Abstract
PURPOSE OF REVIEW
Normal B cells that failed to productively rearrange immunoglobulin V region genes, encoding a functional B cell receptor (BCR) are destined to die. Likewise, the majority of B cell malignancies remain dependent on functional BCR signaling, while in some subtypes BCR expression is missing and, apparently, counterselected. Here we summarize recent the experimental evidence for the importance of BCR signaling and clinical concepts to target the BCR pathway in B cell leukemia and lymphoma.
RECENT FINDINGS
While the dependency on pre-BCR signaling in pre-B acute lymphoblastic leukemia (ALL) seems to be limited to few ALL subtypes (e.g. TCF3-PBX1), most mature B cell lymphomas rely on BCR signaling provided by different stimuli e.g. tonic B cell signaling, chronic (auto)-antigen exposure, and self-binding properties of the BCR. The finding that in chronic lymphocytic leukemia (CLL), BCRs bind to an epitope on the BCR itself unravels a novel concept for CLL pathogenesis.
SUMMARY
Targeting of the B cell receptor tyrosine kinases SYK, BTK, and PI3K achieve promising clinical responses in various mature B cell malignancies and might also be useful in defined subsets of ALL. However, further understanding of the BCR signal integration in the different disease groups are required to accurately predict, which groups of patients will benefit from BCR pathway-inhibition.
Keywords: (pre-)B cell receptor signaling, acute lymphoblastic leukemia, lymphoma
Introduction
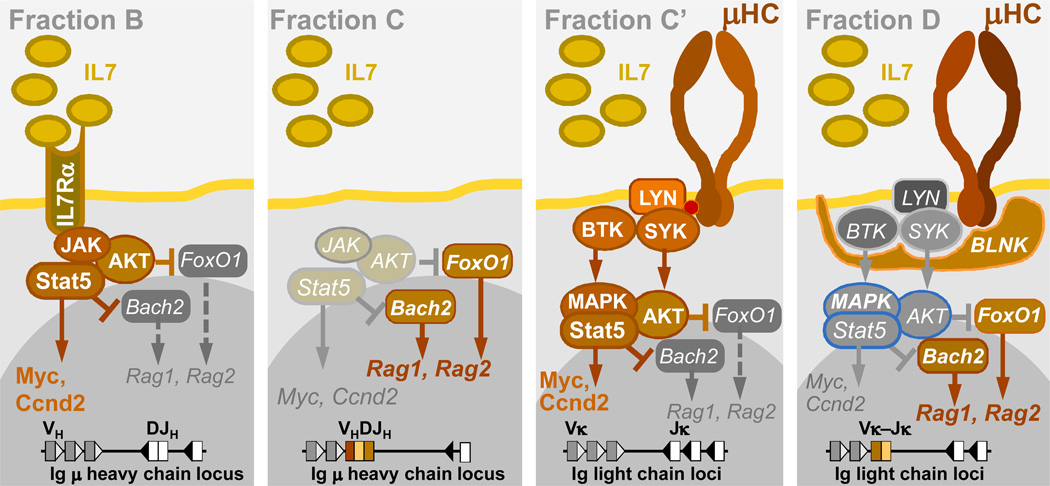
Mature B cells are stringently selected for the continuous expression of a functional B-cell receptor (BCR) throughout their life and loss of BCR expression leads to rapid cell death [1,2]. The majority of B cell malignancies remain dependent on while others lack BCR expression, either due to their maturation state or to selection pressure against the expression of a functional BCR (Table 1) [3–10]. While the BCR is thought to contribute to malignant transformation in most BCR-expressing malignancies, it may serve as a tumor suppressor in BCR-negative B cell malignancies [11–13]. Developing B cells can be classified into different stages according to the expression of differential surface markers (Hardy Fraction A–F) [14]. In Fraction C’, the pre-B cell receptor (pre-BCR) is expressed after successful rearrangement of the μ heavy chain and pairing with the surrogate light chain (VPREB and λ5). The expression causes self-aggregation and results in clonal proliferation and downregulation of pre-BCR components [15]. After further maturation and rearrangement of the light chain, the surrogate light chains of the pre-BCR are replaced by κ or λ light chains, which together with the µ heavy chain form the BCR on mature B cells.
Table 1.
BCR expressing and non-expressing B cell malignancies
| BCR-expressing B cell malignancies | BCR-negative B cell malignancies |
|---|---|
| TCF3-PBX1 acute lymphoblastic leukemia (ALL) [3] | Ph+ and MLL-AF4 ALL [3] |
| Chronic lymphocytic leukemia (CLL) [4–6] | Hodgkin’s lymphoma [8] |
| Diffuse large B cell lymphoma [7] | Immunoblastic lymphoma [7] |
| Mantle cell lymphoma [7] | Post-transplant lymphoma [7] |
| Splenic marginal zone lymphoma [7] | Primary effusion lymphoma [7] |
| Hairy-cell leukemia [7] | Primary mediastinal B cell lymphoma [9] |
| Prolymphocytic leukemia [7] | AIDS-related lymphoma [10] |
| Burkitt's lymphoma [7] | Multiple Myeloma [7] |
| Follicular lymphoma [7] |
BCR signaling
The pre-BCR and BCR utilize very similar signaling components, particularly within the proximal signaling compartment. While both the pre-BCR and the BCR exhibit ligand-independent, ‘tonic’ signaling, mature B cells are typically activated by engagement of the BCR by antigen, for instance in germinal centers. Despite their structural similarities, the outcome of pre-BCR and BCR signaling can be divergent and may depend on expression levels and cellular organization of signaling components, expression of downstream mediators (i.e. transcription factors), and the chromatin structure [16–19].
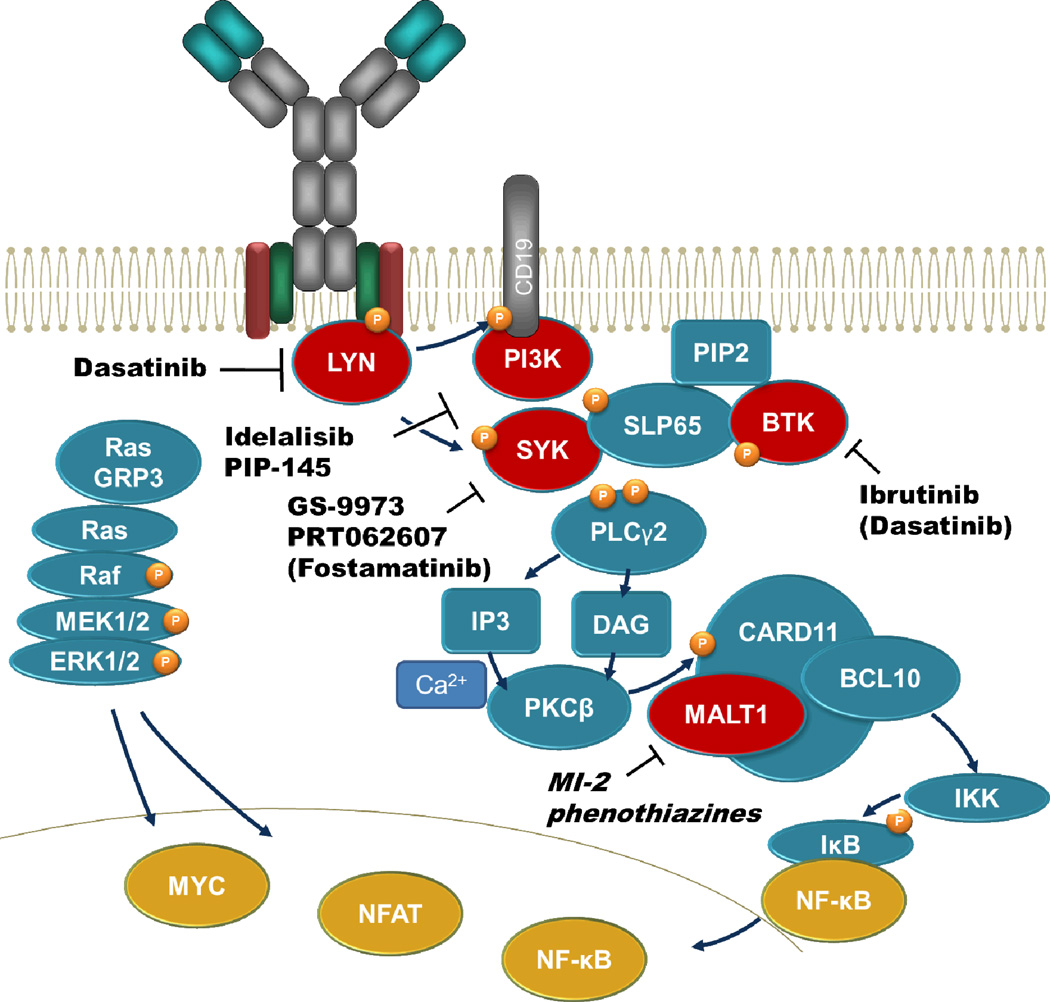
In mature B cells, monovalent antigens fail to activate the BCR. Therefore, activation was assumed to rely on crosslinking of BCRs in order to create clusters [7]. However, recent evidence indicates that in the absence of stimuli, BCRs are clustered in an autoinhibitory conformation and antigen binding disperses these clusters [20,21]. Consequently, Src kinases Lyn, Fyn, and Blk phosphorylate the now accessible immunoreceptor tyrosine-based activation motifs (ITAMs) on Igα (CD79A) and Igβ (CD79B), thereby create a docking site for the tyrosine kinase SYK [22–24]. Activated SYK then phosphorylates direct targets such as the phospholipase Cγ2 (PLCγ2), Bruton's tyrosine kinase (BTK), and the adaptor molecules BLNK and BCAP. Together with the phosphorylated co-receptor CD19, BCAP recruits PI3K to the plasma membrane to generate phosphatidylinositol-3,4,5-trisphosphate (PIP3). This creates a docking site for BTK that, together with SYK, activates PLCγ2. Activated PLCγ2 hydrolyses phosphatidylinositol-4,5-bisphosphate (PIP2) to inositol triphosphate (IP3) and diacylglycerol leading to Ca2+ influx and PKCβ activation. PKCβ phosphorylates and activates CARD11 that together with MALT1 and BCL10 activate NF-κB [25]. PKCβ-mediated phosphorylation of RasGRP3 promotes GTP acquisition of RAS, which activates Raf1 and subsequently the MAP kinases ERK 1/2. Finally, BCR activation results in transcription factor activation including NFAT, NF-κB, and MYC [26]. Further modulation of the signaling outcome is mediated by co-receptors of the BCR that can either dampen signaling by recruitment of inhibitory phosphatases that oppose tyrosine kinase function, i.e. CD22, PECAM1 or promote BCR-downstream signaling through amplifiers of signal transduction (e.g. MEK1-ERK1/2).
Divergent outcomes of pre-B cell receptor signaling at the time of transformation to acute lymphoblastic leukemia
Pre-B cells within the bone marrow represent the normal counterpart of ALL in the vast majority of cases (~90%) [27]. This is surprising because pre-B cells represent a very short-lived transitional subset during early B cell development: Unless rescued through survival signals from a successfully assembled pre- BCR, pre-B cells are destined to die within days [28,29]. During normal early B cell development, the pre-BCR has a dual function in that it first promotes survival and proliferation of large cycling pre-B cells (Fraction C’) and subsequently induces differentiation in small resting (Fraction D) pre-B cells (Figure 2) [14,30]. To which extent these signaling processes cooperate with the different oncogenic lesions in B cell precursors is still under investigation.
Figure 2. Pre-BCR checkpoint control during early B cell development.
Pro-B cells (Fraction B) proliferate under the influence of the IL7 receptor (activation of JAK-STAT5, AKT) until they rearrange Ig VH-DJH gene segments to express a functional μ heavy chain (μ HC) as part of the pre-BCR (Fraction C’). Upon initial cell surface expression (Fraction C’), autonomous pre-BCR signaling induces strong proliferation via activation of SRC kinases (Lyn), SYK, BTK and multiple phosphorylation events in STAT5, AKT and MAPK pathways. Studies in the initial project period demonstrated that subsequent engagement of the pre-BCR linker molecule SLP65 results in global de-phosphorylation in the STAT5, AKT and MAPK pathways, cell cycle arrest and differentiation of pre-B into mature B cells [25].
Genetic defects in the pre-BCR pathway are very common in most ALL subsets –exceptions
The high frequency of defects in the pre-BCR-related signaling molecules in ALL cells identified by us and others suggests that the pre-BCR may counteract malignant transformation. Focal deletions of EBF1, cryptic translocations, point mutations, and monoallelic deletions of PAX5 result in reduced expression of BCR-related genes such as CD19, CD79A, BLNK and CD72. Besides, deletions of BLNK, VPREB1, and IGLL1 as well as SYK, SLP65, CD79B, IRF4, and BTLA are described [3,31]. On the other hand, the pre-BCR also delivers critical survival and proliferation signals and its expression is required for abnormal lymphoproliferation [32]. In addition, previous work demonstrated that the pre-BCR-related tyrosine kinase Syk is required for Myc-mediated transformation of pre-B cells [33]. Our group previously demonstrated that the pre-B cell receptor-related signaling molecule BTK plays a central role in oncogenic signaling of leukemia cells [34]. Based on these findings, it is currently unclear whether pre-BCR signaling is required to enable malignant outgrowth in ALL or functions to suppress leukemogenesis.
The (pre-)BCR tyrosine kinases SYK and BTK as therapeutic targets in B cell malignancies
Future studies to validate (pre-) BCR-related signaling molecules as therapeutic targets are of immediate clinical relevance, because data from four major clinical trials in 2013 demonstrated that targeting of the (pre-) B cell receptor tyrosine kinases SYK and BTK achieves durable clinical responses in various mature B cell malignancies (discussed below). Despite the critical role of pre-BCR signaling in ALL, the clinical successes of Ibrutinib (BTK) and Fostamatinib/GS-9973 (SYK) in mature B cell lymphoma could not be recaptitulated in pre-clinical models for ALL. While ALL cells from some patients are extremely sensitive to BTK/SYK inhibition, ALL cells from other patients are completely resistant to Ibrutinib (BTK) and Fostamatinib/GS-9973 (SYK). Kinase-independent adaptor function as described for BTK in pre B cells may account for this discrepancy [35]. These findings suggest that critical additional information on pathway-specific targeting of pre-BCR signaling molecules is needed to effectively use these and other agents in the treatment of B cell lineage ALL.
Dasatinib selectively kills TCF3-rearranged ALL cells
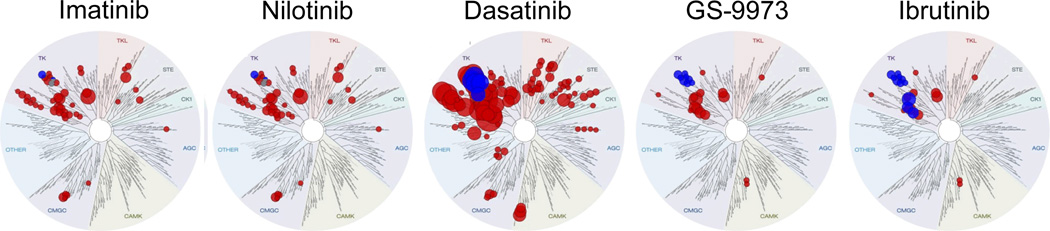
Dasatinib is widely used as ABL1 kinase inhibitor in Ph+ ALL and CML, but unexpectedly showed very strong activity in all TCF3-rearranged cases of ALL (Type 1 ALL). The effect of Dasatinib in TCF3-rearranged ALL cells is not ABL1-dependent because other ABL1 kinase inhibitors (Imatinib, Nilotinib) have no effect on these ALL cells. Interestingly, TCF3-rearranged ALL cells are also sensitive to SYK (GS-9973) and BTK (Ibrutinib) inhibitors, suggesting that these ALL cells are pre-BCR-dependent [36]. Given that TCF3 may directly promote the expression of IgM and κ-LC and reduce the expression of the negative regulator PTPN6, this signaling pathway may be of importance [37]. Recent work demonstrated that besides its activity on BCR-ABL1 and ABL1, Dasatinib is a powerful inhibitor of the pre-BCR tyrosine kinases BTK and the SRC family kinases LYN and BLK [38]. A recent study suggested that the therapeutic effect of Dasatinib on TCF3-rearranged ALL is indeed owing to selective inhibition of BTK and SRC [29,39] (Figure 3). These findings are potentially relevant because (1) they suggest that understanding of the pre-BCR pathway in ALL will allow to identify patients that will benefit from drugs that target pre-BCR signaling (e.g. GS-9973, Ibrutinib). (2) In addition, the FDA-approved ABL1 kinase inhibitor Dasatinib unexpectedly kills Non-Ph+ Type 1 ALL cells that express an active pre-BCR. It will be interesting to determine which other subsets of ALL exhibit a similar dependency on pre-BCR signaling as TCF3-PBX1 ALL.
Figure 3. Spectrum of Dasatinib-targets compared to narrow inhibitors of ABL1 kinase and pre-BCR signaling.
Dendrograms of target-kinases were generated with the TreeSpot software (KinomeScan). Sizes of circles depict inverse Kd values for each kinase target. Red circles are all targets of the individual compound. Among these targets, kinases within the pre-BCR pathway (SYK, BTK, LYN, BLK, SRC) are highlighted in blue.
BCR and its function in B cell lymphoma
The majority of mature B cell lymphoma express a functional B cell receptor. In Burkitt’s lymphoma (BL), BCR expression is required to provide tonic signaling [37,40]. Activating mutations in TCF3 or deleterious lesions of its negative regulator ID3 in BL are associated with increased expression of the BCR, and knockdown of CD79A and SYK was shown to reduce cell survival [37].
For most types of lymphoma, there is strong evidence that the BCR signaling pathway is specifically activated and contributes to pathogenesis (e.g. follicular lymphoma (FL), chronic lymphocytic leukemia CLL, activated B cell type- diffuse large B cell lymphoma (ABC-DLBCL), marginal zone lymphoma (MZL), mantle cell lymphoma (MCL) [7]). These are characterized by the usage of stereotyped, non-random Ig VH segments and chronic activation of the BCR pathway, and for some, ongoing somatic hypermutation during clonal evolution [7,41]. Several different mechanisms contribute to the activation of the BCR signaling pathway in these lymphoma: chronic exogenic antigen stimulation (hepatitis C virus in splenic MZL [42]), chronic auto-antigen stimulation (FL, CLL, mucosa-associated lymphoid tissue lymphoma (MALT) [43–46]), autonomous BCR signaling (CLL [47]), as well as mutations that activate the pathway downstream of the BCR itself (CD79B and CARD11 mutations in ABC-DLBCL [40,48]). Further augmentation of BCR signaling in ABC-DLBCL has been attributed to high expression levels of BCL6, which increases SYK activity by repressing expression of the phosphatase PTPROt [49]. Importantly, removing the BCR stimulus, e.g. by antiviral or antibacterial treatment, results in regression of the lymphoma [50,51], underlining the importance of BCR stimulation in lymphoma development.
Self-recognition in CLL and other lymphoma entities
Recently, novel insight in the signaling from BCR in CLL has led to further understanding of the importance of this pathway in B cell lymphomagenesis. Similar to the pre-BCR or the BCR in non-selected, transitional B cells, CLL BCRs confer autonomous signals by recognizing a peptide within the framework region 2 (FR2) of surface Ig itself [47]. A previous study using phage display libraries identified a peptide (WNWPLPPVRQFS) that was recognized -with different affinity- by the BCR of all tested CLL samples irrespective of IGHV gene use or mutational status but did not bind to the control samples [52]. Homologies between this epitope and a sequence within the BCR itself has led to the identification of a self-bound epitope in FR2 [47]. For CLL, further studies using phage display identified alternative epitopes for autostimulatory mechanisms in CLL [53]. The finding that poly-reactivity of CLL BCRs correlates with inferior clinical outcome, indicates that further stimulation of CLL via autoantigen (in addition to its self-binding) may modulate signaling outcome, i.e. result in increased NF-κB activation and thereby increase the proliferation capacity [52]. A recent study suggests that a subgroup of FL-derived BCR bind themselves, however, whether this is via a similar mechanism – on a single cell level- remains to be determined as well as its clinical impact [45,54].
Treatment options targeting the BCR signaling pathway
Despite clinical improvement, particularly indolent B cell Non-Hodgkin’s lymphoma (B-NHL) lack persistent response rates to current therapies and remain mainly incurable. Given the importance of the BCR signaling pathway in many B-NHLs, drug discovery efforts have focused on the BCR-pathway and several molecular targets have been evaluated in different clinical trials.
SYK inhibition
SYK is a key element of the BCR pathway [55]. The first clinically relevant SYK inhibitor Fostamatinib (also called R406, or the oral form R788; AstraZeneca Pharmaceuticals) is an ATP-competitive inhibitor that has been shown to inhibit BCR signaling in CLL and ABC-DLBCL models [56–58]. In a phase 1 study patients with relapsed hematologic malignancies, fostamatib induced objective response rates (ORR) of 55% in CLL, 24% in DLBCL, 11% in MCL, and 10% in FL [59]. In this study all patients with CLL experienced an increase in circulating lymphocytes following initial treatment, a phenomenon that has also been noted in patients treated with other BCR pathway inhibitors whcih might be due to common signaling events after microenvironmental stimuli [55,60]. However, in vitro kinase assays demonstrated that fostamatinib can also inhibit kinases i.e. FLT3, KIT, Lck, JAK1, and JAK3, with comparable potencies [61]. Other more specific SYK inhibitors such as GS-9973 (Gilead Sciences) [62] and PRT062607 (Portola Pharmaceuticals) are currently tested in clinical trials. In vitro data shows anti-proliferative activity against DLBCL cell lines and inhibition of the BCR signaling pathway [63,64].
BTK inhibition
Ibrutinib (PCI-32765) is an orally available, highly selective kinase inhibitor that irreversibly binds to the C481 residue of BTK and has low off target effects. In Februrary 2013, it was designated a "breakthrough therapy" by the United States Food and Drug Administration (FDA) for the treatment of patients with relapsed or refractory mantle cell lymphoma and Waldenström macroglobulinemia. A Phase 1 study revealed safety and an ORR of 60% in relapsed/refractory aggressive and indolent lymphoma at escalating oral doses [65,66]. In elderly, previously untreated CLL/SLL patients Ibrutinib was found to be safe and effective (71% ORR) in a phase 1b/2 trial [67]. Interestingly, similar to the SYK inhibitor [59], most patients treated with Ibrutinib experienced an initial lymphocytosis as a consequence of a lymphocyte egress from nodal compartments [68], suposedly due to common signaling events form stimuli from the microenvironment [69]. For indolent lymphoma the results are reviewed in [70]. In aggressive lymphoma, ibrutinib revealed a ORR of 68% in MCL [71] and showed substancial response rates in the relapsed, refractory ABC DLBCL (ORR=40%). In contrast, the ORR for GCB DLBCL was only 5%, consistent with the concept that this subgroup is not dependent on BCR-signaling [48]. In addition, combination of Ibrutinib with either Rituximab alone or both, Rituximab (R) and Bendamustine (B) have revealed very promising initial ORRs (Ibrutinib+R 85%; Ibrutinib+BR 92%) [72–74].
For Ibrutinib, mutation of the C481 BTK binding site (BTKC481S) within the tyrosine kinase domain was found in 4 of 13 patients with acquired resistance [75,76]. However, only some of the clinically evident ibrutinib resistant cases can be attributed to mutations in BTK or other genes of the BCR signaling pathway, such as CARD11 [48]. Further mutations in genes that confer resistance to necroptosis RIPK1 Q375* or constititive activation of KRAS via Q61H confered resistance to Ibrutinib in vitro [77].
PI3K inhibition
The PI3K pathway is a promising therapeutic target as it is downstream target of the BCR and the SYK kinase [78]. In addition, its negative regulator PTEN is frequently deleted or deregulated in B-NHL [79]. Initial in vitro studies have indicated that different PI3K inhibitors were promising in CLL [80], ABC DLBCL [48,81] and MCL [82]. In addition, there is evidence that tonic BCR signaling mainly utilized the PI3K pathway, supporting a rationale for PI3K inhibition in Burkitt‘s lymphoma [13,37,83].
Clinically tested PI3K inhibitors are Idelalisib and IPI-145. Idelalisib (formally called GS-1101 or CAL-101) reversibly inhibits the PI3Kδ isoform of the p110 catalytic subunit. Phase I trial in relapsed/refractory CLL revealed that Idelalisib was well tolerated and reduced lymphadenopathy and phosphorylation of the downstream molecule AKT. As described for Fostamatinib and Ibrutinib, Idelalisib also caused a initial increase in lymphocyte count as consequence of a regress form the protective microenvironment. A phase 2 study of Idelalisib in heavily pretreated indolent lymphoma patients revealed a response rate of 57% [84,85]. IPI-145 (formerly INK-1147; Infinity Pharmaceuticals) is another selective PI3K inhibitor that targets both, PI3K-δ and PI3K-γ isoforms and has been evaluated in CLL [86]. The PI3K-γ isoform has additional functions in mediating mast cell activation and chemokine-induced cell trafficking [87,88].
MALT1 inhibition
A compound screen for the ability to inhibit the cleavage function of a dimeric, active form of MALT1 resulted in identification of novel MALT1 inhibitors. These molecules decreased NF-κB signaling and reduced target gene expression and induced apoptosis at low concentrations in ABC-DLBCL but not GC-like DLBCL in vitro and in vivo [89,90]. As some of the used components have already been used in clinical setting for neurological symptoms, they might be useful for the treatment of ABC-DLBCL and other MALT1-dependent lymphomas in the near future.
Conclusion
In summary, recent studies identified tyrosine kinases (SYK, BTK), PI3K, and MALT1 within the BCR pathway as a novel class of therapeutic targets in B cell lymphomas. While the BCR pathway is integrated in oncogenic signalling in the majority of mature B cell lymphomas, this is only the case for a small fraction of B cell lineage acute lymphoblastic leukemia (ALL). Future studies will elucidate the mechanistic basis of divergent outcomes of BCR signaling in B cell malignancies.
Figure 1. The BCR signaling pathway.
After ligation of BCRs, Src kinases phosphorylate ITAMs, where SYK binds and is also activated by phosphorylation. SYK then passes the signal further downstream by phosphorylating SLP65, BTK, and PLCγ2. Furthermore, the PI3K and MAPK signaling pathways are activated. Finally, BCR receptor stimulation leads by sequential phosphorylation events to activation of MYC, NF-κB and NFAT.
Key points.
Reflecting its key role in B cell survival and selection, the majority of B cell malignancies remain dependent on BCR function.
B cell malignancies can be divided into BCR-addicted tumors and malignancies in which the BCR plays a tumor-suppressive role.
Mechanisms contributing to oncogenic activation of the BCR pathway include chronic stimulation by exogenous antigen, autonomous BCR signaling, as well as mutations that activate the pathway downstream of the BCR itself.
Recent clinical trials validated tyrosine kinases (SYK, BTK) and PI3K within the BCR-pathway as a novel class of therapeutic targets in a number of mature B cell malignancies.
Acknowledgements
We would like to thank Hassan Jumaa (Ulm, Germany), Hendrik Veelken (Leiden, Netherlands), Arthur Weiss and André Limnander (UCSF, San Francisco, CA) and Louis M. Staudt (NCI, Bethesda, MD) for critical discussions.
Disclosure of funding:
This work was supported by grants from the National Institutes of Health (NIH) through grants R01CA137060, R01CA139032, R01CA157644, R01CA169458, R01CA172558. Markus Müschen is a Senior Investigator of the Wellcome Trust (101880Z/13/Z) and a scholar of the Leukemia and Lymphoma Society (LLS-1479-11).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
(*) special interest
(**) for outstanding interest
- 1.Kraus M, Alimzhanov MB, Rajewsky N, et al. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 3.Trageser D, Iacobucci I, Nahar R, et al. Pre-B cell receptor-mediated cell cycle arrest in Philadelphia chromosome-positive acute lymphoblastic leukemia requires IKAROS function. J Exp Med. 2009;206:1739–1753. doi: 10.1084/jem.20090004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deglesne PA, Chevallier N, Letestu R, et al. Survival response to B-cell receptor ligation is restricted to progressive chronic lymphocytic leukemia cells irrespective of Zap70 expression. Cancer Res. 2006;66:7158–7166. doi: 10.1158/0008-5472.CAN-06-0085. [DOI] [PubMed] [Google Scholar]
- 5.Mauerer K, Zahrieh D, Gorgun G, et al. Immunoglobulin gene segment usage, location and immunogenicity in mutated and unmutated chronic lymphocytic leukaemia. Br J Haematol. 2005;129:499–510. doi: 10.1111/j.1365-2141.2005.05480.x. [DOI] [PubMed] [Google Scholar]
- 6.Messmer BT, Albesiano E, Efremov DG, et al. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia. J Exp Med. 2004;200:519–525. doi: 10.1084/jem.20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005;5:251–262. doi: 10.1038/nrc1589. [DOI] [PubMed] [Google Scholar]
- 8.Kanzler H, Kuppers R, Hansmann ML, et al. Hodgkin and Reed-Sternberg cells in Hodgkin's disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. J Exp Med. 1996;184:1495–1505. doi: 10.1084/jem.184.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savage KJ, Monti S, Kutok JL, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood. 2003;102:3871–3879. doi: 10.1182/blood-2003-06-1841. [DOI] [PubMed] [Google Scholar]
- 10.Lu P, Yang C, Guasparri I, et al. Early events of B-cell receptor signaling are not essential for the proliferation and viability of AIDS-related lymphoma. Leukemia. 2009;23:807–810. doi: 10.1038/leu.2008.304. [DOI] [PubMed] [Google Scholar]
- 11.Brauninger A, Spieker T, Willenbrock K, et al. Survival and clonal expansion of mutating "forbidden" (immunoglobulin receptor-deficient) epstein-barr virus-infected b cells in angioimmunoblastic t cell lymphoma. J Exp Med. 2001;194:927–940. doi: 10.1084/jem.194.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segal GH, Edinger MG, Owen M, et al. Concomitant delineation of surface Ig, B-cell differentiation antigens, and HLADR on lymphoid proliferations using three-color immunocytometry. Cytometry. 1991;12:350–359. doi: 10.1002/cyto.990120410. [DOI] [PubMed] [Google Scholar]
- 13.Sander S, Calado DP, Srinivasan L, et al. Synergy between PI3K signaling and MYC in Burkitt lymphomagenesis. Cancer Cell. 2012;22:167–179. doi: 10.1016/j.ccr.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 15.Herzog S, Jumaa H. Self-recognition and clonal selection: autoreactivity drives the generation of B cells. Curr Opin Immunol. 2012;24:166–172. doi: 10.1016/j.coi.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Herzog S, Reth M, Jumaa H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat Rev Immunol. 2009;9:195–205. doi: 10.1038/nri2491. [DOI] [PubMed] [Google Scholar]
- 17.Limnander A, Weiss A. Ca-dependent Ras/Erk signaling mediates negative selection of autoreactive B cells. Small GTPases. 2011;2:282–288. doi: 10.4161/sgtp.2.5.17794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta N, DeFranco AL. Lipid rafts and B cell signaling. Semin Cell Dev Biol. 2007;18:616–626. doi: 10.1016/j.semcdb.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rickert RC. New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nat Rev Immunol. 2013;13:578–591. doi: 10.1038/nri3487. [DOI] [PubMed] [Google Scholar]
- 20.Fiala GJ, Kaschek D, Blumenthal B, et al. Pre-clustering of the B cell antigen receptor demonstrated by mathematically extended electron microscopy. Front Immunol. 2013;4:427. doi: 10.3389/fimmu.2013.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Reth M. Oligomeric organization of the B-cell antigen receptor on resting cells. Nature. 2010;467:465–469. doi: 10.1038/nature09357. [DOI] [PubMed] [Google Scholar]
- 22.Saijo K, Schmedt C, Su IH, et al. Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nat Immunol. 2003;4:274–279. doi: 10.1038/ni893. [DOI] [PubMed] [Google Scholar]
- 23.Johnson SA, Pleiman CM, Pao L, et al. Phosphorylated immunoreceptor signaling motifs (ITAMs) exhibit unique abilities to bind and activate Lyn and Syk tyrosine kinases. J Immunol. 1995;155:4596–4603. [PubMed] [Google Scholar]
- 24.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- 25.Shinohara H, Yasuda T, Aiba Y, et al. PKC beta regulates BCR-mediated IKK activation by facilitating the interaction between TAK1 and CARMA1. J Exp Med. 2005;202:1423–1431. doi: 10.1084/jem.20051591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dal Porto JM, Gauld SB, Merrell KT, et al. B cell antigen receptor signaling 101. Mol Immunol. 2004;41:599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Look AT. Oncogenic transcription factors in the human acute leukemias. Science. 1997;278:1059–1064. doi: 10.1126/science.278.5340.1059. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi N, Melchers F. Lambda 5, a new light-chain-related locus selectively expressed in pre-B lymphocytes. Nature. 1986;324:579–582. doi: 10.1038/324579a0. [DOI] [PubMed] [Google Scholar]
- 29.Bicocca VT, Chang BH, Masouleh BK, et al. Crosstalk between ROR1 and the Pre-B cell receptor promotes survival of t(1;19) acute lymphoblastic leukemia. Cancer Cell. 2012;22:656–667. doi: 10.1016/j.ccr.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duy C, Yu JJ, Nahar R, et al. BCL6 is critical for the development of a diverse primary B cell repertoire. J Exp Med. 2010;207:1209–1221. doi: 10.1084/jem.20091299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mullighan CG, Goorha S, Radtke I, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 32.Flemming A, Brummer T, Reth M, et al. The adaptor protein SLP-5 acts as a tumor suppressor that limits pre-B cell expansion. Nat Immunol. 2003;4:38–43. doi: 10.1038/ni862. [DOI] [PubMed] [Google Scholar]
- 33.Wossning T, Herzog S, Kohler F, et al. Deregulated Syk inhibits differentiation and induces growth factor-independent proliferation of pre-B cells. J Exp Med. 2006;203:2829–2840. doi: 10.1084/jem.20060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feldhahn N, Klein F, Mooster JL, et al. Mimicry of a constitutively active pre-B cell receptor in acute lymphoblastic leukemia cells. J Exp Med. 2005;201:1837–1852. doi: 10.1084/jem.20042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Middendorp S, Dingjan GM, Maas A, et al. Function of Bruton's tyrosine kinase during B cell development is partially independent of its catalytic activity. J Immunol. 2003;171:5988–5996. doi: 10.4049/jimmunol.171.11.5988. [DOI] [PubMed] [Google Scholar]
- 36.van der Veer A, van der Velden VH, Willemse ME, et al. Interference with pre-B-cell receptor signaling offers a therapeutic option for TCF3-rearranged childhood acute lymphoblastic leukemia. Blood Cancer J. 2014;4:e181. doi: 10.1038/bcj.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490:116–120. doi: 10.1038/nature11378. This studie provides the first evidence that tonic BCR signaling is important for Burkitt lymphoma.
- 38.Futosi K, Nemeth T, Pick R, et al. Dasatinib inhibits proinflammatory functions of mature human neutrophils. Blood. 2012;119:4981–4991. doi: 10.1182/blood-2011-07-369041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hantschel O, Rix U, Schmidt U, et al. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc Natl Acad Sci U S A. 2007;104:13283–13288. doi: 10.1073/pnas.0702654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang Y, Shaffer AL, 3rd, Emre NC, et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell. 2012;21:723–737. doi: 10.1016/j.ccr.2012.05.024. This article demonstrates preclinical evidence for the rational to combine BCR signaling inhibition with Lenalidomide.
- 41.Bahler DW, Levy R. Clonal evolution of a follicular lymphoma: evidence for antigen selection. Proc Natl Acad Sci U S A. 1992;89:6770–6774. doi: 10.1073/pnas.89.15.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood. 2011;117:1792–1798. doi: 10.1182/blood-2010-06-275818. [DOI] [PubMed] [Google Scholar]
- 43.Dighiero G, Hart S, Lim A, et al. Autoantibody activity of immunoglobulins isolated from B-cell follicular lymphomas. Blood. 1991;78:581–585. [PubMed] [Google Scholar]
- 44.Greiner A, Marx A, Heesemann J, et al. Idiotype identity in a MALT-type lymphoma and B cells in Helicobacter pylori associated chronic gastritis. Lab Invest. 1994;70:572–578. [PubMed] [Google Scholar]
- 45.Sachen KL, Strohman MJ, Singletary J, et al. Self-antigen recognition by follicular lymphoma B-cell receptors. Blood. 2012;120:4182–4190. doi: 10.1182/blood-2012-05-427534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herve M, Xu K, Ng YS, et al. Unmutated and mutated chronic lymphocytic leukemias derive from self-reactive B cell precursors despite expressing different antibody reactivity. J Clin Invest. 2005;115:1636–1643. doi: 10.1172/JCI24387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Duhren-von Minden M, Ubelhart R, Schneider D, et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature. 2012;489:309–312. doi: 10.1038/nature11309. The authors identify a self-binding capacity of the BCR of CLL but not other B cell lymphoma that induces signaling in the absence of further stimulus.
- 48.Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Juszczynski P, Chen L, O'Donnell E, et al. BCL6 modulates tonic BCR signaling in diffuse large B-cell lymphomas by repressing the SYK phosphatase, PTPROt. Blood. 2009;114:5315–5321. doi: 10.1182/blood-2009-02-204362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gisbert JP, Garcia-Buey L, Pajares JM, et al. Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment Pharmacol Ther. 2005;21:653–662. doi: 10.1111/j.1365-2036.2005.02395.x. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura S, Sugiyama T, Matsumoto T, et al. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut. 2012;61:507–513. doi: 10.1136/gutjnl-2011-300495. [DOI] [PubMed] [Google Scholar]
- 52.Binder M, Muller F, Jackst A, et al. B-cell receptor epitope recognition correlates with the clinical course of chronic lymphocytic leukemia. Cancer. 2011;117:1891–1900. doi: 10.1002/cncr.25755. [DOI] [PubMed] [Google Scholar]
- 53.Binder M, Muller F, Frick M, et al. CLL B-cell receptors can recognize themselves: alternative epitopes and structural clues for autostimulatory mechanisms in CLL. Blood. 2013;121:239–241. doi: 10.1182/blood-2012-09-454439. [DOI] [PubMed] [Google Scholar]
- 54.Schulz A, Czerwinski DK, Levy R. B-Cell Receptors Of Follicular Lymphoma Patients Recognize Themselves. Blood (ASH Annu Meet Abstr) 2013;633 [Google Scholar]
- 55.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buchner M, Fuchs S, Prinz G, et al. Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res. 2009;69:5424–5432. doi: 10.1158/0008-5472.CAN-08-4252. [DOI] [PubMed] [Google Scholar]
- 57.Chen L, Monti S, Juszczynski P, et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood. 2008;111:2230–2237. doi: 10.1182/blood-2007-07-100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gobessi S, Laurenti L, Longo PG, et al. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia. 2009;23:686–697. doi: 10.1038/leu.2008.346. [DOI] [PubMed] [Google Scholar]
- 59.Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buchner M, Baer C, Prinz G, et al. Spleen tyrosine kinase inhibition prevents chemokine- and integrin-mediated stromal protective effects in chronic lymphocytic leukemia. Blood. 2010;115:4497–4506. doi: 10.1182/blood-2009-07-233692. [DOI] [PubMed] [Google Scholar]
- 61.Braselmann S, Taylor V, Zhao H, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006;319:998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]
- 62.Sharman J, Klein L, Boxer M, Kolibaba KS, Hawkins MJ, Di Paolo JA, Hu J, Reddy A, Jin F, Melchor-Khan F, Yasenchak CA. Phase 2 Trial Of GS-9973, a Selective Syk Inhibitor, In Chronic Lymphocytic Leukemia (CLL) and Non-Hodgkin Lymphoma (NHL) Blood (ASH Annu Meet Abstr) 2013 #1634. [Google Scholar]
- 63.Cheng S, Coffey G, Zhang XH, et al. SYK inhibition and response prediction in diffuse large B-cell lymphoma. Blood. 2011;118:6342–6352. doi: 10.1182/blood-2011-02-333773. [DOI] [PubMed] [Google Scholar]
- 64.Spurgeon SE, Coffey G, Fletcher LB, et al. The selective SYK inhibitor P505-15 (PRT062607) inhibits B cell signaling and function in vitro and in vivo and augments the activity of fludarabine in chronic lymphocytic leukemia. J Pharmacol Exp Ther. 2013;344:378–387. doi: 10.1124/jpet.112.200832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. In this article, the first clinical study of ibrutinib in patients with relapsed/refractory B-cell malignancies are presented.
- 66.Wiestner A. Targeting B-Cell receptor signaling for anticancer therapy: the Bruton's tyrosine kinase inhibitor ibrutinib induces impressive responses in B-cell malignancies. J Clin Oncol. 2013;31:128–130. doi: 10.1200/JCO.2012.44.4281. [DOI] [PubMed] [Google Scholar]
- 67.O'Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15:48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014 doi: 10.1182/blood-2013-09-527853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Rooij MF, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–2594. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 70.Akinleye A, Furqan M, Adekunle O. Ibrutinib and Indolent B-Cell Lymphomas. Clin Lymphoma Myeloma Leuk. 2013 doi: 10.1016/j.clml.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 71. Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. This article reveals effectiveness of Ibrutinib in relapsed or refractory mantle cell lymphoma.
- 72.Burger JAKM, Wierda WG, Hoellenriegel J, Ferrajoli A, Faderl S, Lerner S, Zacharian G, Huang X, James DF, Buggy JJ, Kantarjian HM, O'Brien SM. The BTK inhibitor ibrutinib (PCI-32765) in combination with rituximab is well tolerated and displays profound activity in high-risk chronic lymphocytic leukemia (CLL) patients. Blood (ASH Annu Meet Abstr) 2012 #187. [Google Scholar]
- 73.Byrd JC, O'Brien S, James DF. Ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:1278–1279. doi: 10.1056/NEJMc1309710. [DOI] [PubMed] [Google Scholar]
- 74.O'Brien S, Barrientos JC, Flinn IW, Barr PM, Burger JA, Navarro T, James DF, Hedrick E, Friedberg JW, Brown JR. Combination of the Bruton's tyrosine kinase (BTK) inhibitor PCI-32765 with bendamustine (B)/rituximab (R) (BR) in patients (pts) with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL): Interim results of a phase Ib/II study. J Clin Oncol. 2012;30 [Google Scholar]
- 75.Chang B, Furman RR Zapatka M, Barrientos MC, Li D, Steggerda S, Eckert K, Francesco M, Woyach JA, Johnson AJ, James DF, Versele M, Byrd JC, Stilgenbauer S, Buggy JJ. Use of tumor genomic profiling to reveal mechanisms of resistance to the BTK inhibitor ibrutinib in chronic lymphocytic leukemia (CLL) Journal of Clinical Oncology, 2013 ASCO Annual Meeting Abstracts. 2013;31 [Google Scholar]
- 76.Burger J, Landau D, Hoellenriegel J, Sougnez C, Schlesner M, Ishaque N, Brors B, Keating MJ, Wierda WG, Cibulskis K, Kantarjian HM, O'Brien SM, Neuberg DS, Zenz T, Get zG, Wu CJ. Clonal Evolution In Patients With Chronic Lymphocytic Leukemia (CLL) Developing Resistance To BTK Inhibition. Blood (ASH Annu Meet Abstr) 2012 #866. [Google Scholar]
- 77.Improgo R, Tiao G, Kiezun A, Wang Y, Werner L, Sougnez C, Tesar B, Fernandes SM, Vartanov AR, Hoang K, Neuberg DS, Getz G, Brown JR. NF-κB Pathway Mutations Modulate Cell Survival and Ibrutinib Response In Chronic Lymphocytic Leukemia. Blood (ASH Annu Meet Abstr) 2012 #670. [Google Scholar]
- 78.Deane JA, Fruman DA. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu Rev Immunol. 2004;22:563–598. doi: 10.1146/annurev.immunol.22.012703.104721. [DOI] [PubMed] [Google Scholar]
- 79.Sakai A, Thieblemont C, Wellmann A, et al. PTEN gene alterations in lymphoid neoplasms. Blood. 1998;92:3410–3415. [PubMed] [Google Scholar]
- 80.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kloo B, Nagel D, Pfeifer M, et al. Critical role of PI3K signaling for NF-kappaB-dependent survival in a subset of activated B-cell-like diffuse large B-cell lymphoma cells. Proc Natl Acad Sci U S A. 2011;108:272–277. doi: 10.1073/pnas.1008969108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rudelius M, Pittaluga S, Nishizuka S, et al. Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood. 2006;108:1668–1676. doi: 10.1182/blood-2006-04-015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Refaeli Y, Young RM, Turner BC, et al. The B cell antigen receptor and overexpression of MYC can cooperate in the genesis of B cell lymphomas. PLoS Biol. 2008;6:e152. doi: 10.1371/journal.pbio.0060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown JRFR, Flinn I, Coutre SE, Wagner-Johnston ND, Kahl BS, Forbes Spurgeon SE, Benson DM, Byrd JC, Peterman S, Johnson DM, Li D, Dansey RD, Jahn MT, Byrd JC. Final results of a phase I study of idelalisib (GS-1101) a selective inhibitor of PI3Kδ, in patients with relapsed or refractory CLL. J Clin Oncol (ASCO abstract) 2013;31(Suppl):7003. [Google Scholar]
- 85. Gopal AK, Kahl BS, de Vos S, et al. PI3Kdelta Inhibition by Idelalisib in Patients with Relapsed Indolent Lymphoma. N Engl J Med. 2014 doi: 10.1056/NEJMoa1314583. This study shows substantial response rates for the PI3K-δ inhibitor Idelalisib in refractory indolent lymphoma.
- 86.Flinn IPM, Kahl B, Horwitz SM, Foss FM, Oki Y, Porcu P, Sweeney J, Allen K, Faia K, Harris P, Dunbar J, Stern HM, Kelly P, O'Brien S. Preliminary Safety and Efficacy Of IPI-145, a Potent Inhibitor Of Phosphoinositide-3-Kinase-δ,γ, In Patients With Chronic Lymphocytic Leukemia. Blood (ASH Annu Meet Abstr) 2013;677 [Google Scholar]
- 87.Hasan AM, Mourtada-Maarabouni M, Hameed MS, et al. Phosphoinositide 3-kinase gamma mediates chemotactic responses of human eosinophils to platelet-activating factor. Int Immunopharmacol. 2010;10:1017–1021. doi: 10.1016/j.intimp.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 88.Lim DH, Cho JY, Song DJ, et al. PI3K gamma-deficient mice have reduced levels of allergen-induced eosinophilic inflammation and airway remodeling. Am J Physiol Lung Cell Mol Physiol. 2009;296:L210–219. doi: 10.1152/ajplung.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fontan L, Yang C, Kabaleeswaran V, et al. MALT1 small molecule inhibitors specifically suppress ABC-DLBCL in vitro and in vivo. Cancer Cell. 2012;22:812–824. doi: 10.1016/j.ccr.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nagel D, Spranger S, Vincendeau M, et al. Pharmacologic inhibition of MALT1 protease by phenothiazines as a therapeutic approach for the treatment of aggressive ABC-DLBCL. Cancer Cell. 2012;22:825–837. doi: 10.1016/j.ccr.2012.11.002. [DOI] [PubMed] [Google Scholar]