Abstract
Window chamber models have been developed and utilized as a means to study the complex microenvironment in which cancers develop, proliferate, and metastasize in small animals. Here we utilize rapid prototyping printer technology to construct a new plastic orthotopic mammary window chamber that is compatible with magnetic resonance imaging, nuclear imaging, and optical imaging. Optical imaging allows for high-resolution cellular and molecular level analysis of tissues; magnetic resonance imaging provides quantitative measures of tumor size, perfusion, diffusion, fat/water content relaxation parameters; and a nuclear imaging technique, called the Beta Imager, supports functional and metabolic imaging. Our demonstration of the multiple imaging capabilities of this model suggests that it can be used as a powerful platform for studying basic cancer biology and developing new cancer therapies.
Keywords: cancer, optical, magnetic resonance, nuclear, imaging, window chamber
Imaging has been vital to our understanding of the molecular biology of disease as well as the development of new therapies. Window chamber models have been developed as a means to study the complex micro-environment in which cancers develop, proliferate, and metastasize (1–5) since these structures enable the visualization of biology at the cellular and molecular levels within a realistic in vivo model. Window chambers are fixed structures placed on animals where the external tissue (e.g., skin, skull) is removed and replaced with a glass coverslip, providing a window into the underlying tissue. This technique allows researchers to follow disease progression or treatment outcome over the course of several days to weeks.
The dorsal skin-fold (DSF) window chamber has been widely utilized to investigate basic cancer biology and tumor development in rodent models (6–11). Numerous studies in DSF models have shown how tumor vasculature differs from normal vasculature and have measured parameters such as vascular permeability, hemoglobin saturation, red cell flux, oxygen tension, and tissue hypoxia. Such parameters have also been studied as biomarkers of therapeutic response (12–17).
Drawbacks of the DSF model are that the approach artificially constrains tumor growth to a thin tissue section and that disease development occurs outside of its natural environment. Most problematically, these constraints have the potential to alter the natural behavior of the disease process and complicate the interpretation of experimental results. Orthotopic window chambers, placed in a region where a particular cancer would normally arise, provide a more realistic model and overcome many disadvantages of ectopic models (18). A recent review article stated that non-orthotopic, subcutaneous models are often not predictive of response when used to test anti-cancer drugs (19). Investigators have therefore developed and studied different orthotopic models. In particular, mammary window chamber (MWC) models have been developed to optically image the microenvironment of breast cancer tumors (20–22).
Optical methods are well suited for imaging window chambers as they can achieve a spatial resolution along with the field-of-view necessary to image cellular detail, vascular development, and many aspects of the tumor microenvironment. However, optical techniques are limited in their depth imaging capability and thus do not take full advantage of the ability to study the three-dimensional tumor growth that occurs within orthotopic models. Optical methods such as fluorescence tomography can quantitatively assess tumor size in three dimensions, but the measurement accuracy is limited. Alternatively, magnetic resonance imaging (MRI) can provide high-resolution and high contrast morphological measurements of tumor size, location, and growth patterns, while nuclear imaging can provide a functional assessment of tumor phenotype. The combination of MRI and intravital optical imaging has been accomplished in a dorsal skin fold model (6, 23–25), and the combination of nuclear and optical imaging has also been demonstrated in a dorsal skin fold model (26). However, multimodality imaging has not been demonstrated to date in a MWC model.
Here we describe the development of an MWC mouse model compatible with optical, MRI, and nuclear imaging. The ability to obtain multiple co-registered imaging measurements in the same animal is demonstrated, and the advantages of applying a multi-modality approach are discussed.
Methods
MWC design
The structures of the MWC and animal holding apparatus were designed using SolidWorks (Dassault Systèmes Solid-Works Corp., Waltham, MA) and fabricated using an Objet Connex 350 rapid prototyping printer (Stratsys Ltd., Edina, MN). The MWC design, shown in Figure 1A, consists of a 13 mm outer diameter annular structure with a groove on the outer surface. The inner portion of the window chamber has a thin lip that supports an 8 mm diameter glass coverslip (Warner Instruments, Hamden, CT). The coverslip is held securely in place with a custom retaining ring, also manufactured with the rapid-prototyping printer, which fits under two small protrusions from the inside wall of the chamber. The retaining ring can be removed by grasping the indentions in the ring with tweezers and compressing the ring to slip it out from underneath the tabs. The coverslip can be removed to allow access to the surface of the mammary fat pad to perform nuclear imaging studies, manipulate the local tumor environment, or to deliver drugs.
Figure 1. MWC model.
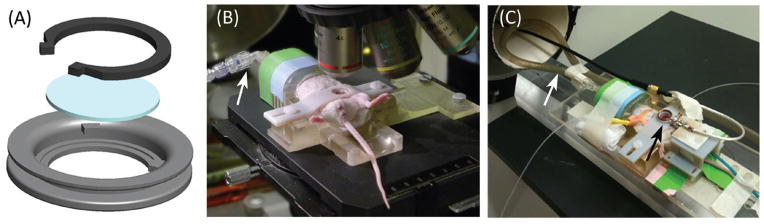
(A) Schematic displaying the components of the MWC, which include the chamber, coverslip, and snap ring. (B) MWC implanted in a mouse and secured in a holder on the microscope (white arrow points to gas anesthesia connection). (C) MWC mouse secured in a holder with custom surface coil (black arrow) centered directly over top of chamber. Anesthesia is supplied (white arrow), and temperature and respiration are monitored throughout the imaging experiments.
MWC implantation
All animal experiments and procedures were reviewed and approved by the University of Arizona Institutional Animal Care and Use Committee. Post-breeder female SCID mice were utilized due to their immune status compatibility with human cell lines and well-developed mammary glands. The chambers were implanted over the fat pad of the fourth mammary gland, located abdominally near the animal’s hind left leg. This site was chosen to reduce issues with respiratory motion artifacts. Surgery to implant the MWC structure was performed under isoflurane anesthesia and analgesics were administered to minimize pain—buprenorphine, (0.1 mg/kg IP/SC) twice a day for three days following surgery. To install the window chambers, the animal’s fur was first depilated around the nipple. A 3–4 mm diameter hole in the skin was then created over the mammary fat pad by pulling up the nipple, cutting skin around the nipple, and gently separating the skin from the underlying tissue. The skin was then stretched around the edge of the chamber as it was placed in the animal. This is a suture-less procedure, where the outer diameter of the chamber is larger than the hole created, which allows the skin to retract into the groove on the edge of the chamber to secure the structure in place. Surgical glue was applied to the periphery of the MWC to help secure the structure. Additional glue was applied at later times when it was beneficial to stabilize the structure to increase the longevity of the window chamber. The entire surgical procedure from induction of anesthesia to recovery was performed in approximately 15 min.
Cell implantation
MDA-MB-231 (triple negative) or MCF-7 (ER+) breast cancer cells from American Type Culture Collection (ATCC) were used in experiments. The cells were tested for authenticity by the University of Arizona Genetics Core using the StemElite system (Promega Corp., Madison, WI). Both cell lines were transfected to express green fluorescent protein (GFP), which allowed identification of tumors using fluorescent microscopy. The cells were grown in T175 flasks in 10% RPMI until they were 60%–90% confluent and were then harvested by trypsinization with 0.25% trypsin2 in 21 mM EDTA in HBSS for 3 min. A trypan blue cytometer assay was used to determine cell number and viability. Only mycoplasma free cells with 90% or greater viability were used for experiments. Estrogen pellets were implanted subcutaneously in mice growing MCF-7 tumors. Two protocols for the implantation of cells and chambers were tested. In the first method, the window chambers were implanted first, and 2 days later 1 × 106 to 5 × 106 GFP expressing cells, either MDA-MB-231 or MCF-7, were injected into the mammary fat pad under the coverslip of the window chamber. To maintain adequate hydration, the animals were given 1 mL saline subcutaneous injections daily starting one day prior to chamber implantation and continuing two days post-cell injection. In the second protocol, the cells were implanted first underneath the nipple into the mammary fat pad. Approximately 1 week later, the window chamber was implanted over the established tumor. Prior to implanting the window chamber, the fur was removed and a fluorescence microscope used to observe the diffuse GFP signal in order to assess the tumor size and location.
Animal housing and care
Mice were housed individually in cages in a temperature and humidity controlled environment with free access to food and water. Yuk-2e was applied daily to the exposed plastic surfaces of the chamber to discourage chewing. Additionally, food treats, such as sunflower seeds, were placed in the cage to distract the animal from chewing on the window chamber.
Imaging
Animals were anesthetized with 2% isoflurane before being placed in the animal holder and maintained on 1% isoflurane with 1 L/min O2 flow rate with slight modifications made based on respiration rate. The window chamber was secured to the holder, shown in Figure 1B, with a cross bar to minimize tissue movement. Temperature was monitored with a rectal probe and maintained at a 37°C (±3°C) with either a heat lamp or circulating warm water pad. Ophthalmic ointment was applied to the eyes. In the case of MRI, where the animal is not visible to the operator, respiration was monitored via a pressure sensor placed at the animal’s chest.
Optical imaging was performed using a Nikon E600 microscope with a C1 confocal attachment (Nikon Instruments Inc., Melville, NY). Bright-field optical microscopy was performed using a quartz halogen lamp coupled to a goose-neck fiber illuminator in an epi-illumination configuration. Fluorescent imaging of GFP was done using a broad-spectrum mercury lamp and a Nikon B-2E/C filter cube. Images were captured on a RETIGA 2000R 12-bit per color-channel cooled CCD with 1600 × 1200 pixels (QImaging, Surrey, BC, Canada). High-resolution confocal images were acquired on the same microscope using the Nikon C1 attachment. Fluorescent contrast agents were administered to demonstrate molecular level targeting and cellular resolution imaging in the model. Albumin labeled with Alexa Fluor 647 (200 μL, 0.055 mg/μL) (Invitrogen, Grand Island, NY) was intravenously injected to highlight vasculature. To assess cell viability, 50 μL of annexin V labeled with Alexa Fluor 647 and 50 μL of propidium iodide (Invitrogen) were injected subcutaneously at the site of the tumor at concentrations of 2 mL/kg and 1 mg/mL, respectively.
MRI of the mammary window chamber model was performed using a Bruker Biospec 7 Tesla small-animal MRI system (Bruker Corporation, Billerica, MA). The animal holder was modified for MRI experiments, as shown in Figure 1C, to hold a single-turn radio frequency (RF) surface coil (Doty Scientific, Columbia, SC) over the center of the window chamber. The holder and attached surface coil were placed inside a linear volume coil centered in the bore of the magnet. The linear volume coil was used for RF excitation while the surface coil was used as the receiver coil. An intraperitoneal injection of 469.01 mg/mL Magnevist (Gd-DTPA) (Bayer HealthCare Pharmaceuticals LLC, Montville, NJ) was administered to demonstrate increased contrast between tumor and surrounding tissue.
A specialized nuclear imaging system, called the Beta Imager (26), was utilized instead of traditional nuclear imaging, which requires specialized hardware and reconstruction techniques. In the Beta Imager technique, a thin scintillator is placed directly in contact with the exposed tissue surface in the window chamber. Due to the shallow depth of the tissue at the surface of the MWC, some positrons can escape from the tissue without annihilating with an electron. The escaped positrons interact with the scintillator to produce visible light photons, which can be detected inside a light shielded box with a cooled CCD, similar to devices used in bioluminescence imaging. The Beta Imager approach has relatively high spatial resolution and sensitivity for imaging radiotracer distributions near the tissue surface. In preparation for imaging, animals were fasted for 12 h, then the coverslip was removed, the exposed tissue was washed with sterile saline, and a thin scintillator material (26) was placed in contact with the tissue. Radiolabeled glucose analog, 0.2 mL of 5–6 mCi 18F-fluorodeoxy-glucose (FDG), was injected intravenously. After imaging, the wound was washed, and the coverslip and retaining ring were replaced. Animals were monitored for several days following imaging for any signs of infection. No cases of infection were observed.
For all imaging modalities, including optical, MR, and nuclear, animal preparation prior to imaging took 15 min. Image acquisition lasted between 5 min to 1 h, depending on the experiment.
Results and discussion
The protocol for implanting cells prior to MWC implantation was 100% successful in growing a viable tumor within the chamber (16 animals). An alternative method of injecting cells after MWC placement was 93% successful (28 animals). Growing the tumor before placing the MWC allows control in selecting tumor size prior to the start of a study and maximizes the time period during which the window chamber remains viable for imaging experiments. No adverse reactions were observed in response to the plastic materials employed nor does the MWC appear to affect tumor growth.
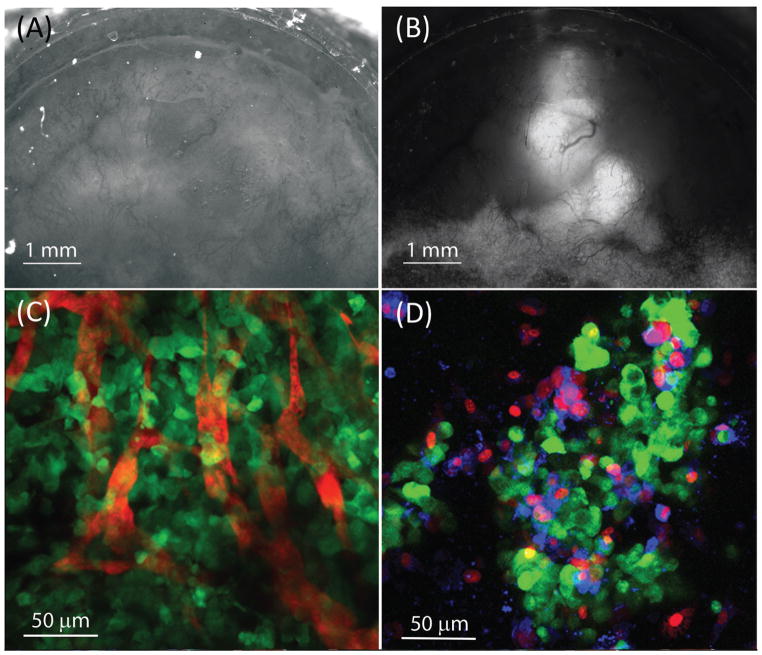
White light imaging of the MWC is shown in Figure 2A, where the mammary fat pad tissue and vascular network can be observed. Fluorescence imaging of the GFP signal from the same MWC is shown in Figure 2B. The location and extent of GFP tumor expression in the MWC can be assessed temporally to monitor growth and visualize tumor angiogenesis.
Figure 2. Optical images of the MWC.
(A) 2× white light epi-illumination image, showing the tissue and blood vessels. (B) 2× wide-field fluorescence image of the same location showing the MDA-MB-231 GFP expressing tumor mass and some of the vascular system, (tumor is 6 days old). (C) Maximum intensity projection of a z-stack of confocal images covering a 60 micron deep section of tissue acquired with a 40× objective after injection of intravascular contrast agent, albumin Alexa Fluor 647 vascular network (red), and MDA-MB-231 GFP cancer cells (green), (tumor is 3 days old). (D) Maximum intensity projection of a z-stack of confocal images acquired with a 40× objective in a region containing MCF-7 GFP tumor cells (green) approximately 1 hour after the injection of the cell-membrane impermeable dye, propidium iodide (red) and phosphatidylserine binding dye, annexin V labeled with Alexa Fluor 647 (blue), (tumor is 7 days old).
Visualization of individual GFP expressing cancer cells was achieved using high-resolution confocal microscopy. The optical sectioning capability of confocal microscopy allowed delineation of individual cancer cells. The images shown in Figure 2, C and D were obtained using a 40× objective. Albumin labeled Alexa Fluor 647 contrast agent was administered intravenously to observe the distribution of the microvasculature in relationship to the cancer cells. A maximum intensity projection image, Figure 2C, was created from a z-stack volume acquisition covering a 60 μm thick section of tissue. Figure 2C shows capillary vessels containing contrast agent (red) among tumor cells (green). The ability to visualize the vascular distribution in relation to the cancer cells has potential value in a number of applications, such as assessing the effectiveness of anti-angiogenic cancer therapies, monitoring the extravasation and biodistribution of cancer drugs, or investigating regional variations in the tumor microenvironment.
An example of the ability to target and assess characteristics of individual cells in vivo is shown in Figure 2D, where propidium iodide and annexin V labeled with Alexa Fluor 647 were injected to distinguish between viable, apoptotic, and necrotic cells. A maximum intensity projection image containing a cluster of GFP expressing tumor cells an hour after staining is shown in Figure 2D. In this assay, early apoptotic cells should have only annexin V (blue) staining of the translocated phosphatidylserine on the extracellular side of the membrane and no propidium iodide (red) staining of the nucleus. Necrotic cells, on the other hand, have both annexin V staining of phosphatidylserine and propidium idodide staining of the nucleus due to the compromised permeability of the cell membrane. Viable cells have no annexin V or propidium iodide staining, but can be delineated as cancer cells based on GFP (green) fluorescence. Cancer cells, necrotic cells, and a few apoptotic cells can be observed in Figure 2D, demonstrating standard in vitro assays can be applied when using this model. In addition to conventional and confocal optical microscopy, the MWC is compatible with alternative techniques such as multi-photon imaging, nonlinear microscopy, and structured illumination.
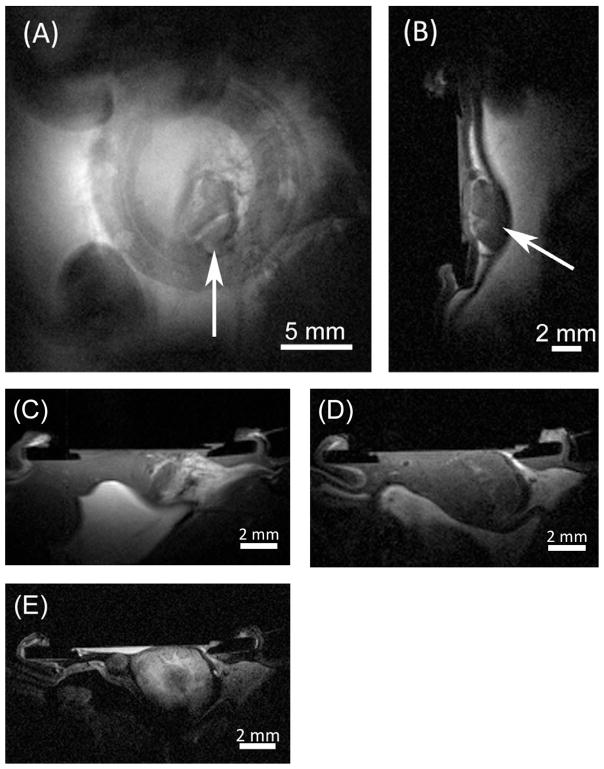
Detailed three-dimensional anatomical visualization of tumors can be obtained using MRI. Figure 3, A and B show en-face (coronal) and cross-sectional (sagittal) T1-weighted slices through a tumor (spin-echo sequence parameters: TR = 250 ms, TE = 10.747 ms, 256 × 256 matrix covering a 2.56 cm × 2.56 cm field of view, 5 mm thick coronal slice and 1.5 mm sagittal slice). A series of slices was acquired to cover the whole extent of the tumor, allowing quantitative assessment of tumor volume with high accuracy. The T1-weighted images have high signal intensity from fat within the mammary fat pad and below the peritoneal lining. The peritoneal lining, forming the boundary between the outer fat/muscle layers and the internal organs, is observed as a distinct dark band between the tumor and internal fat. Both orientations allow visualization of the window chamber structure due to the void in signal where the plastic resides. The MWC structure supports location co-registration between imaging modalities (Supplementary Material).
Figure 3. Magnetic resonance images of the MWC.
(A) Coronal slice with arrow designating location of a 7 day old MDA-MB-231 tumor; (B) corresponding sagittal slice through middle of tumor pressing against peritoneal lining (black band); (C) T1-weighted image showing a 5 day old MDA-MB-231 tumor growing amid (white/higher signal) mammary fat; (D) same tumor at 13 days growing deeper into the body of the mouse; (E) same tumor at 13 days with Gd contrast agent showing darker, potentially necrotic tumor core.
Figures 3C–E show MR images of a tumor in an animal over an 8 day time period (spin-echo sequence parameters: TR = 400 ms, TE = 10.747 ms, 256 × 256 matrix, and 2 averages). The image in Figure 3C was acquired 5 days after cell injection, with a 1 mm axial slice thickness and a 2.56 cm × 2.56 cm FOV. The image in Figure 3D was acquired 13 days post cell injection with a thinner 0.5 mm axial slice thickness but the same FOV. The image in Figure 3E was acquired on the same day as Figure 3D after an injection of Magnevist contrast agent to improve contrast between the tumor and surrounding tissue. Enhanced signal first appeared as a ring around the periphery of the tumor (data not shown). A thinner 0.3 mm axial slice thickness and a smaller 1.92 cm × 1.92 cm FOV with the same 256 × 256 matrix were used to achieve higher spatial resolution in Figure 3E, revealing more detail in the structure of the tumor 15 min after injection, when contrast agent had diffused throughout the tumor. This image also reveals a small tumor nodule that was difficult to identify on the original T1-weighted image obtained without contrast agent.
MRI can also provide quantitative maps of relaxation parameters (T1, T2, and T2*), fat and water content, diffusion properties, and blood perfusion. Moreover, important functional information regarding the vascular permeability and altered metabolism can be obtained by advanced MR imaging and/or spectroscopy techniques. (For quantitative MRI results, see Supplementary Material).
The Beta Imager was used to assess glycolytic metabolism in two different animal tumors. A series of sequential images were acquired with 5 min exposures following intravenous injection of 18F-fluorodeoxy-glucose (FDG). Figure 4, A and B display the FDG signal (red) obtained in 2 animals 42 and 55 min after contrast agent injection, respectively. The signal intensity is overlaid on a co-registered white light reflectance image with the GFP tumor outlined. Figure 4A shows good correlation between the regions of increased metabolism (higher FDG signal) and the location of the GFP tumor signal. Figure 4B shows a lack of FDG signal within the tumor. These results highlight the ability to image variation in the metabolic properties of tumors using MWCs.
Figure 4. Nuclear imaging with FDG.

Nuclear Beta images following intravenous injection of FDG colorized to a red scale, overlaid on a combined white light reflectance and fluorescent GFP image of MDA-MB-231 tumors, with the tumor outline displayed in green. (A) In the first data set, the 5 day old GFP tumor location correlates with the FDG signal. (B) In a second animal, a large portion of a 13 day old tumor does not correlate with the FDG signal.
In conclusion, we have developed an orthotopic breast cancer mammary window chamber model that is compatible with multi-modality imaging. We have demonstrated high-resolution optical imaging, high-resolution MR imaging, and functional nuclear imaging. The different resolutions, imaging depths, and functional capabilities of the modalities complement one another. The utility of the MWC model, coupled with these imaging modalities, provides a powerful platform technology for therapeutic development and studies of basic cancer biology.
Supplementary Material
METHOD SUMMARY.
Mammary window chamber structures were surgically implanted over human breast cancer xenograft tumors grown in the mammary fat pads of mice. The window chamber model enabled imaging of the tumor and surrounding microenvironment with multiple imaging modalities, including optical imaging, magnetic resonance imaging, and functional metabolic imaging.
Acknowledgments
We thank Bethany Skovan and Gillian Paine-Murrieta of the University of Arizona Cancer Center’s Experimental Mouse Shared Service for assistance with surgical implantation of the window chambers. We also thank Christy Howison for her assistance with animal injections. We also acknowledge the Center for Gamma Ray Imaging for maintaining and running the rapid prototyping printer, with a special thanks to Cecile Chaix. This work was supported by the Fenton Maynard Endowment supporting Dr. Gmitro’s research in cancer imaging, NIH Training Grant T32EB000809, and the University of Arizona TRIF Imaging Fellowship Program. This paper is subject to the NIH Public Access Policy.
Footnotes
Supplementary material for this article is available at www.BioTechniques.com/article/114191.
Author contributions
R.S. was responsible for the development of the model. Data collection and imaging were performed by R.S. and H.L. The manuscript was written by R.S. with assistance from A.G.
Competing interests
The authors declare no competing interests.
References
- 1.Fukumura D, Jain RK. Imaging angiogenesis and the microenvironment. APMIS. 2008;116:695–715. doi: 10.1111/j.1600-0463.2008.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodall CM, Sanders AG, Shubik P. Studies of Vascular Patterns in Living Tumors With a Transparent Chamber Inserted in Hamster Cheek Pouch. J Natl Cancer Inst. 1965;35:497–521. doi: 10.1093/jnci/35.3.497. [DOI] [PubMed] [Google Scholar]
- 3.Huang Q, Shan S, Braun RD, Lanzen J, Anyrhanbatla G, Kong G. Noninvasive visualization of tumors in rodent dorsal skin window chambers A novel model for evaluating anti-cancer therapies. Nat Biotechnol. 1999;17:1033–1035. doi: 10.1038/13736. [DOI] [PubMed] [Google Scholar]
- 4.Nims JC, Irwin JW. Chamber Techniques to Study the Microvasculature. Microvasc Res. 1973;5:105–118. doi: 10.1016/s0026-2862(73)80013-5. [DOI] [PubMed] [Google Scholar]
- 5.Papenfuss HD, Gross JF, Intaglietta M, Treeses FA. A Transparent Access Chamber for the Rat Dorsal Skin Fold. Microvasc Res. 1979;18:311–318. doi: 10.1016/0026-2862(79)90039-6. [DOI] [PubMed] [Google Scholar]
- 6.Gmitro AF, Moore S, Gatenby R. In Vivo MRI and Intravital Optical Microscopy of Window Chambers in Mice. Intl Soc Magn Res Med Annu Meet. 2006;14:51. [Google Scholar]
- 7.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koehl GE, Gaumann A, Geissler EK. Intravital microscopy of tumor angiogenesis and regression in the dorsal skin fold chamber: mechanistic insights and preclinical testing of therapeutic strategies. Clin Exp Metastasis. 2009;26:329–344. doi: 10.1007/s10585-008-9234-7. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenbeld HC, Ferarra N, Jain RK, Munn LL. Effect of local anti-VEGF antibody treatment on tumor microvessel permeability. Microvasc Res. 1999;57:357–362. doi: 10.1006/mvre.1998.2140. [DOI] [PubMed] [Google Scholar]
- 10.Monsky WL, Fukumura D, Gohongi T, Ancukiewcz M, Weich HA, Torchilin VP, Yuan F, Jain RK. Augmentation of Transvascular Transport of Macromolecules and Nanoparticles in Tumors Using Vascular Endothelial Growth Factor. Cancer Res. 1999;59:4129–4135. [PubMed] [Google Scholar]
- 11.Skala MC, Fontanella A, Lan L, Izatt JA, Dewhirst MW. Longitudinal optical imaging of tumor metabolism and hemodynamics. J Biomed Opt. 2010;15:011112. doi: 10.1117/1.3285584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angio-genesis and radiotherapy response. Nat Rev Cancer. 2008;8:425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dewhirst MW, Ong ET, Klitzman B, Secomb TW, Vinuya Z, Dodge R, Brizel D, Gross JF. Perivascular Oxygen Tensions in a Transplantable Mammary Tumor Growing in a Dorsal Flap Window Chamber. Radiat Res. 1992;130:171–182. [PubMed] [Google Scholar]
- 14.Kimura H, Braun RD, Ong ET, Hsu R, Secomb TW, Papahadjopoulos D, Hong K, Dewhirst MW. Fluctuations in Red Cell Flux in Tumor Microvessels Can Lead to Transient Hypoxia and Reoxygenation in Tumor Parenchyma. Cancer Res. 1996;56:5522–5528. [PubMed] [Google Scholar]
- 15.Moy AJ, White SM, Indrawan ES, Lotfi J, Nudelman MJ, Costantini SJ, Agarwal N, Jia W, et al. Wide-field functional imaging of blood flow and hemoglobin oxygen saturation in the rodent dorsal window chamber. Microvasc Res. 2011;82:199–209. doi: 10.1016/j.mvr.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai AG, Cabrales P, Winslow RM, Intaglietta M. Microvascular oxygen distribution in awake hamster window chamber model during hyperoxia. 2003;285:H1537–H1545. doi: 10.1152/ajpheart.00176.2003. [DOI] [PubMed] [Google Scholar]
- 17.Makale MT, Lin JT, Calou RE, Tsai AG, Chen PC, Gough DA. Tissue window chamber system for validation of implanted oxygen sensors. Am J Physiol Heart Circ Physiol. 2003;284:H2288–H2294. doi: 10.1152/ajpheart.00721.2002. [DOI] [PubMed] [Google Scholar]
- 18.Richmond A, Su Y. Mouse xenograft models vs GEM models for human cancer therapeutics. Dis Model Mech. 2008;1:78–82. doi: 10.1242/dmm.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Killion JJ, Radinsky R, Fidler I. Orthotopic Models are Necessary to Predict Therapy of Transplantable Tumors in Mice. Cancer Metastasis Rev. 1999;17:279–284. doi: 10.1023/a:1006140513233. [DOI] [PubMed] [Google Scholar]
- 20.Kedrin D, Gligorijevic B, Wyckoff J, Verkhusha VV, Condeelis J, Segall JE, Van Rheenen J. Intravital imaging of metastatic behavior through a Mammary Imaging Window. Nat Methods. 2008;5:1019–1021. doi: 10.1038/nmeth.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shan S, Sorg B, Dewhirst MW. A novel rodent mammary window of orthotopic breast cancer for intravital microscopy. Microvasc Res. 2003;65:109–117. doi: 10.1016/s0026-2862(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 22.Young PA, Nazir M, Szulczewski MJ, Keely PJ, Eliceiri KW. Second-harmonic generation and fluorescence lifetime imaging microscopy through a rodent mammary imaging window. Multiphoton Microscopy in the Biomedical Sciences XII; Proc SPIE; 2012. pp. 822604–822610. [Google Scholar]
- 23.Reitan NK, Thuen M, Goa PE, de Lange Davies C. Characterization of tumor microvascular structure and permeability: comparison between magnetic resonance imaging and intravital confocal imaging. J Biomed Opt. 2010;15:036004. doi: 10.1117/1.3431095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erten A, Wrasidlo W, Scadeng M, Esener S, Hoffman RM, Bouvet M, Makale M. Magnetic resonance and fluorescence imaging of doxorubicin-loaded nanoparticles using a novel in vivo model. Nanomedicine. 2010;6:797–807. doi: 10.1016/j.nano.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaustad JV, Brurberg KG, Simonsen TG, Mollatt CS, Rofstad EK. Tumor Vascularity Assessed By Magnetic Resonance Imaging and Intravital Microscopy Imaging. Neoplasia. 2008;10:354–362. doi: 10.1593/neo.08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Gobar LS, Knowles NG, Liu Z, Gmitro AF, Barrett HH. Direct imaging of radionuclide-produced electrons and positrons with an ultrathin phosphor. J Nucl Med. 2008;49:1141–1145. doi: 10.2967/jnumed.107.040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.