Mann-Whitney test), WKH-F02 (82.6% [43.3% to 108%]; P = 0.024), and WKH-F04 (3.04% [0.94% to 9.36%]; P = 0.0002) (Fig. 1B). The percent-survival distributions differed among the founder populations (P = 0.002, Kruskal-Wallis test), with significantly higher values in WKH-F01 and WKH-F02 than in WKH-F04. These data show that all three founder populations have reduced ART sensitivities in vitro and that the percent-survival phenotype can vary between and within the founder populations. The median (range) percent survival for KH-U was 4.50% (0.12% to 40.0%), and the distribution of percent-survival values clearly shows that KH-U contains a mixture of parasites with normal or reduced ART sensitivity (Fig. 1B). Using data from all of the isolates, the percent survival values and half-lives correlated significantly (r = 0.6965, P < 0.0001, Spearman correlation test).
In Cambodia, mutations in the K13-propeller domain of a kelch protein (PF3D7_1343700) were recently associated with reduced ART sensitivity in vivo and in vitro (7). We investigated whether K13-propeller mutations are associated with founder populations and whether they distinguish parasites with normal or reduced ART sensitivity in KH-U. We found that the WKH-F01, WKH-F02, and WKH-F04 parasites harbored exclusively the C580Y, R539T, and Y493H alleles, respectively, while the KH-C parasites carried the wild-type (WT) allele (Fig. 1B). Within KH-U, the WT allele clearly identified parasites with normal ART sensitivity as having a survival rate of <1%, and the C580Y and Y493H alleles identified parasites with reduced ART sensitivity as having a survival rate of ≥1%. These data indicate that K13-propeller mutations are associated with specific founder populations and clearly segregate parasites with normal or reduced ART sensitivity in KH-U.
To explore whether K13-propeller mutations confer different levels of reduced ART sensitivity in vivo and in vitro, we stratified our data by K13-propeller alleles. The median (range) half-life for WT parasites was 3.67 h (1.58 to 5.58 h, n = 14) and was significantly longer for Y493H (6.26 h [4.49 to 8.54 h], n = 14; P < 0.0001, Mann-Whitney test), C580Y (8.01 h [4.67 to 10.1 h], n = 14; P < 0.0001), and R539T (6.72 h [6.00 to 6.87 h], n = 3; P = 0.003) parasites (Fig. 1C). The half-life values differed between the mutant populations (P = 0.024, Kruskal-Wallis test) and were significantly higher for C580Y parasites than for Y493H and R539T parasites. The median (range) percent survival for WT parasites was 0.41% (0.12% to 0.78%) and was significantly higher for Y493H (3.48% [0.94% to 9.36%]; P < 0.0001, Mann-Whitney test), C580Y (20.7% [2.30% to 90.0%]; P < 0.0001), and R539T (82.6% [43.3% to 108%]; P = 0.003) parasites (Fig. 1D). C580Y and R539T parasites had significantly higher percent-survival values than Y493H parasites, and R539T parasites had higher percent-survival values than C580Y parasites. These data indicate that parasites carrying the same K13-propeller mutation, even those within the same founder population, can differ substantially in their half-lives and percent-survival values, suggesting that genetic or other factors modulate the level of reduced ART sensitivity in vivo and in vitro. The possibility that R539T parasites, which have the highest percent-survival values, clear faster than C580Y parasites may indicate additional mutation-specific effects on parasite clearance or greater loss of fitness in vivo, but this requires further investigation.
In summary, the WKH-F01, WKH-F02, and WKH-F04 founder populations in Pursat have reduced ART sensitivity in vitro and are associated with the C580Y, R539T, and Y493H K13-propeller alleles, respectively. These founder populations and K13-propeller mutations may be associated with different levels of reduced ART sensitivity in vivo and in vitro. Compared to the parasite clearance half-life, the percent-survival phenotype more clearly discriminates parasites with normal or reduced ART sensitivity in KH-U. This is likely because the in vitro RSA0–3 h measures the intrinsic susceptibility of stage-synchronized parasites to ART in the absence of host factors.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the NIAID, NIH, and grants from the Institut Pasteur du Cambodge (International Division, Institut Pasteur, and Banque Natixis).
B.W. was supported by a postdoctoral fellowship from the International Division, Institut Pasteur, and D.M. was supported by the French Ministry of Foreign Affairs.
We thank Roberto Amato, Jennifer Anderson, Char Meng Chuor, Robert Gwadz, Pharath Lim, Sokunthea Sreng, Seila Suon, and Thomas Wellems for their efforts in support of this work.
Footnotes
Published ahead of print 27 May 2014
REFERENCES
- 1.World Health Organization. 2010. Global report on antimalarial efficacy and drug resistance: 2000-2010. World Health Organization, Geneva, Switzerland [Google Scholar]
- 2.World Health Organization. 2010. Guidelines for the treatment of malaria, 2nd ed. World Health Organization, Geneva, Switzerland [Google Scholar]
- 3.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359:2619–2620. 10.1056/NEJMc0805011 [DOI] [PubMed] [Google Scholar]
- 4.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455–467. 10.1056/NEJMoa0808859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amaratunga C, Sreng S, Suon S, Phelps ES, Stepniewska K, Lim P, Zhou C, Mao S, Anderson JM, Lindegardh N, Jiang H, Song J, Su XZ, White NJ, Dondorp AM, Anderson TJ, Fay MP, Mu J, Duong S, Fairhurst RM. 2012. Artemisinin-resistant Plasmodium falciparum in Pursat Province, western Cambodia: a parasite clearance rate study. Lancet Infect. Dis. 12:851–858. 10.1016/S1473-3099(12)70181-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amaratunga C, Mao S, Sreng S, Suon S, Fairhurst RM. 2013. Slow parasite clearance rates in response to artemether in patients with severe malaria. Lancet Infect. Dis. 13:113–114. 10.1016/S1473-3099(12)70347-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Menard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Menard D. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55. 10.1038/nature12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. 2012. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379:1960–1966. 10.1016/S0140-6736(12)60484-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hien TT, Thuy-Nhien NT, Phu NH, Boni MF, Thanh NV, Nha-Ca NT, Thai LE, Thai CQ, Toi PV, Thuan PD, Long LT, Dong LT, Merson L, Dolecek C, Stepniewska K, Ringwald P, White NJ, Farrar J, Wolbers M. 2012. In vivo susceptibility of Plasmodium falciparum to artesunate in Binh Phuoc Province, Vietnam. Malar. J. 11:355. 10.1186/1475-2875-11-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyaw MP, Nyunt MH, Chit K, Aye MM, Aye KH, Lindegardh N, Tarning J, Imwong M, Jacob CG, Rasmussen C, Perin J, Ringwald P, Nyunt MM. 2013. Reduced susceptibility of Plasmodium falciparum to artesunate in southern Myanmar. PLoS One 8(3):e57689. 10.1371/journal.pone.0057689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Char MC, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Nguyen TTN, Ngo VT, Nguyen HP, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, MacInnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WJ, Yeung S, Woodrow CJ, Flegg J, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ. The spread of artemisinin resistance in falciparum malaria. N. Engl. J. Med., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dondorp AM, Fairhurst RM, Slutsker L, Macarthur JR, Breman JG, Guerin PJ, Wellems TE, Ringwald P, Newman RD, Plowe CV. 2011. The threat of artemisinin-resistant malaria. N. Engl. J. Med. 365:1073–1075. 10.1056/NEJMp1108322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flegg JA, Guerin PJ, White NJ, Stepniewska K. 2011. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar. J. 10:339. 10.1186/1475-2875-10-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White NJ. 2011. The parasite clearance curve. Malar. J. 10:278. 10.1186/1475-2875-10-278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miotto O, Almagro-Garcia J, Manske M, Macinnis B, Campino S, Rockett KA, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Duong S, Nguon C, Chuor CM, Saunders D, Se Y, Lon C, Fukuda MM, Amenga-Etego L, Hodgson AV, Asoala V, Imwong M, Takala-Harrison S, Nosten F, Su XZ, Ringwald P, Ariey F, Dolecek C, Hien TT, Boni MF, Thai CQ, Amambua-Ngwa A, Conway DJ, Djimde AA, Doumbo OK, Zongo I, Ouedraogo JB, Alcock D, Drury E, Auburn S, Koch O, Sanders M, Hubbart C, Maslen G, Ruano-Rubio V, Jyothi D, Miles A, O'Brien J, Gamble C, Oyola SO, Rayner JC, Newbold CI, Berriman M, Spencer CC, McVean G, Day NP, White NJ, Bethell D, Dondorp AM, Plowe CV, Fairhurst RM, Kwiatkowski DP. 2013. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat. Genet. 45:648–655. 10.1038/ng.2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopera-Mesa TM, Doumbia S, Chiang S, Zeituni AE, Konate DS, Doumbouya M, Keita AS, Stepniewska K, Traore K, Diakite SA, Ndiaye D, Sa JM, Anderson JM, Fay MP, Long CA, Diakite M, Fairhurst RM. 2013. Plasmodium falciparum clearance rates in response to artesunate in Malian children with malaria: effect of acquired immunity. J. Infect. Dis. 207:1655–1663. 10.1093/infdis/jit082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witkowski B, Amaratunga C, Khim N, Sreng S, Chim P, Kim S, Lim P, Mao S, Sopha C, Sam B, Anderson JM, Duong S, Chuor CM, Taylor WR, Suon S, Mercereau-Puijalon O, Fairhurst RM, Menard D. 2013. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. Lancet Infect. Dis. 13:1043–1049. 10.1016/S1473-3099(13)70252-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amaratunga C, Witkowski B, Khim N, Menard D, Fairhurst RM. 2014. Artemisinin resistance in Plasmodium falciparum. Lancet Infect. Dis. 14:449–450. 10.1016/S1473-3099(14)70777-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manske M, Miotto O, Campino S, Auburn S, Almagro-Garcia J, Maslen G, O'Brien J, Djimde A, Doumbo O, Zongo I, Ouedraogo JB, Michon P, Mueller I, Siba P, Nzila A, Borrmann S, Kiara SM, Marsh K, Jiang H, Su XZ, Amaratunga C, Fairhurst R, Socheat D, Nosten F, Imwong M, White NJ, Sanders M, Anastasi E, Alcock D, Drury E, Oyola S, Quail MA, Turner DJ, Ruano-Rubio V, Jyothi D, Amenga-Etego L, Hubbart C, Jeffreys A, Rowlands K, Sutherland C, Roper C, Mangano V, Modiano D, Tan JC, Ferdig MT, Amambua-Ngwa A, Conway DJ, Takala-Harrison S, Plowe CV, Rayner JC, Rockett KA, Clark TG, Newbold CI, Berriman M, MacInnis B, Kwiatkowski DP. 2012. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature 487:375–379. 10.1038/nature11174 [DOI] [PMC free article] [PubMed] [Google Scholar]