Abstract
Adeno-associated virus (AAV) vectors often undergo long-distance axonal transport after brain injection. This leads to transduction of brain regions distal to the injection site, although the extent of axonal transport and distal transduction varies widely among AAV serotypes. The mechanisms driving this variability are poorly understood. This is a critical problem for applications that require focal gene expression within a specific brain region, and also impedes the utilization of vector transport for applications requiring widespread delivery of transgene to the brain. Here, we compared AAV serotypes 1 and 9, which frequently demonstrate distal transduction, with serotype 8, which rarely spreads beyond the injection site. To examine directional AAV transport in vitro, we used a microfluidic chamber to apply dye-labeled AAV to the axon termini or to the cell bodies of primary rat embryonic cortical neurons. All three serotypes were actively transported along axons, with transport characterized by high velocities and prolonged runs in both the anterograde and retrograde directions. Coinfection with pairs of serotypes indicated that AAV1, 8, and 9 share the same intracellular compartments for axonal transport. In vivo, both AAV8 and 9 demonstrated anterograde and retrograde transport within a nonreciprocal circuit after injection into adult mouse brain, with highly similar distributions of distal transduction. However, in mass-cultured neurons, we found that AAV1 was more frequently transported than AAV8 or 9, and that the frequency of AAV9 transport could be enhanced by increasing receptor availability. Thus, while these serotypes share conserved mechanisms for axonal transport both in vitro and in vivo, the frequency of transport can vary among serotypes, and axonal transport can be markedly increased by enhancing vector uptake. This suggests that variability in distal transduction in vivo likely results from differential uptake at the plasma membrane, rather than fundamental differences in transport mechanisms among AAV serotypes.
Introduction
Adeno-associated virus (AAV), a nonenveloped parvovirus with a single-stranded DNA genome of approximately 4.7 kb (Atchison et al., 1965; Hoggan et al., 1966), provides a popular backbone for recombinant viral vectors. AAV is nonpathogenic and demonstrates relatively low immunogenicity, and AAV vectors can be produced in large quantities with high purity (Samulski et al., 1987; Grieger et al., 2006). AAV is capable of long-term transduction of postmitotic cells, with gene expression observed beyond 6 years in primates (Rivera et al., 2005). These factors have led to extensive use of AAV vectors in the nervous system, where they can focally transduce targeted brain regions via stereotactic injection (Xiao et al., 1997; Cearley and Wolfe, 2006), diffusely transduce large brain volumes via intraventricular (Passini et al., 2003; Karolewski and Wolfe, 2006; Cearley et al., 2008) or intravenous administration (Foust et al., 2009; Gray et al., 2011), or transfer genes to primary cultures of neurons and glia (Royo et al., 2008).
A large number of evolutionarily diverse AAV serotypes have been identified (Gao et al., 2005). When compared directly after brain injection, these serotypes demonstrate distinct nervous cell tropism, transduction strength, and distribution of gene expression (Davidson et al., 2000; Burger et al., 2004; Cearley and Wolfe, 2006, 2007; Li et al., 2006; Taymans et al., 2007; Cearley et al., 2008; Klein et al., 2008). While some serotypes transduce only within a normal range of diffusion, others also transduce cells at a substantial distance from the injection site (Burger et al., 2004; Cearley and Wolfe, 2006, 2007; Klein et al., 2006, 2008; Li et al., 2006; Reimsnider et al., 2007; Sondhi et al., 2007; Taymans et al., 2007; Cearley et al., 2008; Hollis et al., 2008; Hadaczek et al., 2009; Masamizu et al., 2011; Bu et al., 2012). This distal transduction is caused by axonal transport of the intact vector, either via uptake by an axon terminus, retrograde transport to the cell body, and subsequent transduction (Cearley and Wolfe, 2006, 2007; Reimsnider et al., 2007; Sondhi et al., 2007; Taymans et al., 2007; Hollis et al., 2008; Hadaczek et al., 2009; Masamizu et al., 2011; Bu et al., 2012), or via uptake by a projection neuron, anterograde transport along the axonal projection, secretion from the axon terminus, and transduction of a second-order neuron (Cearley and Wolfe, 2007). We previously demonstrated that anterograde AAV9 transport is driven at high velocity by kinesin-2, and that retrograde AAV9 transport is driven by dynein/dynactin within the late endosome/lysosome compartment (Castle et al., 2014), but it remains unclear why the extent of distal transduction varies among AAV serotypes in vivo and whether this variability is caused by a fundamental difference in axonal transport among serotypes. Because we do not understand why distal transduction varies among serotypes or why it is inconsistently observed among experiments, it is not possible to accurately predict where and to what extent gene transfer will occur before injection of AAV. This is a critical problem for the ongoing use of AAV in the brain, particularly when focal administration to a specific brain region is required. This also impedes efforts to harness axonal transport for more widespread distribution of gene transfer, which could ultimately improve AAV-based treatment of neurogenetic disorders that require global correction (Cearley and Wolfe, 2007).
To address these questions, we compared the axonal transport of AAV1, 8, and 9 in vitro and in vivo. These serotypes differ in several important ways. First, they are from different evolutionary clades (Gao et al., 2005), with AAV1 and 9 isolated from humans (clades A and F, respectively) (Atchison et al., 1965; Hoggan et al., 1966; Gao et al., 2004), and AAV8 isolated from the rhesus macaque (clade E) (Gao et al., 2002). These serotypes have unique immune profiles (Gao et al., 2005), and each utilizes a different primary receptor for uptake (Akache et al., 2006; Wu et al., 2006; Bell et al., 2011; Shen et al., 2011). Finally, these serotypes appear to induce distal gene expression with different efficiencies. AAV9 has frequently been observed to undergo robust axonal transport in vivo (Cearley and Wolfe, 2006, 2007; Cearley et al., 2008; Klein et al., 2008; Masamizu et al., 2011). AAV1 often exhibits axonal transport as well (Wang et al., 2003; Burger et al., 2004; Li et al., 2006; Cearley and Wolfe, 2007; Reimsnider et al., 2007; Taymans et al., 2007; Hollis et al., 2008; Hadaczek et al., 2009; Bu et al., 2012), but with weaker distal transduction than AAV9 when directly compared (Cearley and Wolfe, 2007). In contrast, AAV8 often fails to spread beyond the injection site and induce distal transduction (Cearley and Wolfe, 2006; Taymans et al., 2007; Klein et al., 2008).
If this variability among serotypes reflects fundamental differences in axonal transport mechanisms, we would expect to observe strong axonal transport with AAV9 in vitro and in vivo, moderate transport with AAV1, and weak transport with AAV8. However, we found that these three serotypes all undergo robust axonal transport with highly similar velocities, and further, are actively transported within the same intracellular compartments. In addition, AAVs 8 and 9 demonstrate similarly strong anterograde and retrograde transduction when compared directly in vivo. Despite these similarities, AAV1 was observed to undergo more frequent axonal transport than AAV8 or 9 in mass-cultured neurons, and the frequency of AAV9 transport was enhanced by increasing the availability of galactose receptors. Thus, variability in distal transduction in vivo likely reflects a variable frequency of vector transport driven by differences in uptake among serotypes, as the fundamental mechanisms of axonal transport are conserved among these three serotypes of AAV.
Materials and Methods
Microfluidic and mass culture of primary neurons
Two-layer photoresist master molds were designed in AutoCAD software, manufactured by the Stanford Microfluidic Foundry, and used to fabricate polydimethylsiloxane microfluidic chambers as described (Park et al., 2006; Castle et al., 2014). Primary E18 rat cortical neurons were obtained as described (Wilcox and Dichter, 1994; Royo et al., 2008) and were provided courtesy of Marc Dichter (University of Pennsylvania) as tissue byproducts of hippocampal dissection. For microfluidic cultures, chambers were reversibly bonded to poly-L-lysine-coated 50 mm glass fluorodishes (World Precision Instruments), and 300,000 primary neurons cultured on one side of the chamber as described (Castle et al., 2014). For mass cultures, 100,000 primary neurons were cultured on poly-L-lysine-coated 35 mm glass fluorodishes (World Precision Instruments) as described (Castle et al., 2014).
Preparation and validation of dye-labeled AAV vectors
Psuedotyped AAV2/1, AAV2/8, and AAV2/9 vectors were produced by the Penn Vector Core as described (Gao et al., 2000). Each serotype was produced with either a CMV.eGFP or a CMV.turboRFP genome. All vector genomes were single-stranded and contained wild-type AAV2 inverted terminal repeat sequences. Vectors with CMV.eGFP genomes were labeled with the red dyes monofunctional Cyanine 3 (mCy3; GE Healthcare Biosciences) or bis-functional Cyanine 3 (bCy3; GE Healthcare Biosciences). Vectors with CMV.turboRFP genomes were labeled with the green dye Alexa Fluor 488 (AF488; Invitrogen). AAV vectors were concentrated, incubated in dye solution, dialyzed, and spin-purified as described (Castle et al., 2014). The titer of DNAse-resistant particles in each prep was determined via quantitative polymerase chain reaction (qPCR) (Gao et al., 2000). To confirm purity, a Coomassie stain followed by a Western blot using anti-AAV capsid antibody B1 (1:2500; Meridian Life Science) was performed as described (Gao et al., 2000). Normal VP1/2/3 bands were observed for all preps, and all bands colocalized with B1 antibody, indicating that no contaminating proteins were present.
Microfluidic live imaging and analysis
For microfluidic live imaging, 1×109 genome copies (GC) of bCy3- or mCy3-AAV1 or AAV8 were applied to the axon side of a microfluidic neuron culture after 5–6 days in vitro, or 1×1010 GC applied to the cell body side, in a volume of 5 μl. Because only a small percentage of AAV reaches the isolated channel, this selectively applied a maximum titer of 3×107 GC to the axon termini, or 3×108 GC to the cell bodies, with both titers corresponding to an estimated maximum multiplicity of infection of 5,000 GC per cell, as described (Castle et al., 2014). All microfluidic AAV9 data were obtained previously using identical techniques (Castle et al., 2014). Microfluidic GFP-Rab7 data were also obtained previously (Castle et al., 2014), following Amaxa electroporation (Lonza) of 2 μg GFP-Rab7 plasmid (Addgene), as described (Castle et al., 2014). A Leica DMI6000B inverted microscope equipped with a high-speed filter wheel, 63×plan apo oil-immersion objective, climate-controlled chamber, CTR 7000 HS control box, and Hamamatsu Photonics C10600 Orca-R2 camera was utilized for all live imaging of both microfluidic and mass cultures. Microfluidic cultures were imaged from 1 to 4 hr after application of virus. Image series were acquired for 3 min, 3 sec/frame. When observed in real-time, AAV made long, continuous runs that were interrupted by prolonged pauses, and thus a frame interval of 3 sec was chosen to provide an accurate measure of AAV movement without bleaching. Only AAV within the microfluidic groove were imaged. Fluidic isolation under these conditions was confirmed via live imaging of AF488-labeled Dextran (Invitrogen), as well as via qPCR-based detection of viral genomes on the distal side of the chamber, as described (Castle et al., 2014). Hibernate E Low Fluoresence media (Brainbits) was used for all live imaging experiments (Castle et al., 2014).
AAV particles in each microfluidic image series were tracked using Metamorph Software (Leica) and analyzed in Excel software (Microsoft), as described (Castle et al., 2014). Briefly, each AAV punctum that progressed further than 10 μm was tracked. Any sequence of frames in which the punctum moved further than 10 μm was classified as a run (if in the overall direction of movement) or as a reversal (if in reverse of the overall direction of movement). Any sequence of at least 3 frames (9 sec) during each of which the punctum moved below 0.2 μm/sec was classified as a pause. Movement of less than 10 μm between two pauses was not counted as a run and was incorporated into the adjacent pauses. For each AAV punctum, the average forward run velocity, the distance of each run, the total distance traveled, the duration of each pause, and the total time spent paused were quantified.
Statistical analysis of quantified tracking data was performed using Prism software (GraphPad). Sample sizes are found in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/hum). Two sets of five Kruskal–Wallis (KW) tests (Dunn's posttests) were conducted, one set for anterograde-directed populations (Fig. 1) and one for retrograde-directed populations (Fig. 2). Each KW test compared one of the five quantified movement parameters among all four populations (AAV1, AAV8, AAV9, and GFP-Rab7), with the null hypothesis that all populations were identical. For this and all analyses below, KW tests were used when a D'Agostino–Pearson normality test indicated that data were not normally distributed. Distribution of some anterograde parameters was bimodal, and anterograde datasets thus underwent log10 transformation in order to obtain unimodal distributions appropriate for analysis with the KW test.
FIG. 1.
High-speed anterograde transport is highly conserved among AAV serotypes 1, 8, and 9. Anterograde-directed AAV puncta were tracked within the microfluidic groove from 1 to 4 hr after specific application to cell bodies. (A) Histograms of instantaneous velocity, with negative values representing steps in reverse of the overall direction of movement (e.g., a anterograde-directed punctum stepping backward in the retrograde direction). Histograms depict the range of velocities at which each population is capable of moving, as well as the relative frequency of each velocity. (B–F) Box plots portray the quantified axonal transport of anterograde-directed AAVs and GFP-Rab7-labeled late endosomes/lysosomes. The interquartile range and median are represented, with whiskers depicting the 5th–95th percentile and outliers omitted because of large sample sizes. No significant differences were detected among AAV serotypes 1, 8, and 9. All three AAV serotypes were found to move anterograde at significantly higher average velocity (B), make longer individual runs (C), progress further total distance (D), and spend less time paused (F) than GFP-Rab7-labeled late endosomes/lysosomes (all p<0.0001), although no difference was detected in the duration of individual pauses (E). See Supplementary Table S1 for sample sizes. AAV9 and GFP-Rab7 data were obtained previously using identical methods and are included for comparison (Castle et al., 2014). (G) Excerpted frames from live imaging sequences depict representative movement from each population (arrows). Images were inverted and thresholds adjusted for maximum visibility. Full image series are shown in Supplementary Movies S1–S4. AAV, adeno-associated virus.
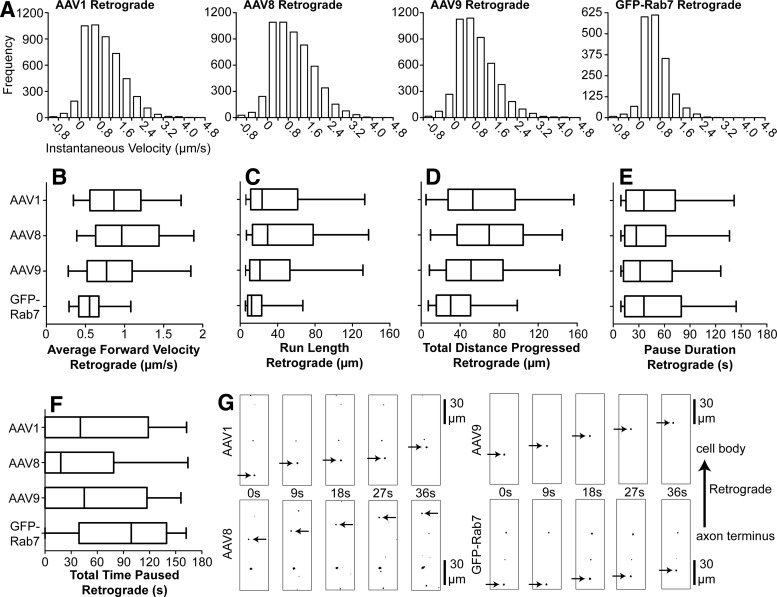
FIG. 2.
AAV serotypes 1, 8, and 9 are targeted for high-speed retrograde transport. Retrograde-directed AAV puncta were tracked within the microfluidic groove from 1 to 4 hr after specific application to axon termini. (A) Histograms of instantaneous velocity, defined as the speed (μm/sec) of movement between two sequential images during a run, with negative values representing steps in reverse of the overall direction of movement (e.g., a retrograde-directed punctum stepping backward in the anterograde direction). Histograms depict the range of velocities at which each population is capable of moving, as well as the relative frequency of each velocity. (B–F) Box plots portray the quantified axonal transport of retrograde-directed AAVs and GFP-Rab7-labeled late endosomes/lysosomes. The interquartile range and median are represented, with whiskers depicting the 5th–95th percentile and outliers omitted because of large sample sizes. AAV8 was found to move retrograde at significantly higher average velocity (B), make longer individual runs (C), progress further total distance (D), and spend less time paused (F) than AAV9 (all p<0.01), although in each case the observed difference was small (see Supplementary Table S1). No significant differences were detected between AAVs 1 and 8 or between AAVs 1 and 9. All three AAV serotypes were found to move retrograde at significantly higher average velocity (B), make longer individual runs (C), progress further total distance (D), and spend less time paused (F) than GFP-Rab7-labeled late endosomes/lysosomes (all p<0.0001). No difference was detected in the duration of individual pauses (E). See Supplementary Table S1 for sample sizes. AAV9 and GFP-Rab7 data were obtained previously using identical methods and are included for comparison (Castle et al., 2014). (G) Excerpted frames from live imaging sequences depict representative movement from each population (arrows). Colors were inverted and thresholds adjusted for maximum visibility. Full image series are shown in Supplementary Movies S5–S8.
Finally, to determine whether the frequency of transport changed over time after specific application of AAV to the cell side or to the axon side of the chamber, each microfluidic culture experiment was divided into six 30 min blocks. The number of retrograde or anterograde AAV puncta that progressed more than 50 μm during each block was calculated, and then divided by the number of image series that were examined. Data from all three serotypes were combined. Four KW tests (Dunn's posttests) were conducted, each comparing among all six 30 min blocks: the frequency of retrograde transport after axon terminus application of AAV, of anterograde transport after axon terminus application, of retrograde transport after cell body application, and of anterograde transport after cell body application, with null hypotheses that all time blocks were identical.
Mass-culture live imaging and analysis
For mass-culture live imaging, 3×109 GC mCy3- or bCy3-AAV1, 8, or 9 and 3×109 GC AF488-AAV1, 8, or 9 were simultaneously applied to mass cultures after 3–4 days in vitro. Cultures were imaged from 1.5 to 4 hr after application of virus. Image series were acquired for 10 min, 3 sec/frame. All AF488-AAV particles that progressed more than 50 μm retrograde or anterograde were counted and scored as either colocalized or uncolocalized with Cy3-AAV. AF488 fluorescence was less intense than Cy3, and thus the brighter Cy3-AAV signal was used to determine colocalization in order to minimize false-negative observations. The total percentages of retrograde and of anterograde AF488-AAV puncta that colocalized with Cy3-AAV were calculated for each culture. As each of the three AF488-AAV serotypes was compared against two Cy3-AAV serotypes, six total comparisons were conducted in each direction. In order to determine whether colocalization varied among pairs of serotypes, two ANOVAs (Student–Newman–Keuls posttests) were conducted, one for anterograde- and one for retrograde-directed AAV. Each ANOVA analyzed the percentage of colocalization among all six serotype comparisons (n=3 cultures per comparison), with the null hypothesis that all serotype combinations were identical.
In order to compare the frequency of transport among serotypes, mass-culture image series were also analyzed by counting the number of Cy3-AAV particles that progressed more than 50 μm retrograde or anterograde, and then dividing this count by the total length of axon analyzed. The frequency of AAV9 transport after treatment of cultures for 2 hr with 30 mU neuraminidase (NA) was determined previously using identical techniques (Castle et al., 2014). To determine whether the frequency of AAV transport varied among serotypes, two ANOVAs (Student–Newman–Keuls posttests) were conducted, one for anterograde- and one for retrograde-directed AAV. Each ANOVA compared the frequency of transport among all three serotypes (n=6 cultures per serotype) as well as AAV9 after NA treatment (n=4 cultures), with the null hypothesis that all conditions were identical.
Finally, in order to determine whether the frequency of AAV transport changed over time in mass culture, these data were analyzed in an identical manner to the microfluidic data described above. Each culture experiment was divided into five 30 min blocks, and the number of AAV particles that progressed more than 50 μm anterograde or retrograde during each block was calculated and divided by the total number of axons that were examined. Data from all three serotypes were combined. Two KW tests (Dunn's posttests) were conducted, one comparing the frequency of retrograde transport among all five 30 min blocks, and the other comparing anterograde transport, with null hypotheses that all time blocks were identical.
Stereotactic injection of AAV vectors in adult mice
Adult C57BL/6 mice were stereotactically injected with AAV2/8.CMV.eGFP or AAV2/9.CMV.eGFP, as described (Heuer et al., 2002; Passini et al., 2002; Cearley and Wolfe, 2006, 2007; Cearley et al., 2008). Briefly, animals were anesthetized with isoflurane, secured in a stereotaxic frame (Kopf), and unilaterally injected through a single drill hole. All procedures were approved by the Children's Hospital of Philadelphia IACUC (protocol no. 397), were performed under anesthesia, and were designed to minimize animal suffering. All mice were injected at approximately 4 months of age. Both male and female mice were used. Injected AAV was obtained from the same preparation that was used for dye labeling and in vitro experiments (above). First, animals were unilaterally injected with 1.8×1010 GC of AAV8 or AAV9 in a volume of 0.5 μl into dentate gyrus (DG), using the following coordinates: 2.2 mm caudal to bregma, 1.5 mm medial to midline, and 1.7 mm ventral to the pial surface (n=3 animals per serotype). Next, animals were unilaterally injected with 9.0×109 GC of AAV8 or AAV9 in a volume of 0.5 μl into entorhinal cortex (EC), using the following coordinates: 4.5 mm caudal to bregma, 3.8 mm medial to midline, and 3.2 mm ventral to the pial surface. AAV was diluted in either phosphate buffered saline (PBS) or NA (2 mU per 0.5 μl inection) before EC injection (n=4 animals per condition). Finally, in order to compare among different mouse strains, 1 μl AAV2/8.GUSB.eGFP (6.4×1010 GC) was unilaterally injected into four different sites along two injection tracts, using the same procedures described above, with one tract targeting the hippocampus (HPC) and thalamus, and the other targeting the cortex and striatum (STR) (Skorupa et al., 1999). A total of four animals of each of the following strains were injected: C57Bl/6, 129, BALB/c, and C3H. The first injection tract was 2.1 mm caudal to bregma, 1.3 mm medial to midline, and 3.2 mm (thalamus) and 1.7 mm (HPC) ventral to the pial surface. The second injection tract was 0.1 mm caudal to bregma, 2.5 mm medial to midline, and 2.5 mm (STR) and 1.1 mm (cortex) ventral to the pial surface.
All animals were processed in an identical manner, as described (Heuer et al., 2002; Passini et al., 2002; Cearley and Wolfe, 2006, 2007; Cearley et al., 2008). Briefly, at 4 weeks postinjection, mice were anesthetized with isoflurane and perfused with 4% paraformaldehyde. Brains were removed and incubated in 4% paraformaldehyde overnight at 4°C, and then incubated 3–5 days in 30% sucrose for cryoprotection. Brains were then mounted in OCT (Sakura), frozen at −20°C, and sectioned coronally at 20 μm thickness using a cryostat (Leica).
Colorimetric in situ hybridization
In order to visualize AAV-transduced cells in cryosectioned brain tissue, colorimetric in situ hybridization (ISH) was performed against the eGFP transgene mRNA sequence, as described (Heuer et al., 2002; Passini et al., 2002; Cearley and Wolfe, 2006, 2007; Cearley et al., 2008). Briefly, eGFP sequence was cut from pZac2.1 plasmid (provided by the University of Pennsylvania Vector Core), inserted into pBluescript II KS cloning plasmid (Stratagene) in both forward and reverse orientations, and linearized to provide sense and antisense templates for run-off transcription (Cearley et al., 2008). Antisense and sense digoxigenin-labeled GFP cRNA riboprobes were then generated using a digoxigenin labeling kit (Roche). Antibody reactivity and probe length were verified via formaldehyde gel (Cearley et al., 2008). After incubation of cryosectioned tissue in riboprobe, cells with bound riboprobe were visualized via antidigoxigenin antibody (Roche) followed by NBP/BCIP stain (Roche). Slides from animals with known AAV-induced eGFP expression were used as positive controls, and slides incubated with sense riboprobe as negative controls. Stained slides were scanned at 20× magnification using an Aperio Scanscope by the Children's Hospital of Philadelphia Pathology Core.
Immunohistochemistry
In order to examine the distribution of AAV-mediated eGFP expression, as well as to determine whether eGFP expression colocalized with the astrocytic marker glial fibrillary acidic protein (GFAP), cryosections immediately adjacent to those stained with ISH were used for standard immunohistochemistry (IHC). Briefly, slides were permeabilized and immunoblocked at room temperature for 1 hr in PBS containing 0.2% Triton X-100 and 2.5% goat serum, incubated overnight at 4°C in 1:1000 chicken anti-eGFP (Molecular Probes) and 1:1000 rabbit anti-GFAP (Millipore) primary antibodies, washed with PBS, incubated at room temperature for 2 hr in 1:500 antichicken AF488 and 1:500 antirabbit AF594 secondary antibodies (Molecular Probes), washed with PBS, and coverslipped with ProLong Gold mounting medium (Life Technologies). Stained slides were imaged using a Leica AF6000 LX upright epifluorescence microscope with 1.5×, 5×, and 20×dry objectives and Leica DFC 360FX CCD camera (Supplementary Figs. S1–S3), or using an Olympus FluoView1000 confocal-scanning laser microscope (Supplementary Fig. S4). Confocal z-stacks were obtained with a 40× oil objective, and maximum intensity projections were generated using ImageJ software.
Results
Long-distance axonal transport is highly conserved among AAV serotypes 1, 8, and 9
Anterograde axonal transport and retrograde axonal transport of AAV were examined independently via microfluidic culture of primary rat E18 cortical neurons. The microfluidic chamber consists of two channels connected by an array of microgrooves (Park et al., 2006). Neurons plated within one channel extend axons across the grooves and into the opposite channel. Each channel also connects to a pair of large wells, through which media, cells, or other factors such as AAV can be applied. When a greater volume of media is added to wells on one side of the chamber, a directional flow of media across the grooves is induced, and AAV that is applied to the channel with less media (termed the isolated channel) cannot move against this flow and thus does not enter the groove and cannot reach the channel with more media (termed the distal channel) (Park et al., 2006; Castle et al., 2014). This permits specific application of AAV to the cell body channel for independent analysis of anterograde transport within the grooves, as well as specific application to the axon terminus channel for analysis of retrograde transport (Park et al., 2006; Castle et al., 2014).
In order to determine whether fundamental differences in axonal transport exist among AAV serotypes 1, 8, and 9, dye-labeled AAV of each serotype was specifically applied to the isolated cell bodies (Fig. 1) or axon termini (Fig. 2), AAV puncta were tracked along axons, movement was quantified, and quantified movement parameters were statistically compared. Late endosomes/lysosomes labeled with GFP-Rab7 were also tracked, providing a baseline measure of intracellular movement.
First, the anterograde movement of each serotype was analyzed after specific application to the cell body (Fig. 1; Supplementary Table S1). All three serotypes were observed to make long anterograde runs at high velocity along axons (Fig. 1G; Supplementary Movies S1–S4). Histograms of instantaneous velocity, which represent the distribution of single velocity steps between sequential frames during forward runs, indicated that all AAV serotypes have a peak frequency of anterograde speed similar to GFP-Rab7, from 0 to 0.8 μm/sec (Fig. 1A). However, all AAV serotypes demonstrated extremely wide distributions, with frequent velocities between 2.4 and 4.4 μm/sec, well beyond what was observed for GFP-Rab7. No significant difference was observed among AAV serotypes in their average forward velocity, run length, total distance progressed, pause duration, or total time paused (Fig. 1B–F). However, GFP-Rab7 was observed to move at significantly slower forward velocity, to make shorter runs, to progress less total distance, and to spend more time paused than all three AAV serotypes (Fig. 1B–F; all p<0.0001). This suggests that AAV1, 8, and 9 are specifically targeted for high-speed anterograde transport in a highly consistent manner.
Next, the retrograde movement of each serotype was quantified after specific application to the axon terminus (Fig. 2; Supplementary Table S1). Again, all three serotypes were observed to make long retrograde runs along axons (Fig. 2G; Supplementary Movies S5–S8). All AAV serotypes exhibited similar distributions of instantaneous velocities in the retrograde direction; these distributions were uniformly broader than those observed for GFP-Rab7, with frequent movement beyond 1.2 μm/sec (Fig. 2A). All three AAV serotypes were observed to move retrograde at significantly higher average forward velocity, to make longer runs, to progress greater total distance, and to spend less time paused than GFP-Rab7 (Fig. 2B–F; all p<0.0001). Although all three AAV serotypes demonstrated similar movement, a small number of statistically significant differences were observed. AAV9 was observed to move at a slower forward velocity (p<0.0001), make shorter runs (p<0.01), and progress less total distance (p<0.01) than AAV8 (Fig. 2B–F). No differences were observed in pause duration or total time paused, and no differences were observed between AAVs 1 and 8 or AAVs 1 and 9. However, these differences among serotypes are minimal when compared with the difference between each serotype and GFP-Rab7 (Fig. 2B–F; Supplementary Table S1), and thus it is clear that AAV1, 8, and 9 are specifically targeted for high-speed retrograde transport, and that this transport is conserved among serotypes.
AAV serotypes 1, 8, and 9 colocalize strongly during long-distance axonal transport
While AAV serotypes 1, 8, and 9 were found to move both retrograde and anterograde in a highly conserved manner, some minor differences were observed (Fig. 2B–F). However, the strong similarities in axonal transport among these serotypes suggested that they utilize the same intracelluar compartments for movement along the axon. To determine whether this was the case, we coinfected mass cultures with pairs of Cy3 (red) and AF488 (green) dye-labeled serotypes. Each serotype examined above (AAV1, 8, or 9) was compared against the other two individually. All AF488-AAV particles that moved more than 50 μm retrograde or anterograde along the axon during 10 min of imaging were classified as either uncolocalized or colocalized with the Cy3-AAV serotype that was coinfected (Fig. 3). Strong and consistent colocalization was observed, with all pairs of serotypes colocalizing approximately 75% of the time during movement in either direction along the axon (Fig. 3; Supplementary Movie S9). No significant differences were observed among combinations of serotypes (p=0.9527 anterograde; p=0.9733 retrograde). AAV9 is known to utilize the late endosome/lysosome for retrograde axonal transport (Castle et al., 2014), and these data indicate not only that AAV1 and 8 do so as well, but also that distinct AAV receptor-mediated entry pathways merge rapidly into this retrograde compartment. Further, AAVs 1, 8, and 9 also utilize a shared intracellular compartment for anterograde axonal transport.
FIG. 3.
AAVs 1, 8, and 9 colocalize strongly and consistently during both retrograde and anterograde transport. From 1.5 to 4 hr after simultaneous application of 3×109 GC bCy3-AAV and 3×109 GC AF488-AAV to a mass culture of 100,000 rat E18 cortical neurons, all AF488-AAV puncta that progressed more than 50 μm retrograde or anterograde over 10 min were counted. Each was classified as either colocalized with a Cy3-AAV punctum or uncolocalized. Each AF488-labeled serotype 1, 8, or 9 was compared against the other two Cy3-labeled serotypes individually. Bars represent the mean among a total of n=3 cultures for each condition, with error bars indicating one standard deviation from the mean. An average n=83 and minimum n=51 AF488-AAV puncta were counted per condition. No significant differences among groups were detected via ANOVA (p=0.9733 and p=0.9527 for retrograde and anterograde, respectively). Representative colocalized movement is shown in Fig. 3; Supplementary Movie S9.
AAV1 undergoes more frequent axonal transport than AAV8 or 9 in vitro
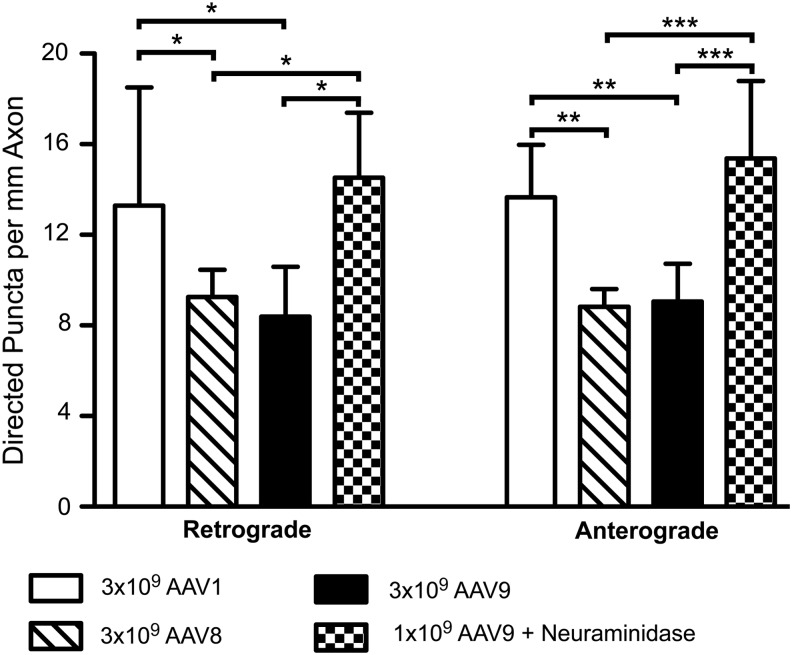
AAVs 1, 8, and 9 appear to be transported identically after endocytosis, and thus the variability in distal transduction in vivo is likely driven by mechanisms acting before this point. One possible mechanism is a difference in uptake at the plasma membrane. That is, although these serotypes may be processed identically once they enter the endosome system, a greater number of endocytosed AAV particles could lead to a greater number of virions undergoing axonal transport, and thus stronger distal transduction. If this is the case, measurable differences in the frequency of axonal transport among serotypes would be expected. We thus compared the frequency of axonal transport among AAVs 1, 8, and 9 in mass-cultured neurons. From 1.5 to 4 hr after application of vector, all Cy3-AAV particles that progressed more than 50 μm retrograde or anterograde along the axon were counted for each serotype, and this number was divided by the length of axon imaged. While no differences were observed between AAVs 8 and 9, AAV1 was transported anterograde (both p<0.01) and retrograde (both p<0.05) with a significantly greater frequency than either of these serotypes (Fig. 4). In addition, one-third the amount of AAV9 was applied to identical cultures that were pretreated with NA, which increases AAV9 uptake by cleaving sialic acid to the more efficient galactose receptor (Bell et al., 2011; Shen et al., 2011). Increasing AAV9 uptake via NA treatment resulted in a significantly greater frequency of both anterograde (p<0.0001) and retrograde (p<0.05) axonal transport of AAV9 than was observed with AAV9 or AAV8 alone (Fig. 4). A significant 59% increase in the uptake of Cy3-AAV9 after NA treatment was confirmed via qPCR against the AAV genome (p=0.0374, unpaired two-tailed t-test). These data provide evidence that differences in uptake at the plasma membrane can lead to differences in the amount of axonal transport among AAV serotypes, even though the mechanisms of this transport may be conserved.
FIG. 4.
AAV1 exhibits more frequent axonal transport than AAV8 or 9 in vitro. 3×109 GC AAV1, 8, or 9 was applied to mass cultures of 100,000 rat E18 cortical neurons, or 1×109 GC AAV9 was applied after treatment of cultures with 30 mU NA for 2 hr. These NA data were obtained previously using identical methods (Castle et al., 2014). From 1.5 to 4 hr after application of vector, the number of AAV puncta progressing more than 50 μm retrograde or anterograde over 10 min of imaging was counted, and then divided by the total length of axon imaged. Stars depict Newman–Keuls posttest significance between groups following ANOVA. *p<0.05; **p<0.01; ***p<0.001. AAV1 and AAV9+NA both made significantly more retrograde and anterograde runs when compared against AAV8 or AAV9 alone (p=0.0150 and p=0.0002, respectively). No significant differences were observed between AAV1 and AAV9+NA, or between AAV8 and AAV9. Bars represent the mean among cultures, with error bars indicating one standard deviation from the mean. AAV1 contains data from n=5 cultures, n=160 retrograde puncta, and n=163 anterograde puncta; AAV8 from n=6, n=106, and n=101; AAV9 from n=6, n=103, and n=107; and AAV9+NA from n=4, n=139, and n=142. NA, neuraminidase.
Retrograde and anterograde axonal transport of AAV have distinct time courses
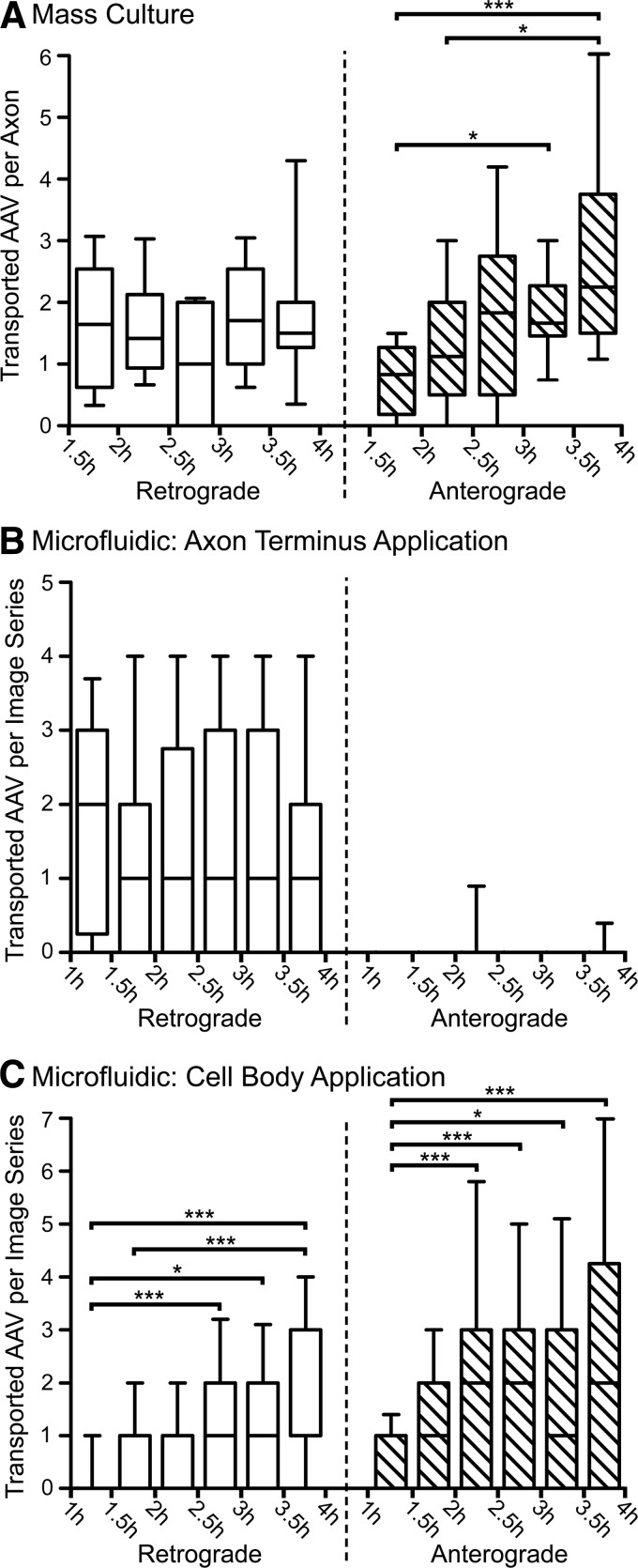
While AAV1, 8, and 9 share the same mechanisms and intracellular compartments for axonal transport, we observed dramatic differences between retrograde- and anterograde-directed AAV puncta (Figs. 1 and 2), which are known to utilize distinct endosomal compartments and molecular motors (Castle et al., 2014). The large quantity of data gathered above allowed for pooling of observations among serotypes, in order to further parse the differences between these two types of transport. In all 3 conditions (mass-culture application, cell body application, and axon terminus application), the number of AAV puncta that moved more than 50 μm during each 30 min block was counted, and then normalized by the number of axons that were imaged (mass culture) or by the number of image series that were acquired (microfluidic culture). Axons within the microfluidic groove frequently overlap and cannot be reliably counted, and thus require normalization by image series rather than axon length or number. Statistical analyses comparing the frequency of retrograde or anterograde axonal transport among all 30 min blocks demonstrated fundamental differences in the time courses of these two types of transport.
Anterograde axonal transport of AAV was observed to increase over time, peaking between 3.5 and 4 hr after mass-culture application of AAV (Fig. 5A; p<0.0001), as well as after cell body application (Fig. 5C; p<0.0001). Furthermore, after cell body application, retrograde AAV transport was also observed to increase over time (Fig. 5C; p<0.0001), indicating that anterograde-directed AAV frequently reverses and returns retrogradely to the cell body. This may be because of the lack of functional synapses within the microfluidic system. In total, approximately 56% of anterograde-directed AAV puncta appear to reverse and return retrogradely to the cell body, as we observed 230 retrograde AAV puncta compared with 410 anterograde AAV puncta.
FIG. 5.
Retrograde transport of AAV peaks rapidly and remains constant, while anterograde transport increases gradually. The number of AAV puncta that progressed more than 50 μm retrograde or anterograde was counted for each axon imaged in mass culture (A) or for each image series obtained from a microfluidic chamber (B and C), and these data were analyzed for changes over time. Data from serotypes 1, 8, and 9 were combined, as no difference among serotypes was observed. Stars represent the significance of Kruskal–Wallis tests (Dunn's posttest). *p<0.05; **p<0.01; ***p<0.001. Boxes represent the median and interquartile range among cultures (A) or image series (B and C), with whiskers indicating the 10th–90th percentile and outliers omitted. In (A), n=18 cultures were analyzed at each time point. In (B) and (C), an average of n=38 and minimum of n=32 image series were analyzed at each time point, with the same series used for both retrograde and anterograde counts. (A) After application of 3×109 GC AAV to mass cultures, there was a significant increase in the frequency of anterograde AAV transport over time (p<0.0001). Retrograde transport peaked rapidly after application and remained constant, with no significant differences detected. The decrease observed from 2.5 to 3 hr after application was not significant (p=0.1692). (B) After application of AAV to the axon terminus side of the microfluidic chamber, retrograde transport of AAV peaked rapidly and remained constant over time, with no significant differences detected (p=0.6491). Anterograde transport of AAV was rare after application to the axon terminus and did not change significantly (p=0.9763). (C) After application of AAV to the cell body side of the microfluidic chamber, both anterograde and retrograde transport of AAV significantly increased over time (both p<0.0001).
Retrograde axonal transport of AAV peaked rapidly, within 1.5 hr of mass-culture application (Fig. 5A) or 1 hr of axon terminus application (Fig. 5B), and did not change significantly over the duration of the experiment (p=0.1692 and p=0.6491, respectively). We observed minimal anterograde transport of AAV after application to the axon terminus (Fig. 5B), suggesting that the retrograde late endosome/lysosome carrier reverses infrequently. Approximately 5% of retrograde-directed AAV puncta appear to return anterograde to the axon terminus, as we observed 18 anterograde AAV puncta compared with 348 retrograde AAV puncta after axon terminus application. This provides further evidence that the anterograde carrier is a late compartment that transports AAV only after a sufficient amount of virus has accumulated in the cell body. In addition, because we observed that 56% of anterograde AAV puncta return retrogradely to the cell body (Fig. 5C), the frequency of retrograde AAV transport after mass-culture application was likely inflated by reversals of anterograde AAV puncta, particularly at later time points. Thus, the nonsignificant decrease in retrograde AAV transport between 2.5 and 3 hr after mass-culture application (Fig. 5A; p=0.1692) may reflect a normal decrease in retrograde transport over time, which is partially masked by an increasing number of anterograde reversals at later time points (Fig. 5C).
Finally, we also examined the frequency of colocalization over time (Fig. 3), comparing the same 30 min time blocks as above (data not shown). No change in colocalization was observed over time for either anterograde (p=0.3962) or retrograde (p=0.8153) AAV transport. This likely reflects a relatively consistent rate of AAV uptake throughout the experiment, as recently endocytosed virions should be less likely to colocalize.
AAV8 and AAV9 undergo similar long-distance retrograde transport in vivo
A clear disparity exists between our in vitro results, which indicated that AAV serotypes 1, 8, and 9 undergo axonal transport in the same compartments and with similar quantified movement, and past studies that have observed substantial differences in axonal transport and distribution of gene transfer among serotypes in vivo (Burger et al., 2004; Cearley and Wolfe, 2006, 2007; Klein et al., 2006, 2008; Li et al., 2006; Taymans et al., 2007; Cearley et al., 2008; Hollis et al., 2008). Thus, we compared the axonal transport of AAV8, which often fails to spread beyond the local injection site in vivo (Cearley and Wolfe, 2006; Taymans et al., 2007; Klein et al., 2008), against AAV9, which frequently demonstrates strong axonal transport and distal transduction (Cearley and Wolfe, 2006, 2007; Cearley et al., 2008; Klein et al., 2008). We utilized axons that project from cell bodies in layer II of EC and terminate in DG, as these projections have been thoroughly studied and are known to be nonreciprocal (Fig. 6B) (van Groen et al., 2003; Witter, 2007).
FIG. 6.
AAV8 and AAV9 exhibit identical retrograde axonal transport after dentate gyrus injection. (A) Adult C57BL/6 mice were unilaterally injected with 1.8×1010 GC AAV8- or AAV9-CMV.eGFP in a volume of 0.5 μl into DG. Four weeks after injection, mice were sacrificed and cryosectioned at 20 μm thickness, and transduced cells were visualized via colorimetric ISH against the eGFP transgene mRNA sequence. Both AAV8- and AAV9-injected mice exhibited strong local transduction throughout HPC, as well as strong distal transduction of ipsilateral EC after retrograde transport of the vector. In addition, retrograde transduction of cells within contralateral HPC (primarily CA3) and contralateral EC was also observed (arrows), indicating that both serotypes can transduce retrogradely over long distances. No fundamental differences in axonal transport were observed between serotypes, although distal transduction of the contralateral hemisphere was stronger for AAV9. All four brain sections in each column are from the same animal. (B) A schematic details the relevant connections among hippocampal and entorhinal brain regions that underlie the axonal transport of AAV and the distal transduction observed here and in Fig. 7. DG, dentate gyrus; EC, entorhinal cortex; GC, genome copies; HPC, hippocampus; ISH, in situ hybridization.
To examine retrograde transport, adult mice were unilaterally injected with AAV8 or AAV9 in DG and sacrificed 4 weeks postinjection, and transduced cells were visualized via ISH against the eGFP transgene mRNA sequence (Fig. 6A). A very low injection volume of 0.5 μl was utilized in order to minimize diffusion and allow for direct examination of axonal transport to distal regions. Both serotypes demonstrated robust retrograde transport, with large numbers of transduced cells observed throughout ipsilateral EC. Axonal transport to contralateral distal sites was also observed, although transport to the contralateral hemisphere was greater for AAV9 than for AAV8 (Fig. 6A, arrows). Transduction in contralateral HPC could be because of either retrograde or anterograde axonal transport, as the two hippocampi are highly interconnected in rodents (Fig. 6B) (Voneida et al., 1981). Transduction in contralateral EC likely reflects retrograde transport, as EC extends a small number of contralateral projections to DG (Fig. 6B) (van Groen et al., 2003; Witter, 2007). Transduction of contralateral EC is notable, as it demonstrates that both AAV8 and AAV9 can retrogradely transduce neurons over a substantial distance of several millimeters.
Finally, IHC against the eGFP transgene product was performed on cryosections taken from the same animals, adjacent to those sections used for ISH (Fig. 3; Supplementary Fig. S1). Expression of eGFP was observed both locally in DG and CA3, as well as distally in ipsilateral and contralateral EC. The distribution of eGFP expression was consistent with ISH staining against AAV mRNA. Local expression of eGFP in the hippocampus was more intense than distal expression in EC. Further, AAV9 appeared to mediate stronger retrograde expression of eGFP in EC, exhibiting more intense fluorescence than AAV8 despite similar distribution. Overall, these data are consistent with our in vitro results, and suggest that both AAV8 and AAV9 can undergo retrograde transport in vivo.
AAV8 and AAV9 undergo similar long-distance anterograde transport in vivo
Next, we sought to compare the anterograde transport of AAV8 and AAV9 via EC injection. As in Fig. 6, adult mice were unilaterally injected with AAV8 or AAV9 and sacrificed 4 weeks postinjection, and ISH was performed (Fig. 7). In addition, AAVs were diluted either with sterile PBS or with NA, in order to determine whether NA could enhance axonal transport in vivo, as was observed in mass-cultured neurons (Fig. 4). However, as a very low injection volume of 0.5 μl was again utilized in order to minimize diffusion, only 2 mU of NA could be injected, much less than the 100 mU that was previously reported to enhance AAV9 transduction of mouse lung in vivo (Bell et al., 2011).
FIG. 7.
AAV8 and AAV9 exhibit identical anterograde axonal transport after entorhinal cortex injection. Adult C57BL/6 mice were unilaterally injected with 9.0×109 GC AAV8- or AAV9-CMV.eGFP in a volume of 0.5 μl into EC. An identical volume and titer of AAV9 was also coinjected with 2 mU NA. Four weeks after injection, mice were sacrificed and cryosectioned at 20 μm thickness, and transduced cells were visualized via colorimetric ISH against the eGFP transgene mRNA sequence. Local EC transduction was strong and similarly distributed in all conditions. Strong distal transduction of DG was observed with both AAV8 and 9, indicating that both serotypes undergo anterograde axonal transport and transduce second-order cell bodies within the granule layer of DG (arrows). Axonal projections from EC to DG are nonreciprocal, and thus DG transduction provides unambiguous evidence of anterograde transport. Distal transduction of CA1 and CA3 was also observed with both serotypes. Although CA3 transduction likely resulted from anterograde transport as well, CA1 transduction could reflect either retrograde or anterograde transport of the vector (Fig. 6B). No enhancement of local or distal transduction was observed after coinjection of AAV9 with NA, which appeared similar to both AAV8 and AAV9 without NA. This may be because of the low concentration of NA that was used. All three sections in each row are from the same animal.
Surprisingly, both AAV8 and AAV9 exhibited strong anterograde transport and robust transduction of DG up to 2 mm rostral to the site of injection (Fig. 7, arrows). Strong transduction of CA3 was also observed with both serotypes. As projections from EC to DG are nonreciprocal (van Groen et al., 2003; Witter, 2007), this provides unambiguous evidence of anterograde transport, secretion of virions, and uptake and transduction of second-order neurons in DG. EC also projects to CA3 (Fig. 6B) (Naber et al., 2001; van Groen et al., 2003), and thus the strong transduction observed in CA3 is likely also because of anterograde transport (Fig. 7). The pattern and strength of transduction were remarkably similar between AAV8 and AAV9 (Fig. 7), suggesting that, as observed in vitro, there is no fundamental difference between these two serotypes after uptake at the plasma membrane, and thus identical injections will produce similar patterns of transduction, at least within the EC/HPC circuitry. NA did not appear to have an effect at this low concentration, as local and distal transduction were identical regardless of whether AAV9 was diluted with PBS or with NA (Fig. 7).
The likelihood that transduction of DG arose via diffusion from the injection site is extremely low, because of the minimal injection volume of 0.5 μl and the substantial distance of nearly 2 mm between these regions. However, the ISH data do not exclude this possibility, as AAV mRNA expression was widespread throughout both the pyrimidal and nonpyrimidal layers of CA1 and CA3 (Fig. 7). To address this possibility, IHC against eGFP was performed on cryosections taken from the same animals, adjacent to those sections used for ISH (Fig. 3; Supplementary Fig. S2). The distribution of eGFP expression was consistent with ISH staining against AAV mRNA. Both serotypes mediated strong local eGFP expression within EC, although AAV8 appeared to transduce lateral EC more strongly than AAV9. Further, intense eGFP fluorescence was observed within the outer molecular layer of DG, reflecting strong transduction of projection neurons in EC, whose axons extend into the molecular layer and terminate on the dendrites of dentate granule cells. Cell bodies within the granule layer were strongly fluorescent, suggesting that both serotypes underwent anterograde transport within transduced EC projection neurons, were released onto dentate granule cells, and induced gene expression within these second-order cells in DG. Although eGFP-positive cells were also observed within CA1 and CA3, fluorescence in these regions was weaker than in DG, and eGFP-positive cells were rarely present within nonpyrimidal layers. Thus, eGFP expression in the granule layer of DG was both more intense and more widespread than in other hippocampal regions positioned between EC and DG, indicating that DG transduction was driven by specific anterograde transport from EC, and not by diffusion from the injection site via adjacent hippocampal layers.
ISH staining of two mice that differed from the overall population provided further evidence that DG transduction was caused by axonal transport, rather than by diffusion from the injection site (Fig. 3; Supplementary Fig. S3A). One AAV8-injected mouse demonstrated ISH-positive cell bodies exclusively in caudal DG and in the injected EC. Further, one AAV9-injected mouse exhibited weak ISH staining within the pyrimidal layer of caudal CA1, but widespread and intense staining in caudal DG and in the injected EC. Thus, DG transduction did not occur via diffusion in either of these animals, as the hippocampal layers positioned between EC and DG did not contain AAV mRNA. Although it is unknown why these two mice differed from the others, it is possible that these injections were more confined to layer II of EC, which contains neurons that project to DG, resulting in less transduction of layers III and IV, which are strongly connected with CA1, CA3, and subiculum (Fig. 6B). Furthermore, although distal transduction of rostral DG was limited in comparison to caudal DG, isolated ISH-positive cells could be observed within rostral DG after both AAV8 and AAV9 injection of EC (Fig. 3; Supplementary Fig. S3B). Not only is this distance well beyond the range of diffusion, but also surrounding rostral HPC layers were completely devoid of ISH signal, indicating unambiguously that AAV can undergo long-distance anterograde transport and transduce second-order cells in DG at least 3 mm from the injection site. It is therefore evident that the widespread ISH staining observed in caudal HPC (Fig. 7) resulted from axonal transport within the hippocampal/entorhinal circuitry, and does not reflect diffusion from the site of injection.
Finally, after both EC and DG injection of AAV8 and AAV9, ISH-positive cells were observed in nonpyrimidal, nongranule cell layers of the HPC, including the stratum oriens, the stratum radiatum, and the stratum lacunosum-moleculare of CA1, 2 and 3, the stratum lucidum of CA3, and the molecular and polymorph layers of DG (Figs. 6 and 7). As expected, transduced cells in the pyrimidal and granule HPC layers demonstrated clear neuronal morphology when examined via cytosolic filling of eGFP (Fig. 3; Supplementary Figs. S1 and S2), consistent with the high density of staining for neuronal nuclei (NeuN) in these layers (Kimoto et al., 2009). However, nonpyrimidal, nongranule layers exhibit relatively lower density of NeuN staining and higher density of staining for GFAP, a filament protein that labels astrocytic processes (Kimoto et al., 2009). Thus, ISH-positive cells in these layers could represent transduced astrocytes or other glial subtypes. Dual IHC against eGFP and GFAP was performed on cryosections from these animals in order to determine whether these transduced cells were astrocytic. However, very few cells in the nonpyrimidal, nongranule layers were positive for eGFP, indicating that transduction of these regions was substantially weaker than transduction of the pyrimidal and granule layers (Supplementary Figs. S1 and S2). No eGFP-positive cells colocalized with GFAP regardless of the AAV serotype or site of injection (Supplementary Fig. S4). Thus, strongly transduced cells in the nonpyrimidal, nongranule layers are not astrocytes. Although this does not exclude oligodendrocytes or microglia, strongly transduced cells in the nonpyrimidal, nongranule layers exhibited characteristic neuronal morphology via cytosolic filling of eGFP (Supplementary Fig. S4). The majority of cells in these layers lacked detectable eGFP expression and thus could not be evaluated via IHC, and it is therefore possible that some ISH-positive cells represent glia. This is unlikely, however, as neither AAV8 nor AAV9 exhibit transduction of GFAP-positive astrocytes or proteolipid-protein-positive oligodendrocytes when evaluated via fluorescent ISH after intraparenchymal injection of adult mice (Cearley and Wolfe, 2006).
Neither local nor distal transduction of AAV8 varies among mouse strains
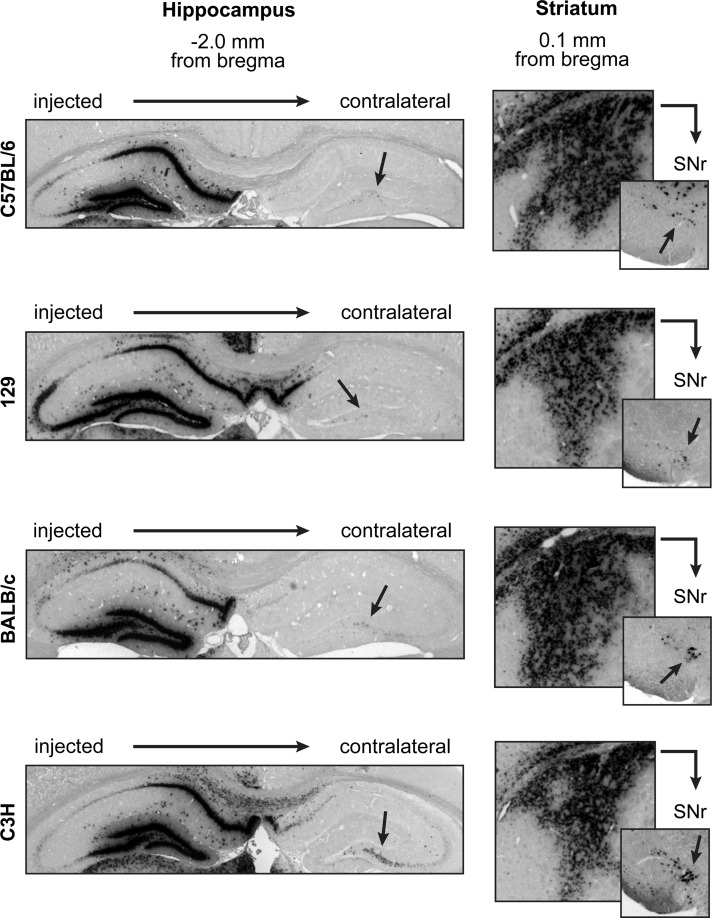
The robust anterograde and retrograde transport of AAV8 was surprising in light of past studies that failed to observe distal transduction with this serotype (Cearley and Wolfe, 2006; Taymans et al., 2007; Klein et al., 2008). One possible explanation was a difference in the animals used, as we observed robust AAV8 transport in C57BL/6 mice (Figs. 6 and 7), while past studies in C3H mice (Cearley and Wolfe, 2006) or Sprague-Dawley rats (Klein et al., 2008) showed limited spread of AAV8 transduction and no evidence of transport. Thus, we sought to compare local and distal AAV8 transduction among C57BL/6, 129, BALB/c, and C3H mouse strains. Each animal received four unilateral injections along two needle tracts: the HPC and the thalamus, and the cortex and the caudate putamen of the STR (Skorupa et al., 1999). As above, animals were sacrificed at 4 weeks and transduced cells were visualized via anti-eGFP ISH. All four mouse strains demonstrated similarly strong local transduction in the HPC and the STR, with further evidence of axonal transport to the contralateral HPC as well as the ipsilateral substantia nigra pars reticulata (SNr) (Fig. 8, arrows). Transduction in the SNr could also have resulted via retrograde transport from the thalamus. For all strains, transduction in the cortex and thalamus was strong but limited, with minimal spread away from the injection site (data not shown). Thus, not only does AAV8 transduce the nervous tissue of each mouse strain with similar efficiency, but also AAV8 can undergo axonal transport and transduce distal brain regions in all four mouse strains. Thus, it is unlikely that differences in experimental animals could account for the variability in local and distal AAV8 transduction among past studies.
FIG. 8.
AAV8 exhibits identical local and distal transduction among mouse strains C57BL/6, 129, BALB/c, and C3H. Adult mice from each strain were unilaterally injected with 1 μl AAV8 in four sites along two injection tracts: the HPC and thalamus, and the cortex and STR. Four weeks after injection, mice were sacrificed and cryosectioned at 20 μm thickness, and transduced cells were visualized via colorimetric ISH against the eGFP transgene mRNA sequence. No differences were observed among the four mouse strains. Transduction of the cortex and thalamus was consistently strong but focal. Transduction of the HPC and STR was more widespread, with evidence of axonal transport to connected brain regions. Neither the pattern nor the overall strength of transduction varied among mouse strains. Axonal transport of vector was also highly conserved among mouse strains, with similar levels of distal transduction observed within contralateral CA3 and ipsilateral SNr among all strains (arrows). Axonal transport of vector to the SNr could have occurred from either the STR or the thalamus infusion of AAV. Images of the SNr are −3.2 mm caudal from bregma.
Discussion
These data indicate that AAV serotypes 1, 8, and 9 share highly conserved mechanisms for both anterograde and retrograde axonal transport in cultured rat embryonic cortical neurons. All three serotypes demonstrated nearly identical velocities of movement, run distances, pause durations, and pause frequencies, and all moved faster than the motile population of Rab7-specific late endosomes/lysosomes. As suggested by the similarity of movement among these serotypes, all three were found to share the same intracellular compartments for axonal transport. Colocalization among serotypes was strong at early time points, suggesting that the different receptor-mediated entry pathways used by each serotype rapidly merge into transport-directed compartments. Despite these similarities, AAV1 exhibited a greater frequency of axonal transport in mass culture. AAV9 also displayed more frequent transport after neurons were pretreated with NA, increasing virion uptake, suggesting that axonal transport is correlated with AAV uptake. Further, we observed differences in the time courses of retrograde and anterograde AAV transport. Retrograde transport peaked within 1 hr and remained constant, while anterograde transport increased gradually over time. This suggests that AAV rapidly enters the retrograde-directed late endosome/lysosome carrier, while entry into the anterograde carrier occurs primarily at later time points after AAV has accumulated within the cell body. Next, we examined the axonal transport of AAV8 and AAV9 in vivo, utilizing the nonreciprocal axons that project from cell bodies in layer II of EC and terminate in DG (van Groen et al., 2003; Witter, 2007). Diffusion was minimized by injecting a very low volume of 0.5 μl, allowing for direct examination of axonal transport to distal brain regions. Retrograde transport of both serotypes to ipsilateral and contralateral EC was observed after DG injection, as well as anterograde transport of both serotypes to DG after EC injection. Although mice were examined 4 weeks after injection, anterograde transfer of Cy3-AAV9 to second-order neurons, as well as retrograde transport to distal brain regions, can occur in less than 24 hr (Cearley and Wolfe, 2007). The strength and localization of distal transduction were both highly similar between the two serotypes in either direction. It thus appears that, so long as both serotypes are endocytosed at sufficient levels, the similarities in axonal transport observed in vitro also hold true in vivo after intraparenchymal injection.
AAV8 and AAV9 both anterogradely transduced second-order DG cells in vivo, but it was not possible to examine anterograde release of AAV in vitro using the microfluidic system. This is because, based on prior measurements (Park et al., 2006), approximately 7.2 μl of medium will flow across the microgrooves from the distal axon channel to the isolated cell body channel over an 8 hr period. This flow is required for fluidic isolation of the cell bodies. Each channel has a volume of approximately 1 μl (Castle et al., 2014), and thus the entire volume of the axon channel will flow into the cell body channel several times over the course of an experiment. AAV that is anterogradely released into the axon channel will therefore be carried back to the AAV-treated cell body channel, and will not be detectable. At 8 hr after specific application of AAV9 to the isolated cell bodies, no AAV genomes were detected within the distal media via qPCR (data not shown). Although a different experimental system must be developed to study the anterograde release of AAV in vitro, the further investigation of this phenomenon could contribute to understanding the trans-synaptic anterograde transport of AAV in vivo.
In light of the strong axonal transport observed with AAV8 in the above systems, it is unclear why other studies have failed to observed distal transduction with this serotype (Cearley and Wolfe, 2006; Taymans et al., 2007; Klein et al., 2008). However, similar variability among studies has occurred in the past. For example, some reported strong axonal transport and distal transduction with AAV5 (Burger et al., 2004; Paterna et al., 2004; Reimsnider et al., 2007; Taymans et al., 2007), while others reported minimal distal transduction with this serotype, despite observing transport of other serotypes under identical conditions (Klein et al., 2006; Li et al., 2006; Hollis et al., 2008). Furthermore, although several studies reported minimal distal transduction with AAV8 (Cearley and Wolfe, 2006; Taymans et al., 2007; Klein et al., 2008), previous work has demonstrated retrograde, but not anterograde, transport of this serotype (Reimsnider et al., 2007; Sondhi et al., 2007; Masamizu et al., 2011). One possible explanation for this variability among studies is the use of different experimental animals. However, we examined AAV8 in four different mouse strains, and observed not only local transduction to be similar among all strains, but nearly identical levels of distal transduction in contralateral HPC and ipsilateral SNr as well (Fig. 8). It is therefore unlikely that animal differences contribute to variability among studies that utilize mice.
In addition to variability among experiments, there is also a well-established variability in distal transduction among AAV serotypes when they are compared directly. For example, after identical adult mouse injections, serotype 9 exhibited strong axonal transport and clear distal transduction, while serotypes 7 and 8 demonstrated a complete absence of distal transduction (Cearley and Wolfe, 2006). Further, AAV1 demonstrated strong retrograde transduction of motor neurons after intramuscular or sciatic nerve injection, while retrograde transduction was minimal after identical peripheral injection of AAVs 2–6 (Hollis et al., 2008). Many other studies have observed similar variability in distal transduction among serotypes (Burger et al., 2004; Klein et al., 2006, 2008; Li et al., 2006; Cearley and Wolfe, 2007; Taymans et al., 2007; Cearley et al., 2008). Our data raise an essential question: if AAV serotypes share the same mechanisms for axonal transport, then what drives this variability among serotypes in the strength and localization of distal transduction in vivo? We hypothesize that the same fundamental mechanism drives both variability among different AAV serotypes within a single experiment, as well as variability among different experiments using the same AAV serotype. As there are no apparent mechanistic differences in the axonal transport of AAV serotypes 1, 8, and 9, it is likely that variability is driven by factors acting before AAV endocytosis. The enhanced frequency of AAV9 transport after NA treatment (Castle et al., 2014), which increases the availability of AAV9 receptors (Bell et al., 2011; Shen et al., 2011), suggests a correlation between AAV uptake and axonal transport. Thus, the observed variability in vivo is likely to be driven by differences in the uptake of AAV at the plasma membrane.
Although most AAVs can use multiple receptors and attachment factors, primary receptors for many AAV serotypes have been identified. For those AAVs used in this study, the major receptors appear to be sialic acid for AAV1 (Wu et al., 2006), the laminin receptor for AAV8 (Akache et al., 2006), and galactose for AAV9 (Bell et al., 2011; Shen et al., 2011). Thus, differences in distal transduction among serotypes could reflect differences in the density of each receptor on either axon termini (for retrograde transport), or on the cell surface of projection neurons (for anterograde transport). That is, if axons projecting into the injection site have a greater density of galactose than of laminin receptors on their terminal ends, then a greater amount of retrograde transduction would be expected with AAV9 than with AAV8. Further, high doses of either serotype would retrogradely transduce these theoretical neurons. However, as the dose is decreased, the serotype with fewer receptors will fall below the level of uptake required for retrograde transduction more quickly, and thus at specific doses distal transduction will be observed for one serotype but not the other. In addition, it appears that the anterograde carrier is a late compartment that transports the virion only after AAV has accumulated in the cell body (Fig. 5). Thus, the level of AAV uptake required at the cell body to achieve anterograde axonal transport and transfer to a distal second-order cell is likely to be much higher than the level of uptake required for transduction of the first-order projection neuron itself. Again, this suggests that if two AAV serotypes are endocytosed at different levels because of differences in receptor density, then only the serotype with greater uptake may anterogradely transduce second-order cells, while both serotypes would transduce the projection neurons themselves at the injection site. Thus, two serotypes may both transduce locally, but only the serotype with greater receptor availability would transduce distally. Further, these effects could be masked by altering the dose, with both serotypes exhibiting anterograde transduction at higher doses, and neither at lower doses. The combinatorial effects of receptor density and injected dose can thus explain the observed variability among serotypes in vivo.
Dose-dependent effects can also partially explain the variability among studies in the axonal transport of specific serotypes, given that the injected dose varies widely among experiments. However, differences in injected brain regions likely play a role in this variability as well. For example, we observed strong anterograde transport after injecting AAV8 into EC (Fig. 7). Although anterograde transport of AAV8 had not been observed previously, this serotype is known to transduce projection neurons in layer II of EC (Broekman et al., 2006), and thus strong uptake of AAV8 by these neurons, and subsequent axonal transport of vector to their projection targets, is not surprising.
These conclusions have substantial implications for the experimental and clinical use of AAV in the nervous system. It is evident that some degree of axonal transport to connected brain regions should be anticipated for all AAV brain injections regardless of the serotype or the site of injection, as this appears to be a fundamental property of AAV vectors. If axonal transport is not desirable, the probability of distal transduction can be reduced by injecting a smaller amount of vector, or by using a serotype with weak CNS tropism such as AAV2 (Davidson et al., 2000; Burger et al., 2004; Klein et al., 2006; Reimsnider et al., 2007; Sondhi et al., 2007; Taymans et al., 2007). In contrast, if increased spread of transduction is desired, a larger amount of vector should be injected, and a serotype with strong CNS tropism such as AAV1, 9, or rh.10 should be utilized (Burger et al., 2004; Cearley and Wolfe, 2006, 2007; Li et al., 2006; Sondhi et al., 2007; Klein et al., 2008). Given the limited knowledge of receptor distribution in the brain, as well as other factors in the extracellular space that could influence AAV uptake, a great deal of further empirical research is needed to fully achieve the goal of confident prediction. The full range of receptors and coreceptors utilized by each serotype must be identified, and the distribution of each receptor must be quantified in sufficient detail to facilitate comparison among different substructures and different subtypes of projection neurons. Thus, substantial work remains before the hypotheses described above can be tested in vivo. Nonetheless, by identifying the shared mechanisms that mediate axonal transport of AAV serotypes 1, 8, and 9, and by examining this transport within a nonreciprocal circuit that can be used for further study of AAV transport, both in vivo and in organotypic slice culture (Dugladze et al., 2001; De Simoni and Yu, 2006), this study indicates that the ability to undergo anterograde and retrograde axonal transport and to subsequently transduce distal brain regions is a fundamental property shared among AAV serotypes.
Supplementary Material
Acknowledgments
We thank T. Clarke, A. Polesky, and E. Cabacungan for expert technical assistance; M. Maronski and M. Dichter (University of Pennsylvania) for providing dissociated rat cortical neurons; and R. Xiao (University of Pennsylvania) for assistance with statistical analysis. This work was supported by NINDS Grants T32NS007413, R01NS038690, and R01NS060698.
Author Disclosure Statement
No competing financial interests exist for any of the authors.
References
- Akache B., Grimm D., Pandey K., et al. (2006). The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J. Virol. 80, 9831–9836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison R.W., Casto B.C., and Hammon W.M. (1965). Adenovirus-associated defective virus particles. Science 149, 754–756 [DOI] [PubMed] [Google Scholar]
- Bell C.L., Vandenberghe L.H., Bell P., et al. (2011). The AAV9 receptor and its modification to improve in vivo lung gene transfer in mice. J. Clin. Invest. 121, 2427–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekman M.L., Comer L.A., Hyman B.T., and Sena-Esteves M. (2006). Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or -2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience 138, 501–510 [DOI] [PubMed] [Google Scholar]
- Bu J., Ashe K.M., Bringas J., et al. (2012). Merits of combination cortical, subcortical, and cerebellar injections for the treatment of Niemann-Pick disease type A. Mol. Ther. 20, 1893–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C., Gorbatyuk O.S., Velardo M.J., et al. (2004). Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol. Ther. 10, 302–317 [DOI] [PubMed] [Google Scholar]
- Castle M.J., Perlson E., Holzbaur E.L., and Wolfe J.H. (2014). Long-distance axonal transport of AAV9 is driven by dynein and kinesin-2 and is trafficked in a highly motile Rab7-positive compartment. Mol. Ther. 22, 554–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley C.N., and Wolfe J.H. (2006). Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol. Ther. 13, 528–537 [DOI] [PubMed] [Google Scholar]
- Cearley C.N., and Wolfe J.H. (2007). A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J. Neurosci. 27, 9928–9940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley C.N., Vandenberghe L.H., Parente M.K., et al. (2008). Expanded repertoire of AAV vector serotypes mediate unique patterns of transduction in mouse brain. Mol. Ther. 16, 1710–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B.L., Stein C.S., Heth J.A., et al. (2000). Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc. Natl. Acad. Sci. USA 97, 3428–3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simoni A., and Yu L.M. (2006). Preparation of organotypic hippocampal slice cultures: interface method. Nat. Protoc. 1, 1439–1445 [DOI] [PubMed] [Google Scholar]
- Dugladze T., Heinemann U., and Gloveli T. (2001). Entorhinal cortex projection cells to the hippocampal formation in vitro. Brain Res. 905, 224–231 [DOI] [PubMed] [Google Scholar]
- Foust K.D., Nurre E., Montgomery C.L., et al. (2009). Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 27, 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G., Qu G., Burnham M.S., et al. (2000). Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo. Hum. Gene Ther. 11, 2079–2091 [DOI] [PubMed] [Google Scholar]
- Gao G.P., Alvira M.R., Wang L., et al. (2002). Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA 99, 11854–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G., Vandenberghe L.H., Alvira M.R., et al. (2004). Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 78, 6381–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G., Vandenberghe L.H., and Wilson J.M. (2005). New recombinant serotypes of AAV vectors. Curr. Gene Ther. 5, 285–297 [DOI] [PubMed] [Google Scholar]
- Gray S.J., Matagne V., Bachaboina L., et al. (2011). Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol. Ther. 19, 1058–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger J.C., Choi V.W., and Samulski R.J. (2006). Production and characterization of adeno-associated viral vectors. Nat. Protoc. 1, 1412–1428 [DOI] [PubMed] [Google Scholar]
- Hadaczek P., Forsayeth J., Mirek H., et al. (2009). Transduction of nonhuman primate brain with adeno-associated virus serotype 1: vector trafficking and immune response. Hum. Gene Ther. 20, 225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer G.G., Passini M.A., Jiang K., et al. (2002). Selective neurodegeneration in murine mucopolysaccharidosis VII is progressive and reversible. Ann. Neurol. 52, 762–770 [DOI] [PubMed] [Google Scholar]
- Hoggan M.D., Blacklow N.R., and Rowe W.P. (1966). Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc. Natl. Acad. Sci. USA 55, 1467–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis E.R., 2nd, Kadoya K., Hirsch M., et al. (2008). Efficient retrograde neuronal transduction utilizing self-complementary AAV1. Mol. Ther. 16, 296–301 [DOI] [PubMed] [Google Scholar]
- Karolewski B.A., and Wolfe J.H. (2006). Genetic correction of the fetal brain increases the lifespan of mice with the severe multisystemic disease mucopolysaccharidosis type VII. Mol. Ther. 14, 14–24 [DOI] [PubMed] [Google Scholar]
- Kimoto H., Eto R., Abe M., et al. (2009). Alterations of glial cells in the mouse hippocampus during postnatal development. Cell Mol. Neurobiol. 29, 1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R.L., Dayton R.D., Leidenheimer N.J., et al. (2006). Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Mol. Ther. 13, 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R.L., Dayton R.D., Tatom J.B., et al. (2008). AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol. Ther. 16, 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.F., Wang R.Z., Meng Q.H., et al. (2006). Intra-ventricular infusion of rAAV1-EGFP resulted in transduction in multiple regions of adult rat brain: a comparative study with rAAV2 and rAAV5 vectors. Brain Res. 1122, 1–9 [DOI] [PubMed] [Google Scholar]
- Masamizu Y., Okada T., Kawasaki K., et al. (2011). Local and retrograde gene transfer into primate neuronal pathways via adeno-associated virus serotype 8 and 9. Neuroscience 193, 249–258 [DOI] [PubMed] [Google Scholar]
- Naber P.A., Lopes da Silva F.H., and Witter M.P. (2001). Reciprocal connections between the entorhinal cortex and hippocampal fields CA1 and the subiculum are in register with the projections from CA1 to the subiculum. Hippocampus 11, 99–104 [DOI] [PubMed] [Google Scholar]
- Park J.W., Vahidi B., Taylor A.M., et al. (2006). Microfluidic culture platform for neuroscience research. Nat. Protoc. 1, 2128–2136 [DOI] [PubMed] [Google Scholar]
- Passini M.A., Lee E.B., Heuer G.G., and Wolfe J.H. (2002). Distribution of a lysosomal enzyme in the adult brain by axonal transport and by cells of the rostral migratory stream. J. Neurosci. 22, 6437–6446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passini M.A., Watson D.J., Vite C.H., et al. (2003). Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice. J. Virol. 77, 7034–7040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterna J.C., Feldon J., and Bueler H. (2004). Transduction profiles of recombinant adeno-associated virus vectors derived from serotypes 2 and 5 in the nigrostriatal system of rats. J. Virol. 78, 6808–6817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimsnider S., Manfredsson F.P., Muzyczka N., and Mandel R.J. (2007). Time course of transgene expression after intrastriatal pseudotyped rAAV2/1, rAAV2/2, rAAV2/5, and rAAV2/8 transduction in the rat. Mol. Ther. 15, 1504–1511 [DOI] [PubMed] [Google Scholar]
- Rivera V.M., Gao G.P., Grant R.L., et al. (2005). Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood 105, 1424–1430 [DOI] [PubMed] [Google Scholar]
- Royo N.C., Vandenberghe L.H., Ma J.Y., et al. (2008). Specific AAV serotypes stably transduce primary hippocampal and cortical cultures with high efficiency and low toxicity. Brain Res. 1190, 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski R.J., Chang L.S., and Shenk T. (1987). A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J. Virol. 61, 3096–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S., Bryant K.D., Brown S.M., et al. (2011). Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. J. Biol. Chem. 286, 13532–13540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorupa A.F., Fisher K.J., Wilson J.M., et al. (1999). Sustained production of beta-glucuronidase from localized sites after AAV vector gene transfer results in widespread distribution of enzyme and reversal of lysosomal storage lesions in a large volume of brain in mucopolysaccharidosis VII mice. Exp. Neurol. 160, 17–27 [DOI] [PubMed] [Google Scholar]
- Sondhi D., Hackett N.R., Peterson D.A., et al. (2007). Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector. Mol. Ther. 15, 481–491 [DOI] [PubMed] [Google Scholar]
- Taymans J.M., Vandenberghe L.H., Haute C.V., et al. (2007). Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum. Gene Ther. 18, 195–206 [DOI] [PubMed] [Google Scholar]
- van Groen T., Miettinen P., and Kadish I. (2003). The entorhinal cortex of the mouse: organization of the projection to the hippocampal formation. Hippocampus 13, 133–149 [DOI] [PubMed] [Google Scholar]
- Voneida T.J., Vardaris R.M., Fish S.E., and Reiheld C.T. (1981). The origin of the hippocampal commissure in the rat. Anat. Rec. 201, 91–103 [DOI] [PubMed] [Google Scholar]
- Wang C., Wang C.M., Clark K.R., and Sferra T.J. (2003). Recombinant AAV serotype 1 transduction efficiency and tropism in the murine brain. Gene Ther. 10, 1528–1534 [DOI] [PubMed] [Google Scholar]
- Wilcox K.S., and Dichter M.A. (1994). Paired pulse depression in cultured hippocampal neurons is due to a presynaptic mechanism independent of GABAB autoreceptor activation. J. Neurosci. 14, 1775–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter M.P. (2007). The perforant path: projections from the entorhinal cortex to the dentate gyrus. Prog. Brain Res. 163, 43–61 [DOI] [PubMed] [Google Scholar]
- Wu Z., Miller E., Agbandje-McKenna M., and Samulski R.J. (2006). Alpha2,3 and alpha2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J. Virol. 80, 9093–9103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Li J., McCown T.J., and Samulski R.J. (1997). Gene transfer by adeno-associated virus vectors into the central nervous system. Exp. Neurol. 144, 113–124 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.