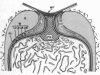
Full text
PDF



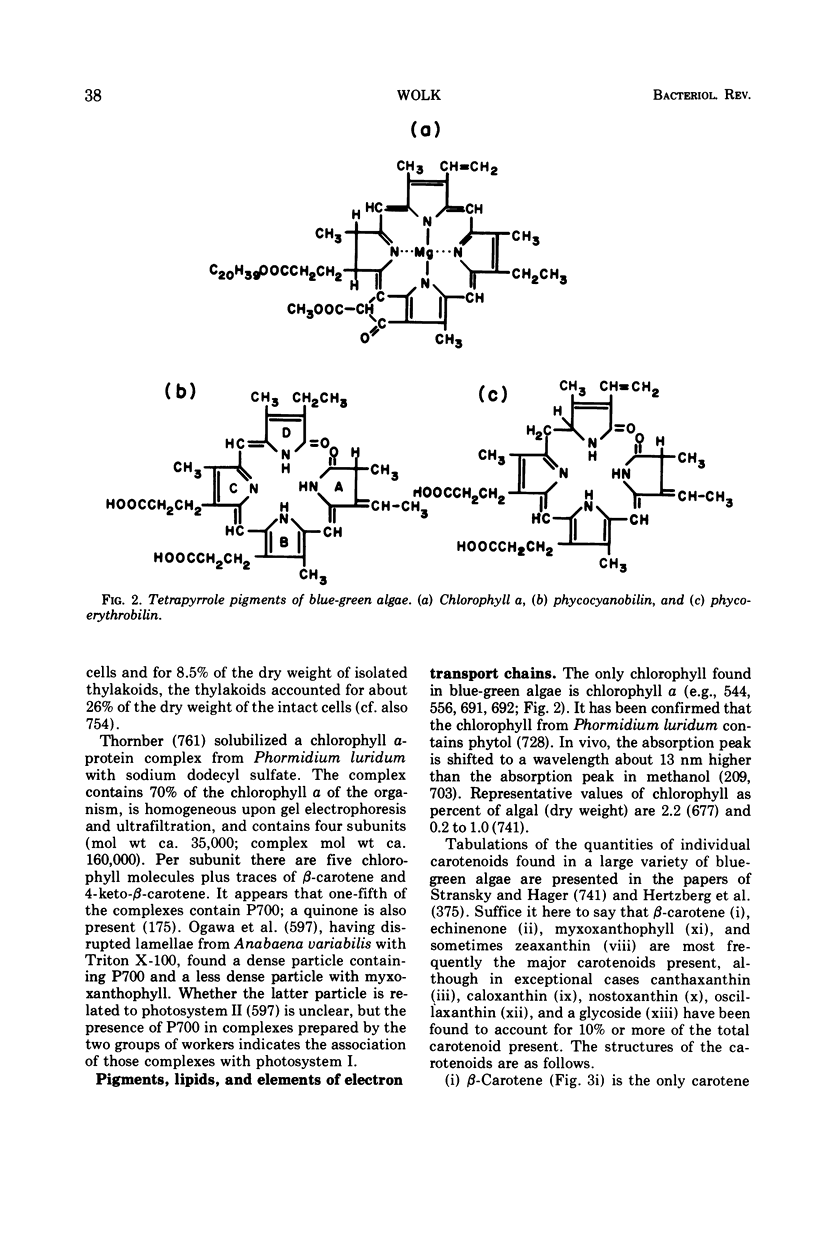
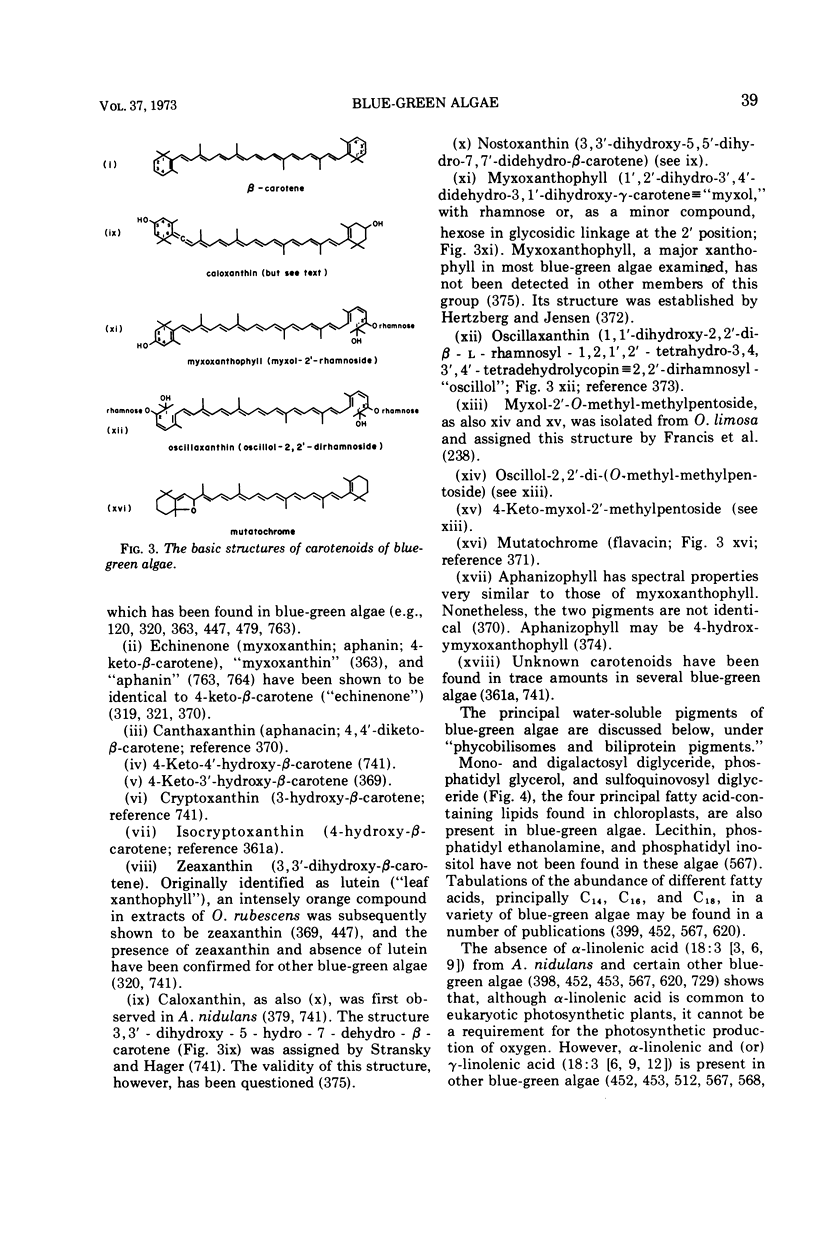
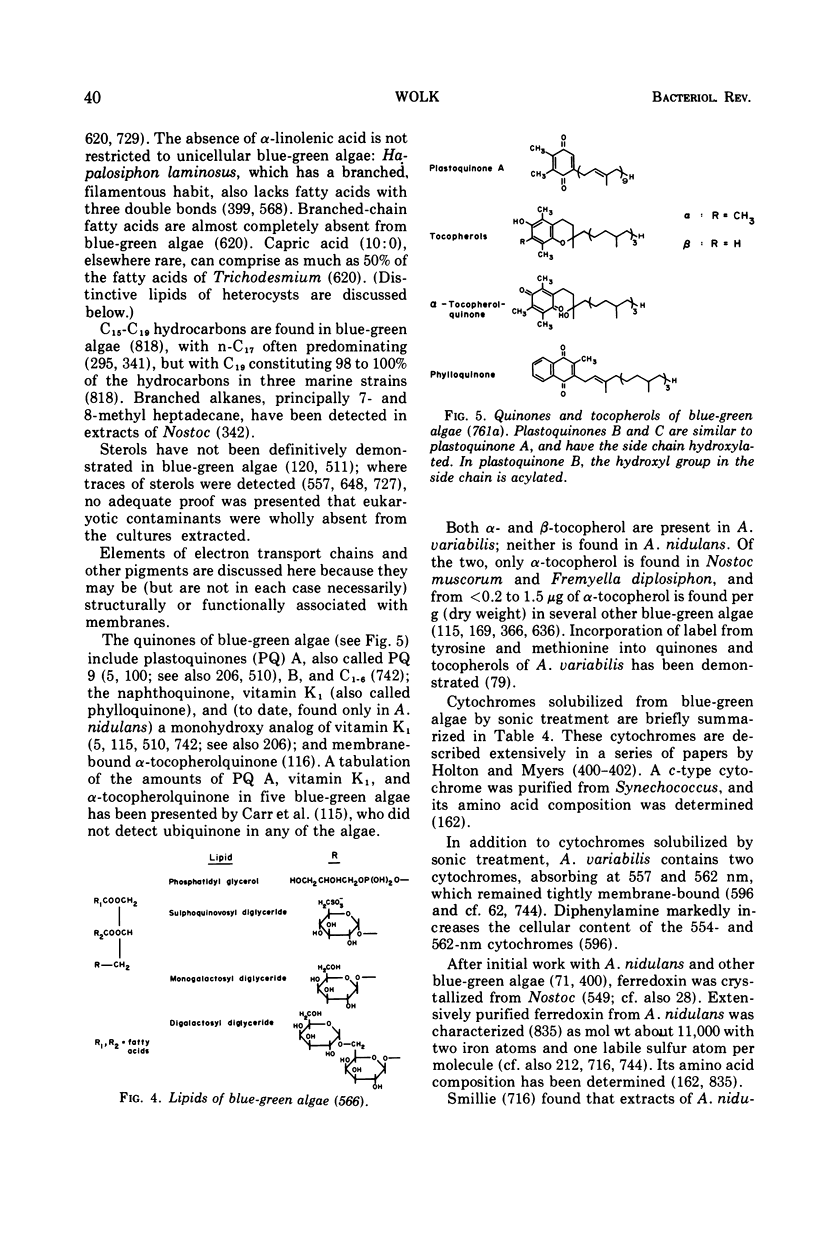


























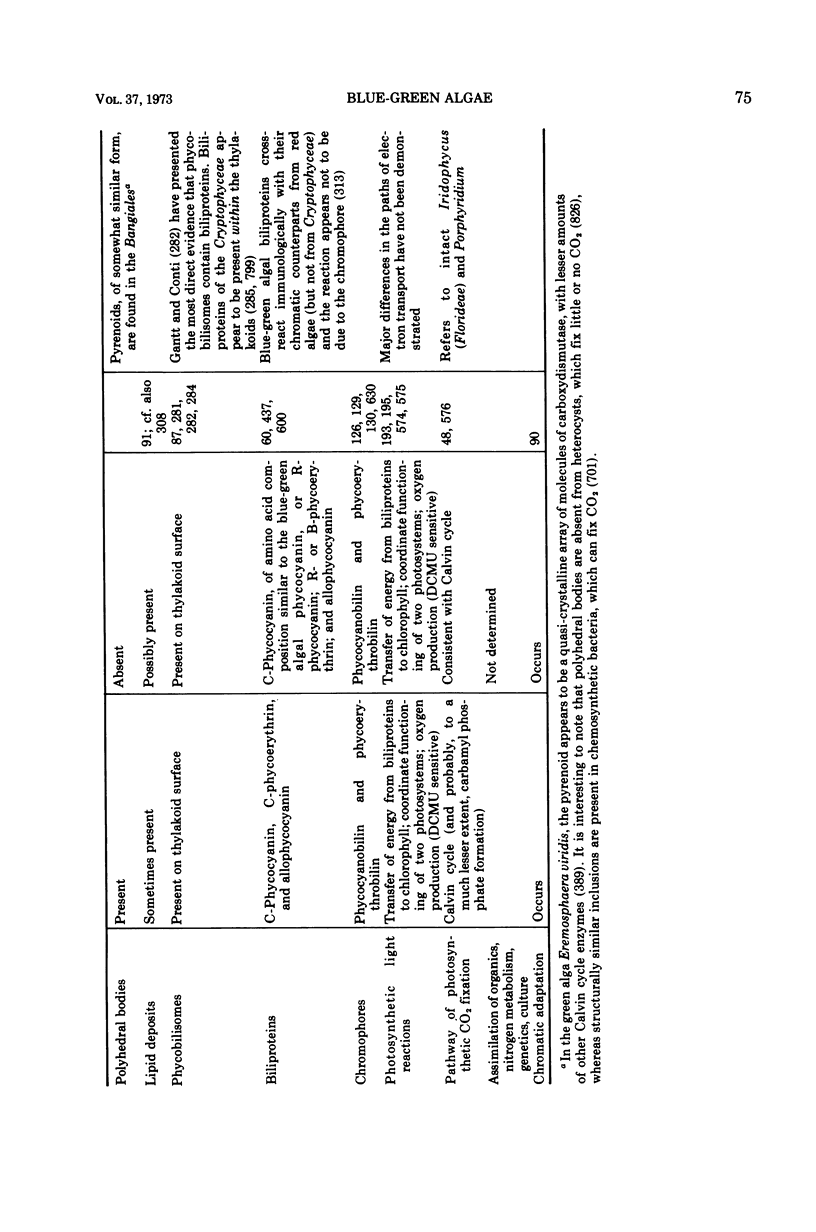


























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON R. K., SKIPPER H. E., REID M. R., SHORT W. A., HOGAN G. L. Studies on the photosynthetic reaction. I. The assimilation of acetate by Nostoc muscorum. J Biol Chem. 1953 Sep;204(1):197–205. [PubMed] [Google Scholar]
- AMBROSE E. J. The movements of fibrocytes. Exp Cell Res. 1961;Suppl 8:54–73. doi: 10.1016/0014-4827(61)90340-8. [DOI] [PubMed] [Google Scholar]
- AMESZ J., DUYSENS L. N. Action spectrum, kinetics and quantum requirement of phosphopyridine nucleotide reduction and cytochrome oxidation in the blue-green alga Anacystis nidulans. Biochim Biophys Acta. 1962 Oct 22;64:261–278. doi: 10.1016/0006-3002(62)90736-9. [DOI] [PubMed] [Google Scholar]
- ARNOLD W., OPPENHEIMER J. R. Internal conversion in the photosynthetic mechanism of blue-green algae. J Gen Physiol. 1950 Mar;33(4):423–435. doi: 10.1085/jgp.33.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph K. W., Haselkorn R. Comparison of the structures of blue-green algal viruses LPP-1M and LPP-2 and bacteriophage T7. Virology. 1972 Mar;47(3):701–710. doi: 10.1016/0042-6822(72)90560-0. [DOI] [PubMed] [Google Scholar]
- Adolph K. W., Haselkorn R. Isolation and characterization of a virus infecting the blue-green alga Nostoc muscorum. Virology. 1971 Nov;46(2):200–208. doi: 10.1016/0042-6822(71)90023-7. [DOI] [PubMed] [Google Scholar]
- Adolph K. W., Haselkorn R. Photosynthesis and the development of blue-green algal virus N-1. Virology. 1972 Feb;47(2):370–374. doi: 10.1016/0042-6822(72)90272-3. [DOI] [PubMed] [Google Scholar]
- Allen C. F., Franke H., Hirayama O. Identification of a plastoquinone and two naphthoquinones in Anacystis nidulans by NMR and mass spectroscopy. Biochem Biophys Res Commun. 1967 Mar 9;26(5):562–568. doi: 10.1016/0006-291x(67)90102-7. [DOI] [PubMed] [Google Scholar]
- Allen M. B., Arnon D. I. Studies on Nitrogen-Fixing Blue-Green Algae. I. Growth and Nitrogen Fixation by Anabaena Cylindrica Lemm. Plant Physiol. 1955 Jul;30(4):366–372. doi: 10.1104/pp.30.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. M. Photosynthetic membrane system in Anacystis nidulans. J Bacteriol. 1968 Sep;96(3):836–841. doi: 10.1128/jb.96.3.836-841.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. M., Smith A. J. Nitrogen chlorosis in blue-green algae. Arch Mikrobiol. 1969;69(2):114–120. doi: 10.1007/BF00409755. [DOI] [PubMed] [Google Scholar]
- Allen M. M., Stanier R. Y. Selective isolation of blue-green algae from water and soil. J Gen Microbiol. 1968 Apr;51(2):203–209. doi: 10.1099/00221287-51-2-203. [DOI] [PubMed] [Google Scholar]
- Allen M. M. Ultrastructure of the cell wall and cell division of unicellular blue-green algae. J Bacteriol. 1968 Sep;96(3):842–852. doi: 10.1128/jb.96.3.842-852.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison F. E., Morris H. J. NITROGEN FIXATION BY BLUE-GREEN ALGAE. Science. 1930 Feb 21;71(1834):221–223. doi: 10.1126/science.71.1834.221-a. [DOI] [PubMed] [Google Scholar]
- Allison R. K., Skipper H. E., Reid M. R., Short W. A., Hogan G. L. Studies on the Photosynthetic Reaction. II. Sodium Formate and Urea Feeding Experiments with Nostoc muscorum. Plant Physiol. 1954 Mar;29(2):164–168. doi: 10.1104/pp.29.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp A. Germination of hormocysts of Scytonema javanicum and the function of blue-green algal heterocysts. Nature. 1968 Nov 23;220(5169):810–810. doi: 10.1038/220810a0. [DOI] [PubMed] [Google Scholar]
- Anderson L. A., Smillie R. M. Binding of chloramphenicol by ribosomes from chloroplasts. Biochem Biophys Res Commun. 1966 May 25;23(4):535–539. doi: 10.1016/0006-291x(66)90762-5. [DOI] [PubMed] [Google Scholar]
- Ankel H., Ankel E., Feingold D. S., Schutzbach J. S. Formation of UDP-D-xylose in algae. Biochim Biophys Acta. 1967 Feb 7;136(1):172–175. doi: 10.1016/0304-4165(67)90339-x. [DOI] [PubMed] [Google Scholar]
- Ankel H., Tischer R. G. UDP-D-glucuronate 4-epimerase in blue-green algae. Biochim Biophys Acta. 1969 Apr 22;178(2):415–419. doi: 10.1016/0005-2744(69)90416-1. [DOI] [PubMed] [Google Scholar]
- Appleby R. S., Safford R., Nichols B. W. The involvement of lecithin and monogalactosyl diglyceride in linoleate synthesis by green and blue-green algae. Biochim Biophys Acta. 1971 Nov 5;248(2):205–211. doi: 10.1016/0005-2760(71)90008-7. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. Ferredoxin and photosynthesis. Science. 1965 Sep 24;149(3691):1460–1470. doi: 10.1126/science.149.3691.1460. [DOI] [PubMed] [Google Scholar]
- Asato Y., Folsome C. E. Temporal genetic mapping of the blue-green alga, Anacystis nidulans. Genetics. 1970 Jul;65(3):407–419. doi: 10.1093/genetics/65.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAN R. C., HASSID W. Z. Assimilation of C14O2 by a photosynthesizing red alga, Iridophycus flaccidum. J Biol Chem. 1955 Jan;212(1):411–425. [PubMed] [Google Scholar]
- BEN-GURION R. Aminopterin as an inducing agent in bacteriocinogenic bacteria. Nature. 1962 Dec 15;196:1121–1121. doi: 10.1038/1961121a0. [DOI] [PubMed] [Google Scholar]
- BERNS D. S., SCOTT E., O'REILLY K. T. C-PHYCOCYANIN: MINIMUM MOLECULAR WEIGHT. Science. 1964 Sep 4;145(3636):1054–1056. doi: 10.1126/science.145.3636.1054. [DOI] [PubMed] [Google Scholar]
- BIRDSEY E. C., LYNCH V. H. Utilization of nitrogen compounds by unicellular algae. Science. 1962 Sep 7;137(3532):763–764. doi: 10.1126/science.137.3532.763. [DOI] [PubMed] [Google Scholar]
- BISWAS B. B., MYERS J. A methyl cytidine from the ribonucleic acid of Anacystis nidulans. Nature. 1960 Apr 16;186:238–239. doi: 10.1038/186238a0. [DOI] [PubMed] [Google Scholar]
- BLACK C. C., FEWSON C. A., GIBBS M. Photochemical reduction of triphosphopyridine nucleotide by cell-free extracts of blue-green algae. Nature. 1963 Apr 6;198:88–88. doi: 10.1038/198088a0. [DOI] [PubMed] [Google Scholar]
- BLACK C. C., SAN PIETRO A., LIMBACH D., NORRIS G. Photosynthetic phosphorylation catalyzed by factors isolated from photosynthetic organisms. Proc Natl Acad Sci U S A. 1963 Jul;50:37–43. doi: 10.1073/pnas.50.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODY M., VATTER A. E. Observations on cellular structures of Porphyridium cruentum. J Biophys Biochem Cytol. 1959 Mar 25;5(2):289–294. doi: 10.1083/jcb.5.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUNT J. S. Isolation of bacteria-free cultures from hormogone-producing blue-green algae. Nature. 1961 Dec 30;192:1275–1276. doi: 10.1038/1921275a0. [DOI] [PubMed] [Google Scholar]
- Bachmayer H., Kreil G. The formation of N-formyl-methionyl-puromycin by intact cells of four different bacteria and a blue-green alga. Biochim Biophys Acta. 1968 Nov 20;169(1):95–102. doi: 10.1016/0005-2787(68)90011-7. [DOI] [PubMed] [Google Scholar]
- Bailey-Watts A. E., Bindloss M. E., Belcher J. H. Freshwater primary production by a blue-green alga of bacterial size. Nature. 1968 Dec 28;220(5174):1344–1345. doi: 10.1038/2201344a0. [DOI] [PubMed] [Google Scholar]
- Batterton J. C., Jr, Van Baalen C. Growth responses of blue-green algae to sodium chloride concentration. Arch Mikrobiol. 1971;76(2):151–165. doi: 10.1007/BF00411789. [DOI] [PubMed] [Google Scholar]
- Bazin M. J. Sexuality in a blue-green alga: genetic recombination in Anacystis nidulans. Nature. 1968 Apr 20;218(5138):282–283. doi: 10.1038/218282a0. [DOI] [PubMed] [Google Scholar]
- Bennett A., Bogorad L. Properties of subunits and aggregates of blue-green algal biliproteins. Biochemistry. 1971 Sep 14;10(19):3625–3634. doi: 10.1021/bi00795a022. [DOI] [PubMed] [Google Scholar]
- Berlyn M. B., Ahmed S. I., Giles N. H. Organization of polyaromatic biosynthetic enzymes in a variety of photosynthetic organisms. J Bacteriol. 1970 Nov;104(2):768–774. doi: 10.1128/jb.104.2.768-774.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns D. S., Edwards M. R. Electron micrographic investigations of C-phycocyanin. Arch Biochem Biophys. 1965 Jun;110(3):511–516. doi: 10.1016/0003-9861(65)90444-3. [DOI] [PubMed] [Google Scholar]
- Berns D. S., Holohan P., Scott E. Urease activity in blue-green algae. Science. 1966 May 20;152(3725):1077–1078. doi: 10.1126/science.152.3725.1077. [DOI] [PubMed] [Google Scholar]
- Berns D. S. Immunochemistry of biliproteins. Plant Physiol. 1967 Nov;42(11):1569–1586. doi: 10.1104/pp.42.11.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns D. S., Morgenstern A. Ultracentrifuge investigation of protein aggregation in dilute solutions of C-phycocyanin. Biochemistry. 1966 Sep;5(9):2985–2990. doi: 10.1021/bi00873a030. [DOI] [PubMed] [Google Scholar]
- Berns D. S., Scott E. Protein aggregation in a thermophilic protein. Phycocyanin from Synechococcus lividus. Biochemistry. 1966 May;5(5):1528–1533. doi: 10.1021/bi00869a012. [DOI] [PubMed] [Google Scholar]
- Biggins J. Photosynthetic Reactions by Lysed Protoplasts and Particle Preparations from the Blue-Green Alga, Phormidium luridum. Plant Physiol. 1967 Oct;42(10):1447–1456. doi: 10.1104/pp.42.10.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins J. Preparation of Metabolically Active Protoplasts and Particle Preparations from the Blue-Green Alga, Phormidium luridum. Plant Physiol. 1967 Oct;42(10):1442–1446. doi: 10.1104/pp.42.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins J. Respiration in blue-green algae. J Bacteriol. 1969 Aug;99(2):570–575. doi: 10.1128/jb.99.2.570-575.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder A., Wilson K., Zuber H. C-phycocyanin from the thermophilic blue-green alga Mastigocladus laminosus, isolation, characterization and subunit composition. FEBS Lett. 1972 Jan 15;20(1):111–116. doi: 10.1016/0014-5793(72)80030-9. [DOI] [PubMed] [Google Scholar]
- Bisalputra T., Bisalputra A. A. The occurrence of DNA fibrils in chloroplasts of Laurencia spectabilis. J Ultrastruct Res. 1967 Jan;17(1):14–22. doi: 10.1016/s0022-5320(67)80016-9. [DOI] [PubMed] [Google Scholar]
- Bisalputra T., Brown D. L., Weier T. E. Possible respiratory sites in a blue-green alga Nostoc sphaericum as demonstrated by potassium tellurite and tetranitro-blue tetrazolium reduction. J Ultrastruct Res. 1969 Apr;27(2):182–197. [PubMed] [Google Scholar]
- Blinks L. R. ACTION SPECTRA OF CHROMATIC TRANSIENTS AND THE EMERSON EFFECT IN MARINE ALGAE. Proc Natl Acad Sci U S A. 1960 Mar;46(3):327–333. doi: 10.1073/pnas.46.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone D. H. Nitrogenase activity and nitrogen assimilation in Anabaena flos-aquae growing in continuous culture. Arch Mikrobiol. 1971;80(3):234–241. doi: 10.1007/BF00410124. [DOI] [PubMed] [Google Scholar]
- Bone D. H. Relationship between phosphates and alkaline phosphatase of Anabaena flos-aquae in continuous culture. Arch Mikrobiol. 1971;80(2):147–153. doi: 10.1007/BF00411879. [DOI] [PubMed] [Google Scholar]
- Botham K. M., Pennock J. F. The biosynthesis of tocopherols and related compounds in the blue-green alga Anabaena variabilis. Biochem J. 1971 Mar;122(1):127–128. doi: 10.1042/bj1220127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothe H. Ferredoxin als Kofaktor der cyclischen Photophosphorylierung in einem zellfreien System aus der Blaualge Anacystis nidulans. Z Naturforsch B. 1969 Dec;24(12):1574–1582. [PubMed] [Google Scholar]
- Bowen C. C., Jensen T. E. Blue-Green Algae: Fine Structure of the Gas Vacuoles. Science. 1965 Mar 19;147(3664):1460–1462. doi: 10.1126/science.147.3664.1460. [DOI] [PubMed] [Google Scholar]
- Bowyer J. W., Skerman V. B. Production of axemic cultures of soil-borne and endophytic blue-green algae. J Gen Microbiol. 1968 Dec;54(2):299–306. doi: 10.1099/00221287-54-2-299. [DOI] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen membranes. Proc Natl Acad Sci U S A. 1966 May;55(5):1048–1056. doi: 10.1073/pnas.55.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell P. F., Nicholas D. J. Some Effects of Sodium on Nitrate Assimilation and N(2) Fixation in Anabaena cylindrica. Plant Physiol. 1967 Jul;42(7):915–921. doi: 10.1104/pp.42.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland B., Walsby A. E. A study of the strength and stability of gas vesicles isolated from a blue-green alga. Arch Mikrobiol. 1971;79(4):327–337. doi: 10.1007/BF00424908. [DOI] [PubMed] [Google Scholar]
- Bunt J. S., Cooksey K. E., Heeb M. A., Lee C. C., Taylor B. F. Assay of algal nitrogen fixation in the marine subtropics by acetylene reduction. Nature. 1970 Sep 12;227(5263):1163–1164. doi: 10.1038/2271163a0. [DOI] [PubMed] [Google Scholar]
- Büchel K. H., Röchling H., Baedelt H GERHARDT B., Trebst A. Hemmung der Photosynthese in Anacystis durch Alkylbenzimidazole. Z Naturforsch B. 1967 May;22(5):535–537. [PubMed] [Google Scholar]
- CALVIN M., LYNCH V. Grana-like structures of Synechococcus cedorum. Nature. 1952 Mar 15;169(4298):455–456. doi: 10.1038/169455b0. [DOI] [PubMed] [Google Scholar]
- CASSEL W. A., HUTCHINSON W. G. Nuclear studies on the smaller Myxophyceae. Exp Cell Res. 1954 Feb;6(1):134–150. doi: 10.1016/0014-4827(54)90155-x. [DOI] [PubMed] [Google Scholar]
- CHAPMAN J. A., SALTON M. R. A study of several blue-green algae in the electron microscope. Arch Mikrobiol. 1962;44:311–322. doi: 10.1007/BF00510951. [DOI] [PubMed] [Google Scholar]
- CRESPI H. L., MANDEVILLE S. E., KATZ J. J. The action of lysozyme on several blue-green algae. Biochem Biophys Res Commun. 1962 Dec 19;9:569–573. doi: 10.1016/0006-291x(62)90127-4. [DOI] [PubMed] [Google Scholar]
- Cannon R. E., Shane M. S., Bush V. N. Lysogeny of a blue-green alga, Plectonema boryanum. Virology. 1971 Jul;45(1):149–153. doi: 10.1016/0042-6822(71)90121-8. [DOI] [PubMed] [Google Scholar]
- Capesius I., Richter G. Enzyme der Polynucleotid-Synthese in pflanzlichen Organismen. I. Isolierung, Reinigung und Charakterisierung der Polynucleotid-Phosphorylase aus der Blaualge Anacystis nidulans. Z Naturforsch B. 1967 Feb;22(2):204–215. [PubMed] [Google Scholar]
- Capesius, Richter G. Enzyme der Polynucleotid-Syntheei pflanzlichen Organsmen. II. Isolierung, Reinigun und Charakterisierung der RNA-Polymerase aus r Blaualge Anacystis niduans. Z Naturforsch B. 1967 Aug;22(8):876–885. [PubMed] [Google Scholar]
- Carr N. G., Hallaway M. Reduction of phenolindo-2,6-dichlorophenol in dark and light by the blue-green alga, Anabaena variabilis. J Gen Microbiol. 1965 Jun;39(3):335–344. doi: 10.1099/00221287-39-3-335. [DOI] [PubMed] [Google Scholar]
- Carr N. G. The occurrence of poly-beta-hydroxybutyrate in the blue-green alga, Chlorogloea fritschii. Biochim Biophys Acta. 1966 Jun 8;120(2):308–310. doi: 10.1016/0926-6585(66)90353-0. [DOI] [PubMed] [Google Scholar]
- Castenholz R. W. Thermophilic blue-green algae and the thermal environment. Bacteriol Rev. 1969 Dec;33(4):476–504. doi: 10.1128/br.33.4.476-504.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L., Bowen C. C. Purification and properties of glycogen isolated from a blue-green alga, Nostoc muscorum. J Bacteriol. 1971 Jan;105(1):331–338. doi: 10.1128/jb.105.1.331-338.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D. J., Cole W. J., Siegelman H. W. Chromophores of allophycocyanin and R-phycocyanin. Biochem J. 1967 Dec;105(3):903–905. doi: 10.1042/bj1050903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D. J., Cole W. J., Siegelman H. W. Cleavage of phycocyanobilin from C-phycocyanin. Biochim Biophys Acta. 1968 Apr 2;153(3):692–698. doi: 10.1016/0005-2728(68)90196-5. [DOI] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Photoactivation of the manganese catalyst of O 2 evolution. I. Biochemical and kinetic aspects. Biochim Biophys Acta. 1971 Nov 2;253(1):167–181. doi: 10.1016/0005-2728(71)90242-8. [DOI] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Photoreactivation of manganese catalyst in photosynthetic oxygen evolution. Biochem Biophys Res Commun. 1967 Jul 10;28(1):89–95. doi: 10.1016/0006-291x(67)90411-1. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y., Gibbs M. Dark and photometabolism of sugars by a blue green alga: Tolypothrix tenuis. Plant Physiol. 1966 Apr;41(4):731–737. doi: 10.1104/pp.41.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho F., Govindjee Low-temperature (4-77 degrees K) spectroscopy of Anacystis: temperature dependence of energy transfer efficiency. Biochim Biophys Acta. 1970 Aug 4;216(1):151–161. doi: 10.1016/0005-2728(70)90167-2. [DOI] [PubMed] [Google Scholar]
- Chua N. H. The methyl viologen-catalyzed Mehler reaction and catalase activity in blue-green algae and Chlamydomonas reinhardi. Biochim Biophys Acta. 1971 Sep 7;245(2):277–287. doi: 10.1016/0005-2728(71)90146-0. [DOI] [PubMed] [Google Scholar]
- Cohen-Bazire G., Kunisawa R., Pfennig N. Comparative study of the structure of gas vacuoles. J Bacteriol. 1969 Nov;100(2):1049–1061. doi: 10.1128/jb.100.2.1049-1061.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Bazire G., Lefort-Tran M. Fixation of phycobiliproteins to photosynthetic membranes by glutaraldehyde. Arch Mikrobiol. 1970;71(3):245–257. doi: 10.1007/BF00410158. [DOI] [PubMed] [Google Scholar]
- Cole W. J., Chapman D. J., Siegelman H. W. The structure and properties of phycocyanobilin and related bilatrienes. Biochemistry. 1968 Aug;7(8):2929–2935. doi: 10.1021/bi00848a033. [DOI] [PubMed] [Google Scholar]
- Cope B. T., Smith U., Crespi H. L., Katz J. J. Studies on the identity of ordinary and deuterio-phycocyanins: end-groups, amino acid compositions, minimum molecular weights, peptide maps. Biochim Biophys Acta. 1967 Apr 11;133(3):446–453. doi: 10.1016/0005-2795(67)90548-x. [DOI] [PubMed] [Google Scholar]
- Correll D. L., Tolbert N. E. Ribonucleic Acid-Polyphosphate From Algae. I. Isolation & Physiology. Plant Physiol. 1962 Sep;37(5):627–636. doi: 10.1104/pp.37.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. M., Fay P. Special aspects of nitrogen fixation by blue-green algae. Proc R Soc Lond B Biol Sci. 1969 Apr 1;172(1029):357–366. doi: 10.1098/rspb.1969.0026. [DOI] [PubMed] [Google Scholar]
- Cox R. M. Physiological studies on nitrogen fixation in the blue-green alga Anabaena cylindrica. Arch Mikrobiol. 1966 Mar 31;53(3):263–276. doi: 10.1007/BF00446673. [DOI] [PubMed] [Google Scholar]
- Craig I. W., Carr N. G. Ribosomes from the blue-green alga Anabeana variabilis. Arch Mikrobiol. 1968;62(2):167–177. doi: 10.1007/BF00410403. [DOI] [PubMed] [Google Scholar]
- Craig I. W., Leach C. K., Carr N. G. Studies with deoxyribonucleic acid from blue-green algae. Arch Mikrobiol. 1969;65(3):218–227. doi: 10.1007/BF00407105. [DOI] [PubMed] [Google Scholar]
- Crespi H. L., Daboll H. F., Katz J. J. The biosynthesis of isotope hybrid proteins for high resolution nuclear magnetic resonance studies by incorporation of [1H]amino acids into fully deuterated algae. Biochim Biophys Acta. 1970 Jan 20;200(1):26–33. doi: 10.1016/0005-2795(70)90039-5. [DOI] [PubMed] [Google Scholar]
- Crespi H. L., Smith U., Gajda L., Tisue T., Ammeraal R. M. Extraction and purification of 1 H, 2 H, and isotope hybrid algal cytochrome, ferredoxin and flavoprotein. Biochim Biophys Acta. 1972 Mar 16;256(3):611–618. doi: 10.1016/0005-2728(72)90196-x. [DOI] [PubMed] [Google Scholar]
- Crespi H. L., Smith U., Katz J. J. Phycocyanobilin. Structure and exchange studies by nuclear magnetic resonance and its mode of attachment in phycocyanin. A model for phytochrome. Biochemistry. 1968 Jun;7(6):2232–2242. doi: 10.1021/bi00846a028. [DOI] [PubMed] [Google Scholar]
- DE D. N., GHOSH S. N. CYTOCHEMICAL EVIDENCE FOR THE APPARENT ABSENCE OF HISTONE IN THE CELLS OF CYANOPHYCEAE. J Histochem Cytochem. 1965 Apr;13:298–298. doi: 10.1177/13.4.298. [DOI] [PubMed] [Google Scholar]
- DEMETER O. Uber Modifikationen bei Cyanophyceen. Arch Mikrobiol. 1956;24(2):105–133. [PubMed] [Google Scholar]
- DREWS G., NIKLOWITZ W. Beitrage zur Cytologie der Blaualgen. II. Zentroplasma und granuläre Einschlüsse von Phormidium uncinatum. Arch Mikrobiol. 1956;24(2):147–162. [PubMed] [Google Scholar]
- DREWS G., NIKLOWITZ W. Beiträge zur Cytologie der Blaualgen. III. Untersuchungen über die granulären Einschlüsse der Hormogonales. Arch Mikrobiol. 1957;25(4):333–351. [PubMed] [Google Scholar]
- DUYSENS L. N. M. Transfer of light energy within the pigment systems present in photosynthesizing cells. Nature. 1951 Sep 29;168(4274):548–550. doi: 10.1038/168548a0. [DOI] [PubMed] [Google Scholar]
- DUYSENS L. N., SWEEP G. Fluorescence spectrophotometry of pyridine nucleotide in photosynthesizing cells. Biochim Biophys Acta. 1957 Jul;25(1):13–16. doi: 10.1016/0006-3002(57)90409-2. [DOI] [PubMed] [Google Scholar]
- Das M., Rabinowitch E., Szalay L. Red drop in the quantum yield of fluorescence of sonicated algae. Biophys J. 1968 Oct;8(10):1131–1137. doi: 10.1016/S0006-3495(68)86544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasilva E. J., Jensen A. Content of -tocopherol in some blue-green algae. Biochim Biophys Acta. 1971 Jul 13;239(2):345–347. doi: 10.1016/0005-2760(71)90180-9. [DOI] [PubMed] [Google Scholar]
- De Souza N. J., Nes W. R. Sterols: isolation from a blue-green alga. Science. 1968 Oct 18;162(3851):363–363. doi: 10.1126/science.162.3851.363. [DOI] [PubMed] [Google Scholar]
- Dietrich W. E., Jr, Thornber J. P. The P700-chlorophyll -protein of a blue-green alga. Biochim Biophys Acta. 1971 Sep 7;245(2):482–493. doi: 10.1016/0005-2728(71)90164-2. [DOI] [PubMed] [Google Scholar]
- Donze M., Haveman J., Schiereck P. Absence of photosystem 2 in heterocysts of the blue-green alga Anabaena. Biochim Biophys Acta. 1972 Jan 21;256(1):157–161. doi: 10.1016/0005-2728(72)90170-3. [DOI] [PubMed] [Google Scholar]
- Duane W. C., Sr, Hohl M. C., Krogmann D. W. Photophosphorylation activity in cell-free preparations of a blue-green alga. Biochim Biophys Acta. 1965 Sep 27;109(1):108–116. doi: 10.1016/0926-6585(65)90095-6. [DOI] [PubMed] [Google Scholar]
- Dunn J. H., Simon R. D., Wolk C. P. Incorporation of amino sugars into walls during heterocyst differentiation. Dev Biol. 1971 Sep;26(1):159–164. doi: 10.1016/0012-1606(71)90115-1. [DOI] [PubMed] [Google Scholar]
- Dunn J. H., Wolk C. P. Composition of the cellular envelopes of Anabaena cylindrica. J Bacteriol. 1970 Jul;103(1):153–158. doi: 10.1128/jb.103.1.153-158.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBEL J. P., COLAS J., MULLER S. Recherches cytochimiques sur les polyphosphates inorganiques contenus dans les organismes vivants. II. Mise au point de méthodes de détection cytochimiques spécifiques des polyphosphates. Exp Cell Res. 1958 Aug;15(1):28–36. doi: 10.1016/0014-4827(58)90059-4. [DOI] [PubMed] [Google Scholar]
- EBEL J. P., COLAS J., MULLER S. Recherches cytochimiques sur les polyphosphates inorganiques contenus dans les organismes vivants. III. Présence de polyphosphates chez divers organismes inférieurs. Exp Cell Res. 1958 Aug;15(1):36–42. doi: 10.1016/0014-4827(58)90060-0. [DOI] [PubMed] [Google Scholar]
- EBEL J. P., MULLER S. Recherches cytochimiques sur les polyphosphates inorganiques contenus dans les organismes vivants. I. Etude de la réaction de métachromasie et des colorations au vert de méthyle et à la pyronine en fonction de la longueur de chine des polyphosphates. Exp Cell Res. 1958 Aug;15(1):21–28. doi: 10.1016/0014-4827(58)90058-2. [DOI] [PubMed] [Google Scholar]
- ECHLIN P., MORRIS I. THE RELATIONSHIP BETWEEN BLUE-GREEN ALGAE AND BACTERIA. Biol Rev Camb Philos Soc. 1965 May;40:143–187. doi: 10.1111/j.1469-185x.1965.tb00800.x. [DOI] [PubMed] [Google Scholar]
- ERWIN J., BLOCH K. BIOSYNTHESIS OF UNSATURATED FATTY ACIDS IN MICROORGANISMS. Science. 1964 Mar 6;143(3610):1006–1012. doi: 10.1126/science.143.3610.1006. [DOI] [PubMed] [Google Scholar]
- EYSTER C. Necessity of boron for Nostoc muscorum. Nature. 1952 Nov 1;170(4331):755–755. doi: 10.1038/170755a0. [DOI] [PubMed] [Google Scholar]
- Edelman M., Swinton D., Schiff J. A., Epstein H. T., Zeldin B. Deoxyribonucleic Acid of the blue-green algae (cyanophyta). Bacteriol Rev. 1967 Dec;31(4):315–331. doi: 10.1128/br.31.4.315-331.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. R., Gantt E. Phycobilisomes of the thermophilic blue-green alga Synechococcus lividus. J Cell Biol. 1971 Sep;50(3):896–900. doi: 10.1083/jcb.50.3.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Hall D. O., Bothe H., Whatley F. R. The stoicheiometry of electron transfer by bacterial and plant ferredoxins. Biochem J. 1968 Dec;110(3):485–489. doi: 10.1042/bj1100485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAY P., FOGG G. E. Studies on nitrogen fixation by bluegreen algae. III. Growth and nitrogen fixation in Chlorogloea fritschii Mitra. Arch Mikrobiol. 1962;42:310–321. doi: 10.1007/BF00422048. [DOI] [PubMed] [Google Scholar]
- FAY P. HETEROTROPHY AND NITROGEN FIXATION IN CHLOROGLOEA FRITSCHII. J Gen Microbiol. 1965 Apr;39:11–20. doi: 10.1099/00221287-39-1-11. [DOI] [PubMed] [Google Scholar]
- FAY P., KUMAR H. D., FOGG G. E. CELLULAR FACTORS AFFECTING NITROGEN FIXATION IN THE BLUE-GREEN ALGA CHLOROGLOEA FRITSCHII. J Gen Microbiol. 1964 May;35:351–360. doi: 10.1099/00221287-35-2-351. [DOI] [PubMed] [Google Scholar]
- FOGG G. E. The comparative physiology and biochemistry of the blue-green algae. Bacteriol Rev. 1956 Sep;20(3):148–165. doi: 10.1128/br.20.3.148-165.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOGG G. E. The production of extracellular nitrogenous substances by a blue-green alga. Proc R Soc Lond B Biol Sci. 1952 Apr 24;139(896):372–397. doi: 10.1098/rspb.1952.0019. [DOI] [PubMed] [Google Scholar]
- FRANK H., LEFORT M., MARTIN H. H. Chemical analysis of a mucopolymer component in cell walls of the blue-green alga Phormidium uncinatum. Biochem Biophys Res Commun. 1962 May 4;7:322–325. doi: 10.1016/0006-291x(62)90200-0. [DOI] [PubMed] [Google Scholar]
- FREDRICKS W. W., JAGENDORF A. T. A SOLUBLE COMPONENT OF THE HILL REACTION IN ANACYSTIS NIDULANS. Arch Biochem Biophys. 1964 Jan;104:39–49. doi: 10.1016/s0003-9861(64)80032-1. [DOI] [PubMed] [Google Scholar]
- FRENKEL A., GAFFRON H., BATTLEY E. H. Photosynthesis and photoreduction by the blue green alga, Synechococcus elongatus, Näg. Biol Bull. 1950 Oct;99(2):157–162. doi: 10.2307/1538735. [DOI] [PubMed] [Google Scholar]
- FREY-WYSSLING A., STECHER H. Uber den feinbau des Nostoc-schleimes. Z Zellforsch Mikrosk Anat. 1954;39(5):515–519. [PubMed] [Google Scholar]
- FUHS G. W. Bau, Verhalten und Bedeutung der kernäquivalenten Strukturen bei Oscillatoria amoena (Kutz.) Gomont. Arch Mikrobiol. 1958;28(3):270–302. [PubMed] [Google Scholar]
- FUHS G. W. Enzymatischer Abbau der Membranen von Oscillatoria amoena (Kutz.) Gomont mit Lysozym. Arch Mikrobiol. 1958;29(1):51–52. [PubMed] [Google Scholar]
- FUJITA Y., HATTORI A. Changes in composition of cellular material during formation of phycobilin chromoproteids in a blue-green alga, Tolypothrix tenuis. J Biochem. 1962 Jul;52:38–42. doi: 10.1093/oxfordjournals.jbchem.a127569. [DOI] [PubMed] [Google Scholar]
- Fay P. Cell differentiation and pigment composition in Anabaena cylindrica. Arch Mikrobiol. 1969;67(1):62–70. doi: 10.1007/BF00413682. [DOI] [PubMed] [Google Scholar]
- Fay P. Photostimulation of nitrogen fixation in Anabaena cylindrica. Biochim Biophys Acta. 1970 Sep 1;216(2):353–356. doi: 10.1016/0005-2728(70)90226-4. [DOI] [PubMed] [Google Scholar]
- Fay P., Stewart W. D., Walsby A. E., Fogg G. E. Is the heterocyst the site of nitrogen fixation in blue-green algae? Nature. 1968 Nov 23;220(5169):810–812. doi: 10.1038/220810b0. [DOI] [PubMed] [Google Scholar]
- Fay P., Walsby A. E. Metabolic activities of isolated heterocysts of the blue-green alga Anabaena cylindrica. Nature. 1966 Jan 1;209(5018):94–95. doi: 10.1038/209094a0. [DOI] [PubMed] [Google Scholar]
- Forrest H. S., VAN Baalen C., Myers J. Occurrence of Pteridines in a Blue-Green Alga. Science. 1957 Apr 12;125(3250):699–700. doi: 10.1126/science.125.3250.699. [DOI] [PubMed] [Google Scholar]
- Fuhs G. W. Spherical subunits in photosynthetic membranes of two cyanophyceae and the bacterium Rhodospirillum rubrum. Arch Mikrobiol. 1966 Sep 8;54(3):253–265. doi: 10.1007/BF00408998. [DOI] [PubMed] [Google Scholar]
- Fujimori E., Pecci J. Dissociation and association of phycocyanin. Biochemistry. 1966 Nov;5(11):3500–3508. doi: 10.1021/bi00875a016. [DOI] [PubMed] [Google Scholar]
- Fujimori E., Pecci J. Distinct subunits of phycoerythrin from Porphyridium cruentum and their spectral characteristics. Arch Biochem Biophys. 1967 Feb;118(2):448–455. doi: 10.1016/0003-9861(67)90373-6. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Myers J. Comparative studies of cytochrome c redox reactions by photochemical lamellar preparations obtained from blue-green, red and green algae, and spinach chloroplasts. Arch Biochem Biophys. 1966 Mar;113(3):738–741. doi: 10.1016/0003-9861(66)90256-6. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Myers J. Cytochrome c redox reactions induced by photochemical system 1 in sonicated preparations of Anabaena cylindrica. Arch Biochem Biophys. 1966 Mar;113(3):730–737. doi: 10.1016/0003-9861(66)90255-4. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Myers J. Hydrogenase and NADP-reduction reactions by a cell-free preparation of Anabaena cylindrica. Arch Biochem Biophys. 1965 Sep;111(3):619–625. doi: 10.1016/0003-9861(65)90243-2. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Myers J. Kinetic analysis of light-induced cytochrome c redox reactions in Anabaena lamellar fragments. Arch Biochem Biophys. 1967 Mar;119(1):8–15. doi: 10.1016/0003-9861(67)90421-3. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Myers J. Light induced redox reactions of cytochrome c by cell-free preparation of anabaena cylindrica. Biochem Biophys Res Commun. 1965 May 18;19(5):604–608. doi: 10.1016/0006-291x(65)90382-7. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Myers J. Light-induced cytochrome c redox reactions by a cell-free preparation of Anabaena cylindrica. Arch Biochem Biophys. 1965 Dec;112(3):519–523. doi: 10.1016/0003-9861(65)90089-5. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Myers J. The 2,6-dichlorophenol indophenol-Hill reaction by a cell-free preparation of Anabaena cylindrica. Arch Biochem Biophys. 1965 Dec;112(3):506–511. doi: 10.1016/0003-9861(65)90087-1. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Tsuji T. Photochemically active chromoprotein isolated from the blue-green alga Anabaena cylindrica. Nature. 1968 Sep 21;219(5160):1270–1271. doi: 10.1038/2191270a0. [DOI] [PubMed] [Google Scholar]
- Fuller R. C., Anderson I. C., Nathan H. A. PTERIDINES IN PHOTOSYNTHESIS. Proc Natl Acad Sci U S A. 1958 Mar;44(3):239–244. doi: 10.1073/pnas.44.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. C., Gibbs M. Intracellular and Phylogenetic Distribution of Ribulose 1,5-Diphosphate Carboxylase and D-Glyceraldehyde-3-Phosphate Dehydrogenases. Plant Physiol. 1959 May;34(3):324–329. doi: 10.1104/pp.34.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEITLER L. Lamelläre Struktur des Chromatoplasmas von Cyanophyceen in mikroskopischen Dimensionen und Baueigentümlichkeiten des Protoplasten von Chroococcus turgidus. Arch Mikrobiol. 1958;29(2):179–188. [PubMed] [Google Scholar]
- GOODWIN T. W. Studies in carotenogenesis. 22. The structure of echinenone. Biochem J. 1956 Jul;63(3):481–483. doi: 10.1042/bj0630481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODWIN T. W., TAHA M. M. A study of the carotenoids echinenone and myxo-xanthin with special reference to their probable identity. Biochem J. 1951 May;48(5):513–514. doi: 10.1042/bj0480513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODWIN T. W. The nature and distribution of carotenoids in some blue-green algae. J Gen Microbiol. 1957 Oct;17(2):467–473. doi: 10.1099/00221287-17-2-467. [DOI] [PubMed] [Google Scholar]
- Galling G. Stimulierung des Aminosäure-Einbaus durch verschiedene Nucleinsäur-Präparationen in zell-- freien Systemen aus Chlorella und Anacystis. Z Naturforsch B. 1967 Jun;22(6):687–688. [PubMed] [Google Scholar]
- Gallon J. R., LaRue T. A., Kurz W. G. Characteristics of nitrogenase activity in broken cell preparations of the blue-green alga Gloeocapsa sp. LB 795. Can J Microbiol. 1972 Mar;18(3):327–332. doi: 10.1139/m72-050. [DOI] [PubMed] [Google Scholar]
- Gantt E., Conti S. F. Granules associated with the chloroplast lamellae of Porphyridium cruentum. J Cell Biol. 1966 Jun;29(3):423–434. doi: 10.1083/jcb.29.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt E., Conti S. F. The ultrastructure of Porphyridium cruentum. J Cell Biol. 1965 Aug;26(2):365–381. doi: 10.1083/jcb.26.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt E., Conti S. F. Ultrastructure of blue-green algae. J Bacteriol. 1969 Mar;97(3):1486–1493. doi: 10.1128/jb.97.3.1486-1493.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt E., Edwards M. R., Provasoli L. Chloroplast structure of the Cryptophyceae. Evidence for phycobiliproteins within intrathylakoidal spaces. J Cell Biol. 1971 Feb;48(2):280–290. doi: 10.1083/jcb.48.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassner E. B. On the Pigment Absorbing at 750 mmu Occurring in Some Blue-Green Algae. Plant Physiol. 1962 Sep;37(5):637–639. doi: 10.1104/pp.37.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile J. H., Maloney T. E. Toxicity and environmental requirements of a strain of Aphanizomenon flos-aquae (L.) Ralfs. Can J Microbiol. 1969 Feb;15(2):165–173. doi: 10.1139/m69-028. [DOI] [PubMed] [Google Scholar]
- Gerhardt B., Trebst A. Photosynthetische Reaktionen in lyophilisierten Zellen der Blaualge Anacystis. Z Naturforsch B. 1965 Sep;20(9):879–889. [PubMed] [Google Scholar]
- Ghosh A. K., Govindjee Transfer of the excitation energy in Anacystis nidulans grown to obtain different pigment ratios. Biophys J. 1966 Sep;6(5):611–619. doi: 10.1016/S0006-3495(66)86681-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N., Cohen-Bazire G., Stanier R. Y. Comparative immunology of algal biliproteins. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3005–3008. doi: 10.1073/pnas.68.12.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N., Cohen-Bazire G. Subunit structure of the phycobiliproteins of blue-green algae. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1398–1401. doi: 10.1073/pnas.68.7.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godnev T. N., Rotfarb R. M., Gvardiian V. N. Ob uchastii protoporfirina i gematina v biosinteze fikotsianinov. Dokl Akad Nauk SSSR. 1966 Aug 11;169(5):1191–1194. [PubMed] [Google Scholar]
- Goedheer J. C. Fluorescence action spectra of algae and bean leaves at room and at liquid nitrogen temperatures. Biochim Biophys Acta. 1965 May 25;102(1):73–89. doi: 10.1016/0926-6585(65)90203-7. [DOI] [PubMed] [Google Scholar]
- Goedheer J. C. On the low-temperature fluorescence spectra of blue-green and red algae. Biochim Biophys Acta. 1968 May 28;153(4):903–906. doi: 10.1016/0005-2728(68)90021-2. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Govindjee, Cederstrand C., Rabinowitch E. Existence of Absorption Bands at 730-740 and 750-760 Millimicrons in Algae of Different Divisions. Science. 1961 Aug 11;134(3476):391–392. doi: 10.1126/science.134.3476.391. [DOI] [PubMed] [Google Scholar]
- Grodzinski B., Colman B. Glycolic Acid Oxidase Activity in Cell-free Preparations of Blue-Green Algae. Plant Physiol. 1970 Jun;45(6):735–737. doi: 10.1104/pp.45.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL W. T., CLAUS G. ULTRASTRUCTURAL STUDIES ON THE BLUE-GREEN ALGAL SYMBIONT IN CYANOPHORA PARADOXA KORSCHIKOFF. J Cell Biol. 1963 Dec;19:551–563. doi: 10.1083/jcb.19.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAXO F. T., BLINKS L. R. Photosynthetic action spectra of marine algae. J Gen Physiol. 1950 Mar;33(4):389–422. doi: 10.1085/jgp.33.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAXO F., O'HEOCHA C., NORRIS P. Comparative studies of chromatographically separated phycoerythrins and phycocyanins. Arch Biochem Biophys. 1955 Jan;54(1):162–173. doi: 10.1016/0003-9861(55)90019-9. [DOI] [PubMed] [Google Scholar]
- HENNINGER M. D., BHAGAVAN H. N., CRANE F. L. COMPARATIVE STUDIES ON PLASTOQUINONES. I. EVIDENCE FOR THREE QUINONES IN THE BLUE-GREEN ALGA, ANACYSTIS NIDULANS. Arch Biochem Biophys. 1965 Apr;110:69–74. doi: 10.1016/0003-9861(65)90155-4. [DOI] [PubMed] [Google Scholar]
- HOCH G., OWENS O. V., KOK B. Photosynthesis and respiration. Arch Biochem Biophys. 1963 Apr;101:171–180. doi: 10.1016/0003-9861(63)90547-2. [DOI] [PubMed] [Google Scholar]
- HOLTON R. W., MYERS J. CYTOCHROMES OF A BLUE-GREEN ALGA: EXTRACTION OF A C-TYPE WITH A STRONGLY NEGATIVE REDOX POTENTIAL. Science. 1963 Oct 11;142(3589):234–235. doi: 10.1126/science.142.3589.234. [DOI] [PubMed] [Google Scholar]
- HOPWOOD D. A., GLAUERT A. M. The fine structure of the nuclear material of a blue-green alga, Anabaena cylindrica Lemm. J Biophys Biochem Cytol. 1960 Dec;8:813–823. doi: 10.1083/jcb.8.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefnar K., Kirschfeld H. Copulation of ultra-violet-irradiated and unirradiated Saccharomyces cells of different ploidy. Nature. 1966 Jul 16;211(5046):310–311. doi: 10.1038/211310b0. [DOI] [PubMed] [Google Scholar]
- Halfen L. N., Castenholz R. W. Gliding in a blue-green alga: a possible mechanism. Nature. 1970 Mar 21;225(5238):1163–1165. doi: 10.1038/2251163a0. [DOI] [PubMed] [Google Scholar]
- Hallier U. W., Park R. B. Photosynthetic Light Reactions in Chemically Fixed Anacystis nidulans, Chlorella pyrenoidosa, and Porphyridium cruentum. Plant Physiol. 1969 Apr;44(4):535–539. doi: 10.1104/pp.44.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Calvin M. Hydrocarbon distribution of algae and bacteria, and microbiological activity in sediments. Proc Natl Acad Sci U S A. 1969 Oct;64(2):436–443. doi: 10.1073/pnas.64.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori A., Myers J. Reduction of nitrate and nitrite by subcellular preparations of Anabaena cylindrica. I. Reduction of nitrite to ammonia. Plant Physiol. 1966 Jun;41(6):1031–1036. doi: 10.1104/pp.41.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haystead A., Robinson R., Stewart W. D. Nitrogenase activity in extracts of heterocystous and non-heterocystous blue-green algae. Arch Mikrobiol. 1970;74(3):235–243. doi: 10.1007/BF00408884. [DOI] [PubMed] [Google Scholar]
- Haystead A., Stewart W. D. Characteristics of the nitrogenase system of the blue-green alga Anabaena cylindrica. Arch Mikrobiol. 1972;82(4):325–336. doi: 10.1007/BF00424936. [DOI] [PubMed] [Google Scholar]
- Herdman M., Carr N. G. Observations on replication and cell division in synchronous cultures of the blue-green alga Anacystis nidulans. J Bacteriol. 1971 Aug;107(2):583–584. doi: 10.1128/jb.107.2.583-584.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman M., Carr N. G. The isolation and characterization of mutant strains of the blue-green alga anacystis nidulans. J Gen Microbiol. 1972 Apr;70(2):213–220. doi: 10.1099/00221287-70-2-213. [DOI] [PubMed] [Google Scholar]
- Herzfeld F., Zillig W. Subunit composition of DNA-dependent RNA polymerase of Anacystis nidulans. Eur J Biochem. 1971 Dec;24(2):242–248. doi: 10.1111/j.1432-1033.1971.tb19676.x. [DOI] [PubMed] [Google Scholar]
- Hirayama O. Lipids and lipoprotein complex in photosynthetic tissues. II. Pigments and lipids in blue-green alga, Anacystis nidulans. J Biochem. 1967 Feb;61(2):179–185. doi: 10.1093/oxfordjournals.jbchem.a128529. [DOI] [PubMed] [Google Scholar]
- Hiyama T., Ke B. A new photosynthetic pigment, "P430": its possible role as the priary electron acceptor of photosystem I. Proc Natl Acad Sci U S A. 1971 May;68(5):1010–1013. doi: 10.1073/pnas.68.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama T., Ke B. Laser-induced reactions of P700 and cytochrome f in a blue-green alga, Plectonema boryanum. Biochim Biophys Acta. 1971 Mar 2;226(2):320–327. doi: 10.1016/0005-2728(71)90099-5. [DOI] [PubMed] [Google Scholar]
- Hoare D. S., Hoare S. L. Feedback regulation of arginine biosynthesis in blue-green algae and photosynthetic bacteria. J Bacteriol. 1966 Aug;92(2):375–379. doi: 10.1128/jb.92.2.375-379.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare D. S., Moore R. B. Photoassimilation of organic compounds by autotrophic blue-green algae. Biochim Biophys Acta. 1965 Nov 29;109(2):622–625. doi: 10.1016/0926-6585(65)90192-5. [DOI] [PubMed] [Google Scholar]
- Holdsworth R. H. The isolation and partial characterization of the pyrenoid protein of Eremosphaera viridis. J Cell Biol. 1971 Nov;51(21):499–513. doi: 10.1083/jcb.51.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm-Hansen O. Ecology, physiology, and biochemistry of blue-green algae. Annu Rev Microbiol. 1968;22:47–70. doi: 10.1146/annurev.mi.22.100168.000403. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Edwards M. R. Fine structure of the thermophilic blue-green alga Synechococcus lividus Copeland. A study of frozen-fractured-etched cells. Can J Microbiol. 1972 Feb;18(2):175–181. doi: 10.1139/m72-028. [DOI] [PubMed] [Google Scholar]
- Holton R. W., Blecker H. H., Stevens T. S. Fatty acids in blue-green algae: possible relation to phylogenetic position. Science. 1968 May 3;160(3827):545–547. doi: 10.1126/science.160.3827.545. [DOI] [PubMed] [Google Scholar]
- Holton R. W., Myers J. Water-soluble cytochromes from a blue-gree alga. II. Physicochemical properties and quantitative relationships of cytochromes C (549, 552, and 554 Anacystis nidulans). Biochim Biophys Acta. 1967 Mar 8;131(2):375–384. doi: 10.1016/0005-2728(67)90151-x. [DOI] [PubMed] [Google Scholar]
- Holton R. W., Myers J. Water-soluble cytochromes from a blue-green alga. I. Extraction, purification, and spectral properties of cytochromes C (549, 552, and 554, Anacystis nidulans). Biochim Biophys Acta. 1967 Mar 8;131(2):362–374. doi: 10.1016/0005-2728(67)90150-8. [DOI] [PubMed] [Google Scholar]
- Honeycutt R. C., Krogmann D. W. A light-dependent oxygen-reducing system from Anabaena variabilis. Biochim Biophys Acta. 1970 Mar 3;197(2):267–275. doi: 10.1016/0005-2728(70)90037-x. [DOI] [PubMed] [Google Scholar]
- Hood W., Carr N. G. A single glyceraldehyde-3-phosphate dehydrogenase active with NAD and NADP in Anabaena variabilis. Biochim Biophys Acta. 1967 Sep 12;146(1):309–311. doi: 10.1016/0005-2744(67)90103-9. [DOI] [PubMed] [Google Scholar]
- Hood W., Carr N. G. Apparent lack of control by repression of arginine metabolism in blue-green algae. J Bacteriol. 1971 Jul;107(1):365–367. doi: 10.1128/jb.107.1.365-367.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood W., Carr N. G. Threonine deaminase and acetolactate synthetase in Anabaena variabilis. Biochem J. 1968 Sep;109(2):4P–4P. doi: 10.1042/bj1090004pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood W., Leaver A. G., Carr N. G. Extracellular nitrogen and the control of arginine biosynthesis in Anabaena variabilis. Biochem J. 1969 Aug;114(1):12P–12P. doi: 10.1042/bj1140012pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton A. A. NADH oxidase in blue-green algae. Biochem Biophys Res Commun. 1968 Sep 6;32(5):839–845. doi: 10.1016/0006-291x(68)90317-3. [DOI] [PubMed] [Google Scholar]
- Howland G. P., Ramus J. Analysis of blue-green and red algal ribosomal-RNAs by gel electrophoresis. Arch Mikrobiol. 1971;76(4):292–298. doi: 10.1007/BF00408526. [DOI] [PubMed] [Google Scholar]
- Hoyt J. W., Soli G. Algal Cultures: Ability To Reduce Turbulent Friction in Flow. Science. 1965 Sep 24;149(3691):1509–1511. doi: 10.1126/science.149.3691.1509. [DOI] [PubMed] [Google Scholar]
- Ilani A., Berns D. S. The effect of ferric ion on phycocyanin fluorescence. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1423–1430. doi: 10.1016/0006-291x(71)90179-3. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Pierson D., Kane J. F., Van Baalen C., Jensen R. A. Documentation of auxotrophic mutation in blue-green bacteria: characterization of a tryptophan auxotroph in Agmenellum quadruplicatum. J Bacteriol. 1972 Jul;111(1):112–118. doi: 10.1128/jb.111.1.112-118.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O., Thurston E. L., Van Baalen C. Effects of selected inhibitors on growth and cell division in Agmenellum. Arch Mikrobiol. 1972;81(1):1–12. doi: 10.1007/BF00715019. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Van Baalen C. Characteristics of a stable, filamentous mutant of a coccoid blue-green alga. J Bacteriol. 1970 Jun;102(3):784–789. doi: 10.1128/jb.102.3.784-789.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES L. W., MYERS J. A COMMON LINK BETWEEN PHOTOSYNTHESIS AND RESPIRATION IN A BLUE-GREEN ALGA. Nature. 1963 Aug 17;199:670–672. doi: 10.1038/199670a0. [DOI] [PubMed] [Google Scholar]
- Jackim E., Gentile J. Toxins of a blue-green alga: similarity to saxitoxin. Science. 1968 Nov 22;162(3856):915–916. doi: 10.1126/science.162.3856.915. [DOI] [PubMed] [Google Scholar]
- Jensen T. E., Clark R. L. Cell wall and coat of the developing akinete of a Cylindrospermum species. J Bacteriol. 1969 Mar;97(3):1494–1495. doi: 10.1128/jb.97.3.1494-1495.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T. E., Sicko L. M. Fine structure of poly-beta-hydroxybutyric acid granules in a blue-green alga, Chlorogloea fritschii. J Bacteriol. 1971 May;106(2):683–686. doi: 10.1128/jb.106.2.683-686.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. D., Haug A., Jost M., Graber D. R. Ultrastructural and conformational changes in gas vacuole membranes isolated from Microcystis aeruginosa. Arch Biochem Biophys. 1969 Dec;135(1):296–303. doi: 10.1016/0003-9861(69)90543-8. [DOI] [PubMed] [Google Scholar]
- Jones D. D., Jost M. Isolation and chemical characterization of gas-vacuole membranes from Microcystis aeruginosa Kuetz. emend. Elenkin. Arch Mikrobiol. 1970;70(1):43–64. doi: 10.1007/BF00691059. [DOI] [PubMed] [Google Scholar]
- Jones L. W., Myers J. Enhancement in the Blue-Green Alga, Anacystis nidulans. Plant Physiol. 1964 Nov;39(6):938–946. doi: 10.1104/pp.39.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost M., Jones D. D. Morphological parameters and macromolecular organization of gas vacuole membranes of Microcystis aeruginosa Kuetz. emend. Elenkin. Can J Microbiol. 1970 Mar;16(3):159–164. doi: 10.1139/m70-028. [DOI] [PubMed] [Google Scholar]
- Jost M., Jones D. D., Weathers P. J. Counting of gas vacuoles by electron microscopy in lysates and purified fractions of Microcystis aeruginosa. Protoplasma. 1971;73(3):329–335. doi: 10.1007/BF01273937. [DOI] [PubMed] [Google Scholar]
- Jost M., Matile P. Zur Charakterisierung der Gasvacuolen der Blaualge Oscillatoria rubescens. Arch Mikrobiol. 1966 Feb 1;53(1):50–58. [PubMed] [Google Scholar]
- KOK B., BEINERT H. The light induced EPR signal of photocatalyst P700. II. Two light effects. Biochem Biophys Res Commun. 1962 Oct 31;9:349–354. doi: 10.1016/0006-291x(62)90053-0. [DOI] [PubMed] [Google Scholar]
- KOK B. On the reversible absorption change at 705 mu in photosynthetic organisms. Biochim Biophys Acta. 1956 Nov;22(2):399–401. doi: 10.1016/0006-3002(56)90172-x. [DOI] [PubMed] [Google Scholar]
- Kale S. R., Bahal M., Talpasayi E. R. Wall development and tetrazolium chloride reduction in heterocysts of blue-green algae, Anabaena ambigua. Experientia. 1970 Jun 15;26(6):605–606. doi: 10.1007/BF01898713. [DOI] [PubMed] [Google Scholar]
- Kao O., Berns D. S. The monomer molecular weight of C-phycocyanin. Biochem Biophys Res Commun. 1968 Nov 8;33(3):457–462. doi: 10.1016/0006-291x(68)90595-0. [DOI] [PubMed] [Google Scholar]
- Kenyon C. N. Fatty acid composition of unicellular strains of blue-green algae. J Bacteriol. 1972 Feb;109(2):827–834. doi: 10.1128/jb.109.2.827-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. N., Stanier R. Y. Possible evolutionary significance of polyunsaturated fatty acids in blue-green algae. Nature. 1970 Sep 12;227(5263):1164–1166. doi: 10.1038/2271164a0. [DOI] [PubMed] [Google Scholar]
- Knaff D. B., Arnon D. I. On two photoreactions in system II of plant photosynthesis. Biochim Biophys Acta. 1971 Mar 2;226(2):400–408. doi: 10.1016/0005-2728(71)90107-1. [DOI] [PubMed] [Google Scholar]
- Kratz W. A., Myers J. Photosynthesis and Respiration of Three Blue-Green Algae. Plant Physiol. 1955 May;30(3):275–280. doi: 10.1104/pp.30.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulasooriya S. A., Lang N. J., Fay P. The heterocysts of blue-green algae. 3. Differentiation and nitrogenase activity. Proc R Soc Lond B Biol Sci. 1972 Jun 6;181(1063):199–209. doi: 10.1098/rspb.1972.0046. [DOI] [PubMed] [Google Scholar]
- Kung S. D., Moscarello M. A., Williams J. P., Nadler H. Renaturation of DNA from the blue-green alga Lyngbya sp. Biochim Biophys Acta. 1971 Mar 11;232(2):252–254. doi: 10.1016/0005-2787(71)90577-6. [DOI] [PubMed] [Google Scholar]
- Kunisawa R., Cohen-Bazire G. Mutations of Anacystis nidulans that affect cell division. Arch Mikrobiol. 1970;71(1):49–59. doi: 10.1007/BF00412234. [DOI] [PubMed] [Google Scholar]
- LAZAROFF N., VISHNIAC W. The effect of light on this developmental cycle of Nostoc muscorum, a filamentous blue-green alga. J Gen Microbiol. 1961 Jul;25:365–374. doi: 10.1099/00221287-25-3-365. [DOI] [PubMed] [Google Scholar]
- LAZAROFF N., VISHNIAC W. The participation of filament anastomosis in the developmental cycle of Nostoc muscorum, a blue-green alga. J Gen Microbiol. 1962 Jun;28:203–210. doi: 10.1099/00221287-28-2-203. [DOI] [PubMed] [Google Scholar]
- LEAK L. V. ELECTRON MICROSCOPIC AUTORADIOGRAPHY INCORPORATION OF H3-THYMIDINE IN A BLUE-GREEN ALGA, ANABAENA SP. J Ultrastruct Res. 1965 Feb;12:135–146. doi: 10.1016/s0022-5320(65)80012-0. [DOI] [PubMed] [Google Scholar]
- LESTER R. L., CRANE F. L. The natural occurrence of coenzyme Q and related compounds. J Biol Chem. 1959 Aug;234(8):2169–2175. [PubMed] [Google Scholar]
- LEVIN E. Y., BLOCH K. ABSENCE OF STEROLS IN BLUE-GREEN ALGAE. Nature. 1964 Apr 4;202:90–91. doi: 10.1038/202090a0. [DOI] [PubMed] [Google Scholar]
- LEVIN E., LENNARZ W. J., BLOCH K. OCCURRENCE AND LOCALIZATION OF ALPHA-LINOLENIC ACID CONTAINING GALACTOLIPIDS IN THE PHOTOSYNTHETIC APPARATUS OF ANABAENA VARIABILLIS. Biochim Biophys Acta. 1964 Aug 5;84:471–474. doi: 10.1016/0926-6542(64)90015-0. [DOI] [PubMed] [Google Scholar]
- LOW E. M. Composition of the nucleic acids of some algae. Nature. 1958 Oct 18;182(4642):1096–1096. doi: 10.1038/1821096a0. [DOI] [PubMed] [Google Scholar]
- Lambein F., Wolk C. P. Structural studies on the glycolipids from the envelope of the heterocyst of Anabaena cylindrica. Biochemistry. 1973 Feb 27;12(5):791–798. doi: 10.1021/bi00729a002. [DOI] [PubMed] [Google Scholar]
- Lamont H. C. Shear-oriented microfibrils in the mucilaginous investments of two motile oscillatoriacean blue-green algae. J Bacteriol. 1969 Jan;97(1):350–361. doi: 10.1128/jb.97.1.350-361.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N. J., Fisher K. A. Variation in the fixation image of "structured granules" in Anabaena. Arch Mikrobiol. 1969;67(2):173–181. doi: 10.1007/BF00409683. [DOI] [PubMed] [Google Scholar]
- Lang N. J. The fine structure of blue-green algae. Annu Rev Microbiol. 1968;22:15–46. doi: 10.1146/annurev.mi.22.100168.000311. [DOI] [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Enzyme activities of the carbon reduction cycle in some photosynthetic organisms. Plant Physiol. 1969 Feb;44(2):295–300. doi: 10.1104/pp.44.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaroff N., Schiff J. Action Spectrum for Developmental Photo-Induction of the Blue-Green Alga Nostoc muscorum. Science. 1962 Aug 24;137(3530):603–604. doi: 10.1126/science.137.3530.603. [DOI] [PubMed] [Google Scholar]
- Leach C. K., Carr N. G. Electron transport and oxidative phosphorylation in the blue-green alga Anabaena variabilis. J Gen Microbiol. 1970 Nov;64(1):55–70. doi: 10.1099/00221287-64-1-55. [DOI] [PubMed] [Google Scholar]
- Leach C. K., Carr N. G. Oxidative phosphorylation in an extract of Anabaena variabilis. Biochem J. 1969 Mar;112(1):125–126. doi: 10.1042/bj1120125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach C. K., Carr N. G. Pyruvate: ferredoxin oxidoreductase and its activation by ATP in the blue-green alga Anabaena variabilis. Biochim Biophys Acta. 1971 Aug 6;245(1):165–174. doi: 10.1016/0005-2728(71)90019-3. [DOI] [PubMed] [Google Scholar]
- Leach C. K., Carr N. G. Reduced nicotinamide-adenine dinucleotide phosphate oxidase in the autotrophic blue-green algae Anabaena variabilis. Biochem J. 1968 Sep;109(2):4P–5P. doi: 10.1042/bj1090004pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak L. V. Fine structure of the mucilaginous sheath of Anabaena sp. J Ultrastruct Res. 1967 Nov;21(1):61–74. doi: 10.1016/s0022-5320(67)80006-6. [DOI] [PubMed] [Google Scholar]
- Leak L. V. Studies on the preservation and organization of DNA-containing regions in a blue-green alga, a ctochemical and ultrastructural study. J Ultrastruct Res. 1967 Oct 10;20(3):190–205. doi: 10.1016/s0022-5320(67)90281-x. [DOI] [PubMed] [Google Scholar]
- Lee J. J., Berns D. S. Protein aggregation. Studies of larger aggregates of C-phycocyanin. Biochem J. 1968 Dec;110(3):457–464. doi: 10.1042/bj1100457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. S., Young A. M., Krogmann D. W. Site-specific inactivation of the photophosphorylation reactions of Anabeana variabilis. Biochim Biophys Acta. 1969 May;180(1):130–136. doi: 10.1016/0005-2728(69)90200-x. [DOI] [PubMed] [Google Scholar]
- Lehmann H., Jost M. Assembly of gas vacuoles in a cell-free system of the blue-green alga Microcystis aeruginosa Kuetz. emend. Elenkin. Arch Mikrobiol. 1972;81(1):100–102. doi: 10.1007/BF00715027. [DOI] [PubMed] [Google Scholar]
- Lex M., Silvester W. B., Stewart W. D. Photorespiration and nitrogenase activity in the blue-green alga, Anabaena cylindrica. Proc R Soc Lond B Biol Sci. 1972 Jan 18;180(1058):87–102. doi: 10.1098/rspb.1972.0007. [DOI] [PubMed] [Google Scholar]
- Lightbody J. J., Krogmann D. W. Isolation and properties of plastocyanin from Anabaena variabilis. Biochim Biophys Acta. 1967 May 9;131(3):508–515. doi: 10.1016/0005-2728(67)90010-2. [DOI] [PubMed] [Google Scholar]
- Lightbody J. J., Krogmann D. W. The role of plastoquinone in the photosynthetic reactions of Anabaena variabilis. Biochim Biophys Acta. 1966 May 12;120(1):57–64. doi: 10.1016/0926-6585(66)90276-7. [DOI] [PubMed] [Google Scholar]
- Loening U. E., Ingle J. Diversity of RNA components in green plant tissues. Nature. 1967 Jul 22;215(5099):363–367. doi: 10.1038/215363a0. [DOI] [PubMed] [Google Scholar]
- Loening U. E. Molecular weights of ribosomal RNA in relation to evolution. J Mol Biol. 1968 Dec;38(3):355–365. doi: 10.1016/0022-2836(68)90391-4. [DOI] [PubMed] [Google Scholar]
- Lorenzen H., Venkataraman G. S. Synchronous cell divisions in Anacystis nidulans Richter. Arch Mikrobiol. 1969;67(3):251–255. doi: 10.1007/BF00411260. [DOI] [PubMed] [Google Scholar]
- Ludlow C. J., Park R. B. Action Spectra for Photosystems I and II in Formaldehyde Fixed Algae. Plant Physiol. 1969 Apr;44(4):540–543. doi: 10.1104/pp.44.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R., Haselkorn R. Comparison of blue-green algae virus LPP-1 and the morphologically related viruses G-3 and coliphage T7. Virology. 1968 Apr;34(4):675–678. doi: 10.1016/0042-6822(68)90088-3. [DOI] [PubMed] [Google Scholar]
- Luftig R., Haselkorn R. Morphology of a virus of blue-green algae and properties of its deoxyribonucleic acid. J Virol. 1967 Apr;1(2):344–361. doi: 10.1128/jvi.1.2.344-361.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R., Haselkorn R. Studies on the structure of blue-green algae virus LPP-1. Virology. 1968 Apr;34(4):664–674. doi: 10.1016/0042-6822(68)90087-1. [DOI] [PubMed] [Google Scholar]
- Lyman H., Siegelman H. W. Large-scale autotrophic culture of Euglena gracilis. J Protozool. 1967 May;14(2):297–299. doi: 10.1111/j.1550-7408.1967.tb02000.x. [DOI] [PubMed] [Google Scholar]
- MACLEAN F. I., FORREST H. S., MYERS J. ORIGIN OF THE SIDE-CHAIN IN PTERIDINES OF THE BIOPTERIN TYPE. Biochem Biophys Res Commun. 1965 Feb 17;18:623–626. doi: 10.1016/0006-291x(65)90801-6. [DOI] [PubMed] [Google Scholar]
- MACLEAN F. I., FUJITA Y., FORREST H. S., MYERS J. PHOTOSYNTHETIC PHOSPHORYLATION: STIMULATION BY PTERIDINES AND A COMPARISON WITH PHOSPHODOXIN. Science. 1965 Aug 6;149(3684):636–638. doi: 10.1126/science.149.3684.636. [DOI] [PubMed] [Google Scholar]
- METZNER I. Zur Chemie und zum submikroskopischen Aufbau der Zellwände, Scheiden und Gallerten von Cyanophyceen. II. Zellmorphologische und zellphysiologische Studien an Cyanophyceen. Arch Mikrobiol. 1955;22(1):45–77. [PubMed] [Google Scholar]
- MOSES V., HOLM-HANSEN O., CALVIN M. Nonphotosynthetic fixation of carbon dioxide by three microorganisms. J Bacteriol. 1959 Jan;77(1):70–78. doi: 10.1128/jb.77.1.70-78.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS J., KRATZ W. A. Relation between pigment content and photosynthetic characteristics in a blue-green algae. J Gen Physiol. 1955 Sep 20;39(1):11–22. doi: 10.1085/jgp.39.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacColl R., Berns D. S., Koven N. L. Effect of salts on C-phycocyanin. Arch Biochem Biophys. 1971 Oct;146(2):477–482. doi: 10.1016/0003-9861(71)90151-2. [DOI] [PubMed] [Google Scholar]
- MacColl R., Lee J. J., Berns D. S. Protein aggregation in C-phycocyanin. Studies at very low concentrations with the photoelectric scanner of the ultracentrifuge. Biochem J. 1971 May;122(4):421–426. doi: 10.1042/bj1220421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino F., Tsuzuki J. Absence of histone in the blue-green alga Anabaena cylindrica. Nature. 1971 Jun 18;231(5303):446–447. doi: 10.1038/231446a0. [DOI] [PubMed] [Google Scholar]
- McSwain B. D., Arnon D. I. Enhancement effects and the identity of the two photochemical reactions of photosynthesis. Proc Natl Acad Sci U S A. 1968 Nov;61(3):989–996. doi: 10.1073/pnas.61.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato S., Werbin H. Excitation and fluorescence spectra of the chromophore associated with the DNA-photoreactivating enzyme from the blue-green alga Anacystis nidulans. Photochem Photobiol. 1972 Jan;15(1):97–100. doi: 10.1111/j.1751-1097.1972.tb06227.x. [DOI] [PubMed] [Google Scholar]
- Moore B. G., Tischer R. G. Biosynthesis of extracellular polysaccharides by the blue-green alga Anabaena flos-aquae. Can J Microbiol. 1965 Dec;11(6):877–885. doi: 10.1139/m65-117. [DOI] [PubMed] [Google Scholar]
- NIKLOWITZ W., DREWS G. Beiträge zur Cytologie der Blaualgen. I. Untersuchungen zur Substruktur von Phormidium uncinatum Gom. Arch Mikrobiol. 1956;24(2):134–146. [PubMed] [Google Scholar]
- NIKLOWITZ W., DREWS G. Beiträge zur Cytologie der Blaualgen. IV. Vergleichende elektronenmikroskopische Untersuchungen zur Substruktur einiger Hormogonales. Arch Mikrobiol. 1957;27(2):150–165. [PubMed] [Google Scholar]
- NULTSCH W. [Separation of chromoproteins by gel filtration]. Biochim Biophys Acta. 1962 May 7;59:213–215. doi: 10.1016/0006-3002(62)90713-8. [DOI] [PubMed] [Google Scholar]
- Neilson A., Rippka R., Kunisawa R. Heterocyst formation and nitrogenase synthesis in Anabaena sp. A kinetic study. Arch Mikrobiol. 1971;76(2):139–150. doi: 10.1007/BF00411788. [DOI] [PubMed] [Google Scholar]
- Neufeld G. J., Riggs A. F. Aggregation properties of C-Phycocyanin from Anacystis nidulans. Biochim Biophys Acta. 1969 May;181(1):234–243. doi: 10.1016/0005-2795(69)90246-3. [DOI] [PubMed] [Google Scholar]
- Neumann Joseph, Ogawa Teruo, Vernon Leo P. Increased rate of cyclic photophophorylation in preparations from Anabaena variabilis cells grown in the presence of diphenylamine. FEBS Lett. 1970 Oct 16;10(4):253–256. doi: 10.1016/0014-5793(70)80641-x. [DOI] [PubMed] [Google Scholar]
- Neushul M. Uniformity of thylakoid structure in a red, a brown, and two blue-green algae. J Ultrastruct Res. 1971 Dec;37(5):532–543. doi: 10.1016/s0022-5320(71)80023-0. [DOI] [PubMed] [Google Scholar]
- Nichols B. W. Fatty acid metabolism in the chloroplast lipids of green and blue-green algae. Lipids. 1968 Jul;3(4):354–360. doi: 10.1007/BF02530939. [DOI] [PubMed] [Google Scholar]
- Nichols B. W., Harris R. V., James A. T. The lipid metabolism of blue-green algae. Biochem Biophys Res Commun. 1965 Jul 26;20(3):256–262. doi: 10.1016/0006-291x(65)90356-6. [DOI] [PubMed] [Google Scholar]
- Nichols B. W., Wood B. J. The occurrence and biosynthesis of gamma-linolenic acid in a blue-green alga,Spirulina platensis. Lipids. 1968 Jan;3(1):46–50. doi: 10.1007/BF02530968. [DOI] [PubMed] [Google Scholar]
- Niemeyer R., Richter G. Schnellmarkierte Polyphosphate und Metaphosphate bei der Glaualge Anacystis niculans. Arch Mikrobiol. 1969;69(1):54–59. [PubMed] [Google Scholar]
- Nishimura M. Energy-and electron-transfer systems in algal photosynthesis. II. Oxidation-reduction reactions of two cytochromes and interactions of two photochemical systems in red algae. Biochim Biophys Acta. 1968 May 28;153(4):838–847. doi: 10.1016/0005-2728(68)90010-8. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Takamiya A. Energy- and electron-transfer systems in algal photosynthesis. I. Actions of two photochemical systems in oxidation-reduction reactions of cytochrome in Porphyra. Biochim Biophys Acta. 1966 May 12;120(1):45–56. doi: 10.1016/0926-6585(66)90275-5. [DOI] [PubMed] [Google Scholar]
- Nultsch W. Effect of desaspidin and DCMU on photokinesis of blue-green algae. Photochem Photobiol. 1969 Aug;10(2):119–123. doi: 10.1111/j.1751-1097.1969.tb07228.x. [DOI] [PubMed] [Google Scholar]
- Nultsch W. Einfluss von Redox-Systemen auf die Bewegungsaktivität und das phototaktische Reaktionsverhalten von Phormidium uncinatum. Arch Mikrobiol. 1968;63(4):295–320. [PubMed] [Google Scholar]
- O'Carra P., Killilea S. D. Subunit structures of C-phycocyanin and C-phycoerythrin. Biochem Biophys Res Commun. 1971 Dec 3;45(5):1192–1197. doi: 10.1016/0006-291x(71)90144-6. [DOI] [PubMed] [Google Scholar]
- OLSON J. M., AMESZ J. Action spectra for fluorescence excitation of pyridine nucleotide in photosynthetic bacteria and algae. Biochim Biophys Acta. 1960 Jan 1;37:14–24. doi: 10.1016/0006-3002(60)90072-x. [DOI] [PubMed] [Google Scholar]
- OWENS O. V., HOCH G. ENHANCEMENT AND DE-ENHANCEMENT EFFECT IN ANACYSTIS NIDULANS. Biochim Biophys Acta. 1963 Sep 24;75:183–186. doi: 10.1016/0006-3002(63)90596-1. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Vernon L. P. Increased content of cytochromes 554 and 562 in Anabaena variabilis cells grown in the presence of diphenylamine. Biochim Biophys Acta. 1971 Jan 12;226(1):88–97. doi: 10.1016/0005-2728(71)90180-0. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Vernon L. P., Mollenhauer H. H. Properties and structure of fractions prepared from Anabaena variabilis by the action Triton X-100. Biochim Biophys Acta. 1969 Feb 25;172(2):216–229. doi: 10.1016/0005-2728(69)90065-6. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Vernon L. P. Properties of partially purified photosynthetic reaction centers from Scenedesmus mutant 6E and Anabaena variabilis grown in the presence of diphenylamine. Biochim Biophys Acta. 1970 Mar 3;197(2):292–301. doi: 10.1016/0005-2728(70)90040-x. [DOI] [PubMed] [Google Scholar]
- Olson J. M. The evolution of photosynthesis. Science. 1970 Apr 24;168(3930):438–446. doi: 10.1126/science.168.3930.438. [DOI] [PubMed] [Google Scholar]
- Osborn M. J. Structure and biosynthesis of the bacterial cell wall. Annu Rev Biochem. 1969;38:501–538. doi: 10.1146/annurev.bi.38.070169.002441. [DOI] [PubMed] [Google Scholar]
- PAYEN J. Les glucides de Phormidium tenue Meneg. Gom. C R Hebd Seances Acad Sci. 1953 May 4;236(18):1811–1813. [PubMed] [Google Scholar]
- PINTNER I. J., PROVASOLI L. Artificial cultivation of a red-pigmented marine blue-green alga, Phormidium persicinum. J Gen Microbiol. 1958 Feb;18(1):190–197. doi: 10.1099/00221287-18-1-190. [DOI] [PubMed] [Google Scholar]
- Padan E., Ginzburg D., Shilo M. The reproductive cycle of cyanophage LPP1-G in Plectonema boryanum and its dependence on photosynthetic and respiratory systems. Virology. 1970 Mar;40(3):514–521. doi: 10.1016/0042-6822(70)90194-7. [DOI] [PubMed] [Google Scholar]
- Padan E., Raboy B., Shilo M. Endogenous dark respiration of the blue-green alga, Plectonema boryanum. J Bacteriol. 1971 Apr;106(1):45–50. doi: 10.1128/jb.106.1.45-50.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Rimon A., Ginzberg D., Shilo M. A thermosensitive cyanophage (LPPI-G) attacking the blue-green alga Plectonema boryanum. Virology. 1971 Sep;45(3):773–776. doi: 10.1016/0042-6822(71)90193-0. [DOI] [PubMed] [Google Scholar]
- Padan E., Shilo M., Kislev N. Isolation of "cyanophages" from freshwater ponds and their interaction with Plectonema boryanum. Virology. 1967 Jun;32(2):234–246. doi: 10.1016/0042-6822(67)90273-5. [DOI] [PubMed] [Google Scholar]
- Padan E., Shilo M., Oppenheim A. B. Lysogeny of the blue-green alga Plectonema boryanum by LPP2-SPI cyanophage. Virology. 1972 Feb;47(2):525–526. doi: 10.1016/0042-6822(72)90294-2. [DOI] [PubMed] [Google Scholar]
- Padan E., Shilo M. Short-trichome mutant of Plectonema boryanum. J Bacteriol. 1969 Feb;97(2):975–976. doi: 10.1128/jb.97.2.975-976.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P. Growth of a photoautotroph, Plectomema boryanum, in the dark on glucose. Can J Microbiol. 1972 Mar;18(3):275–280. doi: 10.1139/m72-043. [DOI] [PubMed] [Google Scholar]
- Papageorgiou G., Govindjee Light-induced changes in the fluorescence yield of chlorophyll a in vivo. I. Anacystis nidulans. Biophys J. 1968 Nov;8(11):1299–1315. doi: 10.1016/S0006-3495(68)86557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou G., Govindjee pH control of the chlorophyll a fluorescence in algae. Biochim Biophys Acta. 1971 Jun 15;234(3):428–432. doi: 10.1016/0005-2728(71)90209-x. [DOI] [PubMed] [Google Scholar]
- Parker P. L., Van Baalen C., Maurer L. Fatty acids in eleven species of blue-green algae: geochemical significance. Science. 1967 Feb 10;155(3763):707–708. doi: 10.1126/science.155.3763.707. [DOI] [PubMed] [Google Scholar]
- Pearce J., Carr N. G. The incorporation and metabolism of glucose by Anabaena variabilis. J Gen Microbiol. 1968 Dec;54(3):451–462. doi: 10.1099/00221287-54-3-451. [DOI] [PubMed] [Google Scholar]
- Pearce J., Carr N. G. The metabolism of acetate by the blue-green algae, Anabaena variabilis and Anacystis nidulans. J Gen Microbiol. 1967 Nov;49(2):301–313. doi: 10.1099/00221287-49-2-301. [DOI] [PubMed] [Google Scholar]
- Pearce J., Leach C. K., Carr N. G. The incomplete tricarboxylic acid cycle in the blue-green alga Anabaena variabilis. J Gen Microbiol. 1969 Mar;55(3):371–378. doi: 10.1099/00221287-55-3-371. [DOI] [PubMed] [Google Scholar]
- Pierson B. K., Castenholz R. W. Bacteriochlorophylls in gliding filamentous prokaryotes from hot springs. Nat New Biol. 1971 Sep 1;233(35):25–27. doi: 10.1038/newbio233025a0. [DOI] [PubMed] [Google Scholar]
- Pigott G. H., Carr N. G. The assimilation of nucleic acid precursors by intact cells and protoplasts of the blue-green alga anacystis nidulans. Arch Mikrobiol. 1971;79(1):1–6. doi: 10.1007/BF00412035. [DOI] [PubMed] [Google Scholar]
- Pikálek P. Attempt to find genetic recombination in Anacystis nidulans. Nature. 1967 Aug 5;215(5101):666–667. doi: 10.1038/215666b0. [DOI] [PubMed] [Google Scholar]
- Pringsheim E. G. THE RELATIONSHIP BETWEEN BACTERIA AND MYXOPHYCEAE. Bacteriol Rev. 1949 Jun;13(2):47–98. doi: 10.1128/br.13.2.47-98.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnett T., Derrenbacker E. C. The amino acid composition of algal cell walls. J Gen Microbiol. 1966 Jul;44(1):105–114. doi: 10.1099/00221287-44-1-105. [DOI] [PubMed] [Google Scholar]
- RAFTERY M. A., OHEOCHA C. AMINO ACID COMPOSITION AND C-TERMINAL RESIDUES OF ALGAL BILIPROTEINS. Biochem J. 1965 Jan;94:166–170. doi: 10.1042/bj0940166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHTER G. Enzymes of polynucleotide synthesis in two photosynthetic organisms. Biochim Biophys Acta. 1963 Jun 25;72:342–344. [PubMed] [Google Scholar]
- RICHTER G. The lack of diphosphofructose aldolase in two photosynthetic organisms: Anacystis nidulans and Rhodopsudomonas spheroides. Biochim Biophys Acta. 1961 Apr 15;48:606–608. doi: 10.1016/0006-3002(61)90065-8. [DOI] [PubMed] [Google Scholar]
- RIS H., SINGH R. N. Electron microscope studies on blue-green algae. J Biophys Biochem Cytol. 1961 Jan;9:63–80. doi: 10.1083/jcb.9.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R., Cheniae G. M. Photoactivation of the manganese catalyst of 0 2 evolution II. A two-guantum mechanism. Biochim Biophys Acta. 1971 Nov 2;253(1):182–186. doi: 10.1016/0005-2728(71)90243-x. [DOI] [PubMed] [Google Scholar]
- Rao K. S., Tulpule P. G. Varietal differences of groundnut in the production of aflatoxin. Nature. 1967 May 13;214(5089):738–739. doi: 10.1038/214738b0. [DOI] [PubMed] [Google Scholar]
- Raven P. H. A multiple origin for plastids and mitochondria. Science. 1970 Aug 14;169(3946):641–646. doi: 10.1126/science.169.3946.641. [DOI] [PubMed] [Google Scholar]
- Reitz R. C., Hamilton J. G. The isolation and identification of two sterols from two species of blue-green algae. Comp Biochem Physiol. 1968 May;25(2):401–416. doi: 10.1016/0010-406x(68)90349-6. [DOI] [PubMed] [Google Scholar]
- Rippka R., Neilson A., Kunisawa R., Cohen-Bazire G. Nitrogen fixation by unicellular blue-green algae. Arch Mikrobiol. 1971;76(4):341–348. doi: 10.1007/BF00408530. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lopez M., Muñoz Calvo M. L., Gomez-Acebo J. The effect of rifamycins in the ultrastructure of Anacystis montana. J Ultrastruct Res. 1971 Sep;36(5):595–602. doi: 10.1016/s0022-5320(71)90017-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lopez M., Vazquez D. Comparative studies on cytoplasmic ribosomes from algae. Life Sci. 1968 Mar 15;7(6):327–336. doi: 10.1016/0024-3205(68)90030-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez-López M., Muñoz M. L., Vazquez D. The effects of the rifamycin antibiotics on algae. FEBS Lett. 1970 Sep 6;9(3):171–174. doi: 10.1016/0014-5793(70)80346-5. [DOI] [PubMed] [Google Scholar]
- Rüdiger W., Carra P. O. Studies on the structures and apoprotein linkages of the phycobilins. Eur J Biochem. 1969 Feb;7(4):509–516. doi: 10.1111/j.1432-1033.1969.tb19637.x. [DOI] [PubMed] [Google Scholar]
- SAFFERMAN R. S., MORRIS M. E. Algal virus: isolation. Science. 1963 May 10;140(3567):679–680. doi: 10.1126/science.140.3567.679. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER I. R., DIENER T. O., SAFFERMAN R. S. BLUE-GREEN ALGAL VIRUS LPP-1: PURIFICATION AND PARTIAL CHARACTERIZATION. Science. 1964 May 29;144(3622):1127–1130. doi: 10.1126/science.144.3622.1127. [DOI] [PubMed] [Google Scholar]
- SCHULZ G. Bewegungsstudien sowie elektronenmikroskopische Membranuntersuchungen an Cyanophyceen. Arch Mikrobiol. 1955;21(4):335–370. [PubMed] [Google Scholar]
- SHATKIN A. J. A chlorophyll-containing cell fraction from the blue-green alga, Anabaena variabilis. J Biophys Biochem Cytol. 1960 Jun;7:583–584. doi: 10.1083/jcb.7.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILBERGER J., HAXO F. Aqueous chlorophyll preparations from blue-green algae. Biol Bull. 1950 Oct;99(2):364–364. doi: 10.1086/BBLv99n2p321. [DOI] [PubMed] [Google Scholar]
- SORIANO S., LEWIN R. A. GLIDING MICROBES: SOME TAXONOMIC RECONSIDERATIONS. Antonie Van Leeuwenhoek. 1965;31:66–79. doi: 10.1007/BF02045876. [DOI] [PubMed] [Google Scholar]
- STANIER R. Y., VAN NIEL C. B. The concept of a bacterium. Arch Mikrobiol. 1962;42:17–35. doi: 10.1007/BF00425185. [DOI] [PubMed] [Google Scholar]
- SUSOR W. A., KROGMANN D. W. HILL ACTIVITY IN CELL-FREE PREPARATIONS OF A BLUE-GREEN ALGA. Biochim Biophys Acta. 1964 Jul 29;88:11–19. doi: 10.1016/0926-6577(64)90150-0. [DOI] [PubMed] [Google Scholar]
- Safferman R. S., Diener T. O., Desjardins P. R., Morris M. E. Isolation and characterization of AS-1, a phycovirus infecting the blue-green algae, Anacystis nidulans and Synechococcus cedrorum. Virology. 1972 Jan;47(1):105–113. doi: 10.1016/0042-6822(72)90243-7. [DOI] [PubMed] [Google Scholar]
- Safferman R. S., Morris M. E., Sherman L. A., Haselkorn R. Serological and electron microscopic characterization of a new group of blue-green algal viruses (LPP-2). Virology. 1969 Dec;39(4):775–780. doi: 10.1016/0042-6822(69)90015-4. [DOI] [PubMed] [Google Scholar]
- Safferman R. S., Schneider I. R., Steere R. L., Morris M. E., Diener T. O. Phycovirus SM-1: a virus infecting unicellular blue-green algae. Virology. 1969 Mar;37(3):386–395. doi: 10.1016/0042-6822(69)90222-0. [DOI] [PubMed] [Google Scholar]
- Saito N., Werbin H. Purification of a blue-green algal deoxyribonucleic acid photoreactiving enzyme. An enzyme requiring light as a physical cofactor to perform its catalytic function. Biochemistry. 1970 Jun 23;9(13):2610–2620. doi: 10.1021/bi00815a008. [DOI] [PubMed] [Google Scholar]
- Sala F., Sensi S., Parisi B. Peptide chain initiation in a species of Nostoc and in chloroplasts of Euglena gracilis. FEBS Lett. 1970 Sep 24;10(2):89–91. doi: 10.1016/0014-5793(70)80423-9. [DOI] [PubMed] [Google Scholar]
- Scheibe J. Photoreversible pigment: occurrence in a blue-green alga. Science. 1972 Jun 2;176(4038):1037–1039. doi: 10.1126/science.176.4038.1037. [DOI] [PubMed] [Google Scholar]
- Scher W. I., Vogel H. J. OCCURRENCE OF ORNITHINE delta-TRANSAMINASE: A DICHOTOMY. Proc Natl Acad Sci U S A. 1957 Sep 15;43(9):796–803. doi: 10.1073/pnas.43.9.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K. C., Bradbeer C., Singh R. N., Wang L. C., Wilson P. W., Burris R. H. NITROGEN FIXATION BY CELL-FREE PREPARATIONS FROM MICROORGANISMS. Proc Natl Acad Sci U S A. 1960 May;46(5):726–733. doi: 10.1073/pnas.46.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram B. L. Structure of phycocyanobilin. Biochem J. 1970 Sep;119(3):15P–15P. doi: 10.1042/bj1190015pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A., Haselkorn R. Growth of the blue-green algae virus LPP-1 under conditions which impair photosynthesis. Virology. 1971 Sep;45(3):739–746. doi: 10.1016/0042-6822(71)90188-7. [DOI] [PubMed] [Google Scholar]
- Sherman L. A., Haselkorn R. LPP-1 infection of the blue-green alga Plectonema boryanum. 3. Protein synthesis. J Virol. 1970 Dec;6(6):841–846. doi: 10.1128/jvi.6.6.841-846.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A., Haselkorn R. LPP-1 infection of the blue-green alga Plectonema boryanum. I. Electron microscopy. J Virol. 1970 Dec;6(6):820–833. doi: 10.1128/jvi.6.6.820-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A., Haselkorn R. LPP-1 infection of the blue-green alga Plectonema boryanum. II. Viral deoxyribonucleic acid synthesis and host deoxyribonucleic acid breakdown. J Virol. 1970 Dec;6(6):834–840. doi: 10.1128/jvi.6.6.834-840.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestakov S. V., Khyen N. T. Evidence for genetic transformation in blue-green alga Anacystis nidulans. Mol Gen Genet. 1970;107(4):372–375. doi: 10.1007/BF00441199. [DOI] [PubMed] [Google Scholar]
- Shilo M. Formation and mode of action of algal toxins. Bacteriol Rev. 1967 Sep;31(3):180–193. doi: 10.1128/br.31.3.180-193.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. M., Decker G. L., Greenawalt J. W. Comparative ultrastructure of the thiobacilli. J Bacteriol. 1970 Feb;101(2):618–627. doi: 10.1128/jb.101.2.618-627.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelman H. W., Chapman D. J., Cole W. J. Enzymatic cleavage of phycocyanobilin. Arch Biochem Biophys. 1967 Oct;122(1):261–261. doi: 10.1016/0003-9861(67)90152-x. [DOI] [PubMed] [Google Scholar]
- Simon R. D. Cyanophycin Granules from the Blue-Green Alga Anabaena cylindrica: A Reserve Material Consisting of Copolymers of Aspartic Acid and Arginine. Proc Natl Acad Sci U S A. 1971 Feb;68(2):265–267. doi: 10.1073/pnas.68.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H. N., Srivastava B. S. Studies on morphogenesis in a blue-green alga. I. Effect of inorganic niteogen sources on developmental morphology of Anabaena doliolum. Can J Microbiol. 1968 Dec;14(12):1341–1346. doi: 10.1139/m68-224. [DOI] [PubMed] [Google Scholar]
- Singh R. N., Tiwari D. N. Induction by ultraviolet irradiation of mutation in the blue-green alga Nostoc linckia (Roth) Born. et Flah. Nature. 1969 Jan 4;221(5175):62–64. doi: 10.1038/221062a0. [DOI] [PubMed] [Google Scholar]
- Smillie R. M. Isolation of two proteins with chloroplast ferredoxin activity from a blue-green alga. Biochem Biophys Res Commun. 1965 Sep 8;20(5):621–629. doi: 10.1016/0006-291x(65)90445-6. [DOI] [PubMed] [Google Scholar]
- Smith A. J., London J., Stanier R. Y. Biochemical basis of obligate autotrophy in blue-green algae and thiobacilli. J Bacteriol. 1967 Oct;94(4):972–983. doi: 10.1128/jb.94.4.972-983.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. M., Brown R. M., Jr, Goldstein D. A., Walne P. L. Culture methods for the blue-green alga Plectonema boryanum and its virus, with an electron microscope study of virus-infected cells. Virology. 1966 Apr;28(4):580–591. doi: 10.1016/0042-6822(66)90243-1. [DOI] [PubMed] [Google Scholar]
- Smith K. M., Brown R. M., Jr, Walne P. L., Goldstein D. A. Electron microscopy of the infection process of the blue-green alga virus. Virology. 1966 Oct;30(2):182–192. doi: 10.1016/0042-6822(66)90094-8. [DOI] [PubMed] [Google Scholar]
- Smith K. M., Brown R. M., Jr, Walne P. L. Ultrastructural and time-lapse studies on the replication cycle of the blue-green algal virus LPP-1. Virology. 1967 Feb;31(2):329–337. doi: 10.1016/0042-6822(67)90178-x. [DOI] [PubMed] [Google Scholar]
- Smith R. V., Evans M. C. Nitrogenase activity in cell-free extracts of the blue-green alga, Anabaena cylindrica. J Bacteriol. 1971 Mar;105(3):913–917. doi: 10.1128/jb.105.3.913-917.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. V., Evans M. C. Soluble nitrogenase from vegetative cells of the blue-green alga Anabaena cylindrica. Nature. 1970 Mar 28;225(5239):1253–1254. doi: 10.1038/2251253a0. [DOI] [PubMed] [Google Scholar]
- Smith R. V., Noy R. J., Evans M. C. Physiological electron donor systems to the nitrogenase of the blue-green alga Anabaena cylindrica. Biochim Biophys Acta. 1971 Nov 2;253(1):104–109. doi: 10.1016/0005-2728(71)90238-6. [DOI] [PubMed] [Google Scholar]
- Smith R. V., Telfer A., Evans M. C. Complementary functioning of nitrogenase components from a blue-green alga and a photosynthetic bacterium. J Bacteriol. 1971 Aug;107(2):574–575. doi: 10.1128/jb.107.2.574-575.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spomer G. G. Molecular diversity of the ribulose-1,5-diphosphate carboxylase from photosynthetic microorganisms. Science. 1968 Aug 2;161(3840):482–485. [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens S. E., Jr, Van Baalen C. Growth characteristics of selected mutants of a coccoid blue-green alga. Arch Mikrobiol. 1970;72(1):1–8. doi: 10.1007/BF00411008. [DOI] [PubMed] [Google Scholar]
- Stewart W. D. Biological and ecological aspects of nitrogen fixation by free-living micro-organisms. Proc R Soc Lond B Biol Sci. 1969 Apr 1;172(1029):367–388. doi: 10.1098/rspb.1969.0027. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Fitzgerald G. P., Burris R. H. Acetylene reduction by nitrogen-fixing blue-green algae. Arch Mikrobiol. 1968;62(4):336–348. doi: 10.1007/BF00425639. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Haystead A., Pearson H. W. Nitrogenase activity in heterocysts of blue-green algae. Nature. 1969 Oct 18;224(5216):226–228. doi: 10.1038/224226a0. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Lex M. Nitrogenase activity in the blue-green alga Plectonema boryanum strain 594. Arch Mikrobiol. 1970;73(3):250–260. doi: 10.1007/BF00410626. [DOI] [PubMed] [Google Scholar]
- Stoddart R. W., Northcote D. H. Metabolic relationships of the isolated fractions of the pectic substances of actively growing sycamore cells. Biochem J. 1967 Oct;105(1):45–59. doi: 10.1042/bj1050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonington O. G., Pettijohn D. E. The folded genome of Escherichia coli isolated in a protein-DNA-RNA complex. Proc Natl Acad Sci U S A. 1971 Jan;68(1):6–9. doi: 10.1073/pnas.68.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stransky H., Hager A. Das Carotinoidmuster und die Verbreitung des lichtinduzierten Xanthophyllcyclus in verschiedenen Algenklassen. IV. Cyanophyceae und Rhodophyceae. Arch Mikrobiol. 1970;72(1):84–96. [PubMed] [Google Scholar]
- Sun E., Barr R., Crane F. L. Comparative Studies on Plastoquinones. IV. Plastoquinones in Algae. Plant Physiol. 1968 Dec;43(12):1935–1940. doi: 10.1104/pp.43.12.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susor W. A., Krogmann D. W. Triphosphopyridine nucleotide photoreduction with cell-free preparations of Anabaena variabilis. Biochim Biophys Acta. 1966 May 12;120(1):65–72. doi: 10.1016/0926-6585(66)90277-9. [DOI] [PubMed] [Google Scholar]
- Swain F. M. Paleomicrobiology. Annu Rev Microbiol. 1969;23:455–472. doi: 10.1146/annurev.mi.23.100169.002323. [DOI] [PubMed] [Google Scholar]
- TAHA E. E., EL REFAIAE Physiological and biochemical studies on nitrogen fixing blue-green algae. I. On the nature of cellular and extracellular nitrogenous substances formed by Nostoc commune. Arch Mikrobiol. 1962;41:307–312. doi: 10.1007/BF00403609. [DOI] [PubMed] [Google Scholar]
- TAHA E. E., ELREFAI M. H. Physiological and biochemical studies on nitrogen fixing blue-green algae. Arch Mikrobiol. 1962;43:67–75. doi: 10.1007/BF00408396. [DOI] [PubMed] [Google Scholar]
- TAYLOR M. M., STORCK R. UNIQUENESS OF BACTERIAL RIBOSOMES. Proc Natl Acad Sci U S A. 1964 Oct;52:958–965. doi: 10.1073/pnas.52.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS J. B., DE ROVER W. On phycocyanin participation in the Hill reaction of the blue-green alga Synechococcus cedrorum. Biochim Biophys Acta. 1955 Mar;16(3):391–395. doi: 10.1016/0006-3002(55)90243-2. [DOI] [PubMed] [Google Scholar]
- TISCHER I. Untersuchungen über die granulären Einschlüsse und das Reduktions-Oxydations-Vermögen der Cyanophyceen. IV. Zellmorphologische und zellphysiologische Studien an Cyanophyceen. Arch Mikrobiol. 1957;27(4):400–428. [PubMed] [Google Scholar]
- TOMITA G., RABINOWITCH E. Excitation energy transfer between pigments in photosynthetic cells. Biophys J. 1962 Nov;2:483–499. doi: 10.1016/s0006-3495(62)86869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F. L., Krogmann D. W. Cytochrome Oxidase Activity in Cell-free Preparations from Blue-Green Algae. Plant Physiol. 1972 Feb;49(2):264–266. doi: 10.1104/pp.49.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichler-Zallen D., Hoch G. Cyclic electron transport in algae. Arch Biochem Biophys. 1967 Apr;120(1):227–230. doi: 10.1016/0003-9861(67)90620-0. [DOI] [PubMed] [Google Scholar]
- Thomas J. Absence of the pigments of photosystem II of photosynthesis in heterocysts of a blue-green alga. Nature. 1970 Oct 10;228(5267):181–183. doi: 10.1038/228181b0. [DOI] [PubMed] [Google Scholar]
- Thomas J. Relationship between age of culture and occurrence of the pigments of photosystem II of photosynthesis in heterocysts of a blue-green alga. J Bacteriol. 1972 Apr;110(1):92–95. doi: 10.1128/jb.110.1.92-95.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornber J. P. Comparison of a chlorophyll a- protein complex isolated from a blue-green alga with chlorophyll-protein complexes obtained from green bacteria and higher plants. Biochim Biophys Acta. 1969 Feb 25;172(2):230–241. doi: 10.1016/0005-2728(69)90066-8. [DOI] [PubMed] [Google Scholar]
- Troxler R. F., Brown A. Biosynthesis of phycocyanin in vivo. Biochim Biophys Acta. 1970 Sep 22;215(3):503–511. doi: 10.1016/0304-4165(70)90100-5. [DOI] [PubMed] [Google Scholar]
- Troxler R. F., Brown A., Lester R., White P. Bile pigment formation in plants. Science. 1970 Jan 9;167(3915):192–193. doi: 10.1126/science.167.3915.192. [DOI] [PubMed] [Google Scholar]
- Troxler R. F., Lester R. Formation, Chromophore Composition, and Labeling Specificity of Cyanidium caldarium Phycocyanin. Plant Physiol. 1968 Oct;43(10):1737–1739. doi: 10.1104/pp.43.10.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsusué Y., Yamakawa T. Chemical structure of oligosaccharides in the blue-green alga, Tolypothrix tenuis. J Biochem. 1965 Dec;58(6):587–594. doi: 10.1093/oxfordjournals.jbchem.a128247. [DOI] [PubMed] [Google Scholar]
- VAN BAALEN C. Vitamin B12 requirement of a marine blue-green alga. Science. 1961 Jun 16;133(3468):1922–1923. doi: 10.1126/science.133.3468.1922. [DOI] [PubMed] [Google Scholar]
- VANBAALEN C., MARLER J. E. CHARACTERISTICS OF MARINE BLUE-GREEN ALGAE WITH URIC ACID AS NITROGEN SOURCE. J Gen Microbiol. 1963 Sep;32:457–463. doi: 10.1099/00221287-32-3-457. [DOI] [PubMed] [Google Scholar]
- VON ZASTROW E. M. Uber die Organisation der Cyanophyceenzelle. Arch Mikrobiol. 1953;19(2):174–205. [PubMed] [Google Scholar]
- Van Baalen C. Aldolase in blue-green algae. Nature. 1965 Apr 10;206(980):193–195. doi: 10.1038/206193a0. [DOI] [PubMed] [Google Scholar]
- Van Baalen C., Brown R. M., Jr The ultrastructure of the marine blue green alga, Trichodesmium erythraeum, with special reference to the cell wall, gas vacuoles, and cylindrical bodies. Arch Mikrobiol. 1969;69(1):79–91. doi: 10.1007/BF00408566. [DOI] [PubMed] [Google Scholar]
- Van Baalen C., Hoare D. S., Brandt E. Heterotrophic growth of blue-gren algae in dim light. J Bacteriol. 1971 Mar;105(3):685–689. doi: 10.1128/jb.105.3.685-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Baalen C. Mutation of the Blue-Green Alga, Anacystis nidulans. Science. 1965 Jul 2;149(3679):70–70. doi: 10.1126/science.149.3679.70. [DOI] [PubMed] [Google Scholar]
- Van Baalen C., O'Donnell R. Action spectra for ultraviolet killing and photoreactivation in the blue-green alga Agmenellum quadruplicatum. Photochem Photobiol. 1972 Mar;15(3):269–274. doi: 10.1111/j.1751-1097.1972.tb07331.x. [DOI] [PubMed] [Google Scholar]
- Van Baalen C. The Photooxidation of Uric Acid by Anacystis nidulans. Plant Physiol. 1965 Mar;40(2):368–371. doi: 10.1104/pp.40.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatarman G. S., Lorenzen H. Biochemical studies on Anacystis nidulans during its synchronous growth. Arch Mikrobiol. 1969;69(1):34–39. doi: 10.1007/BF00408560. [DOI] [PubMed] [Google Scholar]
- Vennesland B., Turkington E. O. The relationship of the Hill reaction to photosynthesis: studies with fluoride-poisoned blue-green algae. Arch Biochem Biophys. 1966 Sep 26;116(1):153–161. doi: 10.1016/0003-9861(66)90023-3. [DOI] [PubMed] [Google Scholar]
- WATANABE A., NISHIGAKI S., KONISHI C. Effect of nitrogen-fixing blue-green algae on the growth of rice plants. Nature. 1951 Oct 27;168(4278):748–749. doi: 10.1038/168748b0. [DOI] [PubMed] [Google Scholar]
- WATANABE A. Production in cultural solution of some amino acids by the atmospheric nitrogen-fixing blue-green algae. Arch Biochem Biophys. 1951 Nov;34(1):50–55. doi: 10.1016/s0003-9861(51)80007-9. [DOI] [PubMed] [Google Scholar]
- WILSON P. W. The comparative biochemistry of nitrogen fixation. Adv Enzymol Relat Subj Biochem. 1952;13:345–375. doi: 10.1002/9780470122587.ch9. [DOI] [PubMed] [Google Scholar]
- WORK E., DEWEY D. L. The distribution of alpha, epsilon-diaminopimelic acid among various micro-organisms. J Gen Microbiol. 1953 Dec;9(3):394–406. doi: 10.1099/00221287-9-3-394. [DOI] [PubMed] [Google Scholar]
- Waaland J. R., Branton D. Gas vacuole development in a blue-green alga. Science. 1969 Mar 21;163(3873):1339–1341. doi: 10.1126/science.163.3873.1339. [DOI] [PubMed] [Google Scholar]
- Waaland J. R., Waaland S. D., Branton D. Gas vacuoles. Light shielding in blue-green algae. J Cell Biol. 1971 Jan;48(1):212–215. doi: 10.1083/jcb.48.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby A. E., Nichols B. W. Lipid composition of heterocysts. Nature. 1969 Feb 15;221(5181):673–674. doi: 10.1038/221673a0. [DOI] [PubMed] [Google Scholar]
- Walsby A. E. Structure and function of gas vacuoles. Bacteriol Rev. 1972 Mar;36(1):1–32. doi: 10.1128/br.36.1.1-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver E. C. Kinetic behavior of EPR signal. I. Induction effects in intact algae. Photochem Photobiol. 1968 Jan;7(1):93–100. doi: 10.1111/j.1751-1097.1968.tb05833.x. [DOI] [PubMed] [Google Scholar]
- Weber H. L., Böck A. Regulation of the chorismic acid branch-point in aromatic acid synthesis in blue-green and green algae. Arch Mikrobiol. 1969;66(3):250–258. doi: 10.1007/BF00412057. [DOI] [PubMed] [Google Scholar]
- Webster G. C., Frenkel A. W. Some Respiratory Characteristics of the Blue-Green Alga, Anabaena. Plant Physiol. 1953 Jan;28(1):63–69. doi: 10.1104/pp.28.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise G., Drews G., Jann B., Jann K. Identification and analysis of a lipopolysaccharide in cell walls of the blue-green alga Anacystis nidulans. Arch Mikrobiol. 1970;71(1):89–98. doi: 10.1007/BF00412238. [DOI] [PubMed] [Google Scholar]
- Werbin H., Rupert C. S. Presence of photoreactivating enzyme in blue-green algal cells. Photochem Photobiol. 1968 Feb;7(2):225–230. doi: 10.1111/j.1751-1097.1968.tb08008.x. [DOI] [PubMed] [Google Scholar]
- Whitton B. A., Carr N. G., Craig I. W. A comparison of the fine structure and nucleic acid biochemistry of chloroplasts and blue-green algae. Protoplasma. 1971;72(2):325–357. doi: 10.1007/BF01279057. [DOI] [PubMed] [Google Scholar]
- Whitton B. A. Extracellular products of blue-green algae. J Gen Microbiol. 1965 Jul;40(1):1–11. doi: 10.1099/00221287-40-1-1. [DOI] [PubMed] [Google Scholar]
- Whitton B. A., Peat A. On Oscillatoria redkei Van Goor. Arch Mikrobiol. 1969;68(4):362–376. doi: 10.1007/BF00408860. [DOI] [PubMed] [Google Scholar]
- Wilcox M. One-dimensional pattern found in blue-green algae. Nature. 1970 Nov 14;228(5272):686–687. doi: 10.1038/228686a0. [DOI] [PubMed] [Google Scholar]
- Wildon D. C., Rees T. A. Metabolism of Glucose-C by Anabaena cylindrica. Plant Physiol. 1965 Mar;40(2):332–335. doi: 10.1104/pp.40.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard J. M., Gibbs M. Purification and characterization of the fructose diphosphate aldolases from Anacystis is nidulans and Saprospira thermalis. Biochim Biophys Acta. 1968 Feb 5;151(2):438–448. doi: 10.1016/0005-2744(68)90112-5. [DOI] [PubMed] [Google Scholar]
- Willard J. M., Gibbs M. Role of Aldolase in Photosynthesis. II Demonstration of Aldolase Types in Photosynthetic Organisms. Plant Physiol. 1968 May;43(5):793–798. doi: 10.1104/pp.43.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard J. M., Schulman M., Gibbs M. Aldolase in Anacystis nidulans and Rhodopseudomonas spheroides. Nature. 1965 Apr 10;206(980):195–195. doi: 10.1038/206195a0. [DOI] [PubMed] [Google Scholar]
- Winters K., Parker P. L., Van Baalen C. Hydrocarbons of blue-green algae: geochemical signfficance. Science. 1969 Jan 31;163(3866):467–468. doi: 10.1126/science.163.3866.467. [DOI] [PubMed] [Google Scholar]
- Wolk C. P. Control of sporulation in a blue-green alga. Dev Biol. 1965 Aug;12(1):15–35. doi: 10.1016/0012-1606(65)90018-7. [DOI] [PubMed] [Google Scholar]
- Wolk C. P. Movement of carbon from vegetative cells to heterocysts in Anabaena cylindrica. J Bacteriol. 1968 Dec;96(6):2138–2143. doi: 10.1128/jb.96.6.2138-2143.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk C. P. Physiological basis of the pattern of vegetative growth of a blue-green alga. Proc Natl Acad Sci U S A. 1967 May;57(5):1246–1251. doi: 10.1073/pnas.57.5.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt J. T., Silvey J. K. Nitrogen fixation by gloeocapsa. Science. 1969 Aug 29;165(3896):908–909. doi: 10.1126/science.165.3896.908. [DOI] [PubMed] [Google Scholar]
- Yamanaka T., Takenami S., Wada K., Okunuki K. Purification and some properties of ferredoxin derived from the blue-green alga, Anacystis nidulans. Biochim Biophys Acta. 1969 May;180(1):196–198. doi: 10.1016/0005-2728(69)90208-4. [DOI] [PubMed] [Google Scholar]
- ZEHNDER A., GORHAM P. R. Factors influencing the growth of Microcystis aeruginosa Kutz, emend, Elenkin. Can J Microbiol. 1960 Dec;6:645–660. doi: 10.1139/m60-077. [DOI] [PubMed] [Google Scholar]
- ZIEGLER I. [Di- and tetrahydropterin in the blue algae Anacystis nidulans]. Biochem Z. 1963;337:62–68. [PubMed] [Google Scholar]
- van Gorkom H. J., Donze M. Localization of nitrogen fixation in Anabaena. Nature. 1971 Nov 26;234(5326):231–232. doi: 10.1038/234231b0. [DOI] [PubMed] [Google Scholar]