Abstract
Specific bacterial species are implicated in the pathogenesis of exacerbations of chronic obstructive pulmonary disease (COPD). However, recent studies of clinically stable COPD patients have demonstrated a greater diversity of airway microbiota, whose role in acute exacerbations is unclear. In this study, temporal changes in the airway microbiome before, at the onset of, and after an acute exacerbation were examined in 60 sputum samples collected from subjects enrolled in a longitudinal study of bacterial infection in COPD. Microbiome composition and predicted functions were examined using 16S rRNA-based culture-independent profiling methods. Shifts in the abundance (≥2-fold, P < 0.05) of many taxa at exacerbation and after treatment were observed. Microbiota members that were increased at exacerbation were primarily of the Proteobacteria phylum, including nontypical COPD pathogens. Changes in the bacterial composition after treatment for an exacerbation differed significantly among the therapy regimens clinically prescribed (antibiotics only, oral corticosteroids only, or both). Treatment with antibiotics alone primarily decreased the abundance of Proteobacteria, with the prolonged suppression of some microbiota members being observed. In contrast, treatment with corticosteroids alone led to enrichment for Proteobacteria and members of other phyla. Predicted metagenomes of particular microbiota members involved in these compositional shifts indicated exacerbation-associated loss of functions involved in the synthesis of antimicrobial and anti-inflammatory products, alongside enrichment in functions related to pathogen-elicited inflammation. These trends reversed upon clinical recovery. Further larger studies will be necessary to determine whether specific compositional or functional changes detected in the airway microbiome could be useful indicators of exacerbation development or outcome.
INTRODUCTION
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are a major source of morbidity, characterized by worsening of respiratory symptoms from the baseline normal variation that require new treatment (http://www.goldcopd.org/). Culture-based evaluations have implicated bacteria in ∼50% of exacerbations (1). While acquisition of new strains of Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis, and Pseudomonas aeruginosa is associated with exacerbations (2, 3), these represent relatively few species compared to the overall diversity of airway microbiota recently described in COPD (4–7).
Detection of bacteria in most prior studies was limited by the use of culture-based methods (8, 9). Application of more sensitive molecular tools has consistently shown a greater diversity of airway microbiota in COPD, despite differences in specimens and platforms across studies (4–7, 10). With increasing appreciation of the fact that the microbial microenvironment can modify the behavior of primary pathogens (11, 12), this invites speculation that other microbiota members may contribute to the pathogenesis of AECOPD.
In this pilot study, we investigated the dynamics of the airway bacterial microbiome in the setting of AECOPD by examining a total of 60 sputum samples collected before, at the onset of, and after an exacerbation. Compositional and functional analyses were performed using 16S rRNA-based culture-independent and in silico predictive metagenomic approaches. The relationships between microbiome composition and clinical factors were examined, including the type of treatment prescribed for exacerbation (antibiotics alone, systemic corticosteroids alone, or both).
MATERIALS AND METHODS
Subjects and sample collection.
The sputum samples analyzed were collected from 12 subjects enrolled in a longitudinal study of bacterial infection in COPD described extensively in previous publications (2, 3, 13). To briefly summarize, the parent study has been conducted at the Buffalo Veterans Affairs Hospital and approved by the VA Western New York Institutional Review Board since 1994. All participants gave written informed consent. Members of a cohort of approximately 50 participants with COPD (and without other lung disease) were followed monthly and whenever they experienced worsening symptoms suggestive of an exacerbation. Expectorated sputum was collected at each visit and processed as previously described (2). This included homogenization with dithiothreitol and plating of aliquots of serial dilutions on blood, chocolate, and MacConkey culture agar. Sputum cell pellets obtained after centrifugation were stored long term at −80°C in a glycerol solution and used for microbiome analyses in this study. Molecular typing of isolates of H. influenzae, M. catarrhalis, and P. aeruginosa was performed as previously described (2) to determine whether a new strain, defined as one that had not been isolated from sputum samples obtained previously (>4 weeks) from an individual patient, was present at exacerbation (2).
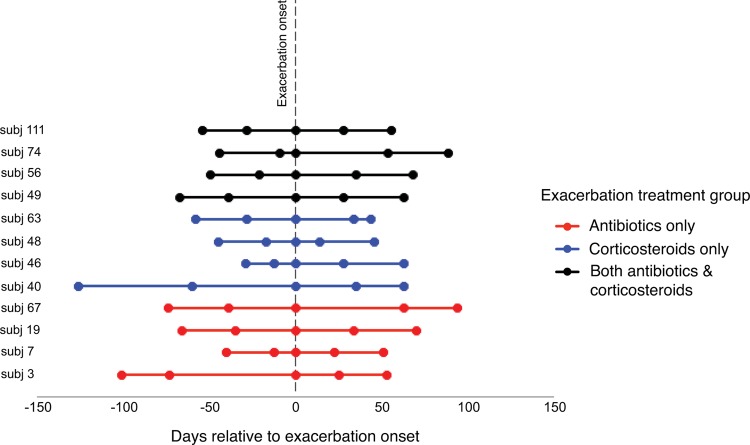
To investigate the exacerbation-related microbiome, subjects and samples were carefully selected for this study to include a diversity of exacerbations, those that were and were not associated with a new strain, and also treatment approaches commonly prescribed in clinical practice (antibiotics only, systemic corticosteroids only, or both). Specimens from five time points per subject were examined (Fig. 1). The specimens spanned an exacerbation-related time period occurring in a given year between 1999 and 2005 (with the specific year depending on the subject). Pre- and postexacerbation samples were those available closest to the exacerbation when the patients were at their baseline respiratory state and on their usual COPD treatment. All subjects were encouraged to seek treatment early for a suspected exacerbation; however, a fixed duration of symptoms was not proscribed. However, all exacerbation samples included in this study were obtained prior to initiation of any specific treatment for the exacerbation. Exacerbation events were determined using predefined criteria described previously (2) and involved evaluation of respiratory symptoms (dyspnea, cough, sputum production, viscosity, and purulence) and their change from the usual state. Pneumonia, if suspected, was ruled out by chest X ray. Decisions about treatments were made by study physicians (S. Sethi and T. Murphy) on the basis of symptoms (especially sputum purulence) and prior to the availability of sputum culture data from the concurrent visit. Antibiotics were prescribed if bacterial infection was thought to be a cause of the exacerbation, whereas steroids only were prescribed if a nonbacterial etiology was suspected.
FIG 1.
Time points of sample collection before, at the onset of, and after an acute COPD exacerbation. The line coloring indicates the type of treatment for the exacerbation that the subject received.
Sample preparations.
DNA was extracted utilizing methods previously described (14) and detailed further in the supplemental material. Briefly, universal 16S rRNA bacterial primers (Bact-27F, Bact-1492R) were used to generate amplicons, which were processed for hybridization to G2 PhyloChip microarrays (Second Genome, South San Francisco, CA) by methods previously described (14, 15). Each sample was hybridized to an individual array, resulting in 60 microbiome profiles. Bacterial taxa were defined as organisms sharing ≥97% 16S rRNA gene sequence homology and classified according to the Hugenholtz taxonomy in the 2011 iteration of the Greengenes database (16).
Data analyses.
Raw array data were processed as previously described (17–19), including correction for background and noise and scaling of fluorescence intensities to those for spiked-in quantitative standards, followed by further data normalization on the basis of the mean intensity of all profiles. The presence of taxa was determined using published methods (19, 20), with slightly modified scoring thresholds based on the scores for spiked-in quantitative standards being applied. Further information is detailed in the supplemental material. The initial normalized data set for all 60 samples combined included ∼4,800 taxa, and this data set was then filtered to include taxa present in at least 10% of samples, yielding for analysis a final data set of ∼3,300 taxa (available in Table S5 in the supplemental material). Log2-transformed intensities, which correlate with the relative abundance of taxa (21), were used in all primary analyses, which were performed using R (version 2.14.1).
Community richness (number of taxa detected), evenness (relative distribution of taxa in a community), and diversity (Shannon and inverse Simpson indices, which consider both the richness and the evenness in the calculation, as well as Faith's phylogenetic diversity, which additionally incorporates phylogenetic information in this calculation [22]), were calculated using the R packages vegan and picante (23, 24). Changes in these metrics were tested using repeated-measures analysis of variance (RM-ANOVA) and paired t tests. Bray-Curtis and Unifrac distance measures (25, 26) were calculated and used in ordination analyses (e.g., nonmetric multidimensional scaling [NMDS]) to assess compositional differences and relationships to clinical factors, including the timing of pre- and postexacerbation sample collection relative to the exacerbation onset date, by distance matrix-based permutational multivariate analysis of variance (PERMANOVA) (27). Taxon-level analyses to identify specific compositional changes between time points were performed using linear models based on moderated t statistics and a paired test design in the R package limma (28). Adjustment for multiple comparisons was applied using the Benjamini-Hochberg method or q values (29). Changes of at least 2-fold (log2 difference ≥ 1.0; unadjusted P < 0.05) were considered notable and significant if the Benjamini-Hochberg-adjusted P value or q value was <0.05. These criteria were extrapolated from thresholds commonly applied to screen for differentially expressed genes in microarray-based studies and that have also been used in prior PhyloChip-based studies (30, 31). For the predictive functional analyses, the PICRUSt software package (32) was used to identify predicted gene families and associated pathways from inferred metagenomes of taxa of interest identified from the compositional analyses.
qPCR and sequencing.
Total 16S rRNA gene copies in samples were determined using methods previously described (14). Species-specific quantitative PCR (qPCR) and Sanger sequencing were also performed to confirm, respectively, array-reported relative abundances and the identities of representative organisms of interest. To further compare and validate the microarray data, 11 samples with sufficient remaining DNA that represented different sampling time points underwent 16S rRNA sequencing using the Illumina MiSeq platform. Details of these experiments are described extensively in the supplemental material.
RESULTS
Relationships between bacterial community composition and clinical associations.
For each subject, two samples from a clinically stable period prior to exacerbation (Pre1 and Pre2; range, 12 to 126 days before exacerbation), a single sample obtained at exacerbation onset before the start of new treatments (Exac), and two samples from after exacerbation when treatments were completed (Post1 and Post2; range, 14 to 94 days after the date of exacerbation onset) were collected (Fig. 1). The characteristics of the subjects, most of whom had moderately severe airflow obstruction (Global Initiative for Chronic Obstructive Lung Disease stage II by criteria published at the time that samples were collected [33]), and the exacerbations are shown in Table 1.
TABLE 1.
Clinical characteristics of subjects and exacerbationsa
| Subject | Age (yr) | FEV1 (%) | Smoking status (pack-yr) | Inhaled steroid use | Clinical score at Exacb | Sputum purulence at Exac | Sputum culture result from Exac sample | Preexisting or new bacterial strain at Exac | Antibiotic for Exac | Systemic steroids for Exac |
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 67 | 91 | Former (10) | Yes | 20 | Purulent | P. aeruginosa | New | Azithromycin | No |
| 7 | 65 | 63 | Former (105) | No | 18 | Purulent | H. influenzae | New | Ofloxacin | No |
| 19 | 51 | 63 | Current (48) | Yes | 19 | Purulent | M. catarrhalis | New | Azithromycin | No |
| 40 | 63 | 56 | Current (112) | Yes | 14 | Mucoid | None | NA | None | Yes |
| 46 | 71 | 48 | Former (125) | No | 13 | Mucopurulent | M. catarrhalis, Klebsiella spp. | Preexisting, NA | None | Yes |
| 48 | 64 | 46 | Current (45) | Yes | 15 | Mucoid | None | NA | None | Yes |
| 49 | 66 | 61 | Former (100) | Yes | 24 | Purulent | Pseudomonas fluorescens | NA | Azithromycin | Yes |
| 56 | 61 | 42 | Current (135) | Yes | 22 | Purulent | H. influenzae | New | Ciprofloxacin | Yes |
| 63 | 80 | 51 | Former (87) | Yes | 13 | Mucoid | H. influenzae, M. catarrhalis, S. pneumoniae | Preexisting for all three | None | Yes |
| 67 | 66 | 48 | Current (50) | No | 23 | Purulent | S. pneumoniae | New | Trimethoprim-sulfamethoxazole | No |
| 74 | 72 | 55 | Former (96) | Yes | 21 | Mucopurulent | H. influenzae, P. aeruginosa | Preexisting, new | Ofloxacin | Yes |
| 111 | 68 | 38 | Current (108) | Yes | 23 | Purulent | M. catarrhalis | New | Gatifloxacin | Yes |
All subjects were Caucasian male veterans. Age, lung function, and smoking status information were collected during a clinically stable state at the time of enrollment in the parent study. Inhaled corticosteroid use status was current for the analyzed time points. FEV1, forced expiratory volume in 1 s; pack-yr, number of packs of cigarettes smoked per day multiplied by the number of years that the person has smoked; NA, not applicable.
See reference 13.
Across all samples examined, bacterial community richness, evenness, and diversity did not differ significantly over time (RM-ANOVA) or when they were considered across pairwise comparisons of sampling time points, e.g., Post1 versus Exac (paired Student's t test). However, the data indicated a trend toward a decrease in diversity following treatment with antibiotics. In contrast, diversity increased in the steroid only-treated group. Similar trends were observed for richness.
To further evaluate relationships between community composition and clinical characteristics, multivariate analyses using PERMANOVA based on a constructed Bray-Curtis distance matrix (27) were performed. No significant relationships were found between community composition and parameters of lung function, symptom severity score at exacerbation, or the time points at which samples were collected (number of days relative to the exacerbation onset date). The latter results indicated that variation among subjects in the timing of sample collection before and after an exacerbation was not a significant factor in the observed compositional differences.
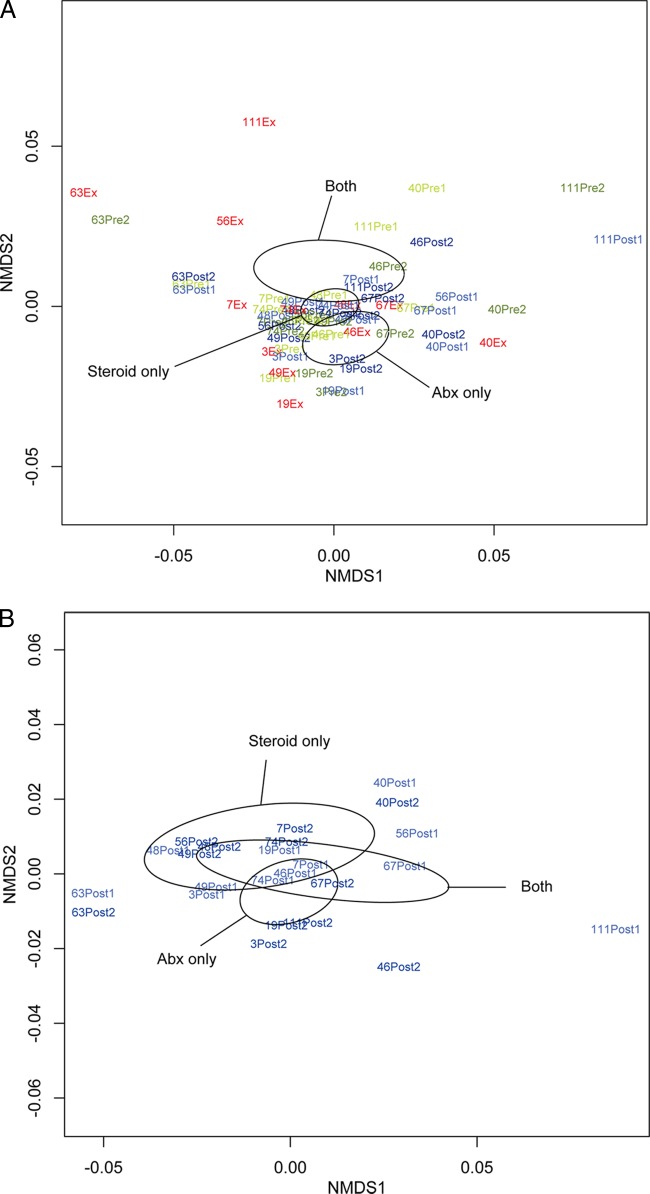
In clinical practice as well as in the parent study, clinical impression regarding the etiology of a COPD exacerbation determines treatment choices. We therefore examined first, using all samples, whether an association existed between the type of exacerbation treatment (antibiotics only, oral steroids only, or both; n = 4 subjects per treatment group) and differences in community composition among samples. A statistically significant relationship between community composition and the treatment administered for exacerbation was observed (P < 0.05; Fig. 2A). When we limited this analysis to only postexacerbation samples, the association remained close to statistically significant (P = 0.08; Fig. 2B), indicating that the preexacerbation and exacerbation samples contribute to the strength of this relationship. These results also suggest that the specific treatment administered for exacerbation has the potential to have a prolonged impact on community composition.
FIG 2.
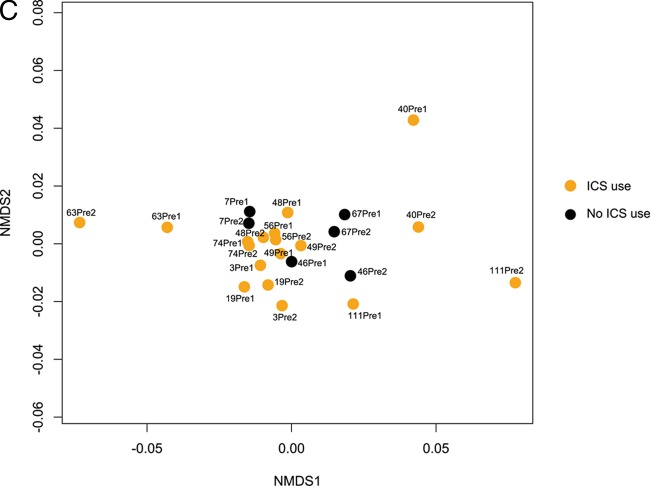
(A) Nonmetric multidimensional scaling (NMDS) ordination demonstrates that samples from all subjects in the study segregate into distinct clusters (indicated by ellipses) on the basis of the type of treatment prescribed for exacerbation (antibiotics [Abx] only, oral steroids only, or both; n = 4 subjects per treatment group; Bray-Curtis distance-based PERMANOVA, P < 0.05). Ellipses represent the 95% confidence interval for the standard error of the distances of samples in each treatment group. (B) An NMDS plot of only postexacerbation treatment samples from all subjects demonstrates that the relationship between sample community composition and treatment type remains strong, though not statistically significant (P = 0.08), when the analysis is limited to only these samples. (C) An NMDS plot of only preexacerbation samples from all subjects shows that chronic use of inhaled corticosteroids may be associated with differences in bacterial community composition (Bray-Curtis distance-based PERMANOVA, P = 0.08).
Chronic use of inhaled corticosteroids (ICSs) also demonstrated a nearly statistically significant relationship with differences in community composition (P = 0.06, based on analysis of all samples; P = 0.08 for analysis limited to only preexacerbation samples; Fig. 2C), indicating that ICSs may impact the community composition across the time points sampled in this study. We also observed a trend toward greater richness and diversity in samples from ICS-exposed subjects than those from non-ICS-exposed subjects, indicating that ICSs, perhaps through their immunosuppressive activity, increase the airway microbiota diversity in COPD patients.
Taxon-level analyses of temporal changes in COPD airway microbiota composition.
To determine the specific taxa that exhibited significant changes in relative abundance during the course of this study, we compared array-reported abundances for each taxon between sequential time points (R package limma). For this set of analyses, we first performed paired time point comparisons using data from all subjects, followed by separate analyses for each exacerbation treatment group (antibiotics only, systemic steroids only, or both) limited to only the postexacerbation time points. Collectively, these results revealed several notable findings.
During the clinically stable period before exacerbation (Pre1-to-Pre2 comparison; Fig. 3A), relatively few taxa exhibited differential relative abundance. Only three taxa showed an increase of ≥2-fold (the largest was a Moraxella species, which showed a 2.6-fold increase; P = 0.001), whereas Neisseriaceae members made up many of the taxa that decreased by ≥2-fold. Overall, these observations suggest relative stability in the airway microbiota composition during a clinically stable period prior to AECOPD in this cohort.
FIG 3.
(A) Volcano plots indicating taxa that are significantly increased (upper right quadrant) or decreased (upper left quadrant) in the pairwise comparisons indicated, using moderated t-test models (R package limma). Results shown are from analyses of all subjects. Dashed lines, significant false discovery rate-adjusted P values and changes in relative abundance of at least 2-fold, or log2 equal to 1. Taxa exhibiting significant changes are colored by phylum-level classification, as shown. Note that in addition to the highlighted taxa, many other microbiota members exhibited smaller-scale changes in abundance, which cumulatively may contribute importantly to microbiome community function, (B) Changes in the relative abundance of taxa from Exac to Post1 and Post1 to Post2, segregated by type of exacerbation treatment (antibiotics only, systemic corticosteroids only, or both; n = 4 subjects in each treatment group).
At exacerbation, it was predominantly members of the Proteobacteria, such as the Moraxellaceae, Pasteurellaceae, Pseudomonadaceae, and Enterobacteriaceae, that were enriched compared to their levels in Pre2 (Table 2). These enriched taxa included other potential respiratory pathogens, in addition to those typically associated with AECOPD. In contrast, taxa that were decreased in abundance at exacerbation relative to their abundance in Pre2 were predominantly Actinobacteria, Clostridia, and Bacteroidia (Table 2). Actinobacteria comprise a large group of metabolically diverse organisms that are prolific producers of secondary metabolites, including those with antimicrobial activity (34), while clade IV and XIVA Clostridia are known inducers of anti-inflammatory T-regulatory cells (35). To speculate, these observations suggest the possibility that members of the microbiome that are depleted at the time of exacerbation may otherwise help maintain community and host immune homeostasis and potentially counteract the detrimental effects of respiratory pathogenic species.
TABLE 2.
Bacterial taxa that demonstrated the largest changes in relative abundance at the onset of exacerbation compared to that at the most recent preexacerbation time point (Pre2)a
| Taxon (class/family/genus) | prokMSA_ID for taxon representative speciesb | Fold changec | P value |
|---|---|---|---|
| Gammaproteobacteria/Moraxellaceae/Acinetobacter | 60270 | +2.9 | 0.01 |
| Gammaproteobacteria/Moraxellaceae/Perlucidibaca | 105696 | +2.7 | 0.02 |
| Gammaproteobacteria/Moraxellaceae/Acinetobacter | 106871 | +2.4 | 0.04 |
| Gammaproteobacteria/Moraxellaceae/Acinetobacter | 101304 | +2.4 | 0.006 |
| Betaproteobacteria/Anaplasmataceae/Ehrlichia | 6030 | +2.2 | 0.02 |
| Gammaproteobacteria/Pasteurellaceae/Actinobacillus | 52835 | +2.1 | 0.04 |
| Alphaproteobacteria/Sphingomonadaceae/Sphingomonas | 89065 | +2.1 | 0.01 |
| Betaproteobacteria/Comamonadaceae/Comamonas | 71872 | +2.0 | 0.03 |
| Gammaproteobacteria/Enterobacteriaceae | 10365 | +2.0 | 0.04 |
| Gammaproteobacteria/Pseudomonadaceae/Pseudomonas | 8434 | +2.0 | 0.01 |
| Bacilli/Bacillaceae/unclassified | 89195 | −1.9 | 0.04 |
| Epsilonproteobacteria/Campylobacteraceae/Campylobacter | 108770 | −1.9 | 0.03 |
| Bacteroidia/Rikenellaceae/unclassified | 101395 | −1.9 | 0.01 |
| Clostridia/Clostridiales family XIII/Mogibacterium | 14167 | −1.8 | 0.002 |
| Actinobacteria/Microbacteriaceae/Agromyces | 12174 | −1.8 | 0.02 |
| Clostridia/Lachnospiraceae/unclassified | 72977 | −1.8 | 0.005 |
| Actinobacteria/ACK-M1/unclassified | 96076 | −1.8 | 0.01 |
| Actinobacteria/Micrococcaceae/Rothia | 58305 | −1.8 | 0.03 |
| Actinobacteria/Nocardiaceae/Rhodococcus | 85556 | −1.8 | 0.04 |
Changes in relative abundance were ≥2-fold (P < 0.05, q ≤ 0.30) at the onset of exacerbation compared to that at the most recent preexacerbation time point (Pre2). The results are based on analysis of all subjects in the study (n = 12 subjects).
prokMSA_ID is the identifier used by the Greengenes database.
Fold change shows the increase or decrease at exacerbation.
The most dramatic changes in community composition were observed after treatment for exacerbation (Exac to Post1). This was supported by a significant difference in the mean Unifrac distance of communities between Exac and Post1 compared to that for communities between Pre2 and Exac, reflecting a significant change in the phylogenetic makeup of the community (P < 0.01; see Fig. S1 in the supplemental material). Among those taxa exhibiting significant alterations in relative abundance, the vast majority decreased in abundance, were primarily members of the Proteobacteria, and included organisms both associated with and not traditionally associated with COPD (P < 0.05, q ≤ 0.12; see Table S1 in the supplemental material).
Striking differences, however, were seen in the effects of different treatments on community composition (Fig. 3B). Treatment with antibiotics alone reduced the abundance of many taxa (mostly Proteobacteria), a number of which exhibited further reductions in relative abundance at the second posttreatment time point (Post1 to Post2). The latter finding supports our data indicating a relationship between treatment type and community composition in posttreatment samples and provides evidence for the prolonged suppressive effects of antibiotics on some microbiota members. For subjects who received any antibiotics (either alone or with steroids), a similar pattern of reduction in microbiota members at Post1 was observed. However, in contrast to those treated with antibiotics only, those treated with both antibiotics and steroids showed a significant increase in abundance of primarily Proteobacteria from Post1 to Post2. This was also reflected in an increase in phylogenetic diversity at Post2 compared to that at Post1 for this group (P < 0.01) and suggests that the recovery of airway community diversity following antibiotic treatment could be influenced by concomitant corticosteroid treatment.
In contrast to antibiotics, treatment with corticosteroids alone led predominantly to enrichment for many taxa, mainly Proteobacteria, but also Bacteroidetes and Firmicutes members (Fig. 3B). Large increases were observed especially for the Enterobacteriaceae (up to 16-fold), Lachnospiraceae, Burkholderiaceae, and Neisseriaceae (see Table S2 in the supplemental material). This was mirrored by a significantly higher mean 16S rRNA copy number, a proxy for bacterial burden, in this group compared to the other treatment groups (RM-ANOVA, P ≤ 0.02; for the steroids-only group versus the antibiotics-only group, P < 0.01; see Fig. S2 in the supplemental material). This indicates that treatment with systemic corticosteroids alone can lead to an increased bacterial burden and an increased abundance of specific airway microbiota.
Relationships between COPD-associated pathogens and other microbiota members.
Previous studies of the gut microbiota have shown that specific organisms in a niche can promote community enrichment for related species and influence colonization susceptibilities (36). To explore this in the airway microbiome context, we evaluated relationships between known COPD-related pathogens and other microbiota members by performing correlation analyses between the detected abundance of taxa representing H. influenzae, P. aeruginosa, or M. catarrhalis and that of all other taxa. Strong positive correlations were observed between H. influenzae and taxa belonging to the same or related bacterial families for this species (R ≥ 0.5, Benjamini-Hochberg-adjusted P < 0.05), e.g., Pasteurellaceae, Enterobacteriaceae, and Pseudomonadaceae (Fig. 4). Most belong to the larger class of Gammaproteobacteria, as well as other classes of Proteobacteria. In contrast, taxa negatively correlated with H. influenzae were primarily members of more phylogenetically distant groups. Similar correlation patterns were observed for P. aeruginosa and M. catarrhalis (data not shown). These observations suggest that enrichment of phylogenetically related organisms in a pathogen-rich airway milieu may represent an important factor in microbiome dynamics associated with the pathogenesis of exacerbations.
FIG 4.
Correlations between the relative abundance of H. influenzae (a member of the Pasteurellaceae/Gammaproteobacteria) and that of all other identified taxa. The analysis was performed using data from all 60 samples in this study. Significant positive and negative correlations are shown (Pearson R ≥ 0.5, Benjamini-Hochberg-adjusted P < 0.05). Positive correlations (red) occur predominantly with members of Pasteurellaceae or closely related bacterial families and classes of Proteobacteria. Negative correlations (blue) occur with bacterial families and classes that phylogenetically are more distant to H. influenzae/Pasteurellaceae. Tree branches are color coded by bacterial class in the key on the right.
Quantitative PCR and sequencing validation studies.
Total 16S rRNA gene copy numbers ranged from 1.9 × 105 to 1.8 × 108 (see Fig. S2 in the supplemental material). While these did not differ significantly over time, the type of treatment for exacerbation was a significant factor in the observed differences (RM-ANOVA, P = 0.02), with the group treated with corticosteroids alone demonstrating higher mean total 16S rRNA copy numbers (for steroids only versus antibiotics only, P < 0.01; for steroids only versus both antibiotics and steroids, P = 0.08).
Sanger sequencing using taxon-specific primers confirmed the identity of M. catarrhalis and organisms representative of other taxons, including species of Corynebacteriaceae, Porphyromonadaceae, Enterobacteriaceae, and Bifidobacteria (see Table S3 in the supplemental material). Species-specific qPCR studies also showed excellent correlations between copy numbers and the array-reported abundances of H. influenzae (R = 0.92, P < 0.03) and P. aeruginosa (R = 0.95, P < 0.01). To further validate array-reported taxon abundances, 16S rRNA sequencing using the Illumina MiSeq platform was performed on 11 samples. Strong correlations between the relative abundance of specific phyla and families detected by both sequencing and array analysis were observed (i.e., Proteobacteria, Actinobacteria, Pasteurellaceae, Moraxellaceae, Enterobacteriaceae; Spearman ρ = 0.7 to 0.9, P < 0.001 to 0.01; see Table S4 in the supplemental material).
Functional analysis of COPD airway microbiome.
Metagenomic profiling of the microbiome affords insights into the functional capacity of the community. However, to do so at high resolution is both expensive and computationally intensive. To explore specifically the predicted functional capacity of the microbiota involved in the changes observed at exacerbation and in response to treatment, we applied a recently developed bioinformatics tool, PICRUSt (32), which predicts the functional capacity of a community based on 16S rRNA data. PICRUSt has been used to predict the presence and relative abundance of gene families in different microbiomes and shown to recapitulate the results of actual metagenomic sequencing efforts from the Human Microbiome Project (32).
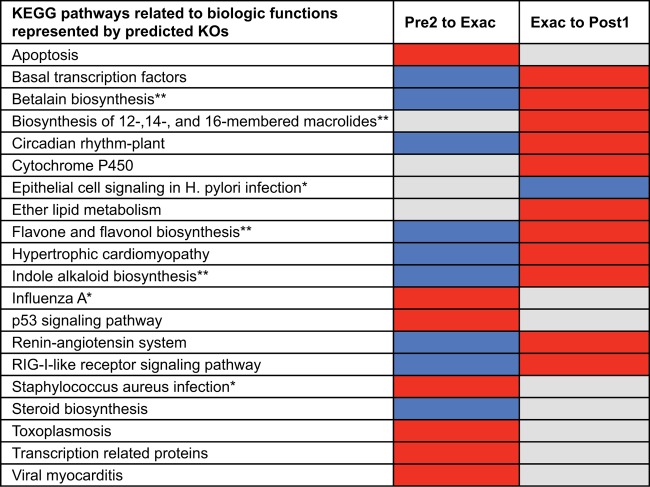
Representative 16S rRNA sequences for taxa that changed in abundance by ≥2-fold were used as PICRUSt inputs. The results predicted a number of KEGG gene orthologs (KOs; Kyoto Encyclopedia of Genes and Genomes, release 67.1) associated with known KEGG pathways to be enriched or depleted within the bacterial community at these time points (Fig. 5). Predicted functions enriched at exacerbation included pathways involved in viral and bacterial infection (e.g., influenza A virus and Staphylococcus aureus infection), as well as those involved in apoptosis and p53 signaling. Conversely, functions predicted to be depleted involved pathways associated with the response to viral infection (e.g., RIG-I-like receptor signaling) and pathways involved in flavonoid and steroid biosynthesis (known anti-inflammatory mediators [37]), as well as betalain and indole alkaloid production (known antimicrobial properties [38, 39]). These observations suggest that a loss of community functions associated with maintenance of microbial homeostasis and regulation of the host immune response is associated with AECOPD. This is further supported by the observation that after treatment, reverse trends were observed. For example, predicted KOs that were enriched for after exacerbation included those involved in the betalain, indole alkaloid, and flavonol biosynthesis pathways. In addition, KOs involved in macrolide biosynthesis were also predicted to be enriched posttreatment. Thus, microbiome enrichment for taxa that encode these anti-inflammatory functional capacities may contribute to recovery from exacerbation.
FIG 5.
KEGG pathways associated with predicted gene functions (KOs) encoded by metagenomes of bacterial taxa that either were increased (red) or decreased (blue) in relative abundance by ≥2-fold at the time of and after exacerbations. Among the microbiome-related functions identified were those involved in the promotion of inflammation by pathogens (*) and the production of antimicrobial and anti-inflammatory compounds (**), functions that were differentially represented at exacerbation compared to postexacerbation.
DISCUSSION
In this study, we extensively examined the dynamics of the bacterial airway microbiome in the setting of AECOPD. In addition to assessment of compositional changes by multiple methods, we further explored the predicted functional capacities encoded by microbiota identified to be potentially important on the basis of observed compositional shifts. Thus, this study represents the most comprehensive effort to date to evaluate the exacerbation-associated microbiome in COPD and serves as a foundation for approaches that could be applied to study larger numbers of patients. Collectively, our findings demonstrate that the COPD airway microbiome is rich in both bacterial species and functional capacity and that identifiable changes in this microbiome are associated with the development, treatment, and resolution of AECOPD.
An important limitation of our study is that the intensive microbiome analyses were performed on 60 samples derived from only a small number of COPD patients. Offsetting this limitation is the longitudinal nature of the study, providing us with valid preexacerbation samples, as well as exacerbation samples prior to any specific treatment for exacerbation. Future studies involving larger patient numbers will be necessary, especially given the heterogeneity of COPD and interest in identifying the best treatment approaches for different phenotypes (40). Despite the cohort size, we were able to capture some of the heterogeneity associated with exacerbations by intentionally including a proportionally even number of subjects treated for exacerbation using regimens prescribed in clinical practice. In this context, we were able to identify microbiome characteristics differentially associated with features of exacerbations in these subjects. Follow-up studies involving larger numbers of carefully phenotyped patients would be of interest.
Several specific findings from our study are important to emphasize and have potential clinical implications to be further investigated. An important overall observation from a microbial community perspective is that many bacterial groups not limited to typical COPD pathogens were among those contributing to the most salient compositional changes observed. These dynamics, which involved Proteobacteria taxa, in addition to those representative of H. influenzae, M. catarrhalis, and P. aeruginosa, suggest potentially important contributions of related microbiota to the etiopathogenesis of AECOPD. Independent analyses confirmed that the abundance of these typical COPD pathogens was highly correlated with the presence of related bacterial phylotypes. While array cross-hybridization at the taxon level is a possible factor in these analyses, it unlikely accounts for the number of distinct groups, characterized at the family level and higher, found to be correlated with these species. Our observations are consistent with previous demonstrations that organisms in an ecosystem can promote enrichment for related species in a like will to like phenomenon, as observed for gut microbiota and susceptibility to pathogen colonization (36). Moreover, quorum-sensing mechanisms between organisms can influence bacterial pathogenicity or resistance to antimicrobials (41, 42). Overall, findings from this and other studies (43) indicate that the context of the microbial community is important to consider in efforts to better understand the pathogenesis of AECOPD.
Another important observation is that intersubject variability in microbial community changes at exacerbation were seen. Similar observations were made in a study using a human model of virus-induced COPD exacerbation (43). In our study, compositional changes at exacerbation in general did not involve very large shifts, although this was not true of all subjects. This is similar to what has been described in exacerbations of cystic fibrosis and non-cystic fibrosis bronchiectasis (44, 45). This suggests that AECOPD could also result from the cumulative effects of smaller-scale changes in community composition that, on the basis of PICRUSt functional predictions, lead not only to an increase in pathogen-elicited inflammation but also the loss of potentially key protective functions in the microbiome. Moreover, organisms do not need to be highly prevalent in a community to have important effects on disease pathogenesis, as has been shown for periodontitis (46). However, two of our subjects (subjects 56 and 111) did exhibit a greater compositional shift at exacerbation, determined on the basis of NMDS analysis (data not shown). Although new bacterial strains were identified in these patients, others in which new strains were also detected did not demonstrate large compositional shifts at exacerbation. Collectively, these observations suggest that there is likely interindividual heterogeneity in the microbiome associated with AECOPD.
Finally, our observations regarding the effects of COPD treatments on microbiota composition have potentially important clinical implications. Inhaled corticosteroids are commonly used in the management of COPD and other airway diseases, and our results suggest that the use of ICSs may alter the microbiome. Whether this has implications for susceptibility to exacerbations or their outcome will require additional study. Also, treatments for exacerbation led to significant changes in community composition, some of which may last beyond the usual time frame of clinical recovery from exacerbations. In particular, oral steroid therapy alone led to community enrichment for many members of the microbiota. This suggests that steroids, even when they are administered systemically, may promote microbial colonization in the airways. Overall, the long-term effects of such treatments on the microbiome in COPD, particularly with recurrent administrations, are unknown.
Strengths of this study include the systematic collection of samples at times encompassing key periods before, at, and after AECOPD. Furthermore, we extended upon the compositional analyses to explore the functional capacity encoded by key microbiome members. Weaknesses include the small number of subjects, the focus on only bacteria in examining the microbiome, and also the use of sputum as a representative specimen. Despite the potential for saliva admixture, collection of sputum represents the only practical approach for repeated airway sampling of COPD patients, particularly during exacerbations, when bronchoscopy may be unsafe. Important microbial insights, including several from this cohort, have been gleaned from analysis of sputum in many studies of COPD (2, 3, 8, 43).
In summary, the results of this study indicate that the context of the airway microbiome needs to be considered in efforts to improve the understanding of the pathogenesis of AECOPD. Given the heterogeneity of COPD, it will be important to study larger numbers of subjects to identify microbiome determinants associated with specific COPD phenotypes and associated exacerbations.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a University of California Tobacco-Related Disease Research Program grant (17FT-0040) and National Institutes of Health grant HL105572 to Y.J.H. and an American Lung Association award to S.V.L.
We gratefully acknowledge Ali Faruqi for guidance in performing PICRUSt analysis of microarray-identified taxa and Emily Cope for technical assistance with Illumina MiSeq sequencing and guidance in the analysis of the sequencing data.
Footnotes
Published ahead of print 21 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00035-14.
REFERENCES
- 1.Sethi S, Murphy TF. 2008. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 359:2355–2365. 10.1056/NEJMra0800353 [DOI] [PubMed] [Google Scholar]
- 2.Sethi S, Evans N, Grant BJ, Murphy TF. 2002. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 347:465–471. 10.1056/NEJMoa012561 [DOI] [PubMed] [Google Scholar]
- 3.Murphy TF, Brauer AL, Eschberger K, Lobbins P, Grove L, Cai X, Sethi S. 2008. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 177:853–860. 10.1164/rccm.200709-1413OC [DOI] [PubMed] [Google Scholar]
- 4.Huang YJ, Kim E, Cox MJ, Brodie EL, Brown R, Wiener-Kronish JP, Lynch SV. 2010. A persistent and diverse airway microbiota present during chronic obstructive pulmonary disease exacerbations. OMICS 14:9–59. 10.1089/omi.2009.0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, Young VB, Toews GB, Curtis JL, Sundaram B, Martinez FJ, Huffnagle GB. 2011. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One 6:e16384. 10.1371/journal.pone.0016384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pragman AA, Kim HB, Reilly CS, Wendt C, Isaacson RE. 2012. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS One 7:e47305. 10.1371/journal.pone.0047305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabrera-Rubio R, Garcia-Núñez M, Setó L, Antó JM, Moya A, Monsó E, Mira A. 2012. Microbiome diversity in the bronchial tracts of patients with chronic obstructive pulmonary disease. J. Clin. Microbiol. 50:3562–3568. 10.1128/JCM.00767-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL. 2006. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am. J. Respir. Crit. Care Med. 173:1114–1121. 10.1164/rccm.200506-859OC [DOI] [PubMed] [Google Scholar]
- 9.Rosell A, Monso E, Soler N, Torres F, Angrill J, Riise G, Zalacain R, Morera J, Torres A. 2005. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch. Intern. Med. 165:891–897. 10.1001/archinte.165.8.891 [DOI] [PubMed] [Google Scholar]
- 10.Sze MA, Dimitriu PA, Hayashi S, Elliott WM, McDonough JE, Gosselink JV, Cooper J, Sin DD, Mohn WW, Hogg JC. 2012. The lung tissue microbiome in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 185:1073–1080. 10.1164/rccm.201111-2075OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan K, Dammel C, Stein J, Rabin H, Surette MG. 2003. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol. Microbiol. 50:1477–1491. 10.1046/j.1365-2958.2003.03803.x [DOI] [PubMed] [Google Scholar]
- 12.Endt K, Stecher B, Chaffron S, Slack E, Tchitchek N, Benecke A, Van Maele L, Sirard JC, Mueller AJ, Heikenwalder M, Macpherson AJ, Strugnell R, von Mering C, Hardt WD. 2010. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog. 6:e1001097. 10.1371/journal.ppat.1001097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sethi S, Wrona C, Eschberger K, Lobbins P, Cai X, Murphy TF. 2008. Inflammatory profile of new bacterial strain exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 177:491–497. 10.1164/rccm.200708-1234OC [DOI] [PubMed] [Google Scholar]
- 14.Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, Woyke T, Allgaier M, Bristow J, Wiener-Kronish JP, Sutherland ER, King TS, Icitovic N, Martin RJ, Calhoun WJ, Castro M, Denlinger LC, Dimango E, Kraft M, Peters SP, Wasserman SI, Wechsler ME, Boushey HA, Lynch SV. 2011. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J. Allergy Clin. Immunol. 127:372–381. 10.1016/j.jaci.2010.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flanagan JL, Brodie EL, Weng L, Lynch SV, Garcia O, Brown R, Hugenholtz P, DeSantis TZ, Andersen GL, Wiener-Kronish JP, Bristow J. 2007. Loss of bacterial diversity during antibiotic treatment of intubated patients colonized with Pseudomonas aeruginosa. J. Clin. Microbiol. 45:1954–1962. 10.1128/JCM.02187-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. 2012. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 6:610–618. 10.1038/ismej.2011.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brodie EL, Desantis TZ, Joyner DC, Baek SM, Larsen JT, Andersen GL, Hazen TC, Richardson PM, Herman DJ, Tokunaga TK, Wan JM, Firestone MK. 2006. Application of a high-density oligonucleotide microarray approach to study bacterial population dynamics during uranium reduction and reoxidation. Appl. Environ. Microbiol. 72:6288–6298. 10.1128/AEM.00246-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeSantis TZ, Brodie EL, Moberg JP, Zubieta IX, Piceno YM, Andersen GL. 2007. High-density universal 16S rRNA microarray analysis reveals broader diversity than typical clone library when sampling the environment. Microb. Ecol. 53:371–383. 10.1007/s00248-006-9134-9 [DOI] [PubMed] [Google Scholar]
- 19.Hazen TC, Dubinsky EA, DeSantis TZ, Andersen GL, Piceno YM, Singh N, Jansson JK, Probst A, Borglin SE, Fortney JL, Stringfellow WT, Bill M, Conrad ME, Tom LM, Chavarria KL, Alusi TR, Lamendella R, Joyner DC, Spier C, Baelum J, Auer M, Zemla ML, Chakraborty R, Sonnenthal EL, D'haeseleer P, Holman HY, Osman S, Lu Z, Van Nostrand JD, Deng Y, Zhou J, Mason OU. 2010. Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science 330:204–208. 10.1126/science.1195979 [DOI] [PubMed] [Google Scholar]
- 20.Handley KM, Wrighton KC, Piceno YM, Andersen GL, DeSantis TZ, Williams KH, Wilkins MJ, N′Guessan AL, Peacock A, Bargar J, Long PE, Banfield JF. 2012. High-density PhyloChip profiling of stimulated aquifer microbial communities reveals a complex response to acetate amendment. FEMS Microbiol. Ecol. 81:188–204. 10.1111/j.1574-6941.2012.01363.x [DOI] [PubMed] [Google Scholar]
- 21.DeSantis TZ, Stone CE, Murray SR, Moberg JP, Andersen GL. 2005. Rapid quantification and taxonomic classification of environmental DNA from both prokaryotic and eukaryotic origins using a microarray. FEMS Microbiol. Lett. 245:271–278. 10.1016/j.femsle.2005.03.016 [DOI] [PubMed] [Google Scholar]
- 22.Faith DP. 1992. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61:1–10. 10.1016/0006-3207(92)91201-3 [DOI] [Google Scholar]
- 23.Oksanen J, Kindt R, Legendre P, O'Hara B, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H. 2008. Vegan: community ecology package. R package, version 1.16-1. R Project for Statistical Computing, Vienna, Austria [Google Scholar]
- 24.Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO. 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26:1463–1464. 10.1093/bioinformatics/btq166 [DOI] [PubMed] [Google Scholar]
- 25.Gotelli N, Ellison A. 2004. A primer of ecological statistics. Sinauer Associates, Inc, Sunderland, MA [Google Scholar]
- 26.Lozupone C, Knight R. 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71:8228–8235. 10.1128/AEM.71.12.8228-8235.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26:32–46. 10.1111/j.1442-9993.2001.01070.pp.x [DOI] [Google Scholar]
- 28.Smyth GK. 2004. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3:Article3. 10.2202/1544-6115.1027 [DOI] [PubMed] [Google Scholar]
- 29.Storey JD, Tibshirani R. 2003. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. U. S. A. 100:9440–9445. 10.1073/pnas.1530509100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sunagawa S, DeSantis TZ, Piceno YM, Brodie EL, DeSalvo MK, Voolstra CR, Weil E, Andersen GL, Medina M. 2009. Bacterial diversity and white plague disease-associated community changes in the Caribbean coral Montastraea faveolata. ISME J. 3:512–521. 10.1038/ismej.2008.131 [DOI] [PubMed] [Google Scholar]
- 31.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139:485–498. 10.1016/j.cell.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. 2013. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 31:814–821. 10.1038/nbt.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pauwels RA, Buist SA, Calverley PM, Jenkins CR, Hurd SS. 2001. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 163:1256–1276. 10.1164/ajrccm.163.5.2101039 [DOI] [PubMed] [Google Scholar]
- 34.Ventura M, Canchaya C, Tauch A, Chandra G, Fitzgerald GF, Chater KF, van Sinderen D. 2007. Genomics of Actinobacteria: tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 71:495–548. 10.1128/MMBR.00005-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. 2011. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331:337–341. 10.1126/science.1198469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stecher B, Chaffron S, Kappeli R, Hapfelmeier S, Freedrich S, Weber TC, Kirundi J, Suar M, McCoy KD, von Mering C, Macpherson AJ, Hardt WD. 2010. Like will to like: abundances of closely related species can predict susceptibility to intestinal colonization by pathogenic and commensal bacteria. PLoS Pathog. 6:e1000711. 10.1371/journal.ppat.1000711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cushnie TP, Lamb AJ. 2011. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 38:99–107. 10.1016/j.ijantimicag.2011.02.014 [DOI] [PubMed] [Google Scholar]
- 38.Vulić JJ, Cebović TN, Canadanović VM, Cetković GS, Djilas SM, Canadanović-Brunet JM, Velićanski AS, Cvetković DD, Tumbas VT. 2013. Antiradical, antimicrobial and cytotoxic activities of commercial beetroot pomace. Food Funct. 4:713–721. 10.1039/c3fo30315b [DOI] [PubMed] [Google Scholar]
- 39.Zoraghi R, Worrall L, See RH, Strangman W, Popplewell WL, Gong H, Samaai T, Swayze RD, Kaur S, Vuckovic M, Finlay BB, Brunham RC, McMaster WR, Davies-Coleman MT, Strynadka NC, Andersen RJ, Reiner NE. 2011. Methicillin-resistant Staphylococcus aureus (MRSA) pyruvate kinase as a target for bis-indole alkaloids with antibacterial activities. J. Biol. Chem. 286:44716–44725. 10.1074/jbc.M111.289033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miravitlles M, Soler-Cataluña JJ, Calle M, Soriano JB. 2013. Treatment of COPD by clinical phenotypes: putting old evidence into clinical practice. Eur. Respir. J. 41:1252–1256. 10.1183/09031936.00118912 [DOI] [PubMed] [Google Scholar]
- 41.Armbruster CE, Hong W, Pang B, Weimer KE, Juneau RA, Turner J, Swords WE. 2010. Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. mBio 1(3):e00102–10. 10.1128/mBio.00102-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sibley CD, Duan K, Fischer C, Parkins MD, Storey DG, Rabin HR, Surette MG. 2008. Discerning the complexity of community interactions using a Drosophila model of polymicrobial infections. PLoS Pathog. 4:e1000184. 10.1371/journal.ppat.1000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molyneaux PL, Mallia P, Cox MJ, Footitt J, Willis-Owen SA, Homola D, Trujillo-Torralbo MB, Elkin S, Kon OM, Cookson WO, Moffatt MF, Johnston SL. 2013. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 188:1224–1231. 10.1164/rccm.201302-0341OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF, Cavalcoli JD, Vandevanter DR, Murray S, Li JZ, Young VB, Lipuma JJ. 2012. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc. Natl. Acad. Sci. U. S. A. 109:5809–5814. 10.1073/pnas.1120577109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tunney MM, Einarsson GG, Wei L, Drain M, Klem ER, Cardwell C, Ennis M, Boucher RC, Wolfgang MC, Elborn JS. 2013. Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am. J. Respir. Crit. Care Med. 187:1118–1126. 10.1164/rccm.201210-1937OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, McIntosh ML, Alsam A, Kirkwood KL, Lambris JD, Darveau RP, Curtis MA. 2011. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 10:497–506. 10.1016/j.chom.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.