Abstract
We used the polymerase chain reaction (PCR) to study which step(s) of the human immunodeficiency virus type 1 (HIV-1) life cycle may be blocked following treatment of HIV-exposed CEM cells with 13B8-2, a monoclonal antibody (mAb) specific for the immunoglobulin (Ig) CDR3-like region of the CD4 molecule and able to inhibit the productive infection of CEM cells by HIV-1. The presence of viral RNA was investigated and found in 13B8-2 mAb-treated CEM cells 30 min after viral exposure; the full-length viral DNA was found at 24 h post-infection. We also found integrated forms of viral DNA at 24 h post-infection. However, the integrated provirus was transcriptionally inactive in 13B8-2 mAb-treated cells, as demonstrated by the absence of spliced HIV-1 mRNA. The lack of HIV transcription under 13B8-2 mAb treatment was confirmed by chloramphenicol acetyltransferase (CAT) assay. We conclude that the inhibition of viral gene transcription accounts for the lack of progeny virions in culture supernatants of cells treated with this anti-CD4 mAb. We also demonstrate that 13B8-2 blocks viral production from chronically infected cells and restores CD4 cell-surface expression on CEM cells containing an integrated provirus(es). We found this effect to be reversible. Moreover, we demonstrate that 13B8-2 mAb treatment is efficient on different HIV-1 and HIV-2 virus isolates. These results may have major implications for the treatment of AIDS.
Full text
PDF





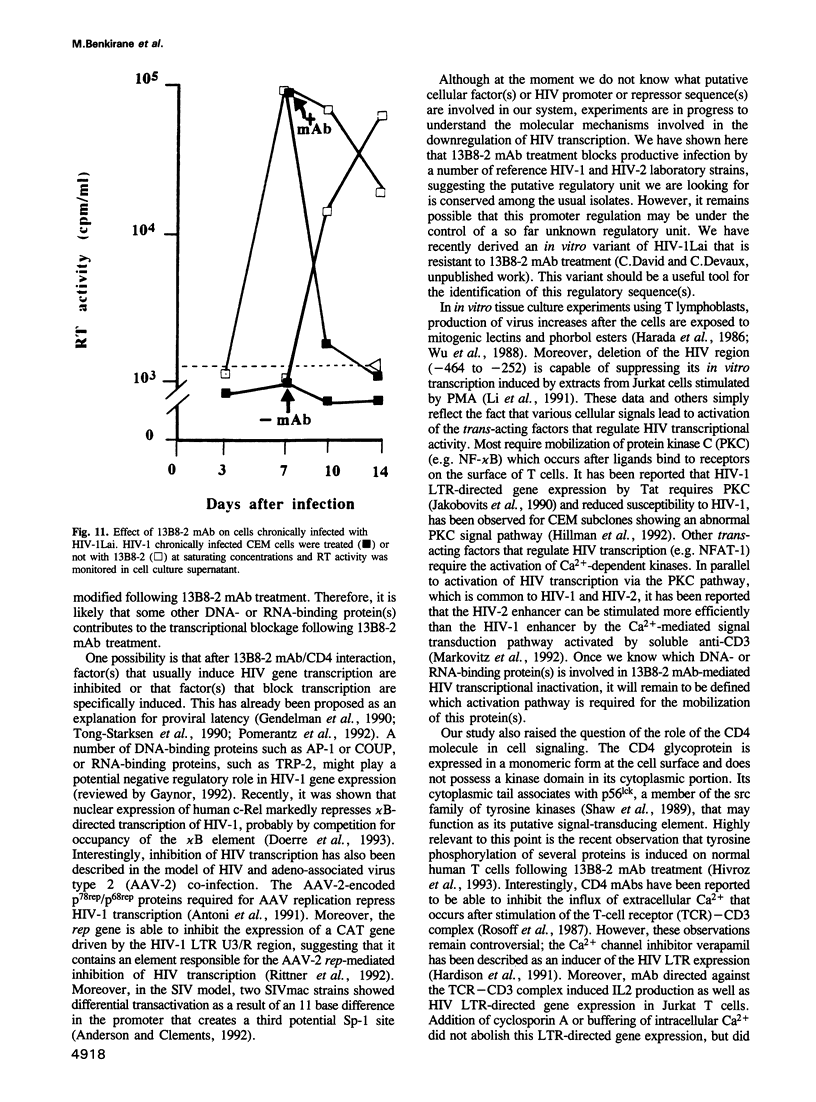



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. G., Clements J. E. Two strains of SIVmac show differential transactivation mediated by sequences in the promoter. Virology. 1992 Dec;191(2):559–568. doi: 10.1016/0042-6822(92)90231-d. [DOI] [PubMed] [Google Scholar]
- Antoni B. A., Rabson A. B., Miller I. L., Trempe J. P., Chejanovsky N., Carter B. J. Adeno-associated virus Rep protein inhibits human immunodeficiency virus type 1 production in human cells. J Virol. 1991 Jan;65(1):396–404. doi: 10.1128/jvi.65.1.396-404.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Blue M. L., Hafler D. A., Daley J. F., Levine H., Craig K. A., Breitmeyer J. B., Schlossman S. F. Regulation of T cell clone function via CD4 and CD8 molecules. Anti-CD4 can mediate two distinct inhibitory activities. J Immunol. 1988 Jan 15;140(2):376–383. [PubMed] [Google Scholar]
- Brown P. O., Bowerman B., Varmus H. E., Bishop J. M. Correct integration of retroviral DNA in vitro. Cell. 1987 May 8;49(3):347–356. doi: 10.1016/0092-8674(87)90287-x. [DOI] [PubMed] [Google Scholar]
- Burkly L. C., Olson D., Shapiro R., Winkler G., Rosa J. J., Thomas D. W., Williams C., Chisholm P. Inhibition of HIV infection by a novel CD4 domain 2-specific monoclonal antibody. Dissecting the basis for its inhibitory effect on HIV-induced cell fusion. J Immunol. 1992 Sep 1;149(5):1779–1787. [PubMed] [Google Scholar]
- Carteron N. L., Schimenti C. L., Wofsy D. Treatment of murine lupus with F(ab')2 fragments of monoclonal antibody to L3T4. Suppression of autoimmunity does not depend on T helper cell depletion. J Immunol. 1989 Mar 1;142(5):1470–1475. [PubMed] [Google Scholar]
- Chen M. Y., Maldarelli F., Karczewski M. K., Willey R. L., Strebel K. Human immunodeficiency virus type 1 Vpu protein induces degradation of CD4 in vitro: the cytoplasmic domain of CD4 contributes to Vpu sensitivity. J Virol. 1993 Jul;67(7):3877–3884. doi: 10.1128/jvi.67.7.3877-3884.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeau P., Benkirane M., Weil R., David C., Emiliani S., Olive D., Mawas C., Serre A., Devaux C. Ig CDR3-like region of the CD4 molecule is involved in HIV-induced syncytia formation but not in viral entry. J Immunol. 1993 Jan 1;150(1):290–301. [PubMed] [Google Scholar]
- Corbeau P., Olive D., Devaux C. Anti-HLA antigen class I heavy chain monoclonal antibodies inhibit human immunodeficiency virus production by peripheral blood mononuclear cells. Eur J Immunol. 1991 Apr;21(4):865–871. doi: 10.1002/eji.1830210402. [DOI] [PubMed] [Google Scholar]
- Dhiver C., Olive D., Rousseau S., Tamalet C., Lopez M., Galindo J. R., Mourens M., Hirn M., Gastaut J. A., Mawas C. Pilot phase I study using zidovudine in association with a 10-day course of anti-CD4 monoclonal antibody in seven AIDS patients. AIDS. 1989 Dec;3(12):835–842. doi: 10.1097/00002030-198912000-00009. [DOI] [PubMed] [Google Scholar]
- Doerre S., Sista P., Sun S. C., Ballard D. W., Greene W. C. The c-rel protooncogene product represses NF-kappa B p65-mediated transcriptional activation of the long terminal repeat of type 1 human immunodeficiency virus. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1023–1027. doi: 10.1073/pnas.90.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. A., Bertonis J. M., Meier W., Johnson V. A., Costopoulos D. S., Liu T., Tizard R., Walker B. D., Hirsch M. S., Schooley R. T. HIV infection is blocked in vitro by recombinant soluble CD4. Nature. 1988 Jan 7;331(6151):76–78. doi: 10.1038/331076a0. [DOI] [PubMed] [Google Scholar]
- Franza B. R., Jr, Josephs S. F., Gilman M. Z., Ryan W., Clarkson B. Characterization of cellular proteins recognizing the HIV enhancer using a microscale DNA-affinity precipitation assay. 1987 Nov 26-Dec 2Nature. 330(6146):391–395. doi: 10.1038/330391a0. [DOI] [PubMed] [Google Scholar]
- Franza B. R., Jr, Rauscher F. J., 3rd, Josephs S. F., Curran T. The Fos complex and Fos-related antigens recognize sequence elements that contain AP-1 binding sites. Science. 1988 Mar 4;239(4844):1150–1153. doi: 10.1126/science.2964084. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Garcia J. V., Miller A. D. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991 Apr 11;350(6318):508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- Gaynor R. Cellular transcription factors involved in the regulation of HIV-1 gene expression. AIDS. 1992 Apr;6(4):347–363. doi: 10.1097/00002030-199204000-00001. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Baca L. M., Turpin J., Kalter D. C., Hansen B., Orenstein J. M., Dieffenbach C. W., Friedman R. M., Meltzer M. S. Regulation of HIV replication in infected monocytes by IFN-alpha. Mechanisms for viral restriction. J Immunol. 1990 Oct 15;145(8):2669–2676. [PubMed] [Google Scholar]
- Gowda S. D., Stein B. S., Mohagheghpour N., Benike C. J., Engleman E. G. Evidence that T cell activation is required for HIV-1 entry in CD4+ lymphocytes. J Immunol. 1989 Feb 1;142(3):773–780. [PubMed] [Google Scholar]
- Gruters R. A., Otto S. A., Al B. J., Verhoeven A. J., Verweij C. L., Van Lier R. A., Miedema F. Non-mitogenic T cell activation signals are sufficient for induction of human immunodeficiency virus transcription. Eur J Immunol. 1991 Jan;21(1):167–172. doi: 10.1002/eji.1830210125. [DOI] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Nakashima H., Kobayashi N., Yamamoto N. Tumor promoter, TPA, enhances replication of HTLV-III/LAV. Virology. 1986 Oct 30;154(2):249–258. doi: 10.1016/0042-6822(86)90451-4. [DOI] [PubMed] [Google Scholar]
- Harbison M. A., Kim S. Y., Gillis J. M., Hammer S. M. Effect of the calcium channel blocker verapamil on human immunodeficiency virus type 1 replication in lymphoid cells. J Infect Dis. 1991 Jul;164(1):53–60. doi: 10.1093/infdis/164.1.53. [DOI] [PubMed] [Google Scholar]
- Hasunuma T., Tsubota H., Watanabe M., Chen Z. W., Lord C. I., Burkly L. C., Daley J. F., Letvin N. L. Regions of the CD4 molecule not involved in virus binding or syncytia formation are required for HIV-1 infection of lymphocytes. J Immunol. 1992 Mar 15;148(6):1841–1846. [PubMed] [Google Scholar]
- Hillman K., Qian J., Siegel J. N., Roderiquez G., Blackburn R., Manischewitz J., Norcross M., Golding H. Reduced susceptibility to HIV-1 infection of ethyl-methanesulfonate-treated CEM subclones correlates with a blockade in their protein kinase C signaling pathway. J Immunol. 1992 Jun 15;148(12):3991–3998. [PubMed] [Google Scholar]
- Hirsch I., Spire B., Tsunetsugu-Yokota Y., Neuveut C., Sire J., Chermann J. C. Differences in replication and cytopathogenicity of human immunodeficiency virus type 1 (HIV-1) are not determined by long terminal repeats (LTR). Virology. 1990 Aug;177(2):759–763. doi: 10.1016/0042-6822(90)90544-2. [DOI] [PubMed] [Google Scholar]
- Hivroz C., Mazerolles F., Soula M., Fagard R., Graton S., Meloche S., Sekaly R. P., Fischer A. Human immunodeficiency virus gp120 and derived peptides activate protein tyrosine kinase p56lck in human CD4 T lymphocytes. Eur J Immunol. 1993 Mar;23(3):600–607. doi: 10.1002/eji.1830230303. [DOI] [PubMed] [Google Scholar]
- Hoxie J. A., Alpers J. D., Rackowski J. L., Huebner K., Haggarty B. S., Cedarbaum A. J., Reed J. C. Alterations in T4 (CD4) protein and mRNA synthesis in cells infected with HIV. Science. 1986 Nov 28;234(4780):1123–1127. doi: 10.1126/science.3095925. [DOI] [PubMed] [Google Scholar]
- Hu W. S., Temin H. M. Retroviral recombination and reverse transcription. Science. 1990 Nov 30;250(4985):1227–1233. doi: 10.1126/science.1700865. [DOI] [PubMed] [Google Scholar]
- Jakobovits A., Rosenthal A., Capon D. J. Trans-activation of HIV-1 LTR-directed gene expression by tat requires protein kinase C. EMBO J. 1990 Apr;9(4):1165–1170. doi: 10.1002/j.1460-2075.1990.tb08223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura I., Koga Y., Oh-Hori N., Onodera K., Kimura G., Nomoto K. Depletion of the surface CD4 molecule by the envelope protein of human immunodeficiency virus expressed in a human CD4+ monocytoid cell line. J Virol. 1989 Sep;63(9):3748–3754. doi: 10.1128/jvi.63.9.3748-3754.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Y., Byrn R., Groopman J., Baltimore D. Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: evidence for differential gene expression. J Virol. 1989 Sep;63(9):3708–3713. doi: 10.1128/jvi.63.9.3708-3713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Barré-Sinoussi F., Nugeyre M. T., Danquet C., Vilmer E., Griscelli C., Brun-Veziret F., Rouzioux C., Gluckman J. C., Chermann J. C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984 Jul 6;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- Leonard J., Parrott C., Buckler-White A. J., Turner W., Ross E. K., Martin M. A., Rabson A. B. The NF-kappa B binding sites in the human immunodeficiency virus type 1 long terminal repeat are not required for virus infectivity. J Virol. 1989 Nov;63(11):4919–4924. doi: 10.1128/jvi.63.11.4919-4924.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P., Hensel M., Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992 Aug;11(8):3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. C., Ross J., Scheppler J. A., Franza B. R., Jr An in vitro transcription analysis of early responses of the human immunodeficiency virus type 1 long terminal repeat to different transcriptional activators. Mol Cell Biol. 1991 Apr;11(4):1883–1893. doi: 10.1128/mcb.11.4.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson J. D., Feinberg M. B., Reyes G. R., Rabin L., Banapour B., Chakrabarti S., Moss B., Wong-Staal F., Steimer K. S., Engleman E. G. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986 Oct 23;323(6090):725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- Markovitz D. M., Smith M. J., Hilfinger J., Hannibal M. C., Petryniak B., Nabel G. J. Activation of the human immunodeficiency virus type 2 enhancer is dependent on purine box and kappa B regulatory elements. J Virol. 1992 Sep;66(9):5479–5484. doi: 10.1128/jvi.66.9.5479-5484.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Rabin L. B., Feinberg M. B., Lieberman M., Kosek J. C., Reyes G. R., Weissman I. L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Yarchoan R., Kageyama S., Broder S. Targeted therapy of human immunodeficiency virus-related disease. FASEB J. 1991 Jul;5(10):2369–2381. doi: 10.1096/fasebj.5.10.1712326. [DOI] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Huang Y. X., Ashkenazi A., Ho D. D. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol. 1992 Jan;66(1):235–243. doi: 10.1128/jvi.66.1.235-243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., Sattentau Q. J., Klasse P. J., Burkly L. C. A monoclonal antibody to CD4 domain 2 blocks soluble CD4-induced conformational changes in the envelope glycoproteins of human immunodeficiency virus type 1 (HIV-1) and HIV-1 infection of CD4+ cells. J Virol. 1992 Aug;66(8):4784–4793. doi: 10.1128/jvi.66.8.4784-4793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Nara P. L., Smit L., Dunlop N., Hatch W., Merges M., Waters D., Kelliher J., Gallo R. C., Fischinger P. J., Goudsmit J. Emergence of viruses resistant to neutralization by V3-specific antibodies in experimental human immunodeficiency virus type 1 IIIB infection of chimpanzees. J Virol. 1990 Aug;64(8):3779–3791. doi: 10.1128/jvi.64.8.3779-3791.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peliska J. A., Benkovic S. J. Mechanism of DNA strand transfer reactions catalyzed by HIV-1 reverse transcriptase. Science. 1992 Nov 13;258(5085):1112–1118. doi: 10.1126/science.1279806. [DOI] [PubMed] [Google Scholar]
- Pomerantz R. J., Seshamma T., Trono D. Efficient replication of human immunodeficiency virus type 1 requires a threshold level of Rev: potential implications for latency. J Virol. 1992 Mar;66(3):1809–1813. doi: 10.1128/jvi.66.3.1809-1813.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz R. J., Trono D., Feinberg M. B., Baltimore D. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell. 1990 Jun 29;61(7):1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- Reimann K. A., Burkly L. C., Burrus B., Waite B. C., Lord C. I., Letvin N. L. In vivo administration to rhesus monkeys of a CD4-specific monoclonal antibody capable of blocking AIDS virus replication. AIDS Res Hum Retroviruses. 1993 Mar;9(3):199–207. doi: 10.1089/aid.1993.9.199. [DOI] [PubMed] [Google Scholar]
- Rieber E. P., Federle C., Reiter C., Krauss S., Gürtler L., Eberle J., Deinhardt F., Riethmüller G. The monoclonal CD4 antibody M-T413 inhibits cellular infection with human immunodeficiency virus after viral attachment to the cell membrane: an approach to postexposure prophylaxis. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10792–10796. doi: 10.1073/pnas.89.22.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittner K., Heilbronn R., Kleinschmidt J. A., Sczakiel G. Adeno-associated virus type 2-mediated inhibition of human immunodeficiency virus type 1 (HIV-1) replication: involvement of p78rep/p68rep and the HIV-1 long terminal repeat. J Gen Virol. 1992 Nov;73(Pt 11):2977–2981. doi: 10.1099/0022-1317-73-11-2977. [DOI] [PubMed] [Google Scholar]
- Robins T., Plattner J. HIV protease inhibitors: their anti-HIV activity and potential role in treatment. J Acquir Immune Defic Syndr. 1993 Feb;6(2):162–170. [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985 Jul;41(3):813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- Rosoff P. M., Burakoff S. J., Greenstein J. L. The role of the L3T4 molecule in mitogen and antigen-activated signal transduction. Cell. 1987 Jun 19;49(6):845–853. doi: 10.1016/0092-8674(87)90622-2. [DOI] [PubMed] [Google Scholar]
- Roth M. J., Schwartzberg P. L., Goff S. P. Structure of the termini of DNA intermediates in the integration of retroviral DNA: dependence on IN function and terminal DNA sequence. Cell. 1989 Jul 14;58(1):47–54. doi: 10.1016/0092-8674(89)90401-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahabuddin M., Volsky B., Hsu M. C., Volsky D. J. Restoration of cell surface CD4 expression in human immunodeficiency virus type 1-infected cells by treatment with a Tat antagonist. J Virol. 1992 Nov;66(11):6802–6805. doi: 10.1128/jvi.66.11.6802-6805.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A. S., Amrein K. E., Hammond C., Stern D. F., Sefton B. M., Rose J. K. The lck tyrosine protein kinase interacts with the cytoplasmic tail of the CD4 glycoprotein through its unique amino-terminal domain. Cell. 1989 Nov 17;59(4):627–636. doi: 10.1016/0092-8674(89)90008-1. [DOI] [PubMed] [Google Scholar]
- Smith D. H., Byrn R. A., Marsters S. A., Gregory T., Groopman J. E., Capon D. J. Blocking of HIV-1 infectivity by a soluble, secreted form of the CD4 antigen. Science. 1987 Dec 18;238(4834):1704–1707. doi: 10.1126/science.3500514. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Campbell K., Haseltine W. A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. 1986 Jul 31-Aug 6Nature. 322(6078):470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- Starcich B., Ratner L., Josephs S. F., Okamoto T., Gallo R. C., Wong-Staal F. Characterization of long terminal repeat sequences of HTLV-III. Science. 1985 Feb 1;227(4686):538–540. doi: 10.1126/science.2981438. [DOI] [PubMed] [Google Scholar]
- Stein B. S., Gowda S. D., Lifson J. D., Penhallow R. C., Bensch K. G., Engleman E. G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987 Jun 5;49(5):659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- Stevenson M., Haggerty S., Lamonica C. A., Meier C. M., Welch S. K., Wasiak A. J. Integration is not necessary for expression of human immunodeficiency virus type 1 protein products. J Virol. 1990 May;64(5):2421–2425. doi: 10.1128/jvi.64.5.2421-2425.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M., Meier C., Mann A. M., Chapman N., Wasiak A. Envelope glycoprotein of HIV induces interference and cytolysis resistance in CD4+ cells: mechanism for persistence in AIDS. Cell. 1988 May 6;53(3):483–496. doi: 10.1016/0092-8674(88)90168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M., Stanwick T. L., Dempsey M. P., Lamonica C. A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990 May;9(5):1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong-Starkesen S. E., Luciw P. A., Peterlin B. M. Signaling through T lymphocyte surface proteins, TCR/CD3 and CD28, activates the HIV-1 long terminal repeat. J Immunol. 1989 Jan 15;142(2):702–707. [PubMed] [Google Scholar]
- Tong-Starksen S. E., Luciw P. A., Peterlin B. M. Human immunodeficiency virus long terminal repeat responds to T-cell activation signals. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6845–6849. doi: 10.1073/pnas.84.19.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong-Starksen S. E., Welsh T. M., Peterlin B. M. Differences in transcriptional enhancers of HIV-1 and HIV-2. Response to T cell activation signals. J Immunol. 1990 Dec 15;145(12):4348–4354. [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Wassmer P., Chan C., Lögdberg L., Shevach E. M. Role of the L3T4-antigen in T cell activation. II. Inhibition of T cell activation by monoclonal anti-L3T4 antibodies in the absence of accessory cells. J Immunol. 1985 Oct;135(4):2237–2242. [PubMed] [Google Scholar]
- Wu F., Garcia J., Mitsuyasu R., Gaynor R. Alterations in binding characteristics of the human immunodeficiency virus enhancer factor. J Virol. 1988 Jan;62(1):218–225. doi: 10.1128/jvi.62.1.218-225.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Haislip A. M., Krogstad P., Chen I. S. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992 Mar;66(3):1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]