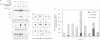Abstract
The product of the c-kit proto-oncogene, denoted Kit/SCF-R, encodes a tyrosine kinase receptor for stem cell factor (SCF). Kit/SCF-R induces proliferation, differentiation or migration of cells within the hematopoietic, gametogenic and melanogenic lineages at different developmental stages. We report here that protein kinase C (PKC) mediates phosphorylation of Kit/SCF-R on serine residues in response to SCF or PMA in intact cells. The phosphorylation inhibits SCF-induced tyrosine autophosphorylation of Kit/SCF-R. In vitro studies showed that PKC phosphorylated the Kit/SCF-R directly on serine residues and inhibited autophosphorylation of Kit/SCF-R, as well as its kinase activity towards an exogenous substrate. The PKC-induced phosphorylation did not affect Kit/SCF-R ligand binding affinity. Inhibition of PKC led to increased SCF-induced tyrosine autophosphorylation, as well as increased SCF-induced mitogenicity. In contrast, PKC was necessary for SCF-induced motility responses, including actin reorganization and chemotaxis. Our data suggest that PKC is involved in a negative feedback loop which regulates the Kit/SCF-R and that the activity of PKC determines whether the effect of SCF will be preferentially mitogenic or motogenic.
Full text
PDF

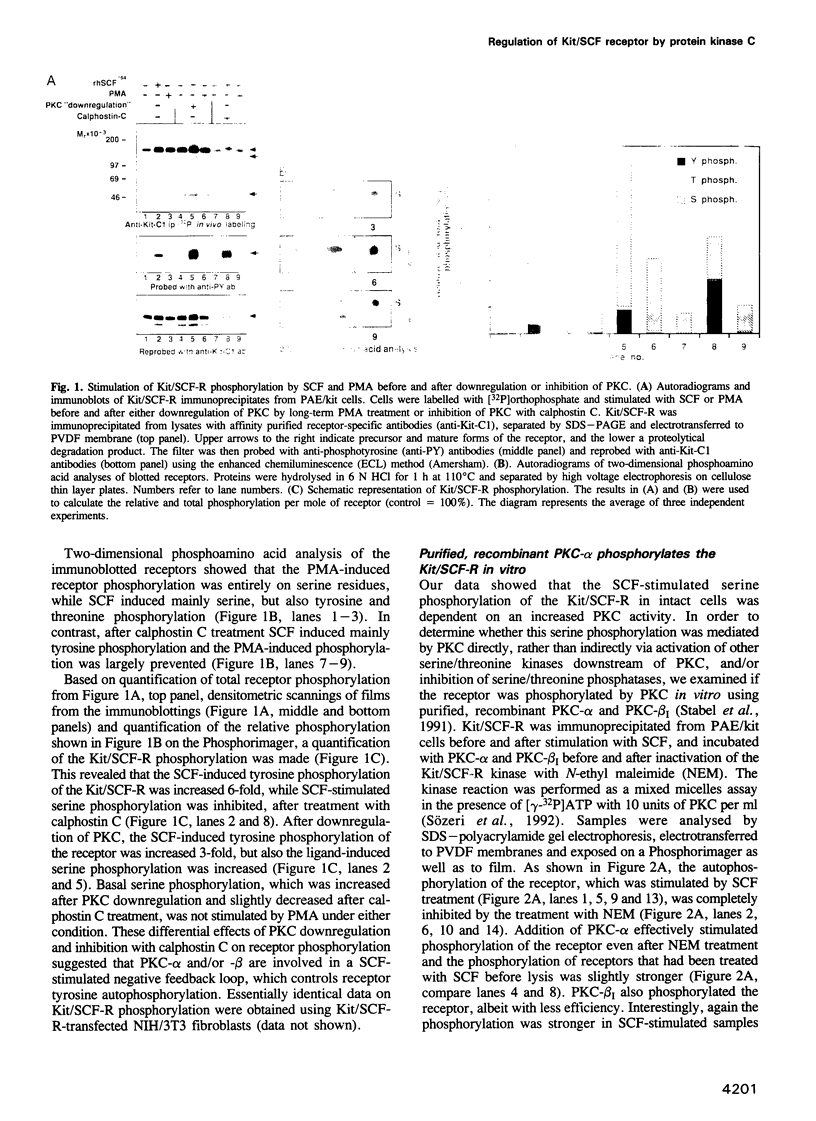
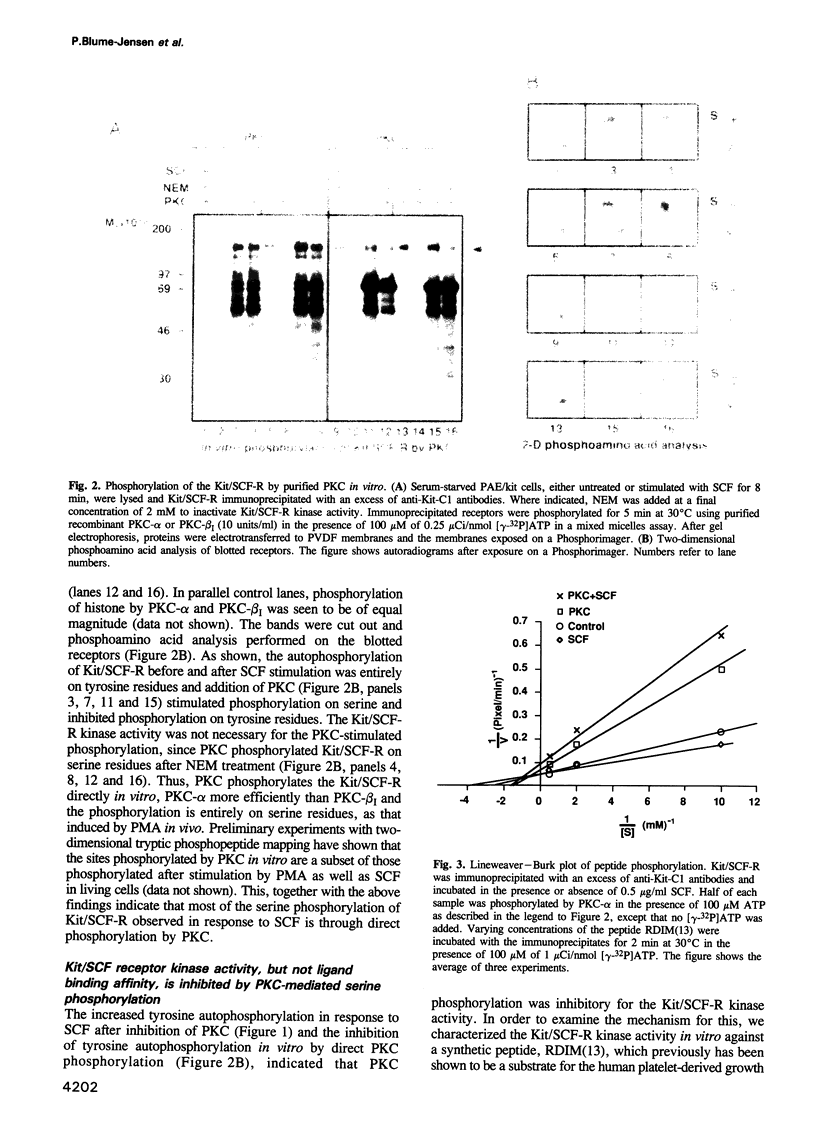
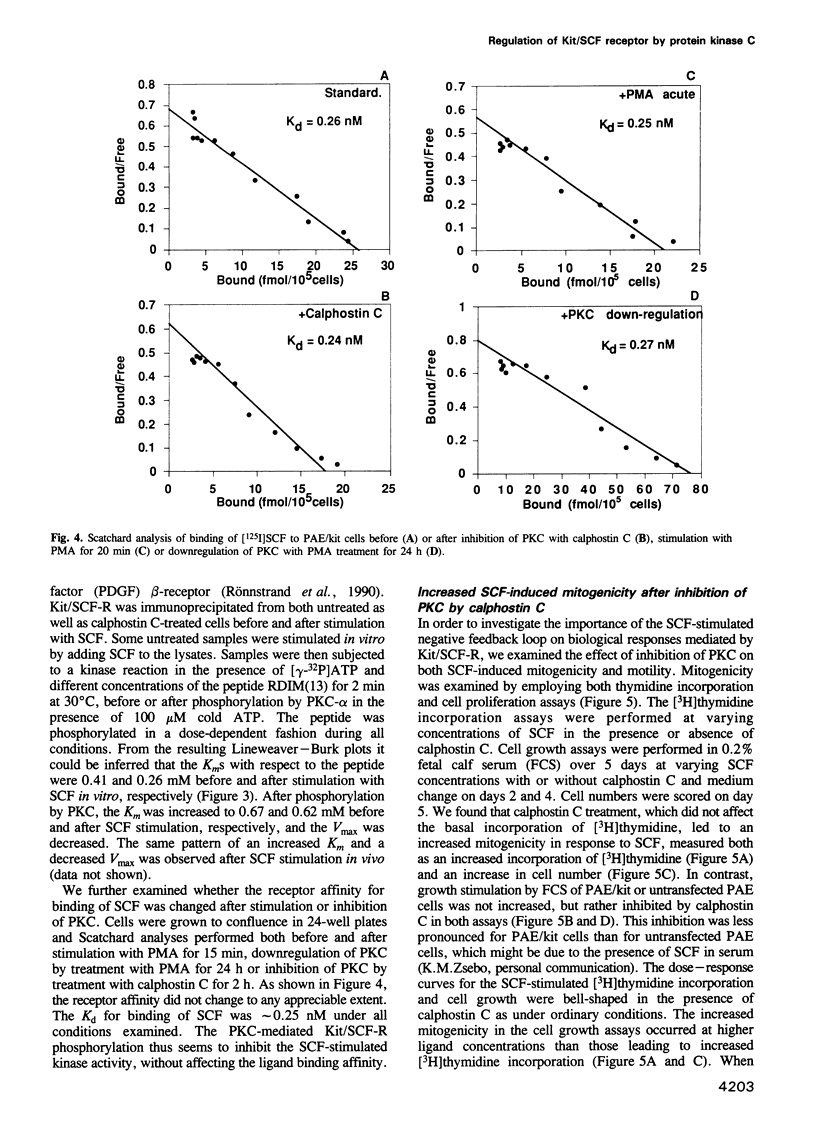
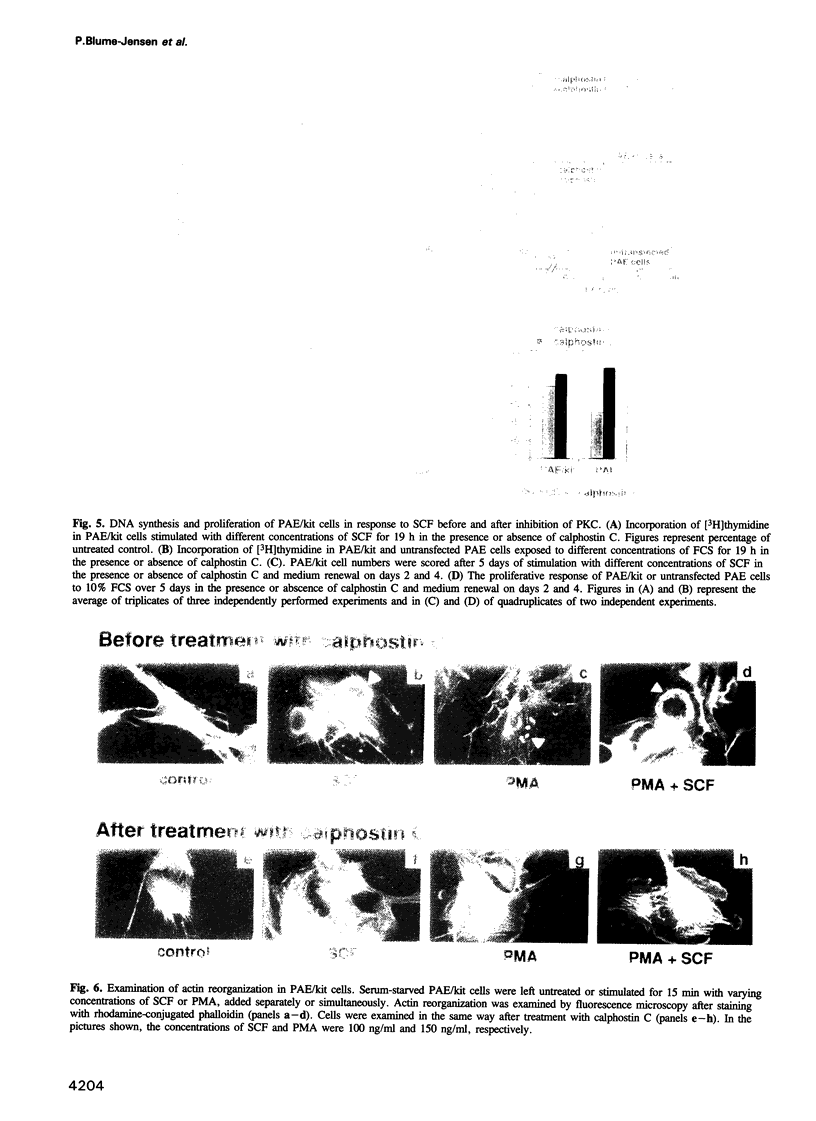
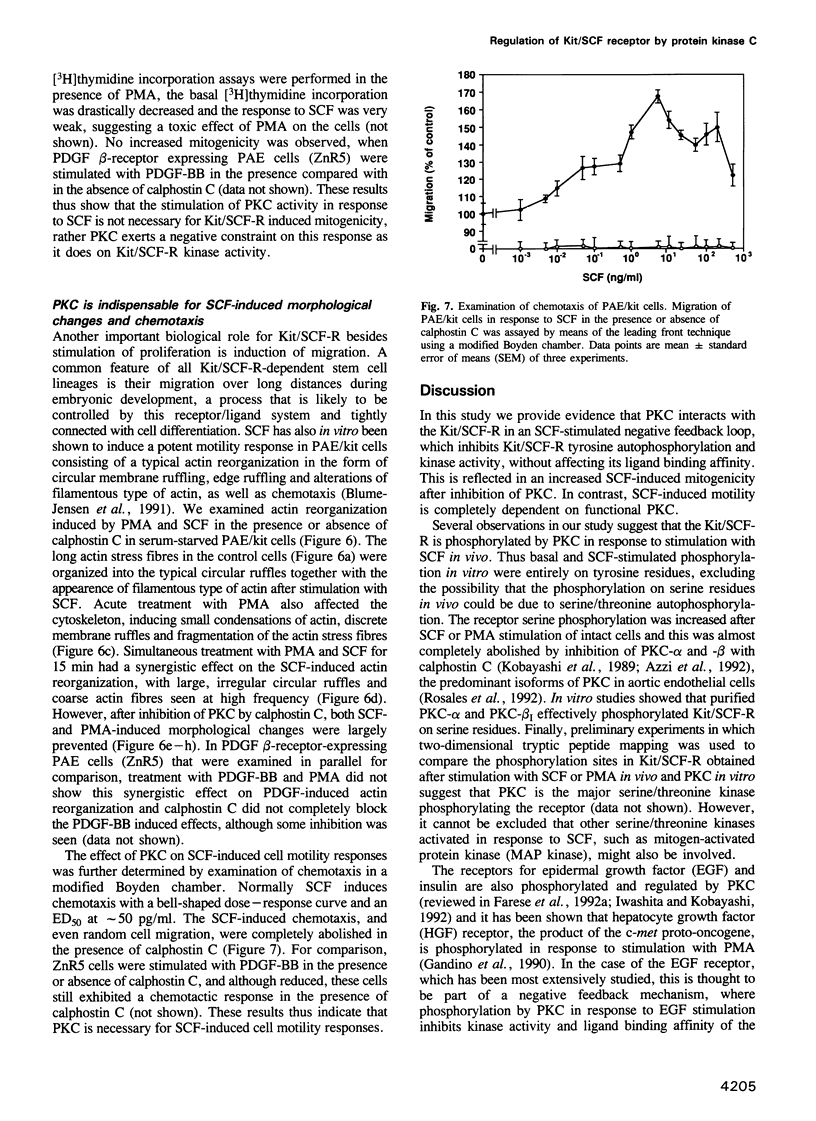




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell. 1992 Nov 27;71(5):713–716. doi: 10.1016/0092-8674(92)90546-o. [DOI] [PubMed] [Google Scholar]
- Alai M., Mui A. L., Cutler R. L., Bustelo X. R., Barbacid M., Krystal G. Steel factor stimulates the tyrosine phosphorylation of the proto-oncogene product, p95vav, in human hemopoietic cells. J Biol Chem. 1992 Sep 5;267(25):18021–18025. [PubMed] [Google Scholar]
- Asaoka Y., Nakamura S., Yoshida K., Nishizuka Y. Protein kinase C, calcium and phospholipid degradation. Trends Biochem Sci. 1992 Oct;17(10):414–417. doi: 10.1016/0968-0004(92)90011-w. [DOI] [PubMed] [Google Scholar]
- Azzi A., Boscoboinik D., Hensey C. The protein kinase C family. Eur J Biochem. 1992 Sep 15;208(3):547–557. doi: 10.1111/j.1432-1033.1992.tb17219.x. [DOI] [PubMed] [Google Scholar]
- Besmer P., Murphy J. E., George P. C., Qiu F. H., Bergold P. J., Lederman L., Snyder H. W., Jr, Brodeur D., Zuckerman E. E., Hardy W. D. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature. 1986 Apr 3;320(6061):415–421. doi: 10.1038/320415a0. [DOI] [PubMed] [Google Scholar]
- Besmer P. The kit ligand encoded at the murine Steel locus: a pleiotropic growth and differentiation factor. Curr Opin Cell Biol. 1991 Dec;3(6):939–946. doi: 10.1016/0955-0674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P., Claesson-Welsh L., Siegbahn A., Zsebo K. M., Westermark B., Heldin C. H. Activation of the human c-kit product by ligand-induced dimerization mediates circular actin reorganization and chemotaxis. EMBO J. 1991 Dec;10(13):4121–4128. doi: 10.1002/j.1460-2075.1991.tb04989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Cai H., Erhardt P., Szeberényi J., Diaz-Meco M. T., Johansen T., Moscat J., Cooper G. M. Hydrolysis of phosphatidylcholine is stimulated by Ras proteins during mitogenic signal transduction. Mol Cell Biol. 1992 Dec;12(12):5329–5335. doi: 10.1128/mcb.12.12.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Pike L. J., Grant G. A., Krebs E. G., Glaser L. Interaction of epidermal growth factor-dependent protein kinase with endogenous membrane proteins and soluble peptide substrate. J Biol Chem. 1983 Mar 10;258(5):2945–2950. [PubMed] [Google Scholar]
- Chabot B., Stephenson D. A., Chapman V. M., Besmer P., Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988 Sep 1;335(6185):88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- Chinkers M., Garbers D. L. Suppression of protein tyrosine kinase activity of the epidermal growth factor receptor by epidermal growth factor. J Biol Chem. 1986 Jun 25;261(18):8295–8297. [PubMed] [Google Scholar]
- Clemens M. J., Trayner I., Menaya J. The role of protein kinase C isoenzymes in the regulation of cell proliferation and differentiation. J Cell Sci. 1992 Dec;103(Pt 4):881–887. doi: 10.1242/jcs.103.4.881. [DOI] [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Dolci S., Williams D. E., Ernst M. K., Resnick J. L., Brannan C. I., Lock L. F., Lyman S. D., Boswell H. S., Donovan P. J. Requirement for mast cell growth factor for primordial germ cell survival in culture. Nature. 1991 Aug 29;352(6338):809–811. doi: 10.1038/352809a0. [DOI] [PubMed] [Google Scholar]
- Downward J., Waterfield M. D., Parker P. J. Autophosphorylation and protein kinase C phosphorylation of the epidermal growth factor receptor. Effect on tyrosine kinase activity and ligand binding affinity. J Biol Chem. 1985 Nov 25;260(27):14538–14546. [PubMed] [Google Scholar]
- Duronio V., Welham M. J., Abraham S., Dryden P., Schrader J. W. p21ras activation via hemopoietin receptors and c-kit requires tyrosine kinase activity but not tyrosine phosphorylation of p21ras GTPase-activating protein. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1587–1591. doi: 10.1073/pnas.89.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Farese R. V., Standaert M. L., Arnold T., Yu B., Ishizuka T., Hoffman J., Vila M., Cooper D. R. The role of protein kinase C in insulin action. Cell Signal. 1992 Mar;4(2):133–143. doi: 10.1016/0898-6568(92)90077-l. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Standaert M. L., Cooper D. R. Protein kinase C downregulation? Nature. 1992 Nov 26;360(6402):305–305. doi: 10.1038/360305c0. [DOI] [PubMed] [Google Scholar]
- Fleischman R. A., Saltman D. L., Stastny V., Zneimer S. Deletion of the c-kit protooncogene in the human developmental defect piebald trait. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10885–10889. doi: 10.1073/pnas.88.23.10885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandino L., Di Renzo M. F., Giordano S., Bussolino F., Comoglio P. M. Protein kinase-c activation inhibits tyrosine phosphorylation of the c-met protein. Oncogene. 1990 May;5(5):721–725. [PubMed] [Google Scholar]
- Geissler E. N., McFarland E. C., Russell E. S. Analysis of pleiotropism at the dominant white-spotting (W) locus of the house mouse: a description of ten new W alleles. Genetics. 1981 Feb;97(2):337–361. doi: 10.1093/genetics/97.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler E. N., Ryan M. A., Housman D. E. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988 Oct 7;55(1):185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Godin I., Deed R., Cooke J., Zsebo K., Dexter M., Wylie C. C. Effects of the steel gene product on mouse primordial germ cells in culture. Nature. 1991 Aug 29;352(6338):807–809. doi: 10.1038/352807a0. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hartwig J. H., Thelen M., Rosen A., Janmey P. A., Nairn A. C., Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992 Apr 16;356(6370):618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Herbst R., Lammers R., Schlessinger J., Ullrich A. Substrate phosphorylation specificity of the human c-kit receptor tyrosine kinase. J Biol Chem. 1991 Oct 25;266(30):19908–19916. [PubMed] [Google Scholar]
- Imamura K., Dianoux A., Nakamura T., Kufe D. Colony-stimulating factor 1 activates protein kinase C in human monocytes. EMBO J. 1990 Aug;9(8):2423-8, 2389. doi: 10.1002/j.1460-2075.1990.tb07418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita S., Kobayashi M. Signal transduction system for growth factor receptors associated with tyrosine kinase activity: epidermal growth factor receptor signalling and its regulation. Cell Signal. 1992 Mar;4(2):123–132. doi: 10.1016/0898-6568(92)90076-k. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Melton D. A. Diffusible factors in vertebrate embryonic induction. Cell. 1992 Jan 24;68(2):257–270. doi: 10.1016/0092-8674(92)90469-s. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989 Jan;176(1):22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi E., Nakano H., Morimoto M., Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989 Mar 15;159(2):548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Lange-Carter C. A., Pleiman C. M., Gardner A. M., Blumer K. J., Johnson G. L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993 Apr 16;260(5106):315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- Larrodera P., Cornet M. E., Diaz-Meco M. T., Lopez-Barahona M., Diaz-Laviada I., Guddal P. H., Johansen T., Moscat J. Phospholipase C-mediated hydrolysis of phosphatidylcholine is an important step in PDGF-stimulated DNA synthesis. Cell. 1990 Jun 15;61(6):1113–1120. doi: 10.1016/0092-8674(90)90074-o. [DOI] [PubMed] [Google Scholar]
- Lev S., Givol D., Yarden Y. A specific combination of substrates is involved in signal transduction by the kit-encoded receptor. EMBO J. 1991 Mar;10(3):647–654. doi: 10.1002/j.1460-2075.1991.tb07993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Matsui Y., Zsebo K., Hogan B. L. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992 Sep 4;70(5):841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- Miyazawa K., Hendrie P. C., Mantel C., Wood K., Ashman L. K., Broxmeyer H. E. Comparative analysis of signaling pathways between mast cell growth factor (c-kit ligand) and granulocyte-macrophage colony-stimulating factor in a human factor-dependent myeloid cell line involves phosphorylation of Raf-1, GTPase-activating protein and mitogen-activated protein kinase. Exp Hematol. 1991 Dec;19(11):1110–1123. [PubMed] [Google Scholar]
- Miyazono K., Hellman U., Wernstedt C., Heldin C. H. Latent high molecular weight complex of transforming growth factor beta 1. Purification from human platelets and structural characterization. J Biol Chem. 1988 May 5;263(13):6407–6415. [PubMed] [Google Scholar]
- Morrison-Graham K., Takahashi Y. Steel factor and c-kit receptor: from mutants to a growth factor system. Bioessays. 1993 Feb;15(2):77–83. doi: 10.1002/bies.950150202. [DOI] [PubMed] [Google Scholar]
- Murphy M., Reid K., Williams D. E., Lyman S. D., Bartlett P. F. Steel factor is required for maintenance, but not differentiation, of melanocyte precursors in the neural crest. Dev Biol. 1992 Oct;153(2):396–401. doi: 10.1016/0012-1606(92)90124-y. [DOI] [PubMed] [Google Scholar]
- Nishikawa S., Kusakabe M., Yoshinaga K., Ogawa M., Hayashi S., Kunisada T., Era T., Sakakura T., Nishikawa S. In utero manipulation of coat color formation by a monoclonal anti-c-kit antibody: two distinct waves of c-kit-dependency during melanocyte development. EMBO J. 1991 Aug;10(8):2111–2118. doi: 10.1002/j.1460-2075.1991.tb07744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nocka K., Tan J. C., Chiu E., Chu T. Y., Ray P., Traktman P., Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 1990 Jun;9(6):1805–1813. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier A. R., Parker P. J. Identification of multiple PKC isoforms in Swiss 3T3 cells: differential down-regulation by phorbol ester. J Cell Physiol. 1992 Aug;152(2):240–244. doi: 10.1002/jcp.1041520204. [DOI] [PubMed] [Google Scholar]
- Otte A. P., Moon R. T. Protein kinase C isozymes have distinct roles in neural induction and competence in Xenopus. Cell. 1992 Mar 20;68(6):1021–1029. doi: 10.1016/0092-8674(92)90074-m. [DOI] [PubMed] [Google Scholar]
- Pears C. J., Parker P. J. Domain interactions in protein kinase C. J Cell Sci. 1991 Dec;100(Pt 4):683–686. doi: 10.1242/jcs.100.4.683. [DOI] [PubMed] [Google Scholar]
- Pessin M. S., Baldassare J. J., Raben D. M. Molecular species analysis of mitogen-stimulated 1,2-diglycerides in fibroblasts. Comparison of alpha-thrombin, epidermal growth factor, and platelet-derived growth factor. J Biol Chem. 1990 May 15;265(14):7959–7966. [PubMed] [Google Scholar]
- Posada J., Cooper J. A. Molecular signal integration. Interplay between serine, threonine, and tyrosine phosphorylation. Mol Biol Cell. 1992 Jun;3(6):583–592. doi: 10.1091/mbc.3.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F. H., Ray P., Brown K., Barker P. E., Jhanwar S., Ruddle F. H., Besmer P. Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family--oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J. 1988 Apr;7(4):1003–1011. doi: 10.1002/j.1460-2075.1988.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith A. D., Ellis C., Lyman S. D., Anderson D. M., Williams D. E., Bernstein A., Pawson T. Signal transduction by normal isoforms and W mutant variants of the Kit receptor tyrosine kinase. EMBO J. 1991 Sep;10(9):2451–2459. doi: 10.1002/j.1460-2075.1991.tb07784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith A. D., Rottapel R., Giddens E., Brady C., Forrester L., Bernstein A. W mutant mice with mild or severe developmental defects contain distinct point mutations in the kinase domain of the c-kit receptor. Genes Dev. 1990 Mar;4(3):390–400. doi: 10.1101/gad.4.3.390. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992 Aug 7;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rosales O. R., Isales C., Nathanson M., Sumpio B. E. Immunocytochemical expression and localization of protein kinase C in bovine aortic endothelial cells. Biochem Biophys Res Commun. 1992 Nov 30;189(1):40–46. doi: 10.1016/0006-291x(92)91522-r. [DOI] [PubMed] [Google Scholar]
- Rottapel R., Reedijk M., Williams D. E., Lyman S. D., Anderson D. M., Pawson T., Bernstein A. The Steel/W transduction pathway: kit autophosphorylation and its association with a unique subset of cytoplasmic signaling proteins is induced by the Steel factor. Mol Cell Biol. 1991 Jun;11(6):3043–3051. doi: 10.1128/mcb.11.6.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E. S. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- Rönnstrand L., Beckmann M. P., Faulders B., Ostman A., Ek B., Heldin C. H. Purification of the receptor for platelet-derived growth factor from porcine uterus. J Biol Chem. 1987 Mar 5;262(7):2929–2932. [PubMed] [Google Scholar]
- Rönnstrand L., Sorokin A., Engström U., Heldin C. H. Characterization of the platelet-derived growth factor beta-receptor kinase activity by use of synthetic peptides. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1333–1340. doi: 10.1016/0006-291x(90)90669-e. [DOI] [PubMed] [Google Scholar]
- Shearman M. S., Heyworth C. M., Dexter T. M., Haefner B., Owen P. J., Whetton A. D. Haemopoietic stem cell development to neutrophils is associated with subcellular redistribution and differential expression of protein kinase C subspecies. J Cell Sci. 1993 Jan;104(Pt 1):173–180. doi: 10.1242/jcs.104.1.173. [DOI] [PubMed] [Google Scholar]
- Spritz R. A., Giebel L. B., Holmes S. A. Dominant negative and loss of function mutations of the c-kit (mast/stem cell growth factor receptor) proto-oncogene in human piebaldism. Am J Hum Genet. 1992 Feb;50(2):261–269. [PMC free article] [PubMed] [Google Scholar]
- Stabel S., Schaap D., Parker P. J. Expression of protein kinase C isotypes using baculovirus vectors. Methods Enzymol. 1991;200:670–673. doi: 10.1016/0076-6879(91)00179-z. [DOI] [PubMed] [Google Scholar]
- Steel K. P., Davidson D. R., Jackson I. J. TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development. 1992 Aug;115(4):1111–1119. doi: 10.1242/dev.115.4.1111. [DOI] [PubMed] [Google Scholar]
- Sözeri O., Vollmer K., Liyanage M., Frith D., Kour G., Mark G. E., 3rd, Stabel S. Activation of the c-Raf protein kinase by protein kinase C phosphorylation. Oncogene. 1992 Nov;7(11):2259–2262. [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Westermark B., Siegbahn A., Heldin C. H., Claesson-Welsh L. B-type receptor for platelet-derived growth factor mediates a chemotactic response by means of ligand-induced activation of the receptor protein-tyrosine kinase. Proc Natl Acad Sci U S A. 1990 Jan;87(1):128–132. doi: 10.1073/pnas.87.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. E., de Vries P., Namen A. E., Widmer M. B., Lyman S. D. The Steel factor. Dev Biol. 1992 Jun;151(2):368–376. doi: 10.1016/0012-1606(92)90176-h. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Kuang W. J., Yang-Feng T., Coussens L., Munemitsu S., Dull T. J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987 Nov;6(11):3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga K., Nishikawa S., Ogawa M., Hayashi S., Kunisada T., Fujimoto T., Nishikawa S. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development. 1991 Oct;113(2):689–699. doi: 10.1242/dev.113.2.689. [DOI] [PubMed] [Google Scholar]
- Zsebo K. M., Williams D. A., Geissler E. N., Broudy V. C., Martin F. H., Atkins H. L., Hsu R. Y., Birkett N. C., Okino K. H., Murdock D. C. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990 Oct 5;63(1):213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- van Zoelen E. J. Receptor-ligand interaction: a new method for determining binding parameters without a priori assumptions on non-specific binding. Biochem J. 1989 Sep 1;262(2):549–556. doi: 10.1042/bj2620549. [DOI] [PMC free article] [PubMed] [Google Scholar]