Abstract
Wolbachia are maternally transmitted endosymbionts that often alter their arthropod hosts’ biology to favor the success of infected females, and they may also serve as a speciation microbe driving reproductive isolation. Two of these host manipulations include killing males outright and reducing offspring survival when infected males mate with uninfected females, a phenomenon known as cytoplasmic incompatibility. Little is known about the mechanisms behind these phenotypes, but interestingly either effect can be caused by the same Wolbachia strain when infecting different hosts. For instance, wRec causes cytoplasmic incompatibility in its native host Drosophila recens and male killing in D. subquinaria. The discovery of prophage WO elements in most arthropod Wolbachia has generated the hypothesis that WO may encode genes involved in these reproductive manipulations. However, PCR screens for the WO minor capsid gene indicated that wRec lacks phage WO. Thus, wRec seemed to provide an example where phage WO is not needed for Wolbachia-induced reproductive manipulation. To enable investigation of the mechanism of phenotype switching in different host backgrounds, and to examine the unexpected absence of phage WO, we sequenced the genome of wRec. Analyses reveal that wRec diverged from wMel approximately 350,000 years ago, mainly by genome reduction in the phage regions. While it lost the minor capsid gene used in standard PCR screens for phage WO, it retained two regions encompassing 33 genes, several of which have previously been associated with reproductive parasitism. Thus, WO gene involvement in reproductive manipulation cannot be excluded and reliance on single gene PCR should not be used to rule out the presence of phage WO in Wolbachia. Additionally, the genome sequence for wRec will enable transcriptomic and proteomic studies that may help elucidate the Wolbachia mechanisms of altered reproductive manipulations associated with host switching, perhaps among the 33 remaining phage genes.
Keywords: Wolbachia, Bacteriophage WO, Endosymbiosis, Genome evolution, Cytoplasmic incompatibility
Introduction
Wolbachia are widespread obligate intracellular α-proteobacteria that infect around 40% of arthropod species (Zug & Hammerstein, 2012) and 47% of filarial nematodes (Ferri et al., 2011). These infection frequencies, if extrapolated to the diversity and abundance of their hosts, make Wolbachia perhaps the most widespread endosymbiont in animals. To maximize its propagation in arthropods, the maternally inherited Wolbachia has evolved an assortment of mechanisms to distort its host’s reproductive system in a manner that enhances the relative production of infected females. These mechanisms include feminization, parthenogenesis, male killing, and cytoplasmic incompatibility (CI), the most common phenotype and one that results in embryonic lethality when matings occur between infected males and uninfected females (Werren, Baldo & Clark, 2008). Females harboring the same Wolbachia strain, meanwhile, can successfully mate and produce infected offspring with either infected or uninfected males, giving these females a selective advantage in populations of mixed infection status.
Interestingly, some Wolbachia strains are multipotent and can induce more than one type of reproductive manipulation depending on the arthropod host it infects (Fujii et al., 2001; Jaenike, 2007). In one striking example, the Wolbachia strain wRec causes CI in its native host, Drosophila recens, but when introgressed into a sibling species, D. subquinaria, it causes male killing (Jaenike, 2007). Moreover in a natural hybrid zone between these same two species, unidirectional CI plays a major role in reducing interbreeding and thus contributes to reproductive isolation between these species (Jaenike et al., 2006; Shoemaker, Katju & Jaenike, 1999). Even though the link between Wolbachia and CI has been known for over 40 years (Yen & Barr, 1971), the mechanisms by which Wolbachia accomplishes its reproductive manipulations remain unknown.
Despite the physical isolation resulting from its intracellular lifestyle, Wolbachia in arthropods are replete with mobile DNA (Wu et al., 2004) including a temperate bacteriophage named WO (Kent & Bordenstein, 2010; Metcalf & Bordenstein, 2012; Masui et al., 2000). It has been speculated that WO may be involved in Wolbachia reproductive manipulations due to the prevalence of ankyrin repeat genes in its genome (Wu et al., 2004), the pervasiveness of phage-encoded bacterial virulence factors (Boyd, 2012), and the frequent occurrence of phage WO in arthropod Wolbachia strains (Gavotte et al., 2007). However, evidence not supportive of this hypothesis includes the observations that CI is inconsistently associated with the presence of phage WO genes (Sanogo, Eitam & Dobson, 2005; Saridaki et al., 2011) and that the penetrance of CI in Nasonia wasps is negatively correlated with densities of phage WO virions, as predicted by the phage density model (Bordenstein et al., 2006; Bordenstein & Bordenstein, 2011). Interestingly, PCR screening for the WO minor capsid gene specified WO’s absence in wRec (Bordenstein & Wernegreen, 2004), even though its closest relatives have large amounts of prophage DNA (Wu et al., 2004; Klasson et al., 2009). Thus, the absence of phage WO in wRec would be a critical example of a Wolbachia strain causing multiple reproductive phenotypes but lacking WO.
To investigate the apparent lack of prophage WO genes and alternative genetic mechanisms behind wRec’s diverse phenotypic influences, we sequenced the wRec genome using next-generation sequencing technology with partial finishing via Sanger sequencing. We determined that although wRec lacks the WO minor capsid gene typically used in diagnostic screens, it does contain a number of prophage WO genes. Thus, the possibility that WO influences Wolbachia reproductive manipulations cannot be eliminated, and those WO genes present in wRec offer a streamlined candidate list of the WO genes that could cause reproductive parasitism. Additionally, the availability of genomic information for a Wolbachia strain that is known to switch reproductive phenotypes will enable genomic, transcriptomic, and proteomic approaches to investigate the mechanisms behind these phenotypes.
Materials & Methods
The wRec genome sequencing reads and annotated contigs can be accessed from NCBI Bioproject PRJNA254527.
Wolbachia strain relatedness
Multi-locus sequence typing (MLST) genes were concatenated and a Bayesian phylogeny was inferred as previously described (Baldo et al., 2006). Briefly, selected fragments of MLST genes (coxA, gatB, fbpA, ftsZ, and hcpA) from Wolbachia strains with complete or nearly complete genome sequences were obtained from GenBank or the sequencing group’s online repositories, concatenated for a total length of 2,079 bp, and aligned with MUSCLE (Edgar, 2004). jModelTest 2 (Darriba et al., 2012) was used to determine the best model of evolution for the set of MLST haplotypes (GTR + I + G), and a Bayesian phylogeny was inferred using Mr. Bayes (Ronquist et al., 2012) with a chain length of 1,100,000, burn-in of 100,000, and subsampling frequency of 200.
Genome sequencing and assembly
DNA was extracted from a pool of 10 female Wolbachia infected Pittsford strain D. recens flies using a Puregene DNA purification kit (Qiagen, Venlo, Limburg). Shotgun sequencing of the wRec genome was conducted using an Illumina Hi-Seq (Vanderbilt Sequencing Core, Nashville, TN) with 100 bp paired end sequencing. Reads were filtered using five available Wolbachia genomes, wBm (Foster et al., 2005), wMel (Wu et al., 2004), wRi (Klasson et al., 2009), wOo (Darby et al., 2012), and wPip (Klasson et al., 2008), by mapping reads to these genomes with length and similarity fractions of 0.5 and keeping all mapped reads, using CLC Genomics Workbench version 6.0.4 (CLC Inc, Aarhus, Denmark). A de novo assembly with a length fraction of 0.5 and similarity fraction of 0.8 was then performed on filtered reads. Sequencing of whole wRec-infected D. recens females produced over 24 million reads, of which nearly 4% matched one or more previously sequenced Wolbachia genomes. De novo assembly of Wolbachia-filtered reads yielded 159 contigs. A de novo assembly of unfiltered reads was also performed and any contigs with a portion of its sequence matching contigs obtained from the filtered assembly were added to scaffolds in a search for novel genes. Separately, reads were mapped to the wMel genome with length and similarity fractions of 0.5 producing a rough consensus sequence to guide assembly of the de novo contigs into scaffolds, which were further refined with Sanger sequencing of PCR amplifications using primers designed to bind either end of putatively adjacent contigs to yield a final draft genome consisting of 43 scaffolds.
Annotation and comparative genomics
The wRec genome was annotated using MicroScope (Vallenet et al., 2009), supplemented with manual curation based on homology with wMel. A comparison of gene-gene identity between wMel and wRec was performed with a reciprocal best BLAST as previously described (Moreno-Hagelsieb & Latimer, 2008). For whole-genome alignments and analyses, wRec scaffolds were concatenated in the order in which the majority of their genes appear in wMel. Whole-genome alignment was performed with Mauve (Darling, Mau & Perna, 2010) and a circular genome plot was created with DNAPlotter (Carver et al., 2009). Manual annotations, BLAST searches, and sequence manipulation were performed with either CLC Genomics Workbench or Geneious V5.5.6 (Biomatters Ltd., Auckland, New Zealand). Ka/Ks rates and ratios were calculated using either single gene or concatenated whole genome CDS alignments with any alignments shorter than 30 amino acids removed (Buschiazzo et al., 2012), using the program DnaSP (Librado & Rozas, 2009). Genomic synteny was assessed with the Cloud Virtual Resource (CloVR) comparative pipeline (Angiuoli et al., 2011) and Sybil synteny gradient viewer (Riley et al., 2012) using the Data Intensive Academic Grid (DIAG) at the University of Maryland. The number of phage and phage-associated genes in Wolbachia genomes was determined based on current GenBank annotations and includes genes in the phage-packaged eukaryotic association module (S Bordenstein, unpublished data).
Results
Taxonomy of wRec
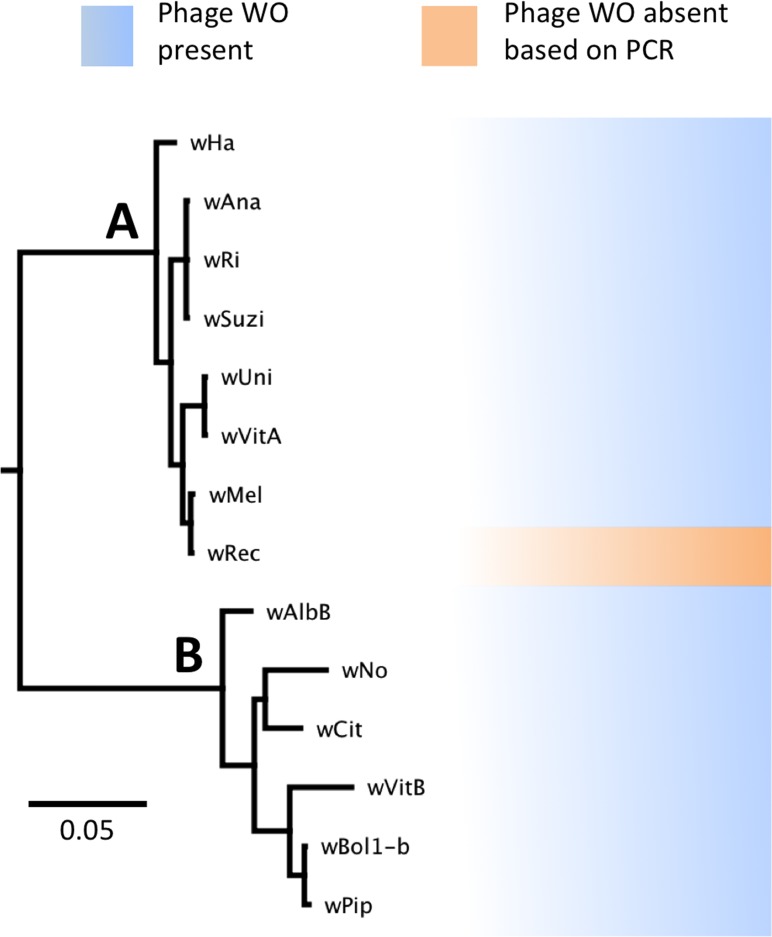
Phylogenetic analysis based on the concatenated multilocus sequence typing (MLST) genes (Baldo et al., 2006) confirms several previous reports that the supergroup A strain wRec is closely related to wMel (Baldo et al., 2006; Werren & Jaenike, 1995; Ioannidis et al., 2007; Gueguen, Onemola & Govind, 2012), a widespread strain infecting D. melanogaster (Fig. 1). In addition, phylogenetic analyses of each individual MLST gene support the same relationship of wMel as the closest sequenced relative to wRec (data not shown). To date, all sequenced Wolbachia genomes in supergroups A and B (Werren, Zhang & Guo, 1995), including wMel (Wu et al., 2004), have contained significant amounts of phage WO DNA. Thus the potential absence of WO in wRec was unexpected and precipitated the genomic analysis described below.
Figure 1. WO phage is present in all sequenced supergroup (A) and (B) Wolbachia strains.
A Bayesian phylogeny based on the concatenated Wolbachia multi-locus sequence typing genes is shown, consisting of selected strains with partial or full genome sequences and wRec. All branches had posterior probabilities of 99% or greater. While all previously sequenced Wolbachia strains in group (A) and (B) possess phage WO elements, wRec (indicated with arrow), was formerly thought to be phage-free.
Genome features of wRec with targeted reduction of prophage WO
Full sequencing statistics and an overview of wRec genome features are listed in Table 1. wRec scaffolds (N = 43) consisted of a total sequence length of 1,126,653 bp containing 1,271 protein coding sequences. 99.7% of all nucleotides in coding sequences shared between wRec and wMel were identical, indicating little divergence between these two closely related genomes despite occupying hosts that diverged >50 million years ago (Ross et al., 2003). Based on a previously established rate of synonymous substitution in Wolbachia of 0.9% per million years (Raychoudhury et al., 2009), the genome-wide percentage of synonymous substitution (0.314%) between wRec and wMel puts their divergence at approximately 350,000 years ago. There were 2,009 single nucleotide polymorphisms (SNPs) between shared coding genes in wMel and wRec, and 599 (29.8%) of these SNPs were synonymous with an average Ka/Ks ratio for each gene of 0.691. The vast majority of genes are highly conserved between wRec and wMel. More than 95% of orthologous gene pairs were 99% identical or greater and only ten gene pairs were less than 98% identical (Table 2). Most of these divergent genes code for hypothetical proteins and ankyrin repeat domain proteins. The wsp surface antigen, a known hypervariable sequence in Wolbachia (Zhou, Rousset & O’Neil, 1998), was also among the less conserved loci. All ten divergent genes contained insertions or deletions compared to wMel in addition to one or more SNPs. Interestingly, four of these divergent genes, two coding for hypothetical proteins, an Ovarian Tumor (OTU)-like cysteine protease, and wsp, had Ka/Ks ratios greater than one (Table 2), suggesting that they are evolving under positive selection, and the proteins they encode may be relevant to strain-specific host interactions. When these four genes were aligned to their homologs in wVitA, the closest relative of wMel and wRec, a roughly equal number of mutations in the OTU protease and wsp genes in each strain matched the sequence in wVitA. However, for the two hypothetical proteins WREC_0649 (WD_0722) and WREC_1268 (WD_1278), the wMel alleles matched wVitA in a majority of cases (18 out of 25 nucleotides and 49 out of 56 nucleotides, respectively), suggesting that the wMel variants were ancestral and that these wRec alleles experienced lineage-specific positive selection during D. recens infection.
Table 1. wRec sequencing and genome statistics.
| Reads | 24,633,972 |
| wRec reads | 955,730 (3.9%) |
| Contigs | 159 |
| Scaffolds | 43 |
| Average coverage | 76.5 |
| Genome size | >1,126,653 bp |
| GC content | 35.2% |
| CDS on scaffolds | 1271 |
| Average CDS length | 764 bp |
| Average intergenic length | 130 bp |
| Transfer RNA’s | 34 |
| Ribosomal RNA’s | 3 (23S, 16S, 5S) |
| Prophage regions | 2 |
Table 2. wMel genes with less than 98% nucleotide identity to their orthologs in wRec.
Genes with a Ka/Ks ratio greater than one are highlighted.
| wMel locus | wRec locus | Function | Pairwise identity (%) |
wRec length |
wMel length |
# SNPs | # Non-synonymous SNPs |
Ka/Ks ratio |
Other changes |
|---|---|---|---|---|---|---|---|---|---|
| WD_0294 | WREC_0283 | Ankyrin repeat domain protein |
89.4 | 1,815 | 1,626 | 4 | 4 | – | 189 bp insertion |
| WD_0443 | WREC_0442 | OTU-like cysteine protease |
97.1 | 927 | 906 | 7 | 6 | 1.59 | 21 bp insertion |
| WD_0550 | WREC_0541 | Ankyrin repeat domain protein *TM domains |
87.4 | 789 | 990 | 2 | 2 | – | 99 bp deletion, C-terminal frameshift, alternate start/stop sites |
| WD_0722 | WREC_0649 | Hypothetical protein *TM domains |
92.0 | 462 | 450 | 25 | 21 | 4.25 | 9 bp insertion, 3 bp insertion |
| WD_0996 | WREC_0956 | Transposase | 89.1 | 744 | 801 | 1 | 0 | 0 | alternate start site, transposase insertion |
| WD_1007 | WREC_0973 | Hypothetical protein |
95.1 | 366 | 351 | 3 | 2 | 0.42 | 15 bp insertion |
| WD_1039 | WREC_1007 | Collagen triple helix repeat protein |
97.5 | 405 | 1,425 | 1 | 1 | – | 9 bp insertion, scaffold break |
| WD_1063 | WREC_1036 | Wsp surface antigen |
97.9 | 708 | 714 | 9 | 8 | 2.55 | 6 bp deletion |
| WD_1278 | WREC_1268 | Hypothetical protein *TM Domain |
92.1 | 2,604 | 2,766 | 56 | 51 | 6.07 | 162 bp deletion |
| WD_1298 | WREC_1289 | RpoD | 97.2 | 1,974 | 1,929 | 10 | 6 | 0.39 | 18 bp insertion, 27 bp insertion |
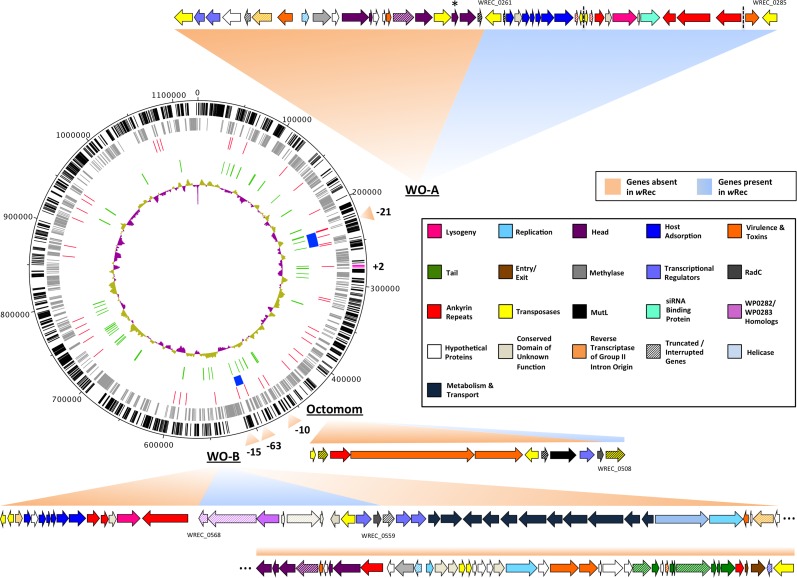
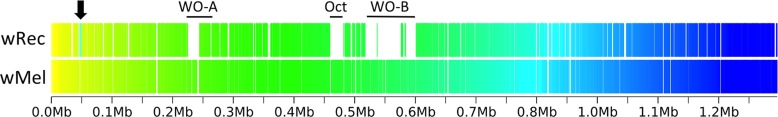
Interestingly, there were only two wRec genes without nucleotide homology to genes in wMel, even when contigs from a de novo assembly of raw host/Wolbachia reads were mapped to scaffolds in a search for additional genes. These two genes, WREC_0318 and WREC_0319, are hypothetical proteins with >95% nucleotide identity to sequences in two other Wolbachia strains, wRi and wHa. Meanwhile, wRec lacked any homologs of 43 wMel genes (Table S1), all but one of which are phage-related (phage genes discussed below). The single non-phage gene without homology in wRec is WD_0032, which codes for a hypothetical protein with 96% similarity to the C-terminus of an ankyrin repeat-containing siRNA binding protein in wRi. As is the case for many Wolbachia genomes, repetitive elements such as transposases and reverse transcriptases are abundant in wRec and have hampered closing of the genome. 77 such repetitive genes were found in wRec, and often appeared at the boundaries of scaffolds (Fig. 2). Although genomic rearrangement between the genomes cannot be completely assessed because the wRec genome is not closed, genes in wRec scaffolds were universally syntenic compared to wMel (Fig. 3), with the exception of a 5 kb region containing WD_0042–WD_0051 (WREC_0853–WREC_0863), consisting of repetitive transposases, reverse transcriptases, hypothetical proteins, and pseudogenes. This region would have been located on the first wRec scaffold if syntenic, but instead is on scaffold 31 (Fig. 3).
Figure 2. wRec genome comparison to wMel.
wRec scaffolds were concatenated in the order in which their genes appear in wMel to produce the circular genome above. Major regions of loss or gain compared to wMel are indicated outside the circle along with the number of genes involved. wRec genome features are indicated within the circle plot as follows (from outside-in): 1 (black): CDS in forward direction, and (magenta) genes not found in wMel; 2 (grey): CDS in reverse direction; 3 (red): scaffold break points; 4 (blue): WO regions; 5 (green): transposases and reverse transcriptases; 6 (purple/gold): GC content variation from average. WO prophage and related regions are shown and genes are categorized by color according to their likely functions and presence/absence in wRec. Locus tags for selected genes are indicated and dashed lines indicates breaks between scaffolds containing WO-A. The minor capsid gene of WO-A, which was used for prior PCR screens, is indicated with an asterisk.
Figure 3. Within-scaffold wRec synteny compared to wMel.
wRec scaffolds were concatenated in the order in which they appear in wMel and within-scaffold synteny was analyzed. Genes are graphed as tick marks colored on a gradient from yellow to blue from left to right with wMel as the reference genome and each wRec gene colored according to the location of its homolog in the wMel genome. White spaces in wRec alone indicate the absence of homologous genes or genes with multiple paralogs whose synteny cannot be established, while white spaces shared by both genomes indicate intergenic regions. A 5 kb region of rearrangement consisting of repetitive elements and hypothetical proteins is noted with an arrow, and phage-related regions are marked.
Prophage WO relics in the genome
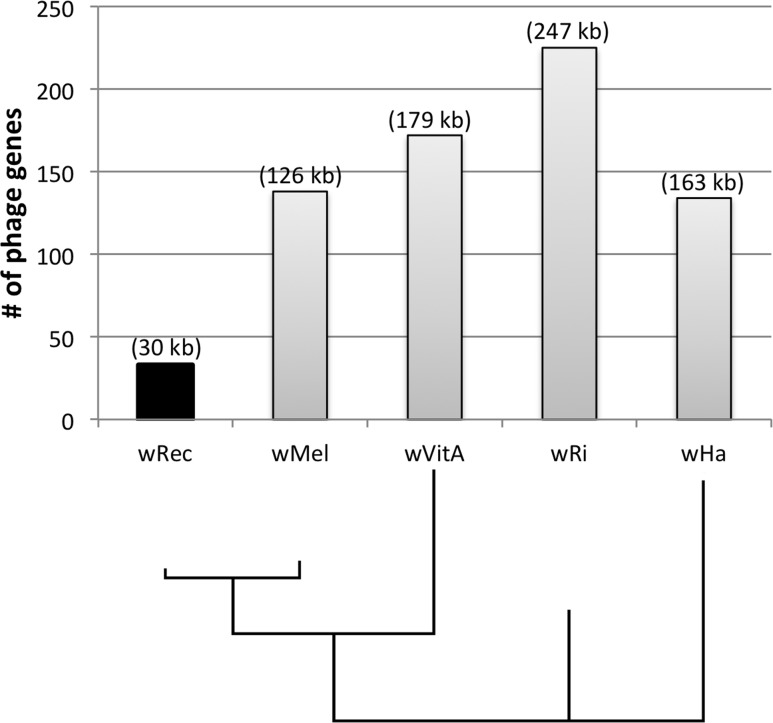
Whole-genome alignment of wRec and wMel revealed three major regions of genome reduction, with wRec lacking a large portion of both phage WO regions present in wMel as well as the entirety of the “Octomom” region (Chrostek et al., 2013) (Fig. 2), with only a bordering reverse transcriptase, WREC_0508 (wMel homolog WD_0506) present. Interestingly, although the minor capsid gene used in prior PCR surveys is absent, wRec does contain two major phage-related regions (Fig. 2). The first is a 19.2 kb region (WREC_0261–WREC_0285) across three scaffolds that is homologous to 21 contiguous genes of wMel WO-A (WD_0276–WD_0296). This region in wRec is syntenic and 99.4% identical to its homologous region in wMel, with two exceptions. The wRec homolog (WREC_0270/WREC_0274) of WD_0285, an ankyrin repeat protein, is fragmented by the insertion of two reverse transcriptases and a gap in the scaffolds, and there is an 189 bp insertion in WREC_0283 (WD_0294), another ankyrin repeat protein. If these two regions are included in the calculation of similarity, then the wRec WO-A phage region is 90.9% identical to the same region in wMel. The second wRec phage region contains 11.3 kb and 7 genes (WREC_0559–WREC_0568) that are syntenic and homologous to part of WO-B in wMel (WD_0625–WD_0632), with 99.5% pairwise identity. Two of these genes are interrupted by premature stop codons and the remaining fragments are annotated as smaller, separate genes. These genes include the orthologs of WD_0630, a hypothetical protein, which is split into three genes in wRec (WREC_0563–WREC_0565), and the ortholog of WD_0632, which is split into the 3,096 bp gene WREC_0567 and 468bp gene WREC_0568. While the head region of WO appears to be absent in wRec, the host adsorption module which is putatively involved in binding to the host surface during phage infection is largely intact, as are a number of ankyrin repeat genes, a transcriptional regulator, and the homologs of WP_02082/WP_0283, two genes in wPip recently proposed as candidate mediators of CI (Beckmann & Fallon, 2013). In summary, the markedly reduced number of phage genes in wRec (N = 33) is the signature feature of the genome compared to its closest relatives, which possess anywhere from 134 (wHa) to 225 (wRi) phage or phage-associated genes (Fig. 4).
Figure 4. Number of phage genes in wRec and its relatives.
The total number of prophage, phage-associated, and WO-like island genes in each Wolbachia genome is plotted above a Bayesian phylogeny of their MLST genes. The approximate total length of phage genes in each genome is noted above each bar.
Discussion
Divergence and genome reduction in wRec
Genome analysis revealed that wMel and wRec are very closely related with an average of 99.7% nucleotide identity in coding regions shared by the two strains. We estimate that wRec and wMel diverged around 350,000 years ago. This estimate raises an interesting biogeographical question: how could Wolbachia have been transferred at this time between the widely allopatric Nearctic D. recens and Afrotropical D. melanogaster? Perhaps a widespread Drosophila-generalist parasitoid played a role in vectoring this endosymbiont between host species, as parasitoid wasps have been previously demonstrated as vectors for Wolbachia transfer (Heath et al., 1999; Vavre, Mouton & Pannebakker, 2009). Molecular evidence suggests that the most recent Wolbachia sweep within D. recens occurred only 50,000 years ago, while D. subquinaria split from D. recens an estimated 600,000 years ago (Shoemaker, Katju & Jaenike, 1999). Thus, the divergence of wMel and wRec from their last common ancestor likely predated the most recent genetic sweep of D. recens, and wRec infected D. recens after its incipient divergence from D. subquinaria (Werren & Jaenike, 1995; Shoemaker, Katju & Jaenike, 1999). Remarkably, these results suggest that wRec may have contributed to reproductive isolation between these two species prior to the last glacial period 110,000–12,000 years ago, when their ranges are thought to have been allopatric (Jaenike et al., 2006). However, we note caution in interpreting the estimated divergence times as variability in mutation rates between bacterial lineages can skew the estimates.
The four wRec genes evolving under positive selection are of particular interest as they may be potential mediators of Wolbachia-host interactions (Table 2). Indeed, wsp is known to be involved in pathogenicity and host interaction (Uday & Puttaraju, 2012) while OTU-like cysteine proteases have deubiquitinase activity facilitating the pathogenicity of intracellular pathogens and viruses (Furtado et al., 2013; Makarova, Aravind & Koonin, 2000). Although the function of the hypothetical proteins is unknown, the presence of transmembrane (TM) domains suggests interaction with the bacterial membrane and potentially its Drosophila host. Additionally, it has previously been speculated that the elevated rate of mitochondrial DNA evolution in D. recens was due to hitchhiking in association with a series of positive selection events in its resident Wolbachia (Shoemaker et al., 2004).
The major difference between the wMel and wRec genomes was the incipient genome reduction of WO prophage regions. Remaining phage WO genes in wRec were often bordered or interrupted by transposases, suggesting that transposase activity may have been involved in the removal and degradation of major portions of WO genomes. Over 100 kb of genetic material, consisting mostly of phage-related genes, has likely been lost in wRec. Unlike the prophages found in wMel (Wu et al., 2004), all of wRec’s WO regions lacked the head genes thought to be necessary for mature virion formation (Metcalf & Bordenstein, 2012), including the orf7 minor capsid protein used in previous PCR tests for WO (Bordenstein & Wernegreen, 2004). The lack of such head genes suggests that wRec has lost the capacity to serve as a source of WO phage to infect other strains of Wolbachia. Future PCR screens may benefit from inclusion of more than one primer set, perhaps adding primers for a gene from the host adsorption module, which is highly conserved across WO prophages. However, it must be cautioned that the presence of multiple and variable degenerate WO haplotypes makes it impossible for any set of one or two primer pairs to detect all haplotypes.
Meanwhile, only 2,009 SNPs were present between the wMel and wRec genomes, indicating that gene deletion has been heavily favored over mutation. Such genome reduction is common in obligate intracellular bacteria, where many genes are expendable due to relaxed selection and there is limited contact with novel gene pools (Casadevall, 2008). Given the predatory nature of intact WO phages (Metcalf & Bordenstein, 2012; Bordenstein et al., 2006; Sanogo & Dobson, 2006), it may have been evolutionarily advantageous for wRec to eliminate the genes required for active phage production. It has been noted from TEM observations and quantitative studies that WO phage can lyse Wolbachia, resulting in an inverse correlation between bacterial and phage densities. Furthermore, because reproductive manipulations are dependent on a critical density of Wolbachia, high phage activity correlates with low expression of CI (Bordenstein et al., 2006). Since wRec exhibits high levels of CI in D. recens (Werren & Jaenike, 1995), while wMel shows lower levels of CI in D. melanogaster (Yamada et al., 2007), it is possible that wRec experiences a higher selective pressure to suppress phage, preserve high bacterial densities, and maintain compatibility with its host’s mating population. Thus, this interaction could be one possible explanation for the major loss of phage genes in wRec that are preserved in wMel.
Although Wolbachia has many more repetitive and mobile elements than most obligate intracellular bacteria (Bordenstein & Reznikoff, 2005) and frequently switches hosts on an evolutionary timescale (Vavre et al., 1999), it is worthwhile to note that there were only two genes in wRec that were not present in wMel. It is possible that these genes were lost in wMel after divergence from its last common ancestor with wRec.
The phage WO hypothesis to explain reproductive parasitism
Because the Octomom region was completely absent in wRec, it is unclear whether wRec lost these genes after diverging from wMel, or whether the genes were acquired by wMel after divergence with their last common ancestor. Given that Octomom is not widespread in supergroup A Wolbachia, the latter possibility is likely. Moreover, although the function of Octomom in reproductive parasitism is unknown, it seems reasonable to conclude that the Octomom region is not needed for reproductive manipulations, as it is completely absent from wRec. Additionally, given the association of Octomom with increased Wolbachia virulence, proliferation, and host viral protection (Chrostek et al., 2013), we would predict that wRec would not possess these phenotypes, and may be a useful strain for confirming these associations.
It is intriguing that some WO genes are conserved in wRec while others were lost. One explanation for their preservation in wRec is that the remaining genes improve Wolbachia fitness. Indeed, prophage sequences code for advantageous virulence factors in a wide array of bacterial species (Brussow, Canchaya & Hardt, 2004). Because previous PCR surveys suggested wRec did not possess phage WO, speculation that WO may be involved in Wolbachia reproductive manipulations has been largely disregarded (Bordenstein & Wernegreen, 2004). However, our sequencing shows that although the phage genomes are not complete, wRec contains many phage-related genes including some that could be involved in CI and/or male-killing. These include at least four ankyrin repeat proteins, whose repetitive domain has been long thought to facilitate Wolbachia-eukaryote interaction (Iturbe-Ormaetxe et al., 2005; Siozios et al., 2013). Additionally, several WO genes in wRec are homologs of genes recently implicated in CI. WREC_0560 is a transcriptional regulator with 88.3% identity at the amino acid level to wtrM in wPipMol, which increases expression of an important regulator of meiosis in Culex mosquitos and is postulated to be a component of the molecular mechanisms of CI (Pinto et al., 2013). WREC_0566–WREC_0568 meanwhile, are homologous to WP_0282 and WP_0283, two genes in wPip that have been implicated in CI due to presence in the proteome of Wolbachia-infected, fertilized mosquito spermathecae, along with their pattern of presence/absence in CI and non-CI strains (Beckmann & Fallon, 2013). Although the wRec homolog of WP_0283 has been truncated by 427 bp, it has 99.8% nucleotide identity to the gene in wMel (WD_0632) and an alternative reading frame enables the transcription of the remaining nucleotides in the same frame as the C-terminus of the homolog in wMel. Whether any of these WO genes are actually involved in Wolbachia host manipulations remains unclear, especially since it is unknown whether the remnants of phage WO are transcribed by wRec. However, the fact that these prophage regions are conserved suggests that they may have a role to play in the biology of Wolbachia.
WO host adsorption genes
In addition to preservation of some potential reproductive manipulation mediators, prophage WO genes WREC_0263–WREC_0269 contain an intact host adsorption module that includes baseplate genes thought to be involved in the binding of WO to its bacterial host and insertion of phage DNA. Indeed, this host adsorption module is nearly universal in WO prophage, with very few degenerate phage haplotypes lacking these genes (Kent et al., 2011). Many intracellular bacteria, including Wolbachia (Rances et al., 2008; Pichon et al., 2009), possess a type IV secretion system that secretes effectors into the host as a common strategy to subvert host-cell functions (Voth, Broederdorf & Graham, 2012). A number of Gram-negative bacteria also possess a phage-like type VI secretion system (Coulthurst, 2013); these include several obligatory intracellular bacterial pathogens, such as Anaplasma and Ehrlichia (Rikihisa & Lin, 2010). Structural analyses have shown this type of secretion system bears a remarkable resemblance to the spike protein of phages (Silverman et al., 2012). Given these similarities, and the fact that the WO host adsorption module is almost universally present in sequenced arthropod Wolbachia (Kent et al., 2011), it is possible that Wolbachia may be using these genes to facilitate host-microbe interactions, as a way to inject CI factors, genes, or other host manipulation particles into its host.
Future studies
The discovery of phage elements in wRec opens up new questions. Additional experiments will be needed to determine whether any conserved phage genes are involved in Wolbachia manipulations of its host biology. In addition, we have seen that using single gene markers of phage WO is not diagnostic of its absence. Thus, unsequenced Wolbachia strains that were thought to be phage-free by PCR assays need reevaluation. Another question that remains is how a single Wolbachia causes multipotent reproductive manipulations in different host backgrounds. The availability of genomic sequence for a multipotent Wolbachia strain will enable future transcriptomic and proteomic studies that could elucidate the genes involved in switching reproductive phenotypes.
Supplemental Information
Missing genes are defined as any wMel genes without BLASTn hits to assembled wRec scaffolds (E-value < 10−10) and also absent when raw wRec/host sequencing reads were mapped to these genes as reference templates.
Acknowledgments
We are grateful to Jennifer Wisecaver for technical assistance and to two reviewers for their helpful feedback.
Funding Statement
This work was supported by NIH R01GM085163 (SRB), NSF DEB1046149 (SRB), NSF DEB-0542094 (JJ), and NIH T32GM07347 (Vanderbilt Medical Scientist Training Program). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Jason A. Metcalf and Seth R. Bordenstein conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Minhee Jo performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Sarah R. Bordenstein performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
John Jaenike contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
NCBI Bioproject PRJNA254527.
References
- Angiuoli et al. (2011).Angiuoli SV, Matalka M, Gussman A, Galens K, Vangala M, Riley DR, Arze C, White JR, White O, Fricke WF. CloVR: a virtual machine for automated and portable sequence analysis from the desktop using cloud computing. BMC Bioinformatics. 2011;12:356. doi: 10.1186/1471-2105-12-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo et al. (2006).Baldo L, Dunning Hotopp JC, Jolley KA, Bordenstein SR, Biber SA, Choudhury RR, Hayashi C, Maiden MC, Tettelin H, Werren JH. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Applied and Environmental Microbiology. 2006;72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann & Fallon (2013).Beckmann JF, Fallon AM. Detection of the Wolbachia protein WPIP0282 in mosquito spermathecae: implications for cytoplasmic incompatibility. Insect Biochemistry and Molecular Biology. 2013;43:867–878. doi: 10.1016/j.ibmb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordenstein & Bordenstein (2011).Bordenstein SR, Bordenstein SR. Temperature affects the tripartite interactions between bacteriophage WO, Wolbachia, and cytoplasmic incompatibility. PLoS ONE. 2011;6:e529. doi: 10.1371/journal.pone.0029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordenstein et al. (2006).Bordenstein SR, Marshall ML, Fry AJ, Kim U, Wernegreen JJ. The tripartite associations between bacteriophage, Wolbachia, and arthropods. PLoS Pathogens. 2006;2:e529. doi: 10.1371/journal.ppat.0020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordenstein & Reznikoff (2005).Bordenstein SR, Reznikoff WS. Mobile DNA in obligate intracellular bacteria. Nature Reviews Microbiology. 2005;3:688–699. doi: 10.1038/nrmicro1233. [DOI] [PubMed] [Google Scholar]
- Bordenstein & Wernegreen (2004).Bordenstein SR, Wernegreen JJ. Bacteriophage flux in endosymbionts (Wolbachia): infection frequency, lateral transfer, and recombination rates. Molecular Biology and Evolution. 2004;21:1981–1991. doi: 10.1093/molbev/msh211. [DOI] [PubMed] [Google Scholar]
- Boyd (2012).Boyd EF. Bacteriophage-encoded bacterial virulence factors and phage-pathogenicity island interactions. Advances in Virus Research. 2012;82:91–118. doi: 10.1016/B978-0-12-394621-8.00014-5. [DOI] [PubMed] [Google Scholar]
- Brussow, Canchaya & Hardt (2004).Brussow H, Canchaya C, Hardt WD. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiology and Molecular Biology Reviews. 2004;68:560–602. doi: 10.1128/MMBR.68.3.560-602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschiazzo et al. (2012).Buschiazzo E, Ritland C, Bohlmann J, Ritland K. Slow but not low: genomic comparisons reveal slower evolutionary rate and higher dN/dS in conifers compared to angiosperms. BMC Evolutionary Biology. 2012;12:8. doi: 10.1186/1471-2148-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver et al. (2009).Carver T, Thomson N, Bleasby A, Berriman M, Parkhill J. DNAPlotter: circular and linear interactive genome visualization. Bioinformatics. 2009;25:119–120. doi: 10.1093/bioinformatics/btn578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall (2008).Casadevall A. Evolution of intracellular pathogens. Annual Review of Microbiology. 2008;62:19–33. doi: 10.1146/annurev.micro.61.080706.093305. [DOI] [PubMed] [Google Scholar]
- Chrostek et al. (2013).Chrostek E, Marialva MS, Esteves SS, Weinert LA, Martinez J, Jiggins FM, Teixeira L. Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genetics. 2013;9:e529. doi: 10.1371/journal.pgen.1003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulthurst (2013).Coulthurst SJ. The Type VI secretion system—a widespread and versatile cell targeting system. Research in Microbiology. 2013;164:640–654. doi: 10.1016/j.resmic.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Darby et al. (2012).Darby AC, Armstrong SD, Bah GS, Kaur G, Hughes MA, Kay SM, Koldkjaer P, Rainbow L, Radford AD, Blaxter ML, Tanya VN, Trees AJ, Cordaux R, Wastling JM, Makepeace BL. Analysis of gene expression from the Wolbachia genome of a filarial nematode supports both metabolic and defensive roles within the symbiosis. Genome Research. 2012;22:2467–2477. doi: 10.1101/gr.138420.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling, Mau & Perna (2010).Darling AE, Mau B, Perna NT. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE. 2010;5:e529. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba et al. (2012).Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar (2004).Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri et al. (2011).Ferri E, Bain O, Barbuto M, Martin C, Lo N, Uni S, Landmann F, Baccei SG, Guerrero R, De Souza Lima S, Bandi C, Wanji S, Diagne M, Casiraghi M. New insights into the evolution of Wolbachia infections in filarial nematodes inferred from a large range of screened species. PLoS ONE. 2011;6:e529. doi: 10.1371/journal.pone.0020843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster et al. (2005).Foster J, Ganatra M, Kamal I, Ware J, Makarova K, Ivanova N, Bhattacharyya A, Kapatral V, Kumar S, Posfai J, Vincze T, Ingram J, Moran L, Lapidus A, Omelchenko M, Kyrpides N, Ghedin E, Wang S, Goltsman E, Joukov V, Ostrovskaya O, Tsukerman K, Mazur M, Comb D, Koonin E, Slatko B. The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biology. 2005;3:e529. doi: 10.1371/journal.pbio.0030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii et al. (2001).Fujii Y, Kageyama D, Hoshizaki S, Ishikawa H, Sasaki T. Transfection of Wolbachia in Lepidoptera: the feminizer of the adzuki bean borer Ostrinia scapulalis causes male killing in the Mediterranean flour moth Ephestia kuehniella. Proceedings of the Royal Society B: Biological Sciences. 2001;268:855–859. doi: 10.1098/rspb.2001.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado et al. (2013).Furtado AR, Essid M, Perrinet S, Balana ME, Yoder N, Dehoux P, Subtil A. The chlamydial OTU domain-containing protein ChlaOTU is an early type III secretion effector targeting ubiquitin and NDP52. Cellular Microbiology. 2013;15:2064–2079. doi: 10.1111/cmi.12171. [DOI] [PubMed] [Google Scholar]
- Gavotte et al. (2007).Gavotte L, Henri H, Stouthamer R, Charif D, Charlat S, Bouletreau M, Vavre F. A Survey of the bacteriophage WO in the endosymbiotic bacteria Wolbachia. Molecular Biology and Evolution. 2007;24:427–435. doi: 10.1093/molbev/msl171. [DOI] [PubMed] [Google Scholar]
- Gueguen, Onemola & Govind (2012).Gueguen G, Onemola B, Govind S. Association of a new Wolbachia strain with, and its effects on, Leptopilina victoriae, a virulent wasp parasitic to Drosophila spp. Applied and Environmental Microbiology. 2012;78:5962–5966. doi: 10.1128/AEM.01058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath et al. (1999).Heath BD, Butcher RD, Whitfield WG, Hubbard SF. Horizontal transfer of Wolbachia between phylogenetically distant insect species by a naturally occurring mechanism. Current Biology. 1999;9:313–316. doi: 10.1016/S0960-9822(99)80139-0. [DOI] [PubMed] [Google Scholar]
- Ioannidis et al. (2007).Ioannidis P, Dunning Hotopp JC, Sapountzis P, Siozios S, Tsiamis G, Bordenstein SR, Baldo L, Werren JH, Bourtzis K. New criteria for selecting the origin of DNA replication in Wolbachia and closely related bacteria. BMC Genomics. 2007;8:182. doi: 10.1186/1471-2164-8-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturbe-Ormaetxe et al. (2005).Iturbe-Ormaetxe I, Burke GR, Riegler M, O’Neill SL. Distribution, expression, and motif variability of ankyrin domain genes in Wolbachia pipientis. Journal of Bacteriology. 2005;187:5136–5145. doi: 10.1128/JB.187.15.5136-5145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenike (2007).Jaenike J. Spontaneous emergence of a new Wolbachia phenotype. Evolution. 2007;61:2244–2252. doi: 10.1111/j.1558-5646.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- Jaenike et al. (2006).Jaenike J, Dyer KA, Cornish C, Minhas MS. Asymmetrical reinforcement and Wolbachia infection in Drosophila. PLoS Biology. 2006;4:e529. doi: 10.1371/journal.pbio.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent & Bordenstein (2010).Kent BN, Bordenstein SR. Phage WO of Wolbachia: lambda of the endosymbiont world. Trends in Microbiology. 2010;18:173–181. doi: 10.1016/j.tim.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent et al. (2011).Kent BN, Funkhouser LJ, Setia S, Bordenstein SR. Evolutionary genomics of a temperate bacteriophage in an obligate intracellular bacteria (Wolbachia) PLoS ONE. 2011;6:e529. doi: 10.1371/journal.pone.0024984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasson et al. (2008).Klasson L, Walker T, Sebaihia M, Sanders MJ, Quail MA, Lord A, Sanders S, Earl J, O’Neill SL, Thomson N, Sinkins SP, Parkhill J. Genome evolution of Wolbachia strain wPip from the Culex pipiens group. Molecular Biology and Evolution. 2008;25:1877–1887. doi: 10.1093/molbev/msn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasson et al. (2009).Klasson L, Westberg J, Sapountzis P, Naslund K, Lutnaes Y, Darby AC, Veneti Z, Chen L, Braig HR, Garrett R, Bourtzis K, Andersson SG. The mosaic genome structure of the Wolbachia wRi strain infecting Drosophila simulans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5725–5730. doi: 10.1073/pnas.0810753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librado & Rozas (2009).Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Makarova, Aravind & Koonin (2000).Makarova KS, Aravind L, Koonin EV. A novel superfamily of predicted cysteine proteases from eukaryotes, viruses and Chlamydia pneumoniae. Trends in Biochemical Sciences. 2000;25:50–52. doi: 10.1016/S0968-0004(99)01530-3. [DOI] [PubMed] [Google Scholar]
- Masui et al. (2000).Masui S, Kamoda S, Sasaki T, Ishikawa H. Distribution and evolution of bacteriophage WO in Wolbachia, the endosymbiont causing sexual alterations in arthropods. Journal of Molecular Evolution. 2000;51:491–497. doi: 10.1007/s002390010112. [DOI] [PubMed] [Google Scholar]
- Metcalf & Bordenstein (2012).Metcalf JA, Bordenstein SR. The complexity of virus systems: the case of endosymbionts. Current Opinion in Microbiology. 2012;15:546–552. doi: 10.1016/j.mib.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Hagelsieb & Latimer (2008).Moreno-Hagelsieb G, Latimer K. Choosing BLAST options for better detection of orthologs as reciprocal best hits. Bioinformatics. 2008;24:319–324. doi: 10.1093/bioinformatics/btm585. [DOI] [PubMed] [Google Scholar]
- Pichon et al. (2009).Pichon S, Bouchon D, Cordaux R, Chen L, Garrett RA, Greve P. Conservation of the type IV secretion system throughout Wolbachia evolution. Biochemical and Biophysical Research Communications. 2009;385:557–562. doi: 10.1016/j.bbrc.2009.05.118. [DOI] [PubMed] [Google Scholar]
- Pinto et al. (2013).Pinto SB, Stainton K, Harris S, Kambris Z, Sutton ER, Bonsall MB, Parkhill J, Sinkins SP. Transcriptional regulation of culex pipiens mosquitoes by Wolbachia influences cytoplasmic incompatibility. PLoS Pathogens. 2013;9:e529. doi: 10.1371/journal.ppat.1003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rances et al. (2008).Rances E, Voronin D, Tran-Van V, Mavingui P. Genetic and functional characterization of the type IV secretion system in Wolbachia. Journal of Bacteriology. 2008;190:5020–5030. doi: 10.1128/JB.00377-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychoudhury et al. (2009).Raychoudhury R, Baldo L, Oliveira DC, Werren JH. Modes of acquisition of Wolbachia: horizontal transfer, hybrid introgression, and codivergence in the Nasonia species complex. Evolution. 2009;63:165–183. doi: 10.1111/j.1558-5646.2008.00533.x. [DOI] [PubMed] [Google Scholar]
- Rikihisa & Lin (2010).Rikihisa Y, Lin M. Anaplasma phagocytophilum and Ehrlichia chaffeensis type IV secretion and Ank proteins. Current Opinion in Microbiology. 2010;13:59–66. doi: 10.1016/j.mib.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley et al. (2012).Riley DR, Angiuoli SV, Crabtree J, Dunning Hotopp JC, Tettelin H. Using Sybil for interactive comparative genomics of microbes on the web. Bioinformatics. 2012;28:160–166. doi: 10.1093/bioinformatics/btr652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist et al. (2012).Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross et al. (2003).Ross CL, Dyer KA, Erez T, Miller SJ, Jaenike J, Markow TA. Rapid divergence of microsatellite abundance among species of Drosophila. Molecular Biology and Evolution. 2003;20:1143–1157. doi: 10.1093/molbev/msg137. [DOI] [PubMed] [Google Scholar]
- Sanogo & Dobson (2006).Sanogo YO, Dobson SL. WO bacteriophage transcription in Wolbachia-infected Culex pipiens. Insect Biochemistry and Molecular Biology. 2006;36:80–85. doi: 10.1016/j.ibmb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Sanogo, Eitam & Dobson (2005).Sanogo YO, Eitam A, Dobson SL. No evidence for bacteriophage WO orf7 correlation with Wolbachia-induced cytoplasmic incompatibility in the Culex pipiens complex (Culicidae: Diptera) Journal of Medical Entomology. 2005;42:789–794. doi: 10.1603/0022-2585(2005)042[0789:NEFBWO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Saridaki et al. (2011).Saridaki A, Sapountzis P, Harris HL, Batista PD, Biliske JA, Pavlikaki H, Oehler S, Savakis C, Braig HR, Bourtzis K. Wolbachia prophage DNA adenine methyltransferase genes in different Drosophila-Wolbachia associations. PLoS ONE. 2011;6:e529. doi: 10.1371/journal.pone.0019708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker et al. (2004).Shoemaker DD, Dyer KA, Ahrens M, Mcabee K, Jaenike J. Decreased diversity but increased substitution rate in host mtDNA as a consequence of Wolbachia endosymbiont infection. Genetics. 2004;168:2049–2058. doi: 10.1534/genetics.104.030890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker, Katju & Jaenike (1999).Shoemaker DD, Katju V, Jaenike J. Wolbachia and the evolution of reproductive isolation between Drosophilla recens and Drosophila subquinaria. Evolution. 1999;53:1157–1164. doi: 10.2307/2640819. [DOI] [PubMed] [Google Scholar]
- Silverman et al. (2012).Silverman JM, Brunet YR, Cascales E, Mougous JD. Structure and regulation of the type VI secretion system. Annual Review of Microbiology. 2012;66:453–472. doi: 10.1146/annurev-micro-121809-151619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siozios et al. (2013).Siozios S, Ioannidis P, Klasson L, Andersson SG, Braig HR, Bourtzis K. The diversity and evolution of Wolbachia ankyrin repeat domain genes. PLoS ONE. 2013;8:e529. doi: 10.1371/journal.pone.0055390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uday & Puttaraju (2012).Uday J, Puttaraju HP. Comparative analysis of Wolbachia surface protein in D. melanoagster, A. tabida and B. malayi. Bioinformation. 2012;8:711–715. doi: 10.6026/97320630008711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallenet et al. (2009).Vallenet D, Engelen S, Mornico D, Cruveiller S, Fleury L, Lajus A, Rouy Z, Roche D, Salvignol G, Scarpelli C, Medigue C. MicroScope: a platform for microbial genome annotation and comparative genomics. Database. 2009;2009:bap021. doi: 10.1093/database/bap021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavre et al. (1999).Vavre F, Fleury F, Lepetit D, Fouillet P, Bouletreau M. Phylogenetic evidence for horizontal transmission of Wolbachia in host-parasitoid associations. Molecular Biology and Evolution. 1999;16:1711–1723. doi: 10.1093/oxfordjournals.molbev.a026084. [DOI] [PubMed] [Google Scholar]
- Vavre, Mouton & Pannebakker (2009).Vavre F, Mouton L, Pannebakker BA. Drosophila-parasitoid communities as model systems for host-Wolbachia interactions. Advances in Parasitology. 2009;70:299–331. doi: 10.1016/S0065-308X(09)70012-0. [DOI] [PubMed] [Google Scholar]
- Voth, Broederdorf & Graham (2012).Voth DE, Broederdorf LJ, Graham JG. Bacterial type IV secretion systems: versatile virulence machines. Future Microbiology. 2012;7:241–257. doi: 10.2217/fmb.11.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren, Baldo & Clark (2008).Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nature Reviews Microbiology. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- Werren & Jaenike (1995).Werren JH, Jaenike J. Wolbachia and cytoplasmic incompatibility in mycophagous Drosophila and their relatives. Heredity. 1995;75(Pt 3):320–326. doi: 10.1038/hdy.1995.140. [DOI] [PubMed] [Google Scholar]
- Werren, Zhang & Guo (1995).Werren JH, Zhang W, Guo LR. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proceedings of the Royal Society B: Biological Sciences. 1995;261:55–63. doi: 10.1098/rspb.1995.0117. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2004).Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, Brownlie JC, Mcgraw EA, Martin W, Esser C, Ahmadinejad N, Wiegand C, Madupu R, Beanan MJ, Brinkac LM, Daugherty SC, Durkin AS, Kolonay JF, Nelson WC, Mohamoud Y, Lee P, Berry K, Young MB, Utterback T, Weidman J, Nierman WC, Paulsen IT, Nelson KE, Tettelin H, O’Neill SL, Eisen JA. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biology. 2004;2:e529. doi: 10.1371/journal.pbio.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada et al. (2007).Yamada R, Floate KD, Riegler M, O’Neill SL. Male development time influences the strength of Wolbachia-induced cytoplasmic incompatibility expression in Drosophila melanogaster. Genetics. 2007;177:801–808. doi: 10.1534/genetics.106.068486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen & Barr (1971).Yen JH, Barr AR. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature. 1971;232:657–658. doi: 10.1038/232657a0. [DOI] [PubMed] [Google Scholar]
- Zhou, Rousset & O’Neil (1998).Zhou W, Rousset F, O’Neil S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proceedings of the Royal Society B: Biological Sciences. 1998;265:509–515. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zug & Hammerstein (2012).Zug R, Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE. 2012;7:e529. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Missing genes are defined as any wMel genes without BLASTn hits to assembled wRec scaffolds (E-value < 10−10) and also absent when raw wRec/host sequencing reads were mapped to these genes as reference templates.