Abstract
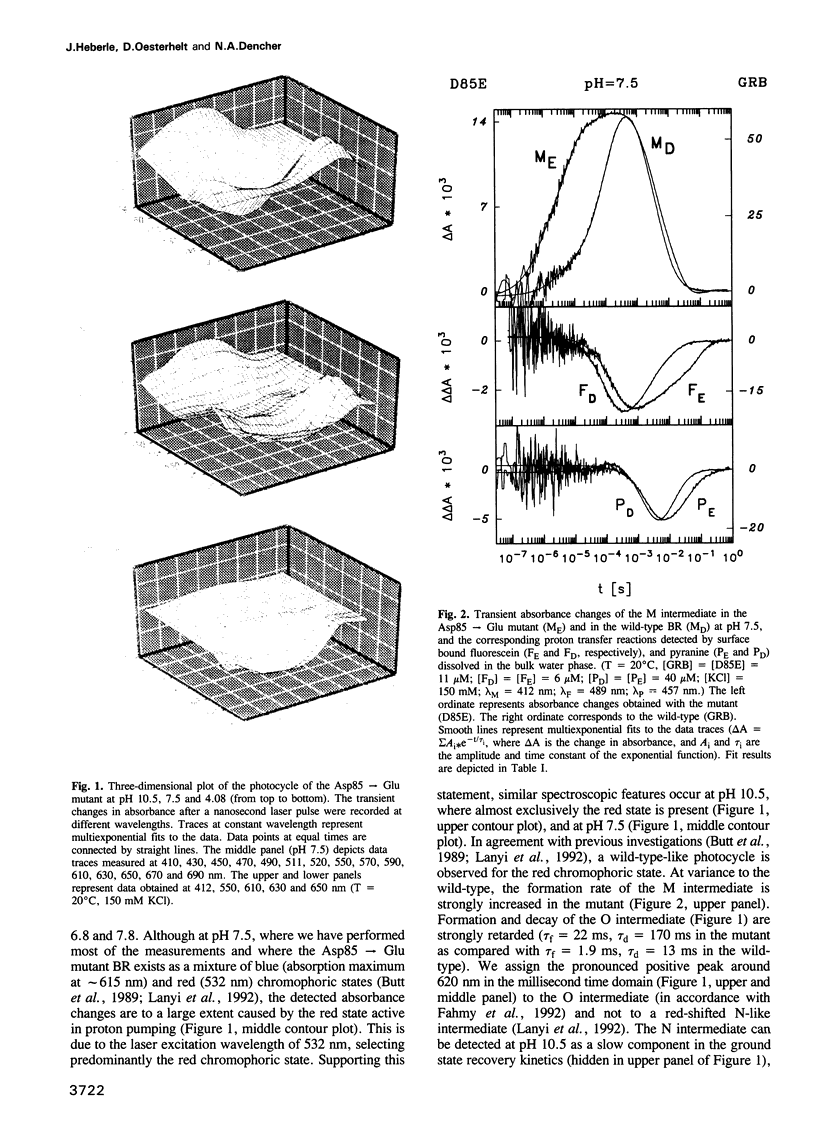
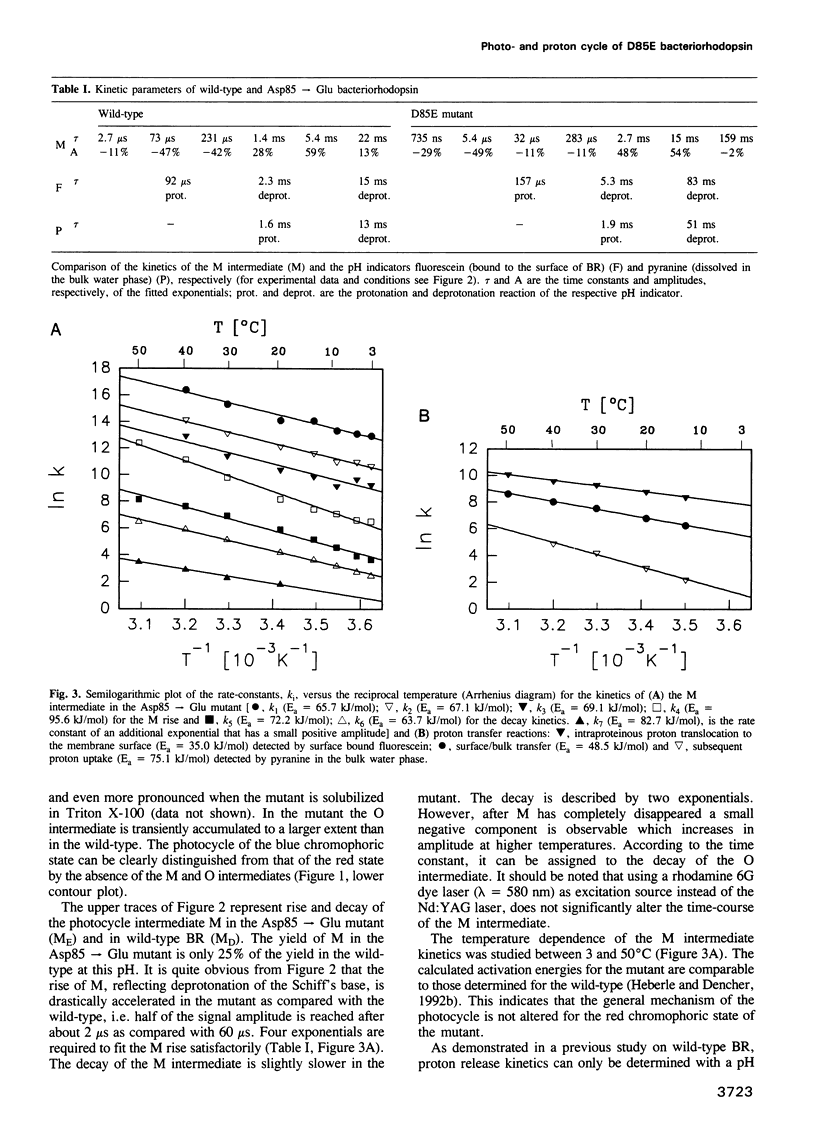
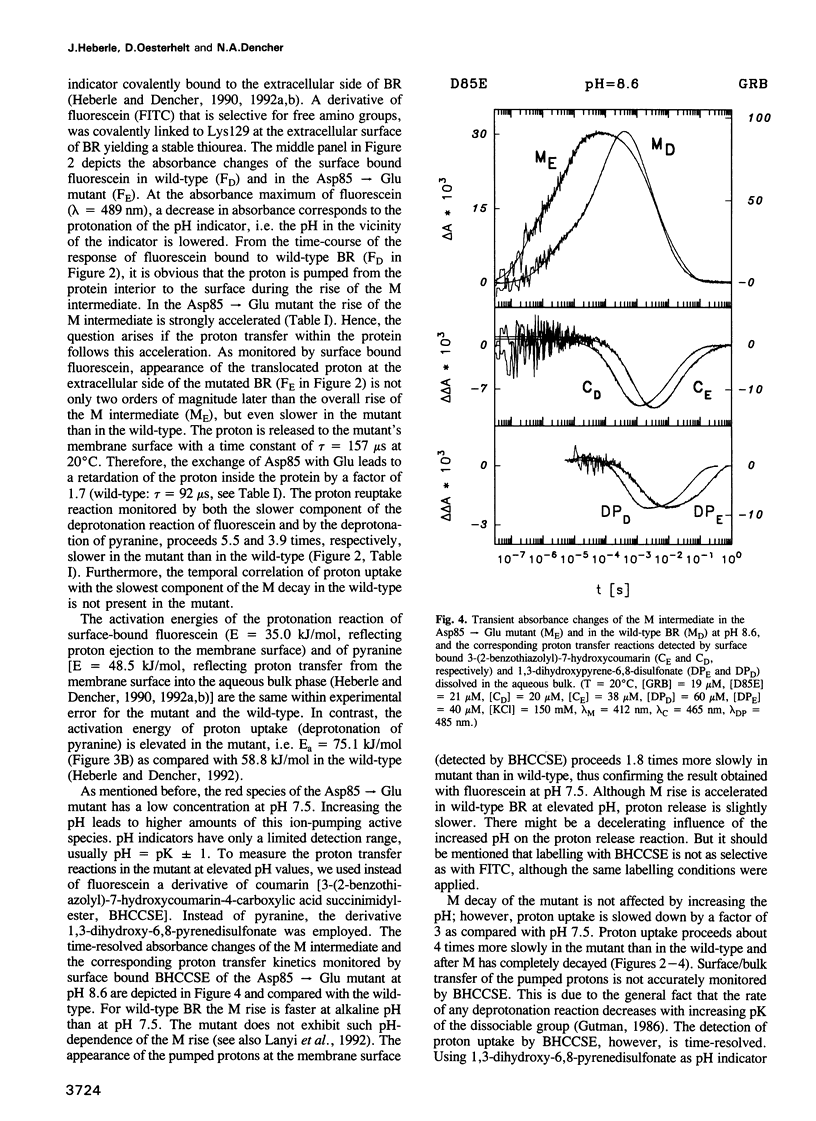
Surface bound pH indicators were applied to study the proton transfer reactions in the mutant Asp85-->Glu of bacteriorhodopsin in the native membrane. The amino acid replacement induces a drastic acceleration of the overall rise of the M intermediate. Instead of following this acceleration, proton ejection to the extracellular membrane surface is not only two orders of magnitude slower than M formation, it is also delayed as compared with the wild-type. This demonstrates that Asp85 not only accepts the proton released by the Schiff's base but also regulates very efficiently proton transfer within the proton release chain. Furthermore, Asp85 might be the primary but is not the only proton acceptor/donor group in the release pathway. The Asp85-->Glu substitution also affects the proton reuptake reaction at the cytoplasmic side, although Asp85 is located in the proton release pathway. Proton uptake is slower in the mutant than in the wild-type and occurs during the lifetime of the O intermediate. This demonstrates a feed-back mechanism between Asp85 and the proton uptake pathway in bacteriorhodopsin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bousché O., Sonar S., Krebs M. P., Khorana H. G., Rothschild K. J. Time-resolved Fourier transform infrared spectroscopy of the bacteriorhodopsin mutant Tyr-185-->Phe: Asp-96 reprotonates during O formation; Asp-85 and Asp-212 deprotonate during O decay. Photochem Photobiol. 1992 Dec;56(6):1085–1095. doi: 10.1111/j.1751-1097.1992.tb09732.x. [DOI] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Marti T., Stern L. J., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 1988 Nov 15;27(23):8516–8520. doi: 10.1021/bi00423a002. [DOI] [PubMed] [Google Scholar]
- Butt H. J., Fendler K., Bamberg E., Tittor J., Oesterhelt D. Aspartic acids 96 and 85 play a central role in the function of bacteriorhodopsin as a proton pump. EMBO J. 1989 Jun;8(6):1657–1663. doi: 10.1002/j.1460-2075.1989.tb03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencher N. A., Dresselhaus D., Zaccai G., Büldt G. Structural changes in bacteriorhodopsin during proton translocation revealed by neutron diffraction. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7876–7879. doi: 10.1073/pnas.86.20.7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckmann S., Ottolenghi M., Pande A., Pande J., Callender R. H. Acid-base equilibrium of the Schiff base in bacteriorhodopsin. Biochemistry. 1982 Sep 28;21(20):4953–4959. doi: 10.1021/bi00263a019. [DOI] [PubMed] [Google Scholar]
- Gerwert K., Souvignier G., Hess B. Simultaneous monitoring of light-induced changes in protein side-group protonation, chromophore isomerization, and backbone motion of bacteriorhodopsin by time-resolved Fourier-transform infrared spectroscopy. Proc Natl Acad Sci U S A. 1990 Dec 15;87(24):9774–9778. doi: 10.1073/pnas.87.24.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman M. Application of the laser-induced proton pulse for measuring the protonation rate constants of specific sites on proteins and membranes. Methods Enzymol. 1986;127:522–538. doi: 10.1016/0076-6879(86)27042-1. [DOI] [PubMed] [Google Scholar]
- Heberle J., Dencher N. A. Bacteriorhodopsin in ice. Accelerated proton transfer from the purple membrane surface. FEBS Lett. 1990 Dec 17;277(1-2):277–280. doi: 10.1016/0014-5793(90)80864-f. [DOI] [PubMed] [Google Scholar]
- Heberle J., Dencher N. A. Surface-bound optical probes monitor protein translocation and surface potential changes during the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5996–6000. doi: 10.1073/pnas.89.13.5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. H., Dencher N. A., Oesterhelt D., Plöhn H. J., Rapp G., Büldt G. Time-resolved X-ray diffraction study of structural changes associated with the photocycle of bacteriorhodopsin. EMBO J. 1991 Mar;10(3):521–526. doi: 10.1002/j.1460-2075.1991.tb07978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K., Tittor J., Váró G., Krippahl G., Oesterhelt D. Influence of the size and protonation state of acidic residue 85 on the absorption spectrum and photoreaction of the bacteriorhodopsin chromophore. Biochim Biophys Acta. 1992 Jan 30;1099(1):102–110. [PubMed] [Google Scholar]
- Marti T., Rösselet S. J., Otto H., Heyn M. P., Khorana H. G. The retinylidene Schiff base counterion in bacteriorhodopsin. J Biol Chem. 1991 Oct 5;266(28):18674–18683. [PubMed] [Google Scholar]
- Mathies R. A., Lin S. W., Ames J. B., Pollard W. T. From femtoseconds to biology: mechanism of bacteriorhodopsin's light-driven proton pump. Annu Rev Biophys Biophys Chem. 1991;20:491–518. doi: 10.1146/annurev.bb.20.060191.002423. [DOI] [PubMed] [Google Scholar]
- Metz G., Siebert F., Engelhard M. Asp85 is the only internal aspartic acid that gets protonated in the M intermediate and the purple-to-blue transition of bacteriorhodopsin. A solid-state 13C CP-MAS NMR investigation. FEBS Lett. 1992 Jun 1;303(2-3):237–241. doi: 10.1016/0014-5793(92)80528-o. [DOI] [PubMed] [Google Scholar]
- Needleman R., Chang M., Ni B., Váró G., Fornés J., White S. H., Lanyi J. K. Properties of Asp212----Asn bacteriorhodopsin suggest that Asp212 and Asp85 both participate in a counterion and proton acceptor complex near the Schiff base. J Biol Chem. 1991 Jun 25;266(18):11478–11484. [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Stern L. J., Engel F., Khorana H. G., Heyn M. P. Substitution of amino acids Asp-85, Asp-212, and Arg-82 in bacteriorhodopsin affects the proton release phase of the pump and the pK of the Schiff base. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1018–1022. doi: 10.1073/pnas.87.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos G., Dencher N. A., Zaccai G., Büldt G. Water molecules and exchangeable hydrogen ions at the active centre of bacteriorhodopsin localized by neutron diffraction. Elements of the proton pathway? J Mol Biol. 1990 Jul 5;214(1):15–19. doi: 10.1016/0022-2836(90)90140-h. [DOI] [PubMed] [Google Scholar]
- Scheiner S., Duan X. Effect of intermolecular orientation upon proton transfer within a polarizable medium. Biophys J. 1991 Oct;60(4):874–883. doi: 10.1016/S0006-3495(91)82121-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppa J., Oesterhelt D. Bacteriorhodopsin mutants of Halobacterium sp. GRB. I. The 5-bromo-2'-deoxyuridine selection as a method to isolate point mutants in halobacteria. J Biol Chem. 1989 Aug 5;264(22):13043–13048. [PubMed] [Google Scholar]
- Subramaniam S., Marti T., Khorana H. G. Protonation state of Asp (Glu)-85 regulates the purple-to-blue transition in bacteriorhodopsin mutants Arg-82----Ala and Asp-85----Glu: the blue form is inactive in proton translocation. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1013–1017. doi: 10.1073/pnas.87.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S., Marti T., Rösselet S. J., Rothschild K. J., Khorana H. G. The reaction of hydroxylamine with bacteriorhodopsin studied with mutants that have altered photocycles: selective reactivity of different photointermediates. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2583–2587. doi: 10.1073/pnas.88.6.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson T. E., Milder S. J., Miercke L. J., Betlach M. C., Shand R. F., Stroud R. M., Kliger D. S. Effects of Asp-96----Asn, Asp-85----Asn, and Arg-82----Gln single-site substitutions on the photocycle of bacteriorhodopsin. Biochemistry. 1991 Sep 24;30(38):9133–9142. doi: 10.1021/bi00102a003. [DOI] [PubMed] [Google Scholar]
- Tittor J., Soell C., Oesterhelt D., Butt H. J., Bamberg E. A defective proton pump, point-mutated bacteriorhodopsin Asp96----Asn is fully reactivated by azide. EMBO J. 1989 Nov;8(11):3477–3482. doi: 10.1002/j.1460-2075.1989.tb08512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimányi L., Váró G., Chang M., Ni B., Needleman R., Lanyi J. K. Pathways of proton release in the bacteriorhodopsin photocycle. Biochemistry. 1992 Sep 15;31(36):8535–8543. doi: 10.1021/bi00151a022. [DOI] [PubMed] [Google Scholar]



