Abstract
We have characterized the molecular nature of mutations in wingless (wg), a segment polarity gene acting during various stages of Drosophila development. Embryo-lethal alleles have undergone mutations in the protein-encoding domain of the gene, including deletions and point mutations of conserved residues. In a temperature sensitive mutation, a conserved cysteine residue is replaced by a serine. In embryo-viable alleles, the wg transcriptional unit is not affected. Immunostaining of mutant embryos shows that the embryo-lethal alleles produce either no wg antigen or a form of the protein that is retained within cells. Interestingly, embryos mutant for the segment polarity gene porcupine show a similar retention of the wg antigen. We have also transfected wild type wg alleles into Drosophila tissue culture cells, which then display wg protein on the cell surface and in the extracellular matrix. In similar experiments with mutant alleles, the proteins are retained in intracellular compartments and appear not to be secreted. These data provide further evidence that wg acts as a secreted factor and suggest that porcupine provides an accessory function for wg protein secretion or transport.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
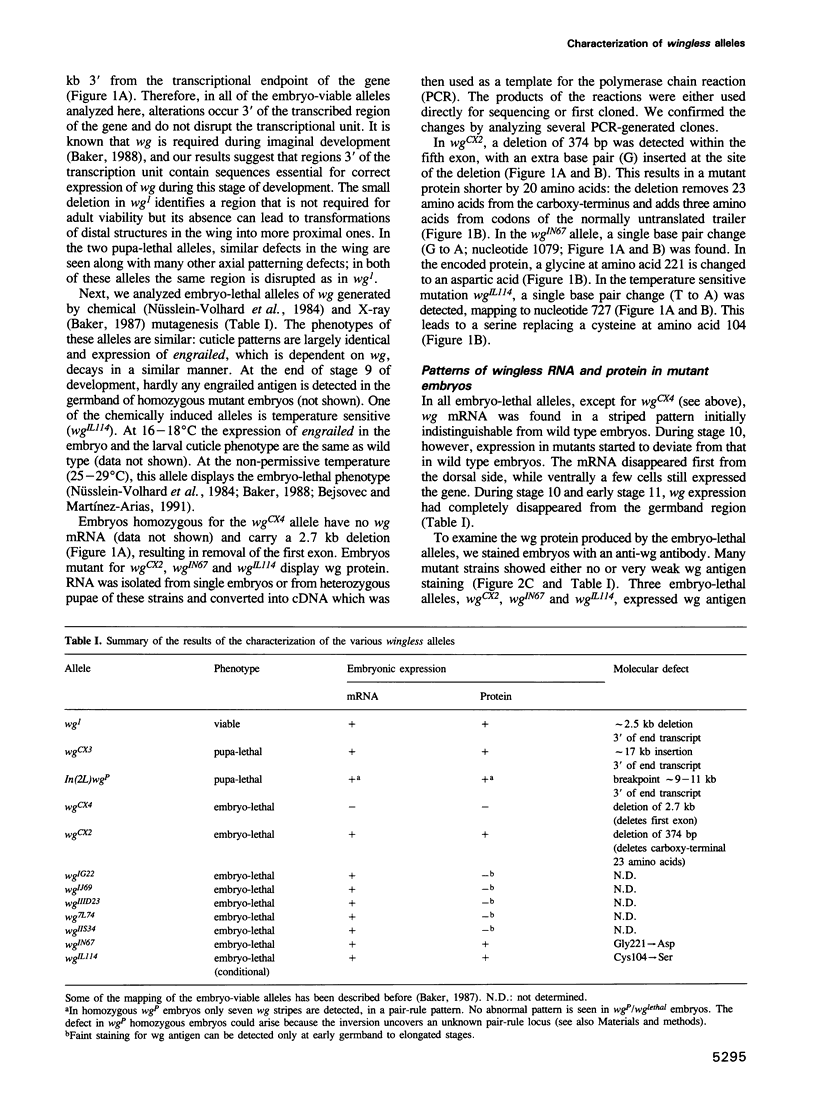
- Baker N. E. Embryonic and imaginal requirements for wingless, a segment-polarity gene in Drosophila. Dev Biol. 1988 Jan;125(1):96–108. doi: 10.1016/0012-1606(88)90062-0. [DOI] [PubMed] [Google Scholar]
- Baker N. E. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J. 1987 Jun;6(6):1765–1773. doi: 10.1002/j.1460-2075.1987.tb02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejsovec A., Martinez Arias A. Roles of wingless in patterning the larval epidermis of Drosophila. Development. 1991 Oct;113(2):471–485. doi: 10.1242/dev.113.2.471. [DOI] [PubMed] [Google Scholar]
- Bradley R. S., Brown A. M. The proto-oncogene int-1 encodes a secreted protein associated with the extracellular matrix. EMBO J. 1990 May;9(5):1569–1575. doi: 10.1002/j.1460-2075.1990.tb08276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Papkoff J., Fung Y. K., Shackleford G. M., Varmus H. E. Identification of protein products encoded by the proto-oncogene int-1. Mol Cell Biol. 1987 Nov;7(11):3971–3977. doi: 10.1128/mcb.7.11.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Wildin R. S., Prendergast T. J., Varmus H. E. A retrovirus vector expressing the putative mammary oncogene int-1 causes partial transformation of a mammary epithelial cell line. Cell. 1986 Sep 26;46(7):1001–1009. doi: 10.1016/0092-8674(86)90699-9. [DOI] [PubMed] [Google Scholar]
- Chakrabarti A., Matthews G., Colman A., Dale L. Secretory and inductive properties of Drosophila wingless protein in Xenopus oocytes and embryos. Development. 1992 May;115(1):355–369. doi: 10.1242/dev.115.1.355. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Di Nardo S. Wingless: from embryo to adult. Trends Genet. 1993 Jun;9(6):189–192. doi: 10.1016/0168-9525(93)90112-u. [DOI] [PubMed] [Google Scholar]
- Couso J. P., Bate M., Martínez-Arias A. A wingless-dependent polar coordinate system in Drosophila imaginal discs. Science. 1993 Jan 22;259(5094):484–489. doi: 10.1126/science.8424170. [DOI] [PubMed] [Google Scholar]
- DiNardo S., Sher E., Heemskerk-Jongens J., Kassis J. A., O'Farrell P. H. Two-tiered regulation of spatially patterned engrailed gene expression during Drosophila embryogenesis. Nature. 1988 Apr 14;332(6165):604–609. doi: 10.1038/332604a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- González F., Swales L., Bejsovec A., Skaer H., Martinez Arias A. Secretion and movement of wingless protein in the epidermis of the Drosophila embryo. Mech Dev. 1991 Aug;35(1):43–54. doi: 10.1016/0925-4773(91)90040-d. [DOI] [PubMed] [Google Scholar]
- Jue S. F., Bradley R. S., Rudnicki J. A., Varmus H. E., Brown A. M. The mouse Wnt-1 gene can act via a paracrine mechanism in transformation of mammary epithelial cells. Mol Cell Biol. 1992 Jan;12(1):321–328. doi: 10.1128/mcb.12.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajewski J., Mason J. O., Varmus H. E. Interaction of Wnt-1 proteins with the binding protein BiP. Mol Cell Biol. 1992 Feb;12(2):784–790. doi: 10.1128/mcb.12.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingensmith J., Noll E., Perrimon N. The segment polarity phenotype of Drosophila involves differential tendencies toward transformation and cell death. Dev Biol. 1989 Jul;134(1):130–145. doi: 10.1016/0012-1606(89)90084-5. [DOI] [PubMed] [Google Scholar]
- Koelle M. R., Talbot W. S., Segraves W. A., Bender M. T., Cherbas P., Hogness D. S. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell. 1991 Oct 4;67(1):59–77. doi: 10.1016/0092-8674(91)90572-g. [DOI] [PubMed] [Google Scholar]
- Krasnow M. A., Saffman E. E., Kornfeld K., Hogness D. S. Transcriptional activation and repression by Ultrabithorax proteins in cultured Drosophila cells. Cell. 1989 Jun 16;57(6):1031–1043. doi: 10.1016/0092-8674(89)90341-3. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Transport of secretory and membrane glycoproteins from the rough endoplasmic reticulum to the Golgi. A rate-limiting step in protein maturation and secretion. J Biol Chem. 1988 Feb 15;263(5):2107–2110. [PubMed] [Google Scholar]
- Lopez A. R., Cook J., Deininger P. L., Derynck R. Dominant negative mutants of transforming growth factor-beta 1 inhibit the secretion of different transforming growth factor-beta isoforms. Mol Cell Biol. 1992 Apr;12(4):1674–1679. doi: 10.1128/mcb.12.4.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martizez Arias A., Baker N. E., Ingham P. W. Role of segment polarity genes in the definition and maintenance of cell states in the Drosophila embryo. Development. 1988 May;103(1):157–170. doi: 10.1242/dev.103.1.157. [DOI] [PubMed] [Google Scholar]
- Mason J. O., Kitajewski J., Varmus H. E. Mutational analysis of mouse Wnt-1 identifies two temperature-sensitive alleles and attributes of Wnt-1 protein essential for transformation of a mammary cell line. Mol Biol Cell. 1992 May;3(5):521–533. doi: 10.1091/mbc.3.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A. P., Moon R. T. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989 Sep 22;58(6):1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- Mercola M., Deininger P. L., Shamah S. M., Porter J., Wang C. Y., Stiles C. D. Dominant-negative mutants of a platelet-derived growth factor gene. Genes Dev. 1990 Dec;4(12B):2333–2341. doi: 10.1101/gad.4.12b.2333. [DOI] [PubMed] [Google Scholar]
- Morata G., Lawrence P. A. The development of wingless, a homeotic mutation of Drosophila. Dev Biol. 1977 Apr;56(2):227–240. doi: 10.1016/0012-1606(77)90266-4. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Wnt genes. Cell. 1992 Jun 26;69(7):1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980 Oct 30;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Brown A. M., Varmus H. E. The int-1 proto-oncogene products are glycoproteins that appear to enter the secretory pathway. Mol Cell Biol. 1987 Nov;7(11):3978–3984. doi: 10.1128/mcb.7.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J. Inducible overexpression and secretion of int-1 protein. Mol Cell Biol. 1989 Aug;9(8):3377–3384. doi: 10.1128/mcb.9.8.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Schryver B. Secreted int-1 protein is associated with the cell surface. Mol Cell Biol. 1990 Jun;10(6):2723–2730. doi: 10.1128/mcb.10.6.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N., Engstrom L., Mahowald A. P. The effects of zygotic lethal mutations on female germ-line functions in Drosophila. Dev Biol. 1984 Oct;105(2):404–414. doi: 10.1016/0012-1606(84)90297-5. [DOI] [PubMed] [Google Scholar]
- Riggleman B., Schedl P., Wieschaus E. Spatial expression of the Drosophila segment polarity gene armadillo is posttranscriptionally regulated by wingless. Cell. 1990 Nov 2;63(3):549–560. doi: 10.1016/0092-8674(90)90451-j. [DOI] [PubMed] [Google Scholar]
- Rijsewijk F., Schuermann M., Wagenaar E., Parren P., Weigel D., Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987 Aug 14;50(4):649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- Rijsewijk F., van Deemter L., Wagenaar E., Sonnenberg A., Nusse R. Transfection of the int-1 mammary oncogene in cuboidal RAC mammary cell line results in morphological transformation and tumorigenicity. EMBO J. 1987 Jan;6(1):127–131. doi: 10.1002/j.1460-2075.1987.tb04729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J., Gennissen A., Nusse R. Isolation and expression of two novel Wnt/wingless gene homologues in Drosophila. Development. 1992 Jun;115(2):475–485. doi: 10.1242/dev.115.2.475. [DOI] [PubMed] [Google Scholar]
- Sharma R. P., Chopra V. L. Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev Biol. 1976 Feb;48(2):461–465. doi: 10.1016/0012-1606(76)90108-1. [DOI] [PubMed] [Google Scholar]
- Struhl G., Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993 Feb 26;72(4):527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Struhl G., Johnston P., Lawrence P. A. Control of Drosophila body pattern by the hunchback morphogen gradient. Cell. 1992 Apr 17;69(2):237–249. doi: 10.1016/0092-8674(92)90405-2. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Vavra S. H., Carroll S. B. The zygotic control of Drosophila pair-rule gene expression. I. A search for new pair-rule regulatory loci. Development. 1989 Nov;107(3):663–672. doi: 10.1242/dev.107.3.663. [DOI] [PubMed] [Google Scholar]
- Wollner D. A., Krzeminski K. A., Nelson W. J. Remodeling the cell surface distribution of membrane proteins during the development of epithelial cell polarity. J Cell Biol. 1992 Feb;116(4):889–899. doi: 10.1083/jcb.116.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M., Nusse R., Johnston P., Lawrence P. A. Distribution of the wingless gene product in Drosophila embryos: a protein involved in cell-cell communication. Cell. 1989 Nov 17;59(4):739–749. doi: 10.1016/0092-8674(89)90020-2. [DOI] [PubMed] [Google Scholar]







