Summary
Disorders of branched-chain amino/keto acid metabolism encompass diverse entities, including maple syrup urine disease (MSUD), the ‘classical’ organic acidurias isovaleric acidemia (IVA), propionic acidemia (PA), methylmalonic acidemia (MMA) and, among others, rarely described disorders such as 2-methylbutyryl-CoA dehydrogenase deficiency (MBDD) or isobutyryl-CoA dehydrogenase deficiency (IBDD). Our focus in this review is to highlight the biochemical basis underlying recent advances and ongoing challenges of long-term conservative therapy including precursor/protein restriction, replenishment of deficient substrates, and the use of antioxidants and anaplerotic agents which refill the Krebs cycle. Ongoing clinical assessments of affected individuals in conjunction with monitoring of disease-specific biochemical parameters remain essential. It is likely that mass spectrometry-based ‘metabolomics’ may be a helpful tool in the future for studying complete biochemical profiles and diverse metabolic phenotypes. Prospective studies are needed to test the effectiveness of adjunct therapies such as antioxidants, ornithine-alpha-ketoglutarate (OKG) or creatine in addition to specialized diets and to optimize current therapeutic strategies in affected individuals. With the individual lifetime risk and degree of severity being unknown in asymptomatic individuals with MBDD or IBDD, instructions regarding risks for metabolic stress and fasting avoidance along with clinical monitoring are reasonable interventions at the current time. Overall, it is apparent that carefully designed prospective clinical investigations and multicenter cohort-controlled trials are needed in order to leverage that knowledge into significant breakthroughs in treatment strategies and appropriate approaches.
Introduction
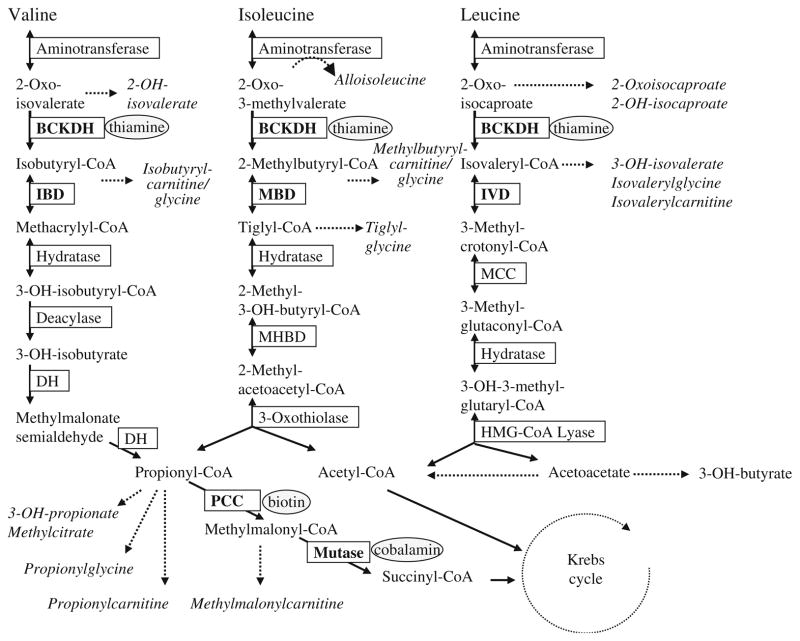
The catabolic pathways of branched-chain amino acids (BCAA) consist of multiple steps including transamination, oxidative decarboxylation and dehydrogenation; inborn errors of these pathways are inherited in an autosomal recessive fashion. These disorders include maple syrup urine disease (MSUD, OMIM 248600), ‘classical’ organic acidurias (isovaleric acidemia, IVA, OMIM 243500, propionic acidemia, PA, OMIM 606054, methylmalonic acidemia, MMA, OMIM 609058), rarely described disorders in the pathways of BCAA degradation, such as 2-methylbutyryl-CoA dehydrogenase deficiency (MBDD, OMIM 610006) or isobutyryl-CoA dehydrogenase deficiency (IBDD, OMIM 611283) and others (see Fig. 1). In this review we opt to provide an overview of distinctive amino and organic acidurias associated with BCAA metabolism, encompassing both mild and severe phenotypes.
Fig. 1.
Pathways of branched-chain amino acid catabolism. Not all steps and intermediates are outlined. Abbreviations employed: OH, hydroxyl; BCKDH, branched-chain ketoacid dehydrogenase; IBD, isobutyryl-CoA dehydrogenase; DH, dehydrogenase; PCC, propionyl-CoA carboxylase; MHBD, 2-methyl-3-hydroxybutyryl-CoA dehydro-genase; MBD, 2-methylbutyryl-CoA dehydrogenase; IVD, isovaleryl-CoA dehydrogenase; MCC, 3-methylcrotonyl-CoA carboxylase. Metabolites that are identified in increased amounts in physiological fluids derived from patients are depicted in italics; enzyme defects discussed here are given in bold, required cofactors are presented in ellipses. Image modified from Zschocke & Hoffmann, Vademecum Metabolicum, Schattauer, Stuttgart, with permission
MSUD is caused by a metabolic block in the early degradation of the BCAA leucine, valine and isoleucine due to defective activity of the branched-chain alpha-keto acid dehydrogenase multienzyme complex (BCKDH). The underlying genes are BckdhA (E1-alpha subunit gene), Bckdhb (E1-beta subunit gene), and DBT (E2 subunit gene). In principle, MSUD can be considered both an amino acidopathy and organic aciduria because of the combined toxic effects of amino acids (AA), particularly leucine, and organic acid intermediates, such as the keto-and hydroxyacid metabolites of BCAA. IVA is caused by a deficiency of isovaleryl CoA dehydrogenase in the catabolism of leucine. PA is caused by propionyl-CoA carboxylase deficiency. MMA is a genetically heterogeneous disorder of methylmalonate or cobalamin (vitamin B12) metabolism, and its ‘classical’ form is the result of methylmalonyl-CoA mutase deficiency. PA and MMA are branched-chain organic acidurias (since the metabolism of valine and isoleucine is impaired along with an accumulation of organic acid intermediates) but they also affect the degradation of methionine, threonine and other propionate-deriving substances.
Inborn errors of BCAA degradation yield a large spectrum of clinical severity from asymptomatic findings (e.g., in individuals with MBDD or IBDD) to multi-organ involvement and life-threatening episodes (in patients with ‘classical’ diseases of BCAA metabolism). Patients with ‘classical’ forms of MSUD, IVA, MMA or PA typically present at early ages with metabolic decompensation. In patients with PA or MMA the phenotype is usually more severe and the clinical spectrum less variable than in subjects with IVA or MSUD (Dionisi-Vici et al. 2006; Simon et al. 2006; Vockley and Ensenauer, 2006; Erdem et al. 2010). Historically, the term ‘classical’ refers to a severe phenotype but we have learned from tandem mass spectrometry-based newborn screening that the mildly affected individuals and patients detected early exhibit considerable overlap in their biochemical abnormalities and that diseases such as MSUD and IVA encompass heterogeneous conditions (Vockley and Ensenauer, 2006, Simon et al. 2006).
However, there are other extremely rare branched-chain organic acidurias which show a heterogeneous phenotype and generally a milder clinical course, compared with the ‘classical’ branched-chain amino-/organic acidurias, particularly those defects affecting distal isoleucine or valine metabolic pathways such as MBDD or IBDD.
MBDD is an inborn defect in isoleucine degradation, caused by mutations in the short/branched-chain acyl-CoA dehydrogenase (ACADSB) gene. More than 20 individuals with MBDD have been reported, most of whom are asymptomatic, although a few have presented with symptoms such as lethargy, hypotonia, retardation, autistic features, hypoglycemia or metabolic acidosis (Gibson et al. 2000a; Kanavin et al. 2007; Sass et al. 2004 and 2008; Van Calcar et al. 2007; Alfardan et al. 2010). The characteristic biochemical feature is elevated pentanoylcarnitine (methylbutyrylcarnitine) and methylbutyrylglycine in biological fluids. In individuals with MBDD a low-protein diet and carnitine supplementation is occasionally implemented with variable results (Matern et al. 2003, Sass et al. 2008). MBDD shows a diverse clinical pattern and milder course compared with the ‘classical’ diseases and may even be a benign metabolic phenotype (Sass et al. 2008).
A further example of a rare atypical branched-chain organic aciduria is IBDD, a disorder of valine degradation due to a defect in the acyl-CoA dehydrogenase (ACAD) 8, now termed the IBD gene. Thus far, at least 22 individuals have been described who are mostly asymptomatic and have been identified via newborn screening with tandem mass spectrometry and urinary excretion of isobutyrylglycine (Koeberl et al. 2003; Sass et al. 2004; Oglesbee et al. 2007). In individuals with persistently elevated C4-acylcarnitine (isobutyryl- and butyrylcarnitine) short-chain acyl-CoA dehydrogenase deficiency is often considered in the differential diagnosis. There are, however, also symptomatic patients including the first patient described with IBDD, a 2-year-old girl who presented with anemia and cardiomyopathy and was successfully treated with oral carnitine (Roe et al. 1998).
Testing for inborn defects of BCAA metabolism is a sequential process involving newborn screening with tandem mass spectrometry for selected disorders, clinical evaluation and follow-up, determination of blood AA and acylcarnitines as well as urinary organic acid analysis, followed by enzyme or molecular studies (Gibson et al. 2000b).
Pathophysiological aspects
The pathophysiology of ‘classical’ branched-chain amino-/organic acidopathies is multi-factorial, including 1) accumulation and toxic effects of specific metabolites and associated intracellular toxicity; 2) secondary metabolic effects including metabolic imbalances, transport competition, and deficiencies of metabolic intermediates, micronutrients etc.; and 3) mitochondrial alterations associated with disturbed energy production and oxidative stress.
Accumulation of toxic metabolites (such as leucine and 2-ketoisocaproic acid in patients with MSUD or methylmalonic acid or propionyl-CoA in MMA or PA, respectively) induces profound metabolic alterations and impairment of energy homeostasis associated with clinical symptoms (Amaral et al. 2010; Deodato et al. 2006). The clinical picture depends on the severity of the underlying disorder and metabolic decompensation, ranging from intermittent symptoms of various degrees, such as hypotonia and failure to thrive to acute life-threatening encephalopathy. In MSUD, leucine and its accumulating metabolites deleteriously alter brain aerobic metabolism by compromising various enzymes, particularly those of the Krebs cycle and the respiratory chain (Ribeiro et al. 2008), which can be followed by apoptosis in neuronal cells (Jouvet et al. 2000). Toxic intermediates such as methylmalonic acid, propionyl-CoA and other by-products of alternative propionate oxidation are able to inhibit various enzyme systems, particularly the oxidative phosphorylation system in the mitochondria, which can lead to a deficit of energy production and multiorgan complications such as neurologic distress, cardiomyopathy or renal disease (Brusque et al. 2002, de Keyzer et al. 2009). Direct effects of toxic metabolites, particularly free organic acids and the various acyl-CoA metabolites, on key enzymes of intermediary metabolism help to explain findings such as acidosis, hypo-glycemia and hyperammonemia in acutely decompensated patients with branched-chain amino-/organic acidopathies. For example, enzyme inhibition can affect α-ketoglutarate dehydrogenase and pyruvate dehydrogenase (Sauer et al. 2008), carbamyl-phosphate synthetase (Martin-Requero et al. 1983), N-acetyl-glutamate synthetase (Stewart and Walser, 1980) creatine kinase and others (Schuck et al. 2004; Scholl-Bürgi et al. 2010).
Alterations of the AA pattern and flow into the central nervous system contribute to the pathophysiology of inborn errors of BCAA catabolism. At the blood-brain barrier (BBB), BCAA share a common transport system with aromatic amino acids (ArAA) and other large neutral AA (LNAA). A rise of BCAA concentrations leads to a decline of brain ArAA concentrations and the neurotransmitters derived from ArAA, particularly serotonin (from tryptophan) and the catechol-amines (from tyrosine and phenylalanine). Physiological plasma leucine concentrations and transport rates across the BBB are essential for normal protein synthesis in brain, generation of ketones, nitrogen shuttling to supplement the glutamate/glutamine cycle between neurons and astrocytes, signaling molecules and regulation of different enzymes (Murin and Hamprecht, 2008). Accumulating metabolites such as methylmalonic acid are transported across the BBB by different transport systems, notably organic acid transporters, whereas the permeability and chemical properties of these metabolites at the mitochondrial membranes are not fully elucidated (Chandler et al. 2009; Sauer et al. 2010). The autonomous intracerebral production of metabolites such as methylmalonate or propionyl-CoA along with the limited efflux transporter capacity of the BBB may lead to a deleterious accumulation of these toxic compounds within the brain if there is an underlying metabolic defect (Kölker et al. 2006; Ballhausen et al. 2009). The so-called ‘trapping hypothesis’ helps to explain neurotoxic findings including stroke-like episodes in patients with organic acidurias (Kölker et al. 2006).
In addition to toxic effects and imbalances of metabolic intermediates, partial depletion of mitochondrial DNA and mitochondrial respiratory impairment along with ultrastructural mitochondrial abnormalities contribute to a cellular energy deficit in tissues of patients with PA or MMA (Schwab et al. 2006; De Keyzer et al. 2009). Moreover, activities of detoxifying enzymes such as catalase are found decreased in fibroblasts of patients with disturbed propionate metabolism (De Keyzer et al. 2009). Inhibition of energy-related enzymes such as creatine kinase by methylmalonic acid has been described in rat cerebral cortex but pre-incubation with antioxidants such as glutathione can prevent this effect (Schuck et al. 2004). Oxidative stress is increased and antioxidant capacity decreased in patients with disorders of BCAA metabolism, such as MSUD, MMA or PA (Barschak et al. 2009; Mc Guire et al. 2009). Oxidative damage may also contribute to the pathophysiology of IVA (Solano et al. 2008) whereas data for antioxidant status in MBDD or IBDD are lacking.
Taken together, several different mechanisms, including direct toxic effects, crucial substrate imbalances and complex mitochondrial dysfunction contribute to the pathophysiology and clinical presentation of the disorders of BCAA metabolism.
Therapeutic strategies
Our focus in this review is to highlight the biochemical basis underlying recent advances and ongoing challenges of long-term conservative therapy including precursor/protein restriction, replenishment of deficient substrates, and the use of antioxidants and anaplerotic agents (i.e., molecules which refill the Krebs cycle).
Emergency treatment, including hemodialysis/hemofiltration, is a key issue and comprehensively described elsewhere (Arbeiter et al. 2010; Deodato et al. 2006; Saudubray et al. 2002; Zand et al. 2008), as are organ and cell transplantation approaches (Barshes et al. 2006; Mc Guire et al. 2008; Strauss et al. 2006).
Essentially, treatment of ‘classical’ branched-chain amino-/organic acidurias aims to restore homeostasis of intermediary metabolism and prevent metabolic decompensation through maintenance of a non-catabolic state. Therapy comprises disease-specific approaches including reduction of toxic metabolites, promotion of anabolism, substrate restriction, replacement of deficient substrates, and stimulation of residual enzyme activity along with activation of alternative pathways (Table 1). Therapeutic strategies must be tailored to the complex pathophysiology and multi-system nature and degree of severity of the various diseases and adapted to individual needs.
Table 1.
Current therapeutic concepts for patients with branched-chain amino/keto acid metabolic defects
| General/specific | Well-established long-term management (see special literature for a detailed description of emergency treatment) | Individualised treatment, transplantation and experimental approaches (including animal data) |
| General | Continuous (long-term) monitoring; combined therapeutic approaches, multidisciplinary care | Antioxidants and essential fatty acids (particularly if low) |
| Prevention of metabolic crises, maintenance of an anabolic state, adequate or high energy diet supplied as carbohydrate and fat to support anabolism | Development of novel, mitochondria-targeted antioxidants | |
| Stimulation of residual activity of deficient enzymes by using their cofactors (in responsive cases) | Competitors for transport of the particular amino acids into the brain | |
| Substrate reduction/protein modified-diet/low-protein diet with disease-specific amino acid supplements (formulas) deficient in the particular precursor amino acids and enriched with micronutrients | Supplementation with alternative energy sources/preservation of cellular energy supply (e.g., creatine, ornithine alpha- ketoglutarate) | |
| Continuous feeding or tube feeding to assure adequate caloric intake if needed | ||
| Reduction of toxic metabolites by using adjunctive medications or procedures if applicable (including even hemodialysis, e.g. in case of hyperammonemic coma) | ||
| Symptomatic treatment during illnesses (e.g., parenteral nutrition, specific drug therapy) | ||
| Treatment of special organ complications (such as renal disease in MMA etc.) | ||
| MSUD | Thiamine in thiamine-responsive MSUD; substitution of valine und isoleucine (according to the individual needs) | Phenylbutyrate (in milder MSUD phenotypes); transplantation of liver/hepatocytes; norleucine |
| MMA | Hydroxocobalamin (usually not cyanocobalamin) in cobalamin-responsive MMA; L-carnitine; intermittent intestinal decontamination, e.g. with non-absorbed antibiotics (to reduce the production of propionate by gut bacteria) | Liver and/or kidney transplantations; growth hormone therapy (limited experience) gene therapy |
| PA | Biotin in biotin-responsive PA; L-carnitine; Intermittent intestinal decontamination, e.g. with non-absorbed antibiotics (to reduce the production of propionate by gut bacteria) | Liver transplantation; gene therapy |
| IVA | L-carnitine; L- glycine (in severe IVA phenotypes) |
The biochemical basis for treatment encompasses 1) maintenance of normal protein synthesis and prevention of protein catabolism; 2) prevention of imbalances or deficiencies of AA and metabolic intermediates; and 3) attenuation of cellular dysfunction, restoration of energy homeostasis and promotion of anabolism.
From the clinical perspective there are two objectives with respect to treatment: Acute-phase treatment and long-term management. After establishing the diagnosis in symptomatic patients treatment of the acute stage gradually shifts to long-term treatment consistent with the patient’s condition. There is a clear benefit from early diagnosis for symptomatic patients with ‘classical’ amino-/organic acidurias (Hörster et al. 2009; Dionisi-Vici et al. 2006; Simon et al. 2006; Ogier de Baulny and Saudubray, 2002; Morton et al. 2002). As the basis of treatment often represents a specific dietary therapy, we have to consider that treatment must comprise careful adjustment of caloric and protein intake along with micronutrient and vitamin supplementation in selected instances (e.g., rare cases of thiamine-responsive MSUD, cobalamin in cobalamin-responsive MMA), carnitine administration and adjunct treatment (e.g., anticonvulsants when seizures form a component of the phenotype) as required for individual needs.
Treatment of patients with ‘classical’ organic acidurias is based on a ‘low-protein adequate/high-energy’ diet combined with disease-specific AA mixtures. These special AA formulas are deprived of the particular precursor AA, whereas the composition of all other AA is usually based on the composition of natural protein in human milk and whole egg. Moreover, the powders are enriched with vitamins, minerals, and some of them with fatty acids and additional precursors of energy. The combination of restriction of natural protein and special medical foods provided in sufficient ratios is geared to support normal growth and development, and corrects nutritional deficiencies in affected patients (Strauss et al. 2010; Yannicelli, 2006). Recently, formulas for patients with MSUD have been designed which are enriched with the AA that compete with BCAA for transport (e.g., tryptophan, tyrosine, phenylalanine, methionine, threonine etc.) and help to maintain physiological AA plasma levels and transport into the brain (Strauss et al. 2010). However, plasma AA imbalances, particularly of essential AA, are common findings in treated patients (Yannicelli, 2006; Barschak et al. 2009). Feeding difficulties are frequent in patients with ‘classical’ branched-chain organic acidurias; reasons for this finding may include muscular hypotonia, nausea, metabolic decompensation, infections, retardation and swallowing difficulties. Up to 50–60% of patients with MMA or PA, for example, require gastrostomy or nasogastric tube feeding, temporarily or exclusively (depending on age, underlying metabolic disorder and severity) (Hörster et al. 2009; Toauti et al. 2006). Additionally, failure to thrive can be seen as a complication in patients with ‘classical’ branched-chain amino-/organic acidurias. This may relate to consumption of protein with low biological value as in a vegetarian-type of diet. It may also be correlated to an over-restriction of a particular precursor AA and natural protein, deficiencies of micronutrients or a higher energy requirement due to chronic illness or inflammation (Strauss et al. 2010; Yannicelli, 2006). Providing energy and protein at intakes required by patients with growth deficiency and catch-up growth needs may result in improved growth and adequate nutritional status (Yannicelli et al. 2003). In patients with PA, changes in plasma AA concentrations have been related to age, nutritional supply and metabolic state (Scholl-Bürgi et al. 2010). A European multicenter study on 183 patients with MMA revealed a huge variation in the management of these patients regarding total and natural protein intake, special AA substitutes and adjunct medication (Zwickler et al. 2008). These findings may be explained by the lack of standardized protocols, different personal experience and therapeutic approaches (e.g., appropriate nutritional intake to high energy). This highlights the need for improved acute-phase and follow-up treatment protocols for patients with ‘classical’ organoacidurias.
Docosahexaenoic acid (DHA) concentrations in plasma of patients with MSUD or MMA may be low and require supplementation (Aldámiz-Echevarría et al. 2006, Mazer et al. 2010). Since myelin abnormalities may be associated with DHA deficiency, nutritional intake of essential fatty acids (EFA) should be monitored (Strauss et al. 2010). Micromanagement of dietary intake such as that described above underscores the critical importance of the presence of well-trained metabolic dietitians closely associated with the metabolic management team.
Adjunct therapies
Carnitine is effective in preventing secondary carnitine deficiency, regenerating the intracellular pool of free coenzyme A (CoA) and in eliminating toxic intermediates such as propionate metabolites (Ogier de Baulny and Saudubray, 2002). The L-form of carnitine is essential for the transport of long-chain fatty acids into the mitochondria for further beta-oxidation. Conversely, accumulating acyl-CoA moieties and organic acids can be cleared from the cell using the carnitine cycle in a reverse direction. Acyl-CoA species, such as propionyl-CoA, exert toxic effects on the mitochondrial energy metabolism through inhibition of the pyruvate dehydrogenase and/or alpha ketoglutarate dehydrogenase complexes, among others, whereas the corresponding carnitine esters are considerably less toxic (Sauer et al. 2008). Additionally, carnitine demonstrates antioxidant capacities via reduction of lipid peroxidation to an extent comparable to that seen with α-tocopherol (Gülcin, 2006). The radical scavenging and iron chelating properties of carnitine further extend its antioxidant spectrum (Gülcin, 2006).
Patients with MMA or PA exhibit a lower oxidative damage level during treatment as compared to their clinical status at disease onset (Ribas et al. 2010). Considering the lower intracellular concentrations of propionic acid, methylmalonic acid and CoA moieties following carnitine application, carnitine therapy may directly reduce oxidative damage and prevent alterations of key enzymes of mitochondrial energy metabolism in affected patients. There is a strong biochemical rational and a broad consensus for the use of carnitine in patients with organic acidurias such as PA, MMA or IVA (Ogier de Baulny et al. 2002; Zwickler et al. 2008). Carnitine supplementation is used in the treatment of carnitine deficiency, which may include systemic primary carnitine deficiency due to carnitine transporter defects, or a secondary carnitine deficiency due to fatty acid oxidation defects (Spiekerkoetter et al. 2009; Nasser et al. 2009). In MSUD patients, the accumulated oxo acids do not trans-esterify with carnitine; accordingly, general carnitine supplementation is not therapeutically useful. Phenylbutyrate, which is used in patients with urea cycle disorders, may reduce plasma concentrations of branched-chain AA and their corresponding oxo acids in individuals with intermediate MSUD (iMSUD) by an increase in residual enzyme activity (Brunetti-Pierri et al. 2010).
The use of various supplements (e.g., dietary, disease-specific AA, vitamin; see Table 1) in treating these disorders is gaining acceptance in the metabolic community and experimental data are promising. Lowering oxidative stress using α-tocopherol (vitamin E) or ascorbate (vitamin C) has been evaluated in animal models of branched-chain amino/organic acidurias (Ribeiro et al. 2008; Wajner et al. 2004). Alpha-tocopherol and creatine prevented the inhibitory effects on the respiratory chain induced by BCAA in cerebral cortex specimens, suggesting that free radicals were involved in these adverse effects of BCAA (Ribeiro et al. 2008). In plasma derived from treated MSUD patients, the total antioxidant reactivity (representing the overall antioxidant capacity of the cell), was significantly lower compared with controls (Barschak et al. 2008). Lowered antioxidant capacity has also been observed in patients with PA or MMA (Mc Guire et al. 2009). Cobalamin supplementation reduces cellular damage in fibroblasts derived from patients with cobalamin responsive MMA (Richard et al. 2009). In patients with MMA or PA, biomarkers of oxidative damage in plasma (i.e., malondialdehyde content, carbonyl formation and sulfhydryl oxidation) were lower following initiation of restricted diet and carnitine supplementation (Ribas et al. 2010). The literature indicates that oxidative stress contributes to the pathophysiology of MMA and PA, and that diet and treatment with carnitine and cobalamin (in vitamin B12-responsive MMA) may improve outcomes through preservation of the cellular antioxidant status (Mc Guire et al. 2009, Ribas et al. 2010). In an in vitro model of renal disease in MMA (i.e., human proximal tubule cells exposed to hydoxycobalamin [c-lactam] and propionic acid), glutathione concentrations are reduced, suggesting oxidative stress due to altered cobalamin metabolism along with methylmalonic acid production. Moreover, distinctive changes of activities of respiratory chain complexes, such as a decrease of complex II and III activities after 21 days of culture are consistent with bioenergetic dysfunction along with oxidative stress in MMA (Sauer et al. 2009).
The brain is particularly susceptible to oxidative stress consistent with its high metabolic demands, high content of polyunsaturated fatty acids in cell membranes and limited capacity for regeneration. The lack of efficiency of conventional antioxidant therapy may be due to the low transport rate of many antioxidants across the BBB and their ineffective distribution within cellular compartments of neural tissue. Alpha-tocopherol and coenzyme Q (e.g., ubiquinone, a major isoprene antioxidant derived from the cholesterol pathway) are found within cell membranes and do not achieve high intracellular concentrations (Szeto, 2006). As mitochondria are most vulnerable to oxidative damage, novel mitochondrial-targeted antioxidants may enhance the effectiveness of antioxidant intervention in the future (Szeto, 2006). Mitochondria-targeted peptides can protect against mitochondrial swelling and apoptosis and have neuroprotective effects, e.g., on dopaminergic neurons in mice, and may be a future therapeutic option for oxidative stress associated with heritable metabolic disorders (Yang et al. 2009).
Alpha-tocopherol and creatine, as alternative energy source, have been both successfully applied in a rat model of MSUD (Ribeiro et al. 2008). Creatine supplementation was also effective in maintaining normal neurotransmission in a rat model of IVA (Ribeiro et al. 2009) but data in human subjects are lacking. In comparison, the use of creatine occasionally is beneficial in patients with primary mitochondrial disorders (Finsterer, 2010).
In patients with PA or MMA, who accumulate propionyl CoA intermediates, anaplerosis from propionyl-CoA is disturbed, and thus this represents a therapeutic target for anaplerotic intervention. Essentially, anaplerotic precursors replenish metabolite levels depleted within the Krebs cycle (Scholl-Bürgi et al. 2010; Brunengraber and Roe, 2006). Considering the various secondary mitochondrial effects in patients with ‘classical’ forms, there may be therapeutic value for anaplerotic substances.
There has also been some limited discussion concerning the utility of ornithine α-ketoglutarate (OKG). OKG is a precursor of AA such as glutamine, arginine and proline and has the ability to supplement the Krebs cycle intermediate alpha-ketoglutarate (‘anaplerotic’ function), since it is composed of 2 moles of ornithine in combination with 1 mole of α-ketoglutarate. An anabolic action of OKG is observed in conditions which are associated with loss of muscle mass, hypercatabolism or malnourished states, such as trauma or sarcopenia, along with positive effects on the action of anabolic hormones such as insulin and growth hormone (Cynober 2004; Walrand, 2010). Nonetheless, clinical or preclinical data on OKG intervention in inherited metabolic diseases has not been reported, and thus this approach remains speculative, yet rational based upon therapeutic efficacy in chronically and acutely malnourished patients.
Moreover, growth hormone has been employed in a limited number of patients with MMA, but not in controlled trials (Kao et al. 2009).
Therapeutic needs and ongoing challenges
In asymptomatic individuals with mild variants of IVA and MSUD the necessity of long-term treatment is yet unclear, but interventions such as prevention of metabolic crisis, careful instructions of affected families and evaluation to identify individuals at risk for metabolic decompensation are recommended (Vockley et al. 2006; Simon et al. 2006).
Therapeutic strategies for patients with rare branched-chain organic acidurias such as MBDD and IBDD are poorly defined because these disorders have a low incidence and the lack of comprehensive long-term outcome data. With the individual life-time risk and degree of severity being unknown in asymptomatic individuals, instructions regarding risks for metabolic stress and fasting avoidance are reasonable interventions at the current time. In addition, a modest protein restriction and carnitine supplementation has been performed, particularly in symptomatic patients or as temporary intervention (Kanavin et al. 2007; Koeberl et al. 2003; Matern et al. 2003; Sass et al. 2008; Van Calcar et al. 2007).
Conclusive data is scarce and careful monitoring is required to distinguish between patients at risk for clinical manifestations, patients with intermittent symptoms that may be retrospectively found in view of the biochemical abnormalities, and individuals with an asymptomatic form or metabolic ‘non-disease’.
Monitoring
Generally, monitoring of affected individuals with ‘classical’ amino-/organic acidurias is comprised of careful observation of the disease course and ongoing therapy adjustment responsive to metabolic demands. Dietary therapy in affected individuals requires close monitoring of nutritional needs and intake (Table 2).
Table 2.
List of metabolic and nutritional assessment parameters for patients with branched-chain amino-/organic acidurias undergoing dietary treatment
| Clinical or laboratory parameter | Measurements |
|---|---|
| Growth | Weight, length/height, head circumference, pubertal status; X-ray for stage of bone maturation and hormonal status if indicated |
| Cardiovascular system | Blood pressure, heart rate. Electrocardiography (ECG) or echocardiography if indicated. |
| Central nervous system | Pediatric neurological examination, psychomotor development (neuro-psychological evaluation), electroencephalography (EEG), nuclear magnetic resonance (NMR) imaging of the central nervous system if indicated. |
| Abdominal organs | Laboratory markers as listed below, abdominal ultrasound if indicated. |
| Nutrient adequacy | 3-day dietary record, laboratory tests |
| Routine blood tests | Blood: Complete blood count (CBC)/differential, osmolarity, blood glucose, ammonia, pH and acid-base analysis, lactic acid, electrolytes, urea, creatinine, uric acid, liver function tests, enzymes such as creatine kinase (CK), lipids. Urine: Urine stix (including ketones) |
| Protein status | Serum or plasma: Total protein, albumin/protein electrophoresis |
| Amino acids (AA) | Serum or plasma: Full AA profile, homocysteine if indicated |
| Organic acids | Urine: Organic acid profile (organic acid/creatinine ratio), organic acids in plasma (e.g., methylmalonic acid) |
| Carnitine status | Free and esterified carnitine in blood (e.g. dried blood, plasma) |
| Micronutrients | Iron/ferritin, iodine, zinc, selenium, essential fatty acids, vitamin status |
Note: Frequencies of test depend on the patient’s clinical status and therapy regime. Additional assessments may be required
Treatment strategies for patients with ‘classic’ branched-chain amino-/organic acidopathies vary considerably between different metabolic centers (Zwickler et al. 2008). As shown in patients with ‘classic’ MMA, long-term complications are found in a high percentage, e.g., 65% of patients exhibit physical and cognitive delays, 28% develop renal failure (Hörster et al. 2009). This highlights the need for improved follow-up treatment protocols for patients with ‘classical’ organoacidurias. Therapeutic goals for metabolic variables, such as daily intake of the individual BCAA, plasma BCAA concentrations, among others, have been established in symptomatic and asymptomatic children with MSUD (Morton et al. 2002). Follow-up for a total of 219 patient years reveals that metabolic decompensation after the newborn period primarily occurs in the course of common illnesses and that clinical status and neurologic function may deteriorate whenever metabolic intoxication occurs (Morton et al. 2002). However, ‘classic’ MSUD can be managed to follow a more benign course associated with normal growth and development (Morton et al. 2002). The heterogeneous condition found in individuals with IVA and their clinical management is still a matter of debate (Castorina et al. 2008; Vockley and Ensenauer, 2006). Individualized therapies along with careful follow-up and long-term compliance are needed for IVA patients at risk for metabolic decompensation.
In MBDD or IBDD the decision concerning therapy initiation with low-dose carnitine and/or moderate protein restriction can be challenging and careful observation of asymptomatic individuals is mandatory. Carnitine supplementation can prevent secondary deficiency, but this has rarely been observed in individuals with MBDD (Sass et al. 2008) and a beneficial effect from a protein-restricted diet remains unclear (Kanavin et al. 2007; Matern et al. 2003; Oglesbee et al. 2007; Van Calcar et al. 2007). Until now no clinical or biochemical markers have been identified which facilitate the assessment of the individual risk and the need for long-term treatment in these patients, again highlighting the absence of well-controlled clinical trials. We therefore recommend careful guidance to the families regarding situations that place patients at risk for metabolic decompensation and a long-term follow-up consisting of extensive outcome data. The latter will provide needed insight into the natural history, metabolic phenotypes and therapeutic needs of individuals with variants of branched-chain organic acidurias such as MBDD and IBDD.
Moreover, a mass spectrometry-based ‘metabolomic’ approach is useful for the simultaneous identification of numerous metabolites in branched-chain amino/keto acid defects. So far this method has been applied to study samples obtained from patients or murine models with MMA, PA, IVA or MSUD (Wikoff et al. 2007; Loots et al. 2005; Loots, 2009; Wu et al. 2004). Recently identified metabolites (e.g., abnormal methylated Krebs cycle intermediates, disease-specific acetylated AA) may serve as an additional link between biochemical abnormalities and clinical conditions. This advanced technique may be a helpful tool in the future for completing biochemical profiles, studying diverse disease entities and monitoring current metabolic status.
Development of new treatment strategies using transgenic and knockout mice
Murine models of classic MUSD or iMSUD have been tested (Homanics et al. 2006). Mice with an iMSUD phenotype survive past weaning and are a useful model for future therapeutic studies (Zinnanti et al. 2009). For example, the nonphysiological AA norleucine has been tested for its ability to prevent accumulation of branched-chain AA, particularly leucine, in the brain. Norleucine supplementation delayed encephalopathy in mutant mice fed with a high-protein diet. Norleucine considerably reduced brain α-ketoisocaproic acid levels and preserved energy metabolism as revealed through near-normal brain pyruvate and α-keto glutaric acid concentrations (Zinnanti et al. 2009). Hepatocyte transplantation in iMSUD mice, with approximately 3% repopulation of the endogenous liver with wild-type hepatocytes, corrects selected neurometabolic abnormalities in both the dopaminergic and serotoninergic systems, and concomitantly increases lifespan and body weight, which points to the potential therapeutic relevance of hepatocyte transplantation in humans (Skvorak et al. 2009a; Skvorak et al. 2009b).
A knock-out model of IVA is not yet available, but novel pathophysiological insights have been obtained by investigating the effects of isovaleric acid and isovalerylglycine on oxidative stress and mitochondrial status in rat brain (Solano et al. 2008). Animal models for MBDD or IBDD are not available.
Mut−/− mice with a severe form of MMA have been successfully treated with hepatocyte-directed delivery of the methylmalonyl-CoA mutase (Mut) gene. Following an intrahepatic injection of adeno-associated virus expressing the murine Mut gene Mut−/− mice were rescued and lived beyond 1 year of age (Carrillo-Carrasco et al. 2010). As secondary mitochondrial alterations occur also in BCAA amino-/organic acidurias such as MMA, new treatment approaches focusing on mitochondrial status and antioxidant defenses can be also characterized in this knock-out mouse model (Chandler et al. 2009; Carrillo-Carrasco et al. 2010). A mouse model of PA (Pcca−/− mice) succumbs to death 24–36 h after birth associated with fatal ketoacidosis (Miyazaki et al. 2001). Pcca gene transfer that provides a postnatal PCCA activity of 10–20% in the liver of a transgenic mouse strain attenuates the fatal ketoacidosis in newborn mice, but a two-step therapy is necessary to maintain survival, particularly when protein intake increases (Miyazaki et al. 2001). Recently, an intrahepatic adeno-associated virus mediated gene transfer for human Pcca was tested in neonatal Pcca−/− mice (Chandler et al. 2010). The authors found a sustained therapeutic effect as demonstrated in a survival rate of 64% approx. and reduction of disease-related metabolites (Chandler et al. 2010). Taken together, in murine models of MMA (Mut−/− mice) and PA (Pcca−/− mice) gene transfer approaches rescue neonatal mice and represent potential future strategies.
Conclusion
Inborn errors of BCAA metabolism cover a diverse spectrum of disorders, including MSUD, IVA, PA, MMA as well as rare entities such as MBDD or IBDD. Clinical severity may range from asymptomatic findings in the latter to life-threatening episodes and multi-organ involvement in patients with ‘classic’ BCAA amino/organic acidopathies.
For patients with ‘classic’ disorders of BCAA, treatment includes: 1) dietary restriction of precursor AA along with optimal nutritional supply; 2) adjunct therapy (e.g., with carnitine for acylcarnitine formation, vitamins as cofactors to increase residual enzyme activity in responsive entities and others); and 3) rapid and effective intervention in instances of metabolic decompensation along with removal of toxic metabolites.
Long-term follow-up is necessary to develop an understanding of the natural history, metabolic phenotypes and therapeutic needs of individuals with variants such as MBDD and IBDD. Currently, clinical or biochemical markers which facilitate the assessment of the individual risks and the necessity of a long-term treatment for affected individuals are scarce and careful instructions regarding situations that risk metabolic decompensation are essential.
Ongoing clinical assessments of affected individuals in conjunction with monitoring of disease-specific biochemical measures and also parameters focusing on mitochondrial function are essential. It remains likely that mass spectrometry-based ‘metabolomics’ may be a helpful tool in the future for completing biochemical profiles, studying diverse phenotypes and monitoring metabolic status.
Prospective studies are needed to test the effectiveness of adjunct substances such as antioxidants, OKG or creatine in addition to a special diet and to optimize current therapeutic strategies in individuals with branched-chain amino/keto acid metabolic defect. Overall, it is apparent that carefully designed clinical investigations (including prospective multicenter cohort studies) are needed in order to leverage that knowledge into significant breakthroughs in treatment strategies in individuals with branched-chain amino/organic acidopathies.
Glossary
- AA
Amino acid(s)
- ACAD
Acyl-CoA dehydrogenase
- ACADSB
Short/branched-chain acyl-CoA dehydrogenase
- ArAA
Aromatic amino acid(s)
- BBB
Blood-brain-barrier
- BCAA
Branched-chain amino acid(s)
- CoA
Coenzyme A
- BCKDH
Branched-chain alpha-keto acid dehydrogenase complex
- DHA
Docosahexaenoic acid
- EFA
Essential fatty acids
- IBDD
Isobutyryl-CoA dehydrogenase deficiency
- iMSUD
Intermediate MSUD
- IVA
Isovaleric acidemia
- LNAA
Large neutral amino acid(s)
- MBDD
2-Methylbutyryl-CoA dehydrogenase deficiency
- MMA
Methylmalonic acidemia
- MSUD
Maple syrup urine disease
- MUT
Methylmalonyl-CoA mutase
- OKG
Ornithine alpha-ketoglutarate
- PA
Propionic acidemia
- PCCA
Propionyl CoA carboxylase alpha polypeptide
Footnotes
Communicated by Jörn Oliver Sass
Competing interest: None declared
Contributor Information
Ina Knerr, Email: ina.knerr@charite.de, Children’s and Adolescents’ Hospital, Otto-Heubner Centrum, Pediatric Metabolic Unit, Charité - Universitätsmedizin, Berlin, Germany, Otto-Heubner-Centrum für Kinder- und Jugendmedizin, Pädiatrische Stoffwechselmedizin, Charité - Universitätsmedizin Berlin, Campus Virchow-Klinikum, Augustenburger Platz 1, D-13353 Berlin, Germany.
Natalie Weinhold, Children’s and Adolescents’ Hospital, Otto-Heubner Centrum, Pediatric Metabolic Unit, Charité - Universitätsmedizin, Berlin, Germany.
Jerry Vockley, Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
K. Michael Gibson, Department of Biological Sciences, Michigan Technological University, Houghton, MI, USA.
References
- Aldámiz-Echevarría L, Sanjurjo P, Elorz J, Prieto JA, Pérez C, Andrade F, Rodríguez-Soriano J. Effect of docosahexaenoic acid administration on plasma lipid profile and metabolic parameters of children with methylmalonic acidaemia. J Inherit Metab Dis. 2006;29:58–63. doi: 10.1007/s10545-006-0182-6. [DOI] [PubMed] [Google Scholar]
- Alfardan J, Mohsen AW, Copeland S, Ellison J, Keppen-Davis L, Rohrbach M, Powell BR, Gillis J, Matern D, Kant J, Vockley J. Characterization of new ACADSB gene sequence mutations and clinical implications in patients with 2-methylbutyrylglycinuria identified by newborn screening. Mol Genet Metab. 2010;100:333–338. doi: 10.1016/j.ymgme.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral AU, Leipnitz G, Fernandes CG, Seminotti B, Schuck PF, Wajner M. Alpha-Ketoisocaproic acid and leucine provoke mitochondrial bioenergetic dysfunction in rat brain. Brain Res. 2010;1324:75–84. doi: 10.1016/j.brainres.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Arbeiter AK, Kranz B, Wingen AM, Bonzel KE, Dohna-Schwake C, Hanssler L, Neudorf U, Hoyer PF, Büscher R. Continuous venovenous haemodialysis (CVVHD) and continuous peritoneal dialysis (CPD) in the acute management of 21 children with inborn errors of metabolism. Nephrol Dial Transplant. 2010;25:1257–1265. doi: 10.1093/ndt/gfp595. [DOI] [PubMed] [Google Scholar]
- Ballhausen D, Mittaz L, Boulat O, Bonafé L, Braissant O. Evidence for catabolic pathway of propionate metabolism in CNS: expression pattern of methylmalonyl-CoA mutase and propionyl-CoA carboxylase alpha-subunit in developing and adult rat brain. Neuroscience. 2009;164:578–587. doi: 10.1016/j.neuroscience.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Barschak AG, Sitta A, Deon M, Barden AT, Dutra-Filho CS, Wajner M, Vargas CR. Oxidative stress in plasma from maple syrup urine disease patients during treatment. Metab Brain Dis. 2008;23:71–80. doi: 10.1007/s11011-007-9077-y. [DOI] [PubMed] [Google Scholar]
- Barschak AG, Sitta A, Deon M, Busanello EN, Coelho DM, Cipriani F, Dutra-Filho CS, Giugliani R, Wajner M, Vargas CR. Amino acids levels and lipid peroxidation in maple syrup urine disease patients. Clin Biochem. 2009;42:462–466. doi: 10.1016/j.clinbiochem.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Barshes NR, Vanatta JM, Patel AJ, Carter BA, O’Mahony CA, Karpen SJ, Goss JA. Evaluation and management of patients with propionic acidemia undergoing liver transplantation: a comprehensive review. Pediatr Transplant. 2006;10:773–781. doi: 10.1111/j.1399-3046.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- Brunengraber H, Roe CR. Anaplerotic molecules: current and future. J Inherit Metab Dis. 2006;29:327–331. doi: 10.1007/s10545-006-0320-1. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Lanpher B, Erez A, Ananieva EA, Islam M, Marini JC, Sun Q, Yu C, Hegde M, Li J, Wynn RM, Chuang DT, Hutson S, Lee B. Phenylbutyrate therapy for maple syrup urine disease. Hum Mol Genet. 2010 Nov 23; doi: 10.1093/hmg/ddq507. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusque AM, Borba Rosa R, Schuck PF, Dalcin KB, Ribeiro CA, Silva CG, Wannmacher CM, Dutra-Filho CS, Wyse AT, Briones P, Wajner M. Inhibition of the mitochondrial respiratory chain complex activities in rat cerebral cortex by methylmalonic acid. Neurochem Int. 2002;40:593–601. doi: 10.1016/s0197-0186(01)00130-9. [DOI] [PubMed] [Google Scholar]
- Carrillo-Carrasco N, Chandler RJ, Chandrasekaran S, Venditti CP. Liver-directed recombinant adeno-associated viral gene delivery rescues a lethal mouse model of methylmalonic acidemia and provides long-term phenotypic correction. Hum Gene Ther. 2010;21:1147–1154. doi: 10.1089/hum.2010.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorina M, Rigante D, Antuzzi D, Sciascia Cannizzaro G, Ricci R. Different outcome in isovaleric acidemia might be related to unsatisfactory diet compliance. Scand J Gastroenterol. 2008;43:767–768. doi: 10.1080/00365520801912128. [DOI] [PubMed] [Google Scholar]
- Chandler RJ, Zerfas PM, Shanske S, Sloan J, Hoffmann V, DiMauro S, Venditti CP. Mitochondrial dysfunction in mut methylmalonic acidemia. FASEB J. 2009;23:1252–1261. doi: 10.1096/fj.08-121848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler RJ, Chandrasekaran S, Carrillo-Carrasco N, Senac JS, Hofherr S, Barry MA, Venditti CP. Adeno-associated virus serotype 8 (AAV8) gene transfer rescues a neonatal lethal murine model of propionic acidemia. Hum Gene Ther. 2010 Oct 15; doi: 10.1089/hum.2010.164. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynober L. Ornithine alpha-ketoglutarate as a potent precursor of arginine and nitric oxide: a new job for an old friend. J Nutr. 2004;134:2858S–2862S. doi: 10.1093/jn/134.10.2858s. [DOI] [PubMed] [Google Scholar]
- De Keyzer Y, Valayannopoulos V, Benoist JF, Batteux F, Lacaille F, Hubert L, Chrétien D, Chadefeaux-Vekemans B, Niaudet P, Touati G, Munnich A, de Lonlay P. Multiple OXPHOS deficiency in the liver, kidney, heart, and skeletal muscle of patients with methylmalonic aciduria and propionic aciduria. Pediatr Res. 2009;66:91–95. doi: 10.1203/PDR.0b013e3181a7c270. [DOI] [PubMed] [Google Scholar]
- Deodato F, Boenzi S, Santorelli FM, Dionisi-Vici C. Methylmalonic and propionic aciduria. Am J Med Genet C Semin Med Genet. 2006;142C:104–112. doi: 10.1002/ajmg.c.30090. [DOI] [PubMed] [Google Scholar]
- Dionisi-Vici C, Deodato F, Röschinger W, Rhead W, Wilcken B. ‘Classical’ organic acidurias, propionic aciduria, methylmalonic aciduria and isovaleric aciduria: long-term outcome and effects of expanded newborn screening using tandem mass spectrometry. J Inherit Metab Dis. 2006;29:383–389. doi: 10.1007/s10545-006-0278-z. [DOI] [PubMed] [Google Scholar]
- Erdem E, Cayonu N, Uysalol E, Yildirmak ZY. Chronic intermittent form of isovaleric acidemia mimicking diabetic ketoacidosis. J Pediatr Endocrinol Metab. 2010;23:503–505. doi: 10.1515/jpem.2010.082. [DOI] [PubMed] [Google Scholar]
- Finsterer J. Treatment of mitochondrial disorders. Eur J Paediatr Neurol. 2010;14:29–44. doi: 10.1016/j.ejpn.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Gibson KM, Burlingame TG, Hogema B, Jakobs C, Schutgens RB, Millington D, Roe CR, Roe DS, Sweetman L, Steiner RD, Linck L, Pohowalla P, Sacks M, Kiss D, Rinaldo P, Vockley J. 2-Methylbutyryl-coenzyme A dehydrogenase deficiency: a new inborn error of L-isoleucine metabolism. Pediatr Res. 2000a;47:830–833. doi: 10.1203/00006450-200006000-00025. [DOI] [PubMed] [Google Scholar]
- Gibson KM, Ugarte M, Fukao T, Mitchell GA. Molecular and enzymatic methods for detection of genetic defects in distal pathways of branched-chain amino acid metabolism. Methods Enzymol. 2000b;324:432–453. doi: 10.1016/s0076-6879(00)24252-3. [DOI] [PubMed] [Google Scholar]
- Gülçin I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006;78:803–811. doi: 10.1016/j.lfs.2005.05.103. [DOI] [PubMed] [Google Scholar]
- Homanics GE, Skvorak K, Ferguson C, Watkins S, Paul HS. Production and characterization of murine models of classic and intermediate maple syrup urine disease. BMC Med Genet. 2006;31:7–33. doi: 10.1186/1471-2350-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörster F, Garbade SF, Zwickler T, Aydin HI, Bodamer OA, Burlina AB, Das AM, De Klerk JB, Dionisi-Vici C, Geb S, Gökcay G, Guffon N, Maier EM, Morava E, Walter JH, Schwahn B, Wijburg FA, Lindner M, Grünewald S, Baumgartner MR, Kölker S. Prediction of outcome in isolated methylmalonic acidurias: combined use of clinical and biochemical parameters. J Inherit Metab Dis. 2009;32:630–639. doi: 10.1007/s10545-009-1189-6. [DOI] [PubMed] [Google Scholar]
- Jouvet P, Rustin P, Taylor DL, Pocock JM, Felderhoff-Mueser U, Mazarakis ND, Sarraf C, Joashi U, Kozma M, Greenwood K, Edwards AD, Mehmet H. Branched chain amino acids induce apoptosis in neural cells without mitochondrial membrane depolarization or cytochrome c release: implications for neurological impairment associated with maple syrup urine disease. Mol Biol Cell. 2000;11:1919–1932. doi: 10.1091/mbc.11.5.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanavin OJ, Woldseth B, Jellum E, Tvedt B, Andresen BS, Stromme P. 2-methylbutyryl-CoA dehydrogenase deficiency associated with autism and mental retardation: a case report. J Med Case Reports. 2007;1:98. doi: 10.1186/1752-1947-1-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CH, Liu MY, Liu TT, Hsiao KJ, Cheng KH, Huang CH, Lin HY, Niu DM. Growth hormone therapy in neonatal patients with methylmalonic acidemia. J Chin Med Assoc. 2009;72:462–467. doi: 10.1016/S1726-4901(09)70408-3. [DOI] [PubMed] [Google Scholar]
- Koeberl DD, Young SP, Gregersen NS, Vockley J, Smith WE, Benjamin DK, Jr, An Y, Weavil SD, Chaing SH, Bali D, McDonald MT, Kishnani PS, Chen YT, Millington DS. Rare disorders of metabolism with elevated butyryl- and isobutyryl-carnitine detected by tandem mass spectrometry newborn screening. Pediatr Res. 2003;54:219–223. doi: 10.1203/01.PDR.0000074972.36356.89. [DOI] [PubMed] [Google Scholar]
- Kölker S, Sauer SW, Surtees RA, Leonard JV. The aetiology of neurological complications of organic acidaemias -a role for the blood-brain barrier. J Inherit Metab Dis. 2006;29:701–704. doi: 10.1007/s10545-006-0415-8. [DOI] [PubMed] [Google Scholar]
- Loots DT. Abnormal tricarboxylic acid cycle metabolites in isovaleric acidaemia. J Inherit Metab Dis. 2009;32:403–411. doi: 10.1007/s10545-009-1071-6. [DOI] [PubMed] [Google Scholar]
- Loots DT, Erasmus E, Mienie LJ. Identification of 19 new metabolites induced by abnormal amino acid conjugation in isovaleric acidemia. Clin Chem. 2005;51:1510–1512. doi: 10.1373/clinchem.2005.048421. [DOI] [PubMed] [Google Scholar]
- Martin-Requero A, Corkey BE, Cerdan S, Walajtys-Rode E, Parrilla RL, Williamson JR. Interactions between alpha-ketoisovalerate metabolism and the pathways of gluconeogenesis and urea synthesis in isolated hepatocytes. J Biol Chem. 1983;258:3673–3681. [PubMed] [Google Scholar]
- Matern D, He M, Berry SA, Rinaldo P, Whitley CB, Madsen PP, van Calcar SC, Lussky RC, Andresen BS, Wolff JA, Vockley J. Prospective diagnosis of 2-methylbutyryl-CoA dehydrogenase deficiency in the Hmong population by newborn screening using tandem mass spectrometry. Pediatrics. 2003;112:74–78. doi: 10.1542/peds.112.1.74. [DOI] [PubMed] [Google Scholar]
- Mazer LM, Yi SH, Singh RH. Docosahexaenoic acid status in females of reproductive age with maple syrup urine disease. J Inherit Metab Dis. 2010;33:121–127. doi: 10.1007/s10545-010-9066-x. [DOI] [PubMed] [Google Scholar]
- Mc Guire PJ, Lim-Melia E, Diaz GA, Raymond K, Larkin A, Wasserstein MP, Sansaricq C. Combined liver-kidney transplant for the management of methylmalonic aciduria: a case report and review of the literature. Mol Genet Metab. 2008;93:22–29. doi: 10.1016/j.ymgme.2007.08.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Guire PJ, Parikh A, Diaz GA. Profiling of oxidative stress in patients with inborn errors of metabolism. Mol Genet Metab. 2009;98:173–180. doi: 10.1016/j.ymgme.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Ohura T, Kobayashi M, Shigematsu Y, Yamaguchi S, Suzuki Y, Hata I, Aoki Y, Yang X, Minjares C, Haruta I, Uto H, Ito Y, Müller U. Fatal propionic acidemia in mice lacking propionyl-CoA carboxylase and its rescue by postnatal, liver-specific supplementation via a transgene. J Biol Chem. 2001;276:35995–35999. doi: 10.1074/jbc.M105467200. [DOI] [PubMed] [Google Scholar]
- Morton DH, Strauss KA, Robinson DL, Puffenberger EG, Kelley RI. Diagnosis and treatment of maple syrup disease: a study of 36 patients. Pediatrics. 2002;109:999–1008. doi: 10.1542/peds.109.6.999. [DOI] [PubMed] [Google Scholar]
- Murín R, Hamprecht B. Metabolic and regulatory roles of leucine in neural cells. Neurochem Res. 2008;33:279–284. doi: 10.1007/s11064-007-9444-4. [DOI] [PubMed] [Google Scholar]
- Nasser M, Javaheri H, Fedorowicz Z, Noorani Z. Carnitine supplementation for inborn errors of metabolism. Cochrane Database Syst Res. 2009 Apr 15;:CD006659. doi: 10.1002/14651858.CD006659.pub2. [DOI] [PubMed] [Google Scholar]
- Ogier de Baulny H, Saudubray JM. Branched-chain organic acidurias. Semin Neonatol. 2002;7:65–74. doi: 10.1053/siny.2001.0087. [DOI] [PubMed] [Google Scholar]
- Oglesbee D, He M, Majumder N, Vockley J, Ahmad A, Angle B, Burton B, Charrow J, Ensenauer R, Ficicioglu CH, Keppen LD, Marsden D, Tortorelli S, Hahn SH, Matern D. Development of a newborn screening follow-up algorithm for the diagnosis of isobutyryl-CoA dehydrogenase deficiency. Genet Med. 2007;9:108–116. doi: 10.1097/gim.0b013e31802f78d6. [DOI] [PubMed] [Google Scholar]
- Ribas GS, Manfredini V, de Mari JF, Wayhs CY, Vanzin CS, Biancini GB, Sitta A, Deon M, Wajner M, Vargas CR. Reduction of lipid and protein damage in patients with disorders of propionate metabolism under treatment: a possible protective role of L-carnitine supplementation. Int J Dev Neurosci. 2010;28:127–132. doi: 10.1016/j.ijdevneu.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Ribeiro CA, Sgaravatti AM, Rosa RB, Schuck PF, Grando V, Schmidt AL, Ferreira GC, Perry ML, Dutra-Filho CS, Wajner M. Inhibition of brain energy metabolism by the branched-chain amino acids accumulating in maple syrup urine disease. Neurochem Res. 2008;33:114–124. doi: 10.1007/s11064-007-9423-9. [DOI] [PubMed] [Google Scholar]
- Ribeiro CA, Leipnitz G, Amaral AU, de Bortoli G, Seminotti B, Wajner M. Creatine administration prevents Na+, K+-ATPase inhibition induced by intracerebroventricular administration of isovaleric acid in cerebral cortex of young rats. Brain Res. 2009;1262:81–88. doi: 10.1016/j.brainres.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Richard E, Jorge-Finnigan A, Garcia-Villoria J, Merinero B, Desviat LR, Gort L, Briones P, Leal F, Pérez-Cerdá C, Ribes A, Ugarte M, Pérez B MMACHC Working Group. Genetic and cellular studies of oxidative stress in methylmalonic aciduria (MMA) cobalamin deficiency type C (cblC) with homocystinuria (MMACHC) Hum Mutat. 2009;30:1558–1566. doi: 10.1002/humu.21107. [DOI] [PubMed] [Google Scholar]
- Roe CR, Cederbaum SD, Roe DS, Mardach R, Galindo A, Sweetman L. Isolated isobutyryl-CoA dehydrogenase deficiency: an unrecognized defect in human valine metabolism. Mol Genet Metab. 1998;65:264–271. doi: 10.1006/mgme.1998.2758. [DOI] [PubMed] [Google Scholar]
- Sass JO, Ensenauer R, Röschinger W, Reich H, Steuerwald U, Schirrmacher O, Engel K, Häberle J, Andresen BS, Mégarbané A, Lehnert W, Zschocke J. 2-Methylbutyryl-coenzyme A dehydrogenase deficiency: functional and molecular studies on a defect in isoleucine catabolism. Mol Genet Metab. 2008;93:30–35. doi: 10.1016/j.ymgme.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Sass JO, Sander S, Zschocke J. Isobutyryl-CoA dehydrogenase deficiency: isobutyrylglycinuria and ACAD8 gene mutations in two infants. J Inherit Metab Dis. 2004;27:741–745. doi: 10.1023/B:BOLI.0000045798.12425.1b. [DOI] [PubMed] [Google Scholar]
- Saudubray JM, Nassogne MC, de Lonlay P, Touati G. Clinical approach to inherited metabolic disorders in neonates: an overview. Semin Neonatol. 2002;7:3–15. doi: 10.1053/siny.2001.0083. [DOI] [PubMed] [Google Scholar]
- Sauer SW, Okun JG, Hoffmann GF, Koelker S, Morath MA. Impact of short- and medium-chain organic acids, acylcarnitines, and acyl-CoAs on mitochondrial energy metabolism. Biochim Biophys Acta. 2008;1777:1276–1282. doi: 10.1016/j.bbabio.2008.05.447. [DOI] [PubMed] [Google Scholar]
- Sauer SW, Opp S, Haarmann A, Okun JG, Kölker S, Morath MA. Long-term exposure of human proximal tubule cells to hydroxycobalamin[c-lactam] as a possible model to study renal disease in methylmalonic acidurias. J Inherit Metab Dis. 2009;32:720–727. doi: 10.1007/s10545-009-1197-6. [DOI] [PubMed] [Google Scholar]
- Sauer SW, Opp S, Mahringer A, Kamiński MM, Thiel C, Okun JG, Fricker G, Morath MA, Kölker S. Glutaric aciduria type I and methylmalonic aciduria: simulation of cerebral import and export of accumulating neurotoxic dicarboxylic acids in in vitro models of the blood-brain barrier and the choroid plexus. Biochim Biophys Acta. 2010;1802:552–560. doi: 10.1016/j.bbadis.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Scholl-Bürgi S, Sass JO, Heinz-Erian P, Amann E, Haberlandt E, Albrecht U, Ertl C, Sigl SB, Lagler F, Rostasy K, Karall D. Changes in plasma amino acid concentrations with increasing age in patients with propionic acidemia. Amino Acids. 2010;38:1473–1481. doi: 10.1007/s00726-009-0356-2. [DOI] [PubMed] [Google Scholar]
- Schuck PF, Rosa RB, Pettenuzzo LF, Sitta A, Wannmacher CM, Wyse AT, Wajner M. Inhibition of mitochondrial creatine kinase activity from rat cerebral cortex by methylmalonic acid. Neurochem Int. 2004;45:661–667. doi: 10.1016/j.neuint.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Schwab MA, Sauer SW, Okun JG, Nijtmans LG, Rodenburg RJ, van den Heuvel LP, Dröse S, Brandt U, Hoffmann GF, Ter Laak H, Kölker S, Smeitink JA. Secondary mitochondrial dysfunction in propionic aciduria: a pathogenic role for endogenous mitochondrial toxins. Biochem J. 2006;398:107–112. doi: 10.1042/BJ20060221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E, Flaschker N, Schadewaldt P, Langenbeck U, Wendel U. Variant maple syrup urine disease (MSUD)-the entire spectrum. J Inherit Metab Dis. 2006;29:716–724. doi: 10.1007/s10545-006-0276-1. [DOI] [PubMed] [Google Scholar]
- Skvorak KJ, Hager EJ, Arning E, Bottiglieri T, Paul HS, Strom SC, Homanics GE, Sun Q, Jansen EE, Jakobs C, Zinnanti WJ, Gibson KM. Hepatocyte transplantation (HTx) corrects selected neurometabolic abnormalities in murine intermediate maple syrup urine disease (iMSUD) Biochim Biophys Acta. 2009a;1792:1004–1010. doi: 10.1016/j.bbadis.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skvorak KJ, Paul HS, Dorko K, Marongiu F, Ellis E, Chace D, Ferguson C, Gibson KM, Homanics GE, Strom SC. Hepatocyte transplantation improves phenotype and extends survival in a murine model of intermediate maple syrup urine disease. Mol Ther. 2009b;17:1266–1273. doi: 10.1038/mt.2009.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano AF, Leipnitz G, De Bortoli GM, Seminotti B, Amaral AU, Fernandes CG, Latini AS, Dutra-Filho CS, Wajner M. Induction of oxidative stress by the metabolites accumulating in isovaleric acidemia in brain cortex of young rats. Free Radic Res. 2008;42:707–715. doi: 10.1080/10715760802311179. [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter U, Lindner M, Santer R, Grotzke M, Baumgartner MR, Boehles H, Das A, Haase C, Hennermann JB, Karall D, de Klerk H, Knerr I, Koch HG, Plecko B, Röschinger W, Schwab KO, Scheible D, Wijburg FA, Zschocke J, Mayatepek E, Wendel U. Treatment recommendations in long-chain fatty acid oxidation defects: consensus from a workshop. J Inherit Metab Dis. 2009;32:498–505. doi: 10.1007/s10545-009-1126-8. [DOI] [PubMed] [Google Scholar]
- Stewart PM, Walser M. Failure of the normal ureagenic response to amino acids in organic acid-loaded rats. Proposed mechanism for the hyperammonemia of propionic and methylmalonic acidemia. J Clin Invest. 1980;66:484–492. doi: 10.1172/JCI109879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss KA, Mazariegos GV, Sindhi R, Squires R, Finegold DN, Vockley G, Robinson DL, Hendrickson C, Virji M, Cropcho L, Puffenberger EG, McGhee W, Seward LM, Morton DH. Elective liver transplantation for the treatment of classical maple syrup urine disease. Am J Transplant. 2006;6:557–564. doi: 10.1111/j.1600-6143.2005.01209.x. [DOI] [PubMed] [Google Scholar]
- Strauss KA, Wardley B, Robinson D, Hendrickson C, Rider NL, Puffenberger EG, Shelmer D, Moser AB, Morton DH. Classical maple syrup urine disease and brain development: principles of management and formula design. Mol Genet Metab. 2010;99:333–345. doi: 10.1016/j.ymgme.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto HH. Mitochondria-targeted peptide antioxidants: novel neuroprotective agents. AAPS J. 2006;8:E521–E531. doi: 10.1208/aapsj080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati G, Valayannopoulos V, Mention K, de Lonlay P, Jouvet P, Depondt E, Assoun M, Souberbielle JC, Rabier D, Ogier de Baulny H, Saudubray JM. Methylmalonic and propionic acidurias: management without or with a few supplements of specific amino acid mixture. J Inherit Metab Dis. 2006;29:288–298. doi: 10.1007/s10545-006-0351-7. [DOI] [PubMed] [Google Scholar]
- Van Calcar SC, Gleason LA, Lindh H, Hoffman G, Rhead W, Vockley G, Wolff JA, Durkin MS. 2-methylbutyryl-CoA dehydrogenase deficiency in Hmong infants identified by expanded newborn screen. WMJ. 2007;106:12–15. [PubMed] [Google Scholar]
- Vockley J, Ensenauer R. Isovaleric acidemia: new aspects of genetic and phenotypic heterogeneity. Am J Med Genet C Semin Med Genet. 2006;142C:95–103. doi: 10.1002/ajmg.c.30089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajner M, Latini A, Wyse AT, Dutra-Filho CS. The role of oxidative damage in the neuropathology of organic acidurias: insights from animal studies. J Inherit Metab Dis. 2004;27:427–448. doi: 10.1023/B:BOLI.0000037353.13085.e2. [DOI] [PubMed] [Google Scholar]
- Walrand S. Ornithine alpha-ketoglutarate: could it be a new therapeutic option for sarcopenia? J Nutr Health Aging. 2010;14:570–577. doi: 10.1007/s12603-010-0109-7. [DOI] [PubMed] [Google Scholar]
- Wikoff WR, Gangoiti JA, Barshop BA, Siuzdak G. Metabolomics identifies perturbations in human disorders of propionate metabolism. Clin Chem. 2007;53:2169–2176. doi: 10.1373/clinchem.2007.089011. [DOI] [PubMed] [Google Scholar]
- Wu JY, Kao HJ, Li SC, Stevens R, Hillman S, Millington D, Chen YT. ENU mutagenesis identifies mice with mitochondrial branched-chain aminotransferase deficiency resembling human maple syrup urine disease. J Clin Invest. 2004;113:434–440. doi: 10.1172/JCI19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhao K, Calingasan NY, Luo G, Szeto HH, Beal MF. Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine neurotoxicity. Antioxid Redox Signal. 2009;11:2095–2104. doi: 10.1089/ars.2009.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannicelli S, Acosta PB, Velazquez A, Bock HG, Marriage B, Kurczynski TW, Miller M, Korson M, Steiner RD, Rutledge L, Bernstein L, Chinsky J, Galvin-Parton P, Arnold GL. Improved growth and nutrition status in children with methylmalonic or propionic acidemia fed an elemental medical food. Mol Genet Metab. 2003;80:181–188. doi: 10.1016/j.ymgme.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Yannicelli S. Nutrition therapy of organic acidaemias with amino acid-based formulas: emphasis on methylmalonic and propionic acidaemia. J Inherit Metab Dis. 2006;29:281–287. doi: 10.1007/s10545-006-0267-2. [DOI] [PubMed] [Google Scholar]
- Zand DJ, Brown KM, Lichter-Konecki U, Campbell JK, Salehi V, Chamberlain JM. Effectiveness of a clinical pathway for the emergency treatment of patients with inborn errors of metabolism. Pediatrics. 2008;122:1191–1195. doi: 10.1542/peds.2008-0205. [DOI] [PubMed] [Google Scholar]
- Zinnanti WJ, Lazovic J, Griffin K, Skvorak KJ, Paul HS, Homanics GE, Bewley MC, Cheng KC, Lanoue KF, Flanagan JM. Dual mechanism of brain injury and novel treatment strategy in maple syrup urine disease. Brain. 2009;132:903–918. doi: 10.1093/brain/awp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwickler T, Lindner M, Aydin HI, Baumgartner MR, Bodamer OA, Burlina AB, Das AM, DeKlerk JB, Gökcay G, Grünewald S, Guffon N, Maier EM, Morava E, Geb S, Schwahn B, Walter JH, Wendel U, Wijburg FA, Müller E, Kölker S, Hörster F. Diagnostic work-up and management of patients with isolated methylmalonic acidurias in European metabolic centres. J Inherit Metab Dis. 2008;31:361–367. doi: 10.1007/s10545-008-0804-2. [DOI] [PubMed] [Google Scholar]