Abstract
Background
Protein Tyrosine Phosphatases (PTPs) are enzymes that catalyze phosphotyrosine dephosphorylation and modulate cell differentiation, growth and metabolism. In mammals, PTPs play a key role in the modulation of canonical pathways involved in metabolism and immunity. PTP1B is the prototype member of classical PTPs and a major target for treating human diseases, such as cancer, obesity and diabetes. These signaling enzymes are, hence, targets of a wide array of inhibitors. Anautogenous mosquitoes rely on blood meals to lay eggs and are vectors of the most prevalent human diseases. Identifying the mosquito ortholog of PTP1B and determining its involvement in egg production is, therefore, important in the search for a novel and crucial target for vector control.
Methodology/Principal Findings
We conducted an analysis to identify the ortholog of mammalian PTP1B in the Aedes aegypti genome. We identified eight genes coding for classical PTPs. In silico structural and functional analyses of proteins coded by such genes revealed that four of these code for catalytically active enzymes. Among the four genes coding for active PTPs, AAEL001919 exhibits the greatest degree of homology with the mammalian PTP1B. Next, we evaluated the role of this enzyme in egg formation. Blood feeding largely affects AAEL001919 expression, especially in the fat body and ovaries. These tissues are critically involved in the synthesis and storage of vitellogenin, the major yolk protein. Including the classical PTP inhibitor sodium orthovanadate or the PTP substrate DiFMUP in the blood meal decreased vitellogenin synthesis and egg production. Similarly, silencing AAEL001919 using RNA interference (RNAi) assays resulted in 30% suppression of egg production.
Conclusions/Significance
The data reported herein implicate, for the first time, a gene that codes for a classical PTP in mosquito egg formation. These findings raise the possibility that this class of enzymes may be used as novel targets to block egg formation in mosquitoes.
Introduction
Tyrosine phosphorylation is part of a complex cell repertoire that first appeared nearly 600-million years ago and is largely responsible for the emergence of the first multicellular animals [1]. Protein tyrosine phosphatases (PTPs) are enzymes that catalyze tyrosine dephosphorylation and regulate central steps of cell biology. The PTP family is composed of four different subfamilies. The active sites of classes I, II and III each harbor a cysteine, which is involved in catalysis. In Class IV, this cysteine is replaced by aspartic acid [2]. Class I Cys-PTPs are the largest group of PTPs and are divided into “classical” and dual specificity phosphatases. Classical phosphatases are enzymes that are strictly devoted to the dephosphorylation of phosphotyrosine residues. Classical PTPs have been further subdivided into receptor PTPs and soluble or non-transmembrane PTPs [3].
Aedes aegypti is the vector of Dengue and yellow fever. Once it feeds on blood, a complex series of signaling events lead to yolk synthesis and egg formation. Synthesized by the female mosquito fat body, vitellogenin (Vg), the main yolk protein, is the ultimate result of a chain of endocrine and signaling events that are still not completely understood. It has been shown that, after a blood meal, the amino-acid concentration in the hemolymph increases and the synthesis of brain-derived signaling molecules, such as insulin-like peptides, is induced [4]–[6]. Such peptides stimulate the ovaries to produce ecdysone, which then induces the fat body to produce Vg. Vg production by the fat body also relies on amino acids derived from blood digestion, which activate the TOR/S6k signaling cascade [7], [8]. Vg is then secreted by the fat body into the hemolymph and taken up by the developing follicles via receptor-mediated endocytosis. In mosquitoes, the interaction of insulin or insulin-like peptides with the mosquito insulin receptor (MIR) triggers the PI3K/Akt pathway and promotes the production of ecdysteroids, the regulation of egg formation and immunity [9], [10]. Furthermore, inhibition of PTPs an antagonist of the insulin pathway decreases ecdysteroid production by mosquito ovaries [9]. Silencing of the Phosphatase and Tensin homologue (PTEN), an antagonist of the PI3K pathway, leads to an increase in egg formation [10]. The above studies suggest the presence of PTPs as modulators of egg formation in mosquitoes, but the genes coding for these enzymes have not yet been identified. It is possible that the inhibition of PTP activity encoded by such genes may ultimately reduce or impair the ability of female mosquitoes to lay eggs, as demonstrated for other components involved in vitellogenesis [11], [12].
In the study reported herein, we conducted a bioinformatics analysis of the A. aegypti genome to identify the mosquito ortholog of PTP1B and determine its involvement in egg formation [13]. The inhibition of these regulators or the blocking of proteins under their transcriptional control can potentially provide new targets for suppression of egg formation and pathogen transmission by mosquitoes [14]. Among the genes that encode mosquito PTPs, AAEL001919 has the highest (53%) identity with human PTP1B. The treatment of blood-fed mosquitoes with classical PTP inhibitors or the silencing of this gene through RNAi partially blocked egg production. Thus, AAEL001919 may present a potential target for the control of tyrosine phosphorylation in mosquitoes and may ultimately be used to decrease mosquito reproduction.
Results
In silico analysis of soluble PTP sequences
The search for PTP sequences in the Aedes aegypti genome has led to the discovery of 8 PTP genes coding for 10 proteins belonging to Class I soluble PTPs: AAEL001046, AAEL001919-PA, AAEL001919-PB, AAEL003108, AAEL005492, AAEL008528-PA, AAEL008528-PB, AAEL010234, AAEL010914 and AAEL011434 (Figure 1). Among the genes that code for classical PTPs in Aedes, two, AAEEL001919 and AAEL008528, presented mRNA splice variants denoted as RA or RB. For each of these genes, the distinguishing character was the presence of at least one classical PTP domain (Figure 1). Some PTPs showed accessory domains, including AAEL003108, that harbored adaptor domains, including FERM and PDZ, which are crucial for signal transduction (Figure 1). Several of these sequences also showed the presence of inactive or absent PTP domains, the significance of which can be linked to the role of pseudo-phosphatases in signaling. Therefore, these sequences may function as adaptor molecules that link pathways rather than fill catalytic roles [15].
Figure 1. Domain architecture of A. aegypti Protein Tyrosine Phosphatases.
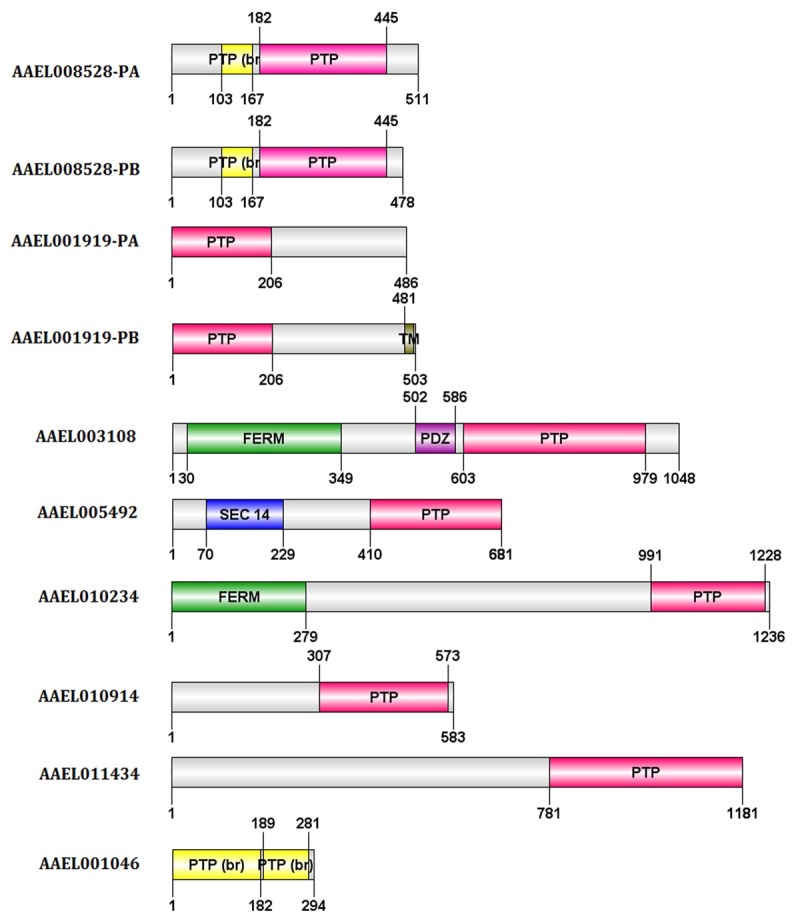
The ten Protein Tyrosine Phosphatase sequences from A. aegypti were analyzed for the presence biologically active domains. The domains were identified using a conserved sequence search as mentioned in the methods. Domain architecture was mapped onto the sequence using the DOG 1.0 protein-structure illustrator (DOG 1.0: illustrator of protein domain structures). Nomenclature used is as follows: PTP - Protein Tyrosine Phosphatase domain, PTP (br) - Absent Protein Tyrosine Phosphatase domain, SEC14 - Phosphatidylinositol transfer protein domain, FERM - F for 4.1 protein, E for ezrin, R for radixin and M for moesin, cytoskeletal association domain (includes the FA: FERM-associated domain) PDZ - also called DHR or GLGF, adaptor domain.
Analysis of PTP sequences and their specific motifs
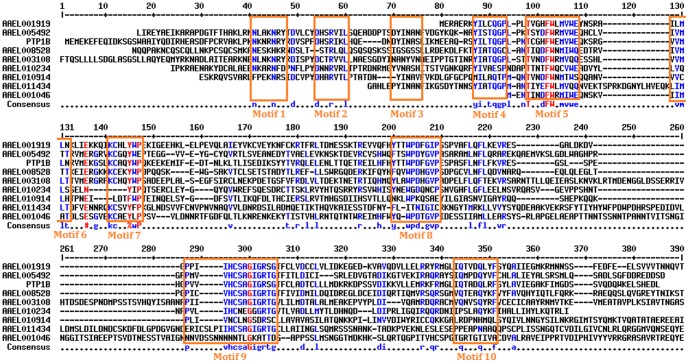
As the PTP domain is defined by ten sequence and structural motifs [16], the sequences of interest were analyzed in detail for PTP motifs. Of the ten motifs, special attention was given to the active-site motifs, motifs 1, 8, 9 and 10. The other six motifs (2, 3, 4, 5, 6, 7) form the core of the PTP protein and are implicated in the thermodynamic stabilities of the molecules. Sequence conservation in the four active-site motifs was, therefore, used as a tool to classify the PTP sequences into active or inactive PTP domains (Table 1 and Table 2). PTP activity is critically dependent on the nucleophilic cysteine present in Motif 9 (also called the HCS motif) [17], [18]. Motif 1 (also called the KNRY loop), while not directly involved in the reaction per se, is crucial for recruiting the phosphotyrosine into the PTP active site and distinguishes PTPs from serine/threonine phosphatases [19].
Table 1. Analysis of PTP catalytic sequences.
| Protein Accession number | Number of PTP domains | PTP domain Boundary (amino acid sequence number) | Sequence of Motif 1, Motif 8, Motif 9 and Motif 10 | Motifs active/inactive | PTP domain predicted active/inactive |
| AAEL001046 | 2 | PTP ABSENT (1–182) | INACTIVE/DOMAIN ABSENT | INACTIVE | INACTIVE |
| PTP ABSENT (189–281) | INACTIVE/DOMAIN ABSENT | INACTIVE | INACTIVE | ||
| AAEL001919-PA | 1 | 1–206 | (ABSENT) YTTWPDFGIP PIIHCSAGIGRSGT IQTVDQLYF | INACTIVE ACTIVE ACTIVE ACTIVE | ACTIVE |
| AAEL001919-PB | 1 | 1–206 | (ABSENT) YTTWPDFGIP PIIHCSAGIGRSGT IQTVDQLYF | INACTIVE ACTIVE ACTIVE ACTIVE | ACTIVE |
| AAEL003108 | 1 | 603–979 | NLNKNRY YLAWPDHGVP PIIHCSAGIGRTG VQNVSQYRF | ACTIVE ACTIVE ACTIVE ACTIVE | ACTIVE |
| AAEL005492 | 1 | 410–681 | NLAKNRY FTSWPDYGVP PMVVHCSAGIGRT IQMPDQYVF | ACTIVE ACTIVE ACTIVE ACTIVE | ACTIVE |
| AAEL008528-PA | 2 | PTP (ABSENT) 103–167 | INACTIVE/DOMAIN ABSENT | INACTIVE | INACTIVE |
| PTP (205–445) | NESKHKR FQVWPDHGVP PICVHCSAGIGRT VQTEAQYKF | INACTIVE ACTIVE ACTIVE ACTIVE | ACTIVE | ||
| AAEL008528-PB | 2 | PTP (ABSENT) 103–167 | INACTIVE/DOMAIN ABSENT | INACTIVE | INACTIVE |
| PTP (205–445) | NESKHKR FQVWPDHGVP PICVHCSAGIGRT VQTEAQYKF | INACTIVE ACTIVE ACTIVE ACTIVE | ACTIVE | ||
| AAEL010234 | 1 | 991–1228 | NKARNF YNEWGDQNCP PPVLIHCNEGGGRTPSLAQYKF | ACTIVE INACTIVE INACTIVE INACTIVE | INACTIVE |
| AAEL010914 | 1 | 307–573 | KNRSID LWPKQSA NCLNGSDRSC DPNHMQL | INACTIVE INACTIVE INACTIVE INACTIVE | INACTIVE |
| AAEL011434 | 1 | 781–1181 | QSKNRY FPDWPDHRSP PIIHCSAGIGRTG VQNSEQYEL | ACTIVE ACTIVE ACTIVE ACTIVE | ACTIVE |
Table 2. Analysis of the superposition Mosquito PTPs with human PTP1B.
| Protein | Amino Acids Modeled | Template PTP structure Name/PDB ID | Percentage Sequence Identity | Root Mean Square Deviation (RMSD) from PTP1B structure (2AZR)(C-α RMSD) (Å) |
| AAEL001919 | 229 | TCPTP (2I1Y) | 34% | 0.283 |
| AAEL005492 | 299 | MEG2/PTPN9(2PA5) | 47% | 1.546 |
| AAEL011434 | 283 | PCPTP1/PTPR (2A8B) | 29% | 2.323 |
| AAEL003108 | 262 | PTPH1/PTPN3(2B49) | 46% | 1.510 |
| AAEL008528 | 309 (PTP domain) | SHP2/PTPN11 (3B7O) | 60% | 1.1412 |
| 188 (Absent PTP domain) | CD45 (1YGU) | 19% | --- |
Of the ten PTP sequences analyzed, the following seven showed the presence of at least one active PTP domain: AAEL001919-PA, AAEL001919-PB, AAEL005492, AAEL011434, AAEL008528-PA, AAEL008528-PB and AAEL003108 (Table 1 and Figure 2). The active domains of the splicing variants of AAEL001919 and AAEL008528 were identical (Table 1 and Figure 2). A multiple sequence alignment of these sequences with human PTP1B showed the presence of all ten conserved PTP motifs (Figures 1 and 4). The sequences of these proteins were subsequently used to obtain molecular models to enable further study of their structure-function relationships.
Figure 2. Multiple sequence alignments of the A. aegypti PTP domains with the PTP domain of human PTP1B.
Regions of high sequence similarity (>90\%) are highlighted in red; regions of moderate sequence identity (50–90%) are highlighted in blue. The ten motifs defining a PTP domain are marked in red boxes. These are in accordance with the nomenclature of Andersen et al. [59].
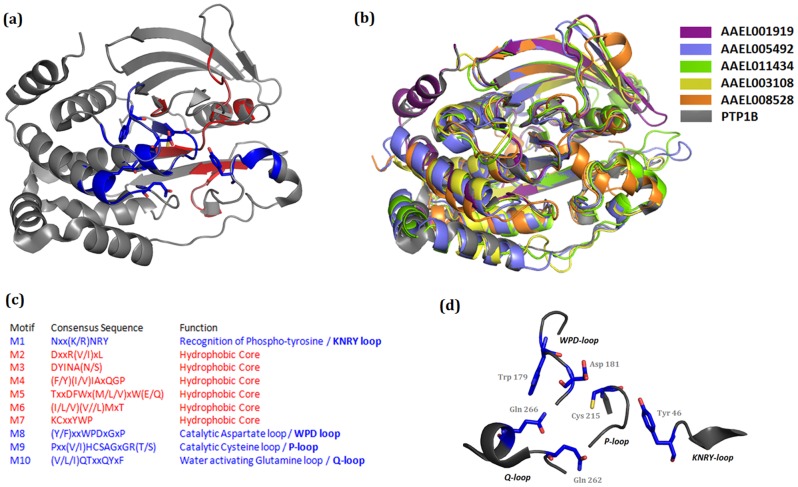
Figure 4. Superimposition of A. aegypti ‘active’ PTP model structures with PTP1B as the reference.
(A) The classical PTP-domain as seen in PTP1B (PDB ID: 2AZR). The ten motifs defining the conserved PTP domain are highlighted (B) Superimposition of the active PTP-domains of Aedes agypti onto the structure of PTP1B (C) Sequence conservation defining the conserved motifs of the PTP domain. Four motifs viz., M1, M8, M9 and M10 harbour the active site residues of the PTP domain. The sequence of these motifs as seen in the Aedes aypti PTP domains are mentioned in Table 1. (D) Active site of the PTP-domain as seen in PTP1B.
Structural analysis of mosquito PTP domains
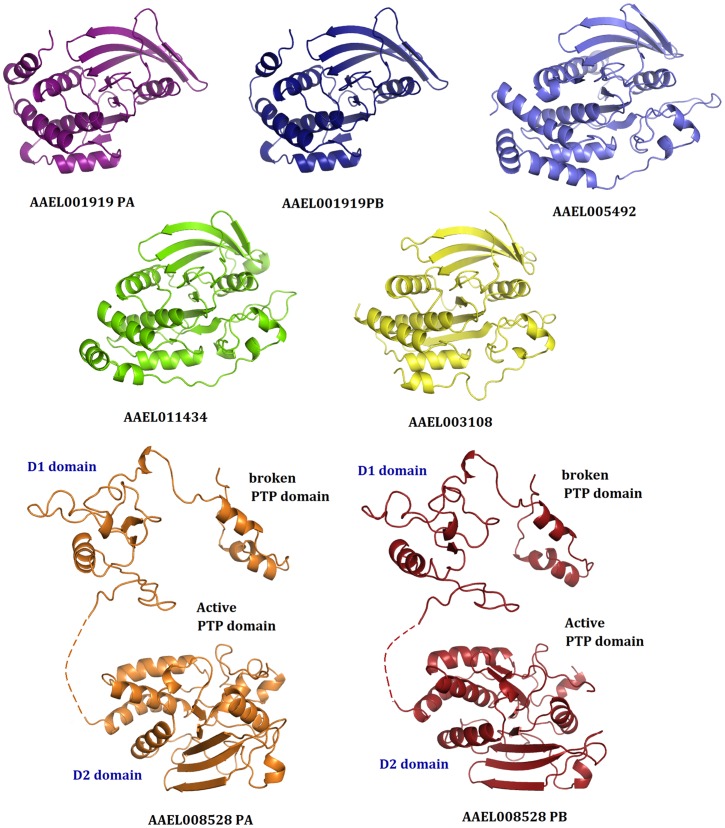
Molecular models of the five active PTP domains from the above seven different proteins were obtained using the previously solved homologous PTP-1B structure as a template (Figure 3, Table 1). Each of the structures showed the classical PTP fold with a twisted β-sheet composed of eight β-strands at the center flanked by eight α-helices. The active-site cysteine was observed at the center of the active site flanked by the general acid aspartate from Motif 8. Motif 10 glutamines were also observed at the active site (Figure 4). A superposition of these structures with human PTP1B showed that the geometry of the central active site is extremely conserved (Figure 4 and Table 2). This result is consistent with the information obtained from multiple sequence alignments which showed that the ten motifs to be extremely conserved (Figure 2).
Figure 3. Structural models of the ‘active’ PTP sequences from A. aegypti.
All proteins showed the classical PTP fold with a twisted β sheet at the center surrounded by α helices. Because of its low sequence conservation, the absent PTP domain of AAEL008528 could not be modelled completely. Only one model is shown for genes with splicing variants because their PTP domains are identical. For additional details, please check Figure 4.
Evolutionary analysis of mosquito PTP sequences
A phylogram of the A. aegypti PTP domains was made using the previously characterized PTPs of humans (Homo sapiens) and flies (Drosophila melanogaster) (Figure 5). These genomes were chosen because of their closeness with the mosquito genome (D. melanogaster) and for their extensive biochemical and structural characterizations (H. sapiens). The phylogram was generated using a multiple sequence alignment at the CLUSTALW web-server. The neighbor-joining algorithm was used to obtain the evolutionary tree using the TREEVIEW software package [20]. From the phylogram, it was evident that both active and inactive A. aegypti PTP domains were closely related to the vertebrate and fly PTP domains. The well-formed PTP domain of AAEL008528 was observed to be closest to the D. melanogaster Corkscrew PTP. Belonging to the SHP1 family, these PTPs are characterized by the presence of SH2 in addition to their tyrosine phosphatase domains. Interestingly, the absent PTP domain of AAEL008528 also clustered near these proteins (along with the absent N-terminal PTP domain of AAEL001046), indicating a plausible role for these absent PTP domains as adapter modules. The single PTP domains AAEL05492, AAEL003108 and AAEL001919 clustered separately into three different groups, which were closest to the D. melanogaster DPTP52F, dm PTPMEG, dmPTP61F and H. sapiens MAG2, MEG1, PTP1B, respectively. The phylogram also indicated that the inactive and active PTPs from A. aegypti clustered separately, but the proximity of the inactive PTP sequences to the other PTP homologues indicates that these could function as signaling molecules, much like adaptor proteins. The gene AAEL011434 was also described in the analysis conducted by Hatzihristidis et al. 2013 [21]. However, in Drosophila, this enzyme is a negative modulator of the MAPK pathway and is, thus, not considered in this study as a true member of the classical PTP group [22].
Figure 5. Evolutionary analysis of Aedes PTP sequences with the well-characterized PTPs from H. sapiens and D. melanogaster.
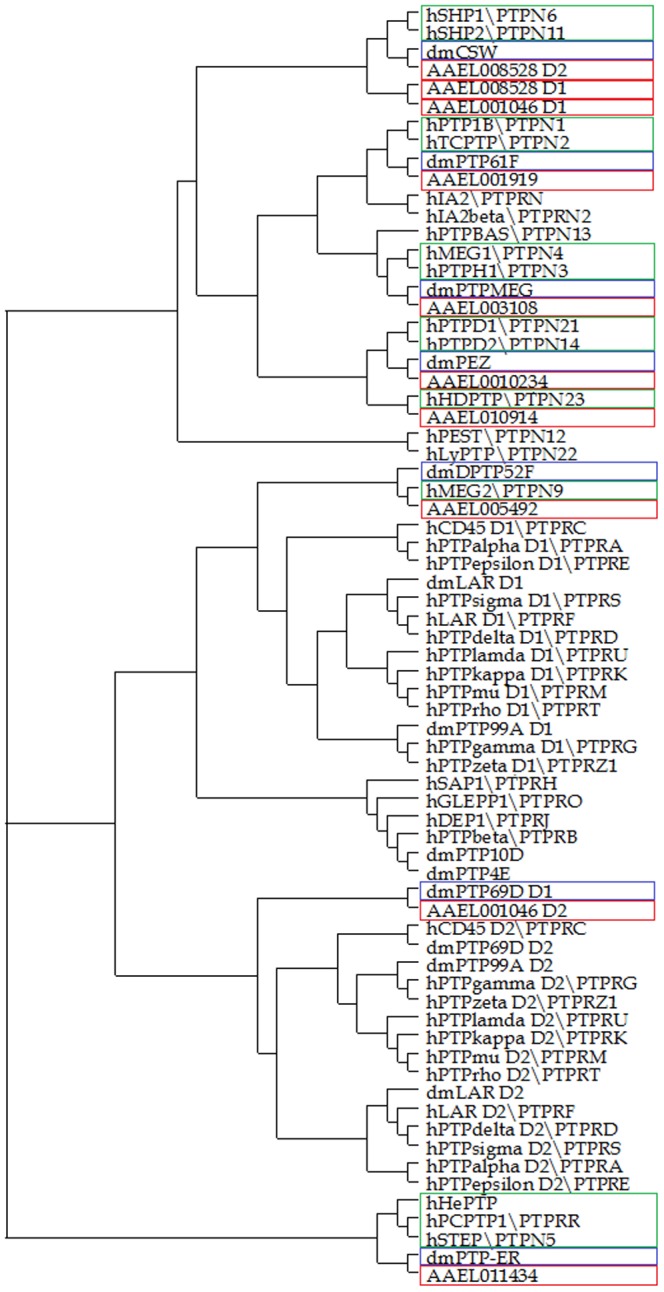
PTP domains from A. aegypti and from sequences derived from human and fly (names starting with “h” and “dm”) were clustered genomes. The A. aegypti active PTP domains are highlighted in red, while the closest human and fly PTPs are highlighted in blue and green, respectively.
PTP involvement in mosquito egg formation
The involvement of a complex set of intracellular signaling pathways in the regulation of Vg synthesis and egg formation has been described in the literature [23]. To determine the involvement of PTPs in mosquito reproduction, we initially evaluated the expression in different tissues of the 4 PTP genes that code for active enzymes (AAEL005492, AAEL008528, AAEL003108, and AAEL001919). AAEL011434 was not analyzed because this gene was identified as a PTP after the major experiments were completed and because it seems to be a dual-specificity phosphatase that modulates MAPKs [22]. AAEL003108 and AAEL001919 are the only PTPs detectable in all tissues isolated from sucrose-fed mosquitoes (Figure 6). In contrast, AAEL005492 had very low concentration in all tissues. Curiously, AAEL008528 is significantly expressed in the mosquito head, concomitant with a transient increase in phosphotyrosine phosphorylation that may involve fluctuations in the expression levels of AAEL008528 [24]. AAEL008528 is orthologous to Drosophila corkscrew, a PTP that was discovered in mutations related to defects in embryonic development [25], [26]. AAEL003108 clusters with the Drosophila dmPTPMEG. PTPMEG is a cytoplasmic PTP containing FERM (F for 4.1 protein, E for ezrin, R for radixin and M for moesin) and PDZ (P for postsynaptic density protein (PSD95)), D for Drosophila disc large-tumor suppressor (Dlg1), and Z for zonula occludens-1 protein (zo-1)) domains. AAEL001919 clusters with the Drosophila PTP dmPTP61F [29], [30], [31]. In mammalian T cells, PTP (TCPTP) and PTP1B share a high level of structural similarity but display different functions, which are defined through the presence of specific non-catalytic domains that alter enzyme localization within the cell.
Figure 6. Expression levels of classical PTPs in Aedes tissues.
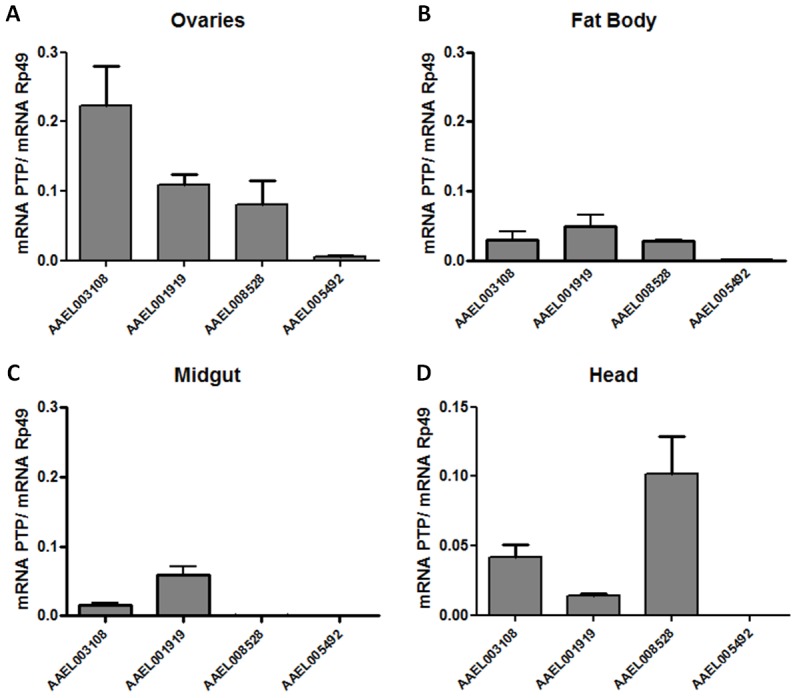
Female mosquitoes (20–30 insects) were maintained on 10% sucrose ad libitum and were collected at four and seven days after emergence from the pupal stage. They were dissected, and RNA was extracted from different tissues. Expression levels of PTPs were measured using qPCR. The following tissues were evaluated: (A) Ovaries; (B) Fat body; (C) Midgut and (D) Head. Expression levels of the following genes were evaluated: AAEL003108, AAEL001919, AAEL008528, AAEL005492. The latter were normalized against the expression of the mosquito Rp49 gene. Data are the means ± S.E.M of three different experiments.
AAEL003108 clusters with the Drosophila dmPTPMEG. PTPMEG is a cytoplasmic PTP containing FERM (F for 4.1 protein, E for ezrin, R for radixin and M for moesin) and PDZ (P for postsynaptic density protein (PSD95)), D for Drosophila disc large-tumor suppressor (Dlg1), and Z for zonula occludens-1 protein (zo-1)) domains. In Drosophila, the vertebrate homologs of PTPN3 (PTPH1) and PTPN4 (MEG1) are expressed in the nervous system, where they are involved in the regulation of axon projections [27], [28]. PTP 003108 was not further addressed in the following experiments no matter it is clearly more expressed in the ovaries due to two reasons: its lack of function in the mammalian models and its lack of effect on early silencing experiments of egg formation and [28]
AAEL001919 clusters with the Drosophila PTP dmPTP61F [29], [30], [31]. In mammalian T cells, PTP (TCPTP) and PTP1B share a high level of structural similarity but display different functions, which are defined through the presence of specific non-catalytic domains that alter enzyme localization within the cell. PTP activity against pNPP using tissues from sucrose-fed mosquitoes is highly sensitive to sodium orthovanadate (data not shown). In addition, in our pNPP assays with mosquito tissues, the inclusion of a peptide that contains a phosphotyrosine dephosphorylated by the mammalian PTP1B leads to a 40–90% inhibition of total PTP activity (Figure S1), indicating the presence of catalytically active mosquito enzymes possessing the same biochemical properties as mammalian PTPs.
The above overall analysis suggests that AAEL001919 is likely to be the mosquito ortholog of mammalian PTP1B and Drosophila dmPTP61F. To determine the role of AAEL001919 in egg formation, we performed several experiments. Because Aedes is an anautogenous mosquito, we evaluated the impact of blood feeding on aspects of PTP function. Initially, we evaluated the effect of blood feeding on total PTP activity using pNPP as substrate (Figure 7). Blood feeding induced a decrease in total enzyme activity at 24 hours in all tissues except the mosquito head. Major changes in PTP activity were detected in both the mosquito midgut and the fat body during the first 72 hours following the blood meal.
Figure 7. PTP activity in mosquito tissues after blood feeding.
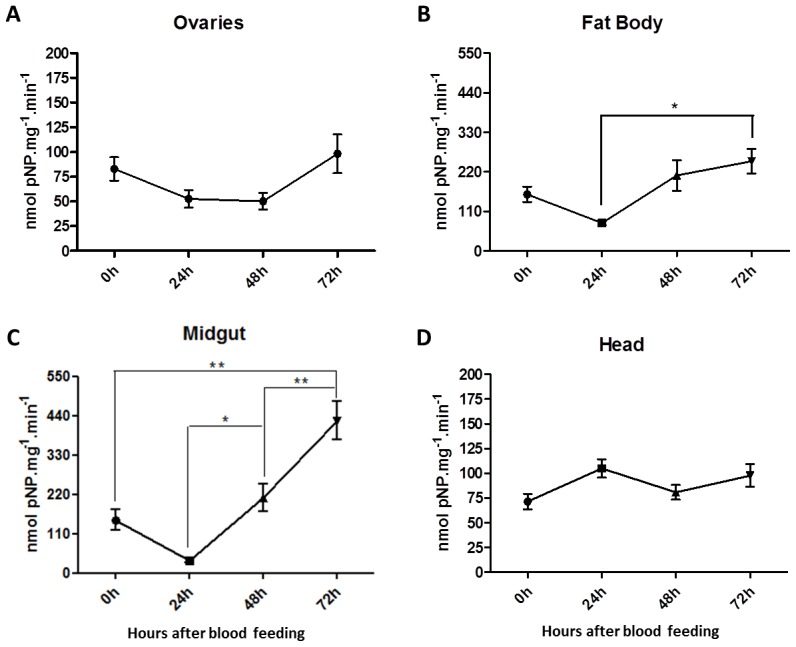
Five- and seven-day old female mosquitoes (20–30 insects) were fed blood on rabbit skin were dissected 24 h, 48 h and 72 h later. Tissues were assayed for PTP activity using pNPP as the substrate. The following tissues were evaluated: (A) ovary; (B) fat body; (C) midgut and (D) head. The control specimens (0 h) were maintained in 10% sucrose ad libitum and were dissected with one of the three points. Normalized data from five experiments were analyzed by one-way analysis of variance (ANOVA) and by a post-test Tukey's Multiple Comparison Test, p<0.05 (A) p = 0.0476, (B) p = 0.0293, (C) p = 0.0001, (D) p = 0.1494.
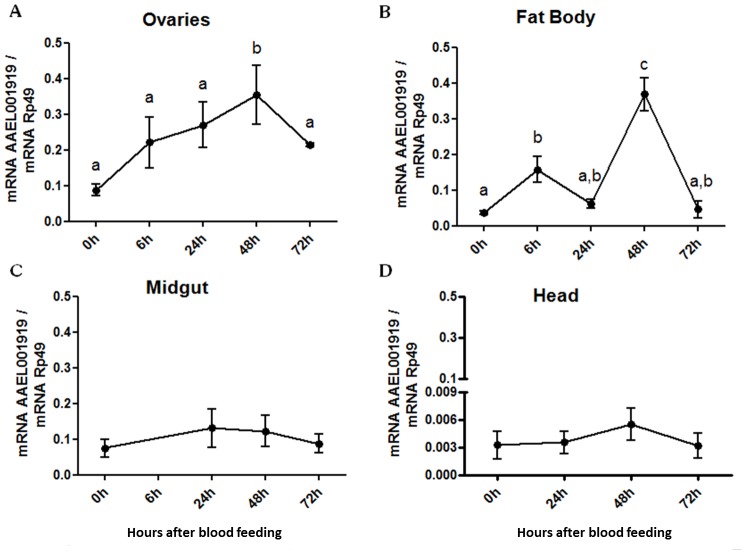
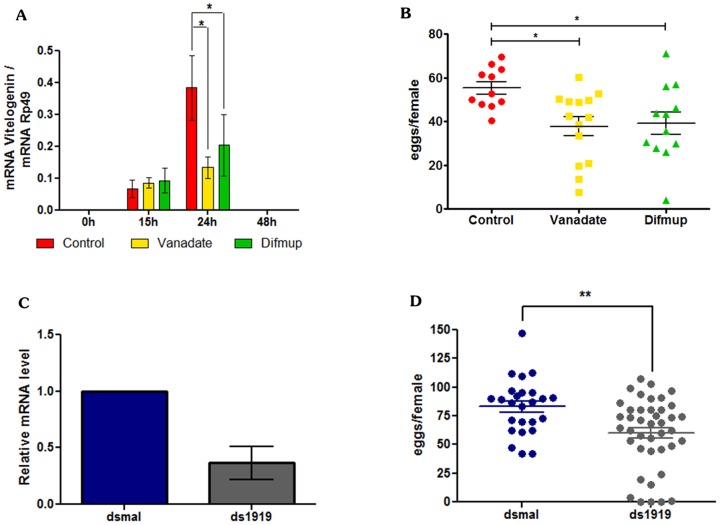
Subsequently, we analyzed the expression of AAEL001919 in mosquito tissues. Blood feeding induced a parallel increase in AAEL001919 expression in the ovaries and the fat body, with major peaks at 48 hours in both tissues (Figure 8). Finally, including the classical PTP inhibitor vanadate or the PTP substrate DiFMUP in a blood meal modified the dynamics of Vg synthesis in the mosquito fat body (Figure 9A). It also decreased the total number of eggs laid by females treated in this manner (Figure 9B). This effect on egg formation was mimicked by RNAi silencing of AAEL001919 (Figures 9C and 9D). Taken together, these results indicate the involvement of the PTP gene AAEL001919 in egg formation in A. aegypti.
Figure 8. Expression of AAEL001919 after blood feeding.
Five- and seven-day old female mosquitoes (20–30 insects) were naturally fed with rabbit blood and dissected 24 h, 48 h and 72 h later. The following tissues were evaluated for AAEL001919 gene expression: (A) Ovaries; (B) Fat body; (C) Midgut and (D) Head. Non-blood fed mosquitoes (0 h) were maintained on 10% sucrose ad libitum and were concomitantly dissected at any one of the three time points. Normalized data from five experiments were analyzed by one-way analysis of variance (ANOVA) and by a post-test Tukey's Multiple Comparison Test (a, b, c p<0.05). Groups assigned with the same letter (a, b or c) indicate that they do not show statistically significant differences among them.
Figure 9. Effect of PTP inhibitors and silencing action of the AAEL001919 gene on egg formation in Aedes aegypti.
(A) Five- and seven-day old females were artificially fed with rabbit blood supplemented with the PTP inhibitor vanadate or the PTP substrate Difmup. Following a blood meal, fat bodies were dissected from females at the indicated times, and the expression of Vg mRNA was measured by qPCR and normalized against Rp49 gene expression. (B) Females from the experiments shown in A were placed in individual tubes, and the eggs laid were quantified on subsequent days after the blood meal. (C) One- and two-day old females (5 insects) were injected with either 140 ng of AAEL001919 RNAi or dsMal. AAEL001919 expression levels were quantified three days after RNAi injection as shown. (D) Injected mosquitoes from the experiment shown in panel C were naturally blood-fed three days later, and the number of eggs laid following the blood meal was quantified. Normalized data from four experiments were analyzed by One-way ANOVA (panels A and B) and Student's t-test (Panel D) with significance levels set at p<0.05. Groups assigned with the same letter (a, b or c) indicate that they do not show statistically significant difference among them. Different letters in different groups indicate that such groups show significant differences among themselves (Panels A and B). Asterisks indicate (Panel B) indicate a significant difference among groups (student T- test, p<0.05).
Discussion
In mammalian cells, the down regulation of insulin pathway is promoted by the activation of either PTEN or PTP1B. PTP1B is classically described as an enzyme involved in the dephosphorylation of phosphotyrosine-containing substrates, especially the insulin receptors in tissues such as liver, muscle and adipocytes. PTP1B usually associates with IR and IRS1 and dephosphorylates these targets. Other classical targets of mammalian PTP1B are the substrates downstream of the leptin receptor. These findings have established PTP1B as a major target for the treatment of diabetes and obesity and have triggered great efforts from many laboratories to develop specific inhibitors of this enzyme [3]. Moreover the combination of trapping mutants with chips containing a collection of cellular peptides has confirmed the prediction of the phosphorylation of the following five high-ranking PTP1B substrates in mammalian cells: PLC-γ1, Gab1, SHP2, EGFR and SHP1 [32], [33]. Furthermore, this strategy has demonstrated, for the first time, that TC-PTP, an enzyme that is closely related to PTP1B, differs functionally from PTP1B and that their substrate specificities overlap only partially. Thus, these findings imply a broader range of functions for PTP1B and PTP1B-like enzymes, indicating that they are not solely involved in the regulation of glucose homeostasis. Several strategies have been employed in the literature to evaluate the role of PTP1B in cell biology [32], [33]. These strategies include overexpression of the enzyme in cultured cells, its knockout in mice, or the physical association of proteins with the active site of the enzyme using substrate-trapping mutants and analysis of the results using mass spectrometry [32]–[35]. Thus, PTP1B is, in fact, a pleiotropic enzyme that acts as a master regulator of several cell-signaling networks [32], [33]. It is possible that vector biology may benefit from such findings by using a similar approach to block PTP1B activity in mosquitoes and, thus, avoid pathogen transmission.
The insulin pathway is involved in diverse aspects of Dipteran biology, including lifespan, immunity and egg formation [29], [30], [31]. As mentioned above, the insulin pathway in mosquitoes acts as an ambivalent modulator of metabolism and immunity [9], [36]–[39]. Several members of the canonical pathways activated by insulin in mosquitoes have been described in recent years, but little is known about negative regulators of this pathway [6], [40]. Such regulators may include PTPs, which are master regulators of tyrosine dephosphorylation. In mosquitos, insulin and insulin-like peptides also modulate the proliferation of immune cells known as hemocytes [39]. The inhibition of these regulators or blocking the expression of proteins under their transcriptional control can potentially provide new targets for molecular modeling and for blocking egg formation and pathogen transmission by mosquitoes [14]. Thus, identifying negative modulators of such pathways is an epidemiologically relevant task. Activation of the PI3K/Akt pathway by down-regulation of a specific Aedes PTEN (AaegPTEN6) leads to an increase in oviposition [10]. Furthermore, PTEN overexpression has been shown to extend the mosquito lifespan and increase resistance to P. falciparum development [40].
The pharmacological inhibition of PTPs by pervanadate and the silencing of AAEL001919 by RNAi decreased egg production (Figure 9). It is important to mention that pervanadate has been previously shown to affect ecdysone production by mimicking insulin effects on mosquito ovaries [9]. However, the same study showed that pervanadate treatment exhibits a bell-shaped activity curve, whereby either low or high doses of the compound can inhibit ecdysone production [9]. These results suggest that PTPs modulate the phosphorylation states of proteins in this pathway. This effect is different from the effects of PTEN silencing, which ultimately activate the PI3K-Akt branch of the insulin signaling pathway and increase egg production. PTENs are lipid phosphatases whose predominant enzymatic activity appears to be the dephosphorylation of phosphoinositides, ultimately leading to the shutdown of the insulin pathway. This is not the case with PTP1B, whose substrates are largely within but not restricted to components of the insulin pathway [32], [33].
In the present study, we evaluated mosquito PTP structure. We have started with all the genes coding for classical enzymes from this family, but four enzymes were more specifically addressed (Figures 1 to 6). AAEL008528 is involved in transducing signals from the receptor tyrosine kinase Torso. Corkscrew and AAEL008528 are also orthologs of the human gene PTPN11, which encodes the PTP SHP2 protein. Mutations in PTPN11 are related to the development of human diseases known as NOONAN and LEOPARD syndromes. These two syndromes have similar effects but differ in their underlying mechanisms, which result in a gain of function in the case of NOONAN and a loss of function in LEOPARD. The role of this mosquito PTP through differently available phenotypes should be investigated in the futures. Regarding AAEL003108 in Drosophila, the vertebrate homologs of PTPN3 (PTPH1) and PTPN4 (MEG1) are expressed in the nervous system, where they are involved in the regulation of axon projections [27], [28]. Despite their presence in mammalian T cells, the roles of PTPN3 and PTN4 are not clear, but their silencing does not affect proper signaling in this model [27], [28]. The Drosophila TCPTP/PTP1B ortholog dmPTP61F codes for two splicing variants that are differentially localized in the cell. The isoform localized in the endoplasmic reticulum is shown to be able to regulate insulin signaling in vivo [29]. Mutants lacking both PTP61F variants display a decrease both in mean life span and in female fecundity [29]. Furthermore, it has been shown previously that PTP61F dephosphorylates the Drosophila insulin receptor in S2 cells in vitro and acts as a negative regulator of the Drosophila JAK/STAT pathway [30], [31].
To ensure that our observations can be interpreted as the enzymatic behavior of PTPs, appropriate controls were performed including enzyme activity initially using pNPP, a generic substrate for phosphatases [24], [39]–[46]. Assays were performed in the presence or absence of micromolar levels of orthovanadate, a classical PTP inhibitor. Under these conditions, this inhibitor always blocked 40–90% of the overall activity, either with pNPP or a specific peptide substrate for PTP1B (Figure S1). To reveal specific effects of PTP1B on mosquito biology, particularly in events during the vitellogenic phase, we studied egg laying in the absence or presence of vanadate and detected a vanadate-induced decrease in egg formation. Surprisingly, even at high concentrations of the inhibitor, only 15–25% of the PTP activity was abolished in the presence of this molecule. Despite this, no structurally or conformationally relevant differences exist between PTP1B in human and A. aegypti, an observation also supported by the structural approach conducted at the beginning of this study (Figures 1 to 4, Tables 1 and 2). This inhibitor-susceptibility difference may open paths for the design of specific inhibitors for different groups of organisms. Furthermore, these results reinforce the idea that PTPs play a key role in the oogenesis of A. aegypti.
Analysis of tyrosine phosphoproteomics in Drosophila S2 or the knockdown of PTP61F in Drosophila Schneider cells led to the identification of several dPTP61F substrates [34]. Comparison of those results with another study addressing the role of Albeson tyrosine kinase (Abl) and Abl interacting protein (Abi) in Drosophila oogenesis revealed Abi/Abl complex as a direct target of dPTP61F [34], [47]. Abl phosphorylates Abi, which is also a direct target of dPTP61F-mediated dephosphorylation. Thus, the silencing of dPTP61F resulted in an increase of Abl phosphorylation and activity and an increase in Abi activity. Abi functions as a substrate adaptor protein for Abl and a core member of the SCAR/WAVE complex, relaying signals from Rac to Arp2/3 and regulating actin dynamics. Tyrosine phosphorylation of SCAR/WAVE by Abl is required for actin polymerization [47]. Thus, the silencing of AAEL001919 in Aedes may lead to an impairment of cytoskeleton dynamics associated with egg formation that ultimately blocked egg production. Regarding the effect of AAEL001919 on Vg synthesis (Figure 9) we speculate that such effect may occur through the up regulation of enzyme expression (Figure 8B). RT-PCR data obtained here were also analyzed in comparison with the RNA-seq results published by another group [48]. Such results regarding the expression of PTP genes described in the present study match each other. Thus the analysis of all PTP genes reported here indicate a similar increase on AAEL001919 after a blood meal but the exact point in time when this occurs is difficult to define due to different approaches used by both studies. Nevertheless, AAEL001919 as reported by Bonizzoni et al. 2011 is the major PTP gene affected by a blood meal [48].
In conclusion the analysis of Aedes classical PTPs reported here led to the identification of a protein encoded by the AAEL001919 gene and showing great structural similarity to human and Drosophila PTP1B. This protein may be a major negative regulator of the insulin pathway in mosquitoes, but its direct targets remain unknown, and their identity was not addressed in the present investigation. Insulin receptors (IRs) usually present high conservation, especially in the kinase catalytic domain that is essential for their activation, but the PTP1B target region (that contains tyrosine phosphorylation sites) is considered the most variable part of these receptors. The high level of similarity in PTP1B is interesting because the IR variable region would be expected to guide selective pressure on PTP1B for the purpose of concomitant changes [41]. The differences in inhibitor susceptibility among PTPs may allow drug design through a strategy that generates a drug that only affects the insect phosphatase or its substrates, such that it is inactive in mammals. In D. melanogaster, the ortholog of PTP1B, the dmPTP61F gene, generates two products that differ only by a small C-terminal sequence and that contain the same membrane-binding domain. This difference allows the product containing this specific domain to become anchored to the endoplasmic reticulum, whilst the product that lacks the specific domain exhibits nuclear localization [41], [42]. Interestingly, in mammals, the membrane-bound PTP1B has been reported to have an inhibitory effect on the insulin signaling pathway, leading to insulin resistance, and has also been shown to be capable of causing an increase in blood glucose [41], [42]. In contrast, the PTP1B protein with the truncated membrane-binding C-terminal domain can still lead to insulin resistance but is unable to activate SREBP-1 [42]–[44]. In conclusion, while it is likely that AAEL001919 regulates glucose metabolism and immunity in mosquitoes, the present study clearly demonstrates its involvement in egg formation in Aedes aegypti females. Furthermore, this enzyme demonstrates potential use as a target for designing specific inhibitors that block mosquito egg development and vector-mediated disease transmission.
Materials and Methods
Reagents
Tris, glycine, acrylamide, bis-acrylamide, TEMED, DMSO, DTT, bovine serum albumin, sodium vanadate, okadaic acid, Folin reagent and pNPP were obtained from Sigma-Aldrich Company (St. Louis, MO, USA). Prestained Full-Range Rainbow molecular weight standards were obtained from Amersham Biosciences (Buckinghamshire, England). Ethanol, Triton X-100 and DMSO were obtained from Merck (Darmstadt, Germany).
Ethics statement
All animal care and experimental protocols were conducted following the guidelines of the institutional care and use committee (Committee for Evaluation of Animal Use for Research from the Federal University of Rio de Janeiro, CAUAP-UFRJ) and the NIH Guide for the Care and Use of Laboratory Animals (ISBN 0-309-05377-3). The protocols were approved by CAUAP-UFRJ under registry IBQM067-05/16. Technicians dedicated to the animal facility at the Institute of Medical Biochemistry (UFRJ) carried out all aspects related to rabbit husbandry under strict guidelines to insure careful and consistent handling of the animals.
Mosquito rearing and blood meals
Aedes aegypti (Liverpool Black Eye strain) were raised in an insectary at the Federal University of Rio de Janeiro, Brazil, under a 12-h light/dark cycle at 28°C and 60–80% relative humidity. Larvae were fed with dog food (Pedigree Junior), and adults were maintained in a cage and given a solution of 10% sucrose ad libitum. Five- to seven-day old females were used in the experiments. Mosquitoes were naturally fed on rabbits' ear veins or were artificially fed with heparinized rabbit blood. Artificial feeding was performed using water-jacketed artificial feeders maintained at 37°C and sealed with parafilm membranes. Whenever indicated blood was supplemented with PTP inhibitors.
Phosphatase assay using para-Nitrophenylphosphate (pNPP)
The tissues were homogenized in buffer containing 20 mM of sodium acetate (pH 4.0). The protein concentration of each extract was determined by the Lowry method. The homogenates were used to perform the phosphatase assays, using 0.5–3 µg of protein, depending on the tissue, and using p-nitrophenylphosphate (pNPP) as substrate. Enzyme activity was measured in the presence or absence of 100 µM sodium orthovanadate, used as tyrosine phosphatase inhibitor, to determine the specific activities of PTPs. The reactions were conducted at 37°C for 60 minutes and were stopped by adding 2 N NaOH. In some experiments, we included 0.1 mM of PTP 1B substrate II (Glu-Leu-Glu-Phe-pTyr-Met-Asp-Tyr-Asp-Tyr-Glu) (Calbiochem) in addition to pNPP. Further conditions were as described previously [24], [49].
In silico analysis of Aedes PTPs
Identification and classification of tyrosine phosphatases (PTPs) in mosquitoes were accomplished by searching conserved domains from Pfam. The search for conserved domains (CDs) employed FAT [50], a program developed by our group. It is a HMMER filter and blast manager. First, proteins predicted (version 1.3) by Vector-Base in the A. aegypti genome were downloaded (https://www.vectorbase.org/download/aedes-aegypti-liverpoolpeptidesaaegl13fagz). Using FAT, the sequences were filtered using characteristic domains of PTP families (Class I soluble classical PTPs used Y_phosphatase domain PF00102). The sequences obtained were compared using blast with databases such as nr, Swiss-prot and a custom human tyrosine phosphatases database. Lastly, hmmscan searched for other conserved domains in these previously filtered proteins. While this manuscript was in preparation, we became aware of another publication that described a specific sequence-based method for the automatic classification of PTPs present in 65 genomes, including A. aegypti [21]. The same classical PTPs described in that study were confirmed by the strategy described here.
Analysis of PTP sequences, protein models and active-site modeling
In silico approaches were used to ascertain the function and the evolutionary homologs of Aedes PTPs. Because proteins with high sequence and structural similarity may possess the same biological functions, the in silico approach was used to find the closest homologs of PTPs in an effort to obtain homology gene models [50]. Sequences were used to search the homologue sequence space for related proteins using the NCBI BLAST server [50]. A BLOSUM62 matrix was used for coring of the sequences, with an expected threshold of 10 and a sampling size of 3 amino-acids at a time. The non-redundant protein database was used for searching the sequence space without filtering out low-complexity regions. To identify the homologues with known protein structures, the PSI-BLAST tool was used against the freely available Protein Data Bank. PSI-BLAST allowed for better sensitivity and enabled us to find the closest five structures, which could be used in homology modeling. These five sequences were used for a multiple sequence alignment using the MULTALIGN server [51]–[53]. In several instances, the Aedes PTP and the homologous PTP sequences had gaps, so the modeler could not be effectively used for homology modeling. In these cases, the automated Protein Homology/analogy Recognition Engine was used to find the respective homology models [54]. The reliability of the models was checked via submission to the WHAT IF server [55], [56]. The protein structures were visualized and superimposed using PyMOL software (DeLano Scientific LLC). The various domains in the sequences were identified using a combination of online servers, including the Conserved residue Function Prediction Server [56] and the Conserved domain Database at the NCBI [57]. The active site of the Protein-Tyrosine-Phosphatase domain was ascertained using the Catalytic Site Atlas [58] as well as the siteFinder web-based tool [59]. The ten motifs that define the PTP domain were identified and used, as defined in the PTP database [60]. Secondary-structure prediction for the sequence corresponding to the PTP domain was obtained from the Psipred server [61]. Sequences of human and fly (Drosophila melanogaster) PTPs were obtained from the non-redundant Protein Database at NCBI and used for construction of dendrograms using the CLUSTALW web server and the TREEVIEW software package.
RNA extraction and qPCR analysis
RNA was extracted from each sample using TRIzol reagent (Life Technologies). RNA quantification was accomplished using a NanoDrop-3300 (Thermo Scientific) device before cDNA synthesis was performed. Briefly, 1 µg of RNA was treated with RNase-free DNase (Fermentas) to avoid genomic DNA contamination. Subsequently, cDNA synthesis was performed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems), according to the manufacturer's protocol. Transcript levels were determined by Real Time PCR using a SYBR-Green-based method. The quantitative PCR assays were employed a StepOnePlus Real Time PCR System (Applied Biosystems) using PowerSYBR Green PCR Master Mix (Applied Biosystems). Gene expressions, as evaluated by mRNA levels, were calculated by normalization to the levels of Ribosomal protein 49 (rp49) mRNA (accession number AAT45939), which served as the endogenous control in each individual sample, and taking into consideration the respective efficiency of each pair of primers. The comparative ΔΔCt method was then used to calculate relative gene expression levels, and all standard errors were calculated based on ΔCt, as described in the Applied Biosystems User Bulletin #2 (http://www3.appliedbiosystems.com/cms/groups/mcb_support/documents/generaldocuments/cms_040980.pdf). The primers used presented an efficiency of at least 95%. The primer pairs corresponding to the analyzed genes were as follows: AAEL001919 (fwd 5′ GATTGGCG AAGAGCACAAATTG, rvs 5′ TAATCGGAACGTCCTTTTGC 3′), AAEL005492 (fwd 5′ GTGATAGTAATGACCACTCG 3′, rvs 5′ ACCTGATAGCATCCATATTC 3′), AAEL008528 (fwd 5′ GGGTCCAATTTGTGTCCAC 3′, rvs 5′ GATCTCGCAGTCCAGACC 3′), AAEL003108 (fwd 5′ATGGTACAACAGGAAAGCAG 3′, rvs 5′GATGGAGAATCCTTCAGACA 3′), AAEL010234 (fwd 5′ TAGATTTACGGTGGCGGAC 3′, rvs 5′ GCTTGCGTCTGGTTCTTTTC 3′), AAEL010914 (fwd 5′ TGGCAGTATGGTGGATAGC 3′, rvs 5′ CAACCGTTCAACCTCCTTG 3′), AAEL001046(fwd 5′AAGCAACCGAACTTTGTTGG 3′, rvs 5′CCCGTTGAGTTGGTCT GATT 3′), Rp49(fwd 5′TCAACCCCCGTTCGAACA 3′, rvs 5′CCGTAACCGATGTTTGGC3′), Vg(fwd 5′ TGAATTTGTCACCCCCGATC 3′, rvs 5′ TTCACGCTTGACACATTCCTG 3′).
dsRNA synthesis and injections
Double-stranded RNA was synthesized using a MEGAscript RNAi kit (Ambion, Austin, TX, USA), according to manufacturer's instructions. 138 nL of 3 µg/µL dsRNA solution re-suspended in water was injected into the thorax of cold-anesthetized 1-to-2-day-old female mosquitoes. dsRNA injections employed a nanoject II nanoliter injector (Drummond Scientific). Primers' sequences used for template amplification for dsRNA synthesis were as follows: RNAi1919F 5′ TAATACGACTCACTATAGGGTGCCACCGTTACCTAAGGAC 3′ and RNAi1919R 5′ TAATACGACTCACTATAGGGCTGGGCTAGACACTGCTTCC 3′. These primers contained a T7 polymerase binding sequence, required for dsRNA synthesis. As a control, we used maltose-binding protein (mal) from Escherichia coli dsRNA (dsmal). This sequence was inserted into a pBlueScript KS+ (Stratagene) and was amplified using the T7 minimal promoter primer (5′ TAATACGACTCACTATAGGG 3′) for template generation and dsRNA synthesis.
Evaluation of the Role of PTP on Egg Oviposition
In some experiments mosquitoes were fed with 10% sucrose enriched either with the 0.1 mM of the PTP inhibitor sodium orthovanadate or the PTP substrate DifMUP. In these experiments cages with 10–15 mosquitoes were fed with blood in the presence or absence of the mentioned PTP modulators and 72 hrs later the number of laid eggs was evaluated for the whole cage. At least three different cages were used in each experiment. Therefore each plotted point on the graphics (Figure 9B) is the number of eggs in a single cage divided by the number of females present on that cage. RNAi silencing of AAEL001919 was also used as a strategy to block PTP activity. In each of these experiments a total of 30 mosquitoes where either injected with dsMal or ds1919 as indicated in figure legend. Following injection groups of 2 mosquitoes were kept in separate in Falcon tubes in order to decrease mortality. The plotted results indicate the total number of eggs laid in each Falcon divided by 2 female mosquitoes.
Statistical Analysis
All experiments were performed at least in triplicate. The results are presented as the means and standard errors of the mean. Normalized data were analyzed by One-way ANOVA or Student's t-test using GraphPad Prism software.
Supporting Information
Effect of a tyrosine phosphorylated peptide on PTP activity towards pNPP on different tissues of A. aegypti . Tissues dissected from 10% sucrose-fed females (were homogenized and enzyme activity towards pNPP was assayed in the presence or absence of 0.1 mM of PTP 1B substrate II. After 30 minutes at 37°C, the reactions were halted by adding 1∶8,5 volumes of 2 M NaOH. Results represent the standard two determinations in triplicate and mean deviation.
(TIF)
Acknowledgments
We acknowledge the technical support of Katia Anastacio Laia, Jaciara Miranda Freire and Geane Cleia Braz. This work is dedicated to the memory of Dr. Alexandre A. Peixoto.
Funding Statement
This work was supported by FAPERJ (Fundacao Carlos Chagas Filho de Apoio a Pesquisa do Estado do Rio de Janeiro), CNPq (Conselho Nacional de Desenvolvimento Cientifico e Tecnologico) and INCT-EM (Instituto Nacional de Ciencia e Tecnologia em Entomologia Molecular). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lim WA, Pawson T (2010) Phosphotyrosine signaling: evolving a new cellular communication system. Cell 142 5: 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sacco F, Perfetto L, Castagnoli L, Cesareni G (2012) The human phosphatase interactome: An intricate family portrait. FEBS Lett 586 17: 2732–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, et al. (2004) Protein tyrosine phosphatases in the human genome. Cell 117 6: 699–711. [DOI] [PubMed] [Google Scholar]
- 4. Brown MR, Graf R, Swiderek KM, Fendley D, Stracker TH, et al. (1998) Identification of a steroidogenic neurohormone in female mosquitoes. J Biol Chem 273 7: 3967–3971. [DOI] [PubMed] [Google Scholar]
- 5. Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, et al. (2008) An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti . Proc Natl Acad Sci U S A 105 15: 5716–5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gulia-Nuss M, Robertson AE, Brown MR, Strand MR (2011) Insulin-like peptides and the target of rapamycin pathway coordinately regulate blood digestion and egg maturation in the mosquito Aedes aegypti . PLoS One 6 5: e20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hansen IA, Sieglaff DH, Munro JB, Shiao SH, Cruz J, et al. (2007) Forkhead transcription factors regulate mosquito reproduction. Insect Biochem Mol Biol 37 9: 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hansen IA, Attardo GM, Roy SG, Raikhel AS (2005) Target of rapamycin-dependent activation of S6 kinase is a central step in the transduction of nutritional signals during egg development in a mosquito. J Biol Chem 280 21: 20565–20572. [DOI] [PubMed] [Google Scholar]
- 9. Riehle MA, Brown MR (1999) Insulin stimulates ecdysteroid production through a conserved signaling cascade in the mosquito Aedes aegypti . Insect Biochem Mol Biol 29 10: 855–860. [DOI] [PubMed] [Google Scholar]
- 10. Arik AJ, Rasgon JL, Quicke KM, Riehle MA (2009) Manipulating insulin signaling to enhance mosquito reproduction. BMC Physiol 9: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kokoza VA, Raikhel AS (2011) Targeted gene expression in the transgenic Aedes aegypti using the binary Gal4-UAS system. Insect Biochem Mol Biol 41 8: 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kokoza V, Ahmed A, Cho WL, Jasinskiene N, James AA, et al. (2000) Engineering blood meal-activated systemic immunity in the yellow fever mosquito, Aedes aegypti . Proc Natl Acad Sci U S A 97 16: 9144–9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barr AJ, Ugochukwu E, Lee WH, King ON, Filippakopoulos P, et al. (2009) Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell 136 2: 352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franz AW, Sanchez-Vargas I, Adelman ZN, Blair CD, Beaty BJ, et al. (2006) Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti . Proc Natl Acad Sci U S A 103 11: 4198–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tonks NK (2009) Pseudophosphatases: grab and hold on. Cell 139 3: 464–5. [DOI] [PubMed] [Google Scholar]
- 16. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23 21: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 17. Asthagiri D, Dillet V, Liu T, Noodleman L, Van Etten RL, et al. (2002) Density functional study of the mechanism of a tyrosine phosphatase: I. Intermediate formation. J Am Chem Soc 124 34: 10225–10235. [DOI] [PubMed] [Google Scholar]
- 18. Zhang ZY (1998) Protein-tyrosine phosphatases: biological function, structural characteristics, and mechanism of catalysis. Crit Rev Biochem Mol Biol 33 1: 1–52. [DOI] [PubMed] [Google Scholar]
- 19. Madan LL, Gopal B (2011) Conformational basis for substrate recruitment in protein tyrosine phosphatase 10D. Biochemistry 50 46: 10114–10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Page RDM (1996) TreeView: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12 4: 357–358. [DOI] [PubMed] [Google Scholar]
- 21. Hatzihristidis T, Liu S, Pryszcz L, Hutchins AP, Gabaldón T, et al. (2013) PTP-central: A comprehensive resource of protein tyrosine phosphatases in eukaryotic genomes. Methods 65 2: 156–164. [DOI] [PubMed] [Google Scholar]
- 22. Karim FD, Rubin GM (1999) PTP-ER, a novel tyrosine phosphatase, functions downstream of Ras1 to downregulate MAP kinase during Drosophila eye development. Mol Cell 3 6: 741–750. [DOI] [PubMed] [Google Scholar]
- 23. Roy SG, Raikhel AS (2011) The small GTPase Rheb is a key component linking amino acid signaling and TOR in the nutritional pathway that controls mosquito egg development. Insect Biochem Mol Biol 41 1: 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jablonka W, Senna R, Nahu T, Ventura G, Menezes L, et al. (2011) A transient increase in total head phosphotyrosine levels is observed upon the emergence of Aedes aegypti from the pupal stage. Mem Inst Oswaldo Cruz 106 5: 546–552. [DOI] [PubMed] [Google Scholar]
- 25. Perkins LA, Larsen I, Perrimon N (1992) Corkscrew encodes a putative protein tyrosine phosphatase that functions to transduce the terminal signal from the receptor tyrosine kinase torso. Cell 70 2: 225–236. [DOI] [PubMed] [Google Scholar]
- 26. Oishi K, Zhang H, Gault WJ, Wang CJ, Tan CC, et al. (2009) Phosphatase-defective LEOPARD syndrome mutations in PTPN11 gene have gain-of-function effects during Drosophila development. Hum Mol Genet 18 1: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Whited JL, Robichaux MB, Yang JC, Garrity PA (2007) Ptpmeg is required for the proper establishment and maintenance of axon projections in the central brain of Drosophila . Development 134 1: 43–53. [DOI] [PubMed] [Google Scholar]
- 28. Bauler TJ, Hendriks WJ, King PD (2008) The FERM and PDZ domain-containing protein tyrosine phosphatases, PTPN4 and PTPN3, are both dispensable for T cell receptor signal transduction. PLoS One 3 12: e4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buszard BJ, Johnson TK, Meng TC, Burke R, Warr CG, et al. (2013) The nucleus- and endoplasmic reticulum-targeted forms of protein tyrosine phosphatase 61F regulate Drosophila growth, life span, and fecundity. Mol Cell Biol 33 7: 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu CL, Buszard B, Teng CH, Chen WL, Warr CG, et al. (2011) Dock/Nck facilitates PTP61F/PTP1B regulation of insulin signalling. Biochem J 439 1: 151–159. [DOI] [PubMed] [Google Scholar]
- 31. Baeg GH, Zhou R, Perrimon N (2005) Genome-wide RNAi analysis of JAK/STAT signaling components in Drosophila. Genes Dev 19 16: 1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferrari E, Tinti M, Costa S, Corallino S, Nardozza AP, et al. (2011) Identification of new substrates of the protein-tyrosine phosphatase PTP1B by Bayesian integration of proteome evidence. J Biol Chem 286 6: 4173–4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mertins P, Eberl HC, Renkawitz J, Olsen JV, Tremblay, et al. (2008) Investigation of protein-tyrosine phosphatase 1B function by quantitative proteomics. Mol Cell Proteomics 7 9: 1763–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang YC, Lin SY, Liang SY, Pan KT, Chou CC, et al. (2008) Tyrosine phosphoproteomics and identification of substrates of protein tyrosine phosphatase dPTP61F in Drosophila S2 cells by mass spectrometry-based substrate trapping strategy. J Proteome Res 7 3: 1055–1066. [DOI] [PubMed] [Google Scholar]
- 35. Hilger M, Bonaldi T, Gnad F, Mann M (2009) Systems-wide analysis of a phosphatase knock-down by quantitative proteomics and phosphoproteomics. Mol Cell Proteomics 8: 1908–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Riehle MA, Fan Y, Cao C, Brown MR (2006) Molecular characterization of insulin-like peptides in the yellow fever mosquito, Aedes aegypti: expression, cellular localization, and phylogeny. Peptides 27 11: 2547–2560. [DOI] [PubMed] [Google Scholar]
- 37. Pakpour N, Corby-Harris V, Green GP, Smithers HM, Cheung KW, et al. (2012) Ingested human insulin inhibits the mosquito NF-κB-dependent immune response to Plasmodium falciparum . Infect Immun 80 6: 2141–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Helbling P, Graf R (1998) Localization of the mosquito insulin receptor homolog (MIR) in reproducing yellow fever mosquitoes (Aedes aegypti). J Insect Physiol 44 12: 1127–1135. [DOI] [PubMed] [Google Scholar]
- 39. Castillo J, Brown MR, Strand MR (2011) Blood feeding and insulin-like peptide 3 stimulate proliferation of hemocytes in the mosquito Aedes aegypti . PLoS Pathog 7 10: e1002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hauck ES, Antonova-Koch Y, Drexler A, Pietri J, Pakpour N, et al. (2013) Overexpression of phosphatase and tensin homolog improves fitness and decreases Plasmodium falciparum development in Anopheles stephensi . Microbes Infect 15 12: 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tiganis T (2013) PTP1B and TCPTP–nonredundant phosphatases in insulin signaling and glucose homeostasis. FEBS J 280 2: 445–458. [DOI] [PubMed] [Google Scholar]
- 42. McLaughlin S, Dixon JE (1993) Alternative splicing gives rise to a nuclear protein tyrosine phosphatase in Drosophila. J Biol Chem 268 10: 6839–6842. [PubMed] [Google Scholar]
- 43. Shimizu S, Ugi S, Maegawa H, Egawa K, Nishio Y, et al. (2003) Protein-tyrosine phosphatase 1B as new activator for hepatic lipogenesis via sterol regulatory element-binding protein-1 gene expression. J Biol Chem 278 44: 43095–43101. [DOI] [PubMed] [Google Scholar]
- 44. Ugi S, Shi K, Nishio Y, Shimizu S, Guo B, et al. (2009) Membrane localization of protein-tyrosine phosphatase 1B is essential for its activation of sterol regulatory element-binding protein-1 gene expression and consequent hypertriglyceridaemia. J Biochem 146 4: 541–547. [DOI] [PubMed] [Google Scholar]
- 45. Silveira AB, Castro-Santos J, Senna R, Logullo C, Fialho E, et al. (2006) Tick vitellin is dephosphorylated by a protein tyrosine phosphatase during egg development: effect of dephosphorylation on VT proteolysis. Insect Biochem Mol Biol 36 3: 200–209. [DOI] [PubMed] [Google Scholar]
- 46. Fialho E, Silveira AB, Masuda H, Silva-Neto MA (2002) Oocyte fertilization triggers acid phosphatase activity during Rhodnius prolixus embryogenesis. Insect Biochem Mol Biol 32 8: 871–280. [DOI] [PubMed] [Google Scholar]
- 47. Huang CH, Lin TY, Pan RL, Juang JL (2007) The involvement of Abl and PTP61F in the regulation of Abi protein localization and stability and lamella formation in Drosophila S2 cells. J Biol Chem 282 44: 32442–32452. [DOI] [PubMed] [Google Scholar]
- 48. Bonizzoni M, Dunn WA, Campbell CL, Olson KE, Dimon MT, et al. (2011) RNA-seq analyses of blood-induced changes in gene expression in the mosquito vector species, Aedes aegypti . BMC Genomics 12: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gazos-Lopes F, Mesquita RD, Silva-Cardoso L, Senna R, Silveira AB, et al. (2012) Glycoinositolphospholipids from Trypanosomatids subvert nitric oxide production in Rhodnius prolixus salivary glands. PLoS One 7 10: e47285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mesquita RD, Seabra Junior ES, Matos ES (2011) FAT-Functional Analysis Tool; patented at the Brazilian national trade mark office, # 11083–6.
- 51. Wood TC, Pearson WR (1999) Evolution of protein sequences and structures. J Mol Biol 291 4: 977–995. [DOI] [PubMed] [Google Scholar]
- 52. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 17: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Corpet F (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16 22: 10881–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kelley LA, Sternberg MJ (2009) Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc 4 3: 363–371. [DOI] [PubMed] [Google Scholar]
- 55. Vriend G (1990) WHAT IF: a molecular modeling and drug design program. J Mol Graph 8 1: 52–29, 52-6, 29. [DOI] [PubMed] [Google Scholar]
- 56. Wass MN, Sternberg MJ (2008) ConFunc–functional annotation in the twilight zone. Bioinformatics 24 6: 798–806. [DOI] [PubMed] [Google Scholar]
- 57. Marchler-Bauer A, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, et al. (2009) CDD: specific functional annotation with the Conserved Domain Database. Nucleic Acids Res 37 Database issue: D205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Porter CT, Bartlett GJ, Thornton JM (2004) The Catalytic Site Atlas: a resource of catalytic sites and residues identified in enzymes using structural data. Nucleic Acids Res 32 Database issue: D129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Innis CA (2007) siteFiNDER|3D: a web-based tool for predicting the location of functional sites in proteins. Nucleic Acids Res 35 Web Server issue: W489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Andersen JN, Mortensen OH, Peters GH, Drake PG, Iversen LF, et al. (2001) Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol Cell Biol 21 21: 7117–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McGuffin LJ, Bryson K, Jones DT (2000) The PSIPRED protein structure prediction server. Bioinformatics 16 4: 404–405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of a tyrosine phosphorylated peptide on PTP activity towards pNPP on different tissues of A. aegypti . Tissues dissected from 10% sucrose-fed females (were homogenized and enzyme activity towards pNPP was assayed in the presence or absence of 0.1 mM of PTP 1B substrate II. After 30 minutes at 37°C, the reactions were halted by adding 1∶8,5 volumes of 2 M NaOH. Results represent the standard two determinations in triplicate and mean deviation.
(TIF)