Abstract
Human sex determining region Y-box 2 (SOX2) is an important transcriptional factor involved in the pluripotency and stemness of human embryonic stem cells. SOX2 plays important roles in maintaining cancer stem cell activities of melanoma and cancers of the brain, prostate, breast, and lung. SOX2 is also a lineage survival oncogene for squamous cell carcinoma of the lung and esophagus. Spontaneous cellular and humoral immune responses against SOX2 present in cancer patients classify it as a tumor-associated antigen (TAA) shared by lung cancer, glioblastoma, and prostate cancer among others. In this study, B-cell epitopes were predicted using computer-assisted algorithms. Synthetic peptides based on the prediction were screened for recognition by serum samples from cancer patients using ELISA. Two dominant B-cell epitopes, SOX2:52-87 and SOX2:98-124 were identified. Prostate cancer, glioblastoma and lung cancer serum samples that recognized the above SOX2 epitopes also recognized the full-length protein based on Western blot. These B-cell epitopes may be used in assessing humoral immune responses against SOX2 in cancer immunotherapy and stem cell-related transplantation.
Keywords: iPS cell, pluripotent stem cell, cancer stem cell, autoantibody, biomarkers
Introduction
SOX2 is a single-exon gene located on chromosome 3q26.3-q27 and encodes a 317 amino acid protein with a characteristic high mobility group (HMG) DNA-binding domain [1]. SOX2 belongs to the SOX family of transcriptional factors that include at least 14 members such as SOX1, SOX4, SOX9, SOX11 and so on [2]. SOX2 is highly expressed in the nervous system [3] during embryonic development but is down-regulated when neural cells exit the cell cycle and differentiate [4]. SOX2 is also found in adult granule cells of the cerebellum [3], brain, testis, and to a less extent the alimentary canal and prostate [5]. SOX2 is probably best known for its role together with OCT4, KLF4 and c-MYC [6,7], or OCT4, NANOG, and LIN28 [8] in reprogramming human and mouse fibroblasts into induced pluripotent stem cells or iPS cells. SOX2 expressions are found in cancerous cells that have acquired stem cell properties or the so-called cancer stem cells. For example, pediatric brain tumors including medulloblastomas and glioblastomas contained stem cell like populations with high levels of SOX2 expression. This population of cells formed neurospheres that can be passaged at clonal density and are able to self-renew [9]. SOX2 was also upregulated in adult medulloblastoma and glioblastoma [10,11] and believed to play roles to maintain tumor stemness properties and tumorigenicity. Similarly, SOX2 overexpression was found in cancer stem cells from prostate cancer [12], lung cancer [13], breast cancer [14], esophageal cancer [15], and melanoma [16] that shared the same neuroectodermal lineage in development. For example, not only was SOX2 overexpressed in a prostate cancer population with stem cell properties, but ectopic overexpression of SOX2 contributed to the acquired stemness properties including androgen independence in vitro [17,18]. In squamous cell carcinomas of the lung, SOX2 expression was required for proliferation and anchorage-independent growth of lung cell lines; while ectopic expression of SOX2 was able to transform immortalized tracheobronchial epithelial cells, showing expression of markers of both squamous differentiation and pluripotency [15]. In human lung adenocarcinomas, SOX2 was specifically expressed in a side population with stem cell properties in vitro; while SOX2 knockdown of LHK2 side population cells by gene-specific siRNA completely abrogated tumorigenicity in vivo [13].
SOX2 is also known for inducing spontaneous antibody and T cell responses in various cancer patients, and therefore qualified as a TAA. A striking feature of the spontaneous immunity against SOX2 is that autoantibodies (autoAb) are even detected with serum dilutions of up to 1:106 in some cancer patients [5,19]. High-titer and class-switched autoAb were observed in 15% of small cell lung cancer (SCLC), 23% of non-small cell lung cancer (NSCLC), 22% of breast cancer, and 19% of ovarian cancer (Ali Gure, unpublished data). T cell responses against SOX2 were also demonstrated in more than 70% monoclonal gammopathy of unknown significance and SCLC patients [20], which were associated with a better prognosis [19].
With the potential implications of autoAb against SOX2 as biomarkers in cancer detection, responses to therapy, and prognosis as well as monitoring teratoma formation in transplantation of pluripotent stem cells, B cell epitopes from human SOX2 were identified and validated in this study.
Materials and methods
Prediction of B-cell epitopes using computer-assisted algorithm
The hydrophilicity/hydrophobicity analysis of SOX2 was calculated based on the Kyte-Doolittle method using a window size of 9. The program of RVP-NET: real value prediction of solvent accessibility at web site http://gibk26.bse.kyutech.ac.jp/jouhou/shandar/netasa/rvp-net/ was used for predicting secondary structures of the SOX2 protein. A window of 12 amino acid residues was used to obtain the solvent accessibility plot using the prediction program.
Preparation of recombinant proteins and synthetic peptides
Recombinant SOX2 protein was purified from bacteria as previously reported [5]. Synthetic peptides used in this study were made using a solid-phase method on a peptide synthesizer at Genscript Inc (Piscataway, NJ). The molecular weight of individual peptide was evaluated by mass spectrometry to confirm the identity before product release from the manufacturer. Peptides were re-suspended in DMSO solution at 20 mg/ml and stored at –20°C until use.
Detection of SOX2 autoAb in patients’ serum samples and confirmation with Western blot
Sera from patients with glioblastoma, prostate cancer, and lung cancer were collected at the UCLA medical center under approved IRB protocols. All serum samples were from histologically confirmed cancer patients; however, clinical stages of each sample varied from patient to patient. Serum samples were stored at –20°C until being analyzed.
Antigen-coated plates were prepared using 250 ng/well purified SOX2 protein or 60 ng/well SOX2 synthetic peptides of 20-36 amino acid residues in 100 µL carbonate bicarbonate buffer. These antigens were adsorbed overnight to coat a 96-well MaxiSorp plate (Nunc, Denmark) at 4°C. Control plates were coated with 60 ng/well of a ß-galactosidase peptide. Plates were blocked with 5% Fetal Bovine Serum (FBS) in PBST (PBS plus 0.05% Tween-20) for at least 2 hours, washed with PBST, and loaded with 100 µL of diluted serum samples. All serum samples were diluted at 1:10, 1:20, and 1:50 with PBST containing 5% FBS unless otherwise specified. Each sample at the three different dilutions was loaded onto pre-coated ELISA plates. After a 2-hour incubation at room temperature, plates were washed, and loaded with secondary antibodies (goat anti-human immunoglobulin conjugated with horseradish peroxidase, Sigma Co., St. Louis, MO) diluted with 5% FBS in PBST. Plates were developed after a one-hour incubation, and absorbance at 450 nm was read by using an ELISA reader. The cut-off value was defined as the mean optimal density (OD) value plus 3 times standard derivations of healthy donors (HD). If an OD value against a peptide exceeded the cut-off from at least 2 of the 3 dilutions, the sample was regarded as positive.
Western blotting analysis was performed by SDS-PAGE followed by electro-transfer of proteins onto low fluorescence Immobilon-FL PVDF membrane (Millipore, MA). Immunoblots were probed using diluted patients’ serum samples reactive to the peptides based on ELISA screening. The secondary Ab used was goat anti-human IgG conjugated with horse redish peroxidase as previously described [21]. Immunoblot was developed using Pierce ECL Plus reagent (Thermo Scientific, IL) and visualized using Typhoon 9410 Imager (GE Healthcare, Pittsburgh, PA) at UCLA Biological Chemistry Imaging Facility. Images were analyzed with ImageQuant software.
Results
Prediction of B-cell epitopes from cancer/stem cell antigen SOX2
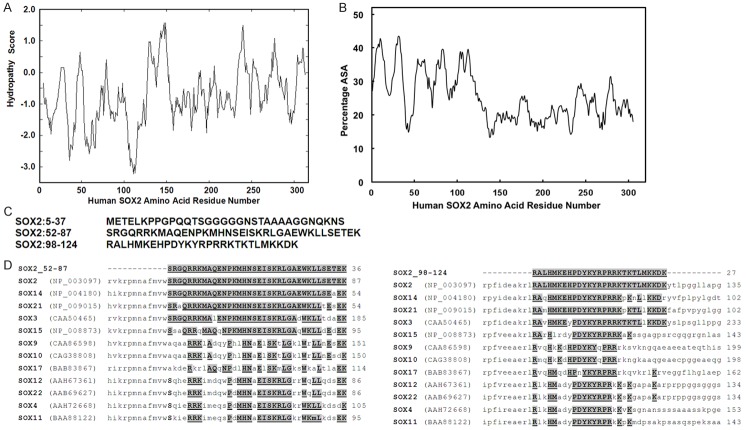
The 317-amino acid protein, SOX2 has a hydrophilic N-terminal domain and a relatively hydrophobic C-terminal domain based on hydropathical profiles predicted using the Kyte-Doolittle method [22] (Figure 1A). Using a window size of 9, negative peaks suggest the likelihood of hydrophobic regions of globular proteins. It is known that linear epitopes of small sequence segments are usually peptides conferring relatively loose conformations, such as the beta-turn and coil conformations of hydrophobic regions [23], and most linear epitopes consisted of at least 7 amino acid residues, we thus used a window of 12 amino acid residues to obtain the solvent accessibility plot (Figure 1B). Essentially, the N-terminal hydrophilic domain was predicted to be exposed on the surface of the protein, which was accessible to the solvent while the C-terminal domain was likely buried in the interior of the protein. Therefore, the N-terminal might have direct contact with immunoglobulin molecules on the surface of a plasma B cell and contain B-cell epitopes recognized by Ab present in patients’ sera. Three peptides of 20-40 amino acid residues with highest predicted ASA scores were synthesized (Figure 1C). Homology alignments based on the peptide sequences show both peptide fragments had significant (> 80%) homologies with some other members of the SOX gene family, such as SOX3, SOX14, and SOX21 (Figure 1D).
Figure 1.
A. A hydropathical profile predicted using the Kyte-Doolittle method with a window size of 9 amino acid residues; B. Computer-assisted prediction of B cell epitopes from human SOX2; C. Peptides synthesized for screening B cell epitopes from SOX2; D. Human SOX family members that share homology with the identified B cell epitopes are highlighted. Initial alignment of SOX proteins and B-cell epitopes was performed using ClustalW2 alignment tool from EMBL-EBI website (www.ebi.ac.uk/tools/msa/clustalw2). Accession numbers for SOX proteins are provided in parentheses.
Identification of SOX2-derived B cell epitopes recognized by Ab present in patients’ serum samples
To identify the B-cell epitopes among the peptides, which were recognized by serum-derived Ab, serum samples from 19 patients with glioblastoma, 19 patients with prostate cancer, and 5 patients with lung cancer were used to screen for recognition of the three synthetic peptides. Eight healthy donors were used to establish the cuttoff value for a seropositve reaction, which is the average OD (OD agasint control peptide subtracted from OD against the peptide of interest) from the healthy donors plus three times of the standard derivation as previously reported in similar experiments. Recognition of two peptides, SOX2:52-87 and SOX2:98-124, was observed in 1 glioblastoma patient (#2783, Figure 2A) and 2 prostate cancer patients (#1038 and 1131, Figure 2B). None of the samples recognized SOX2:5-37 in the screening effort (data not shown).
Figure 2.
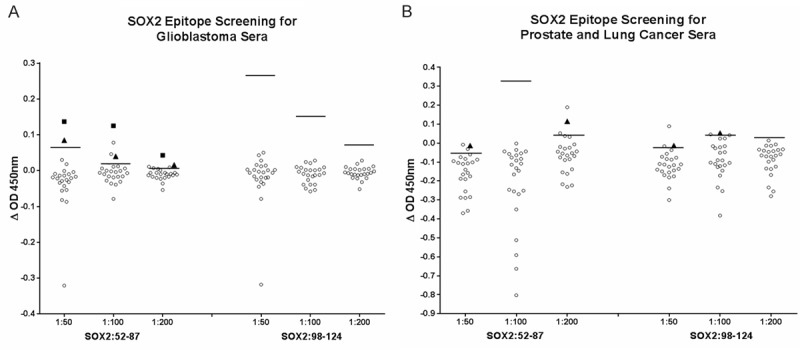
Screening of patients’ serum samples for recognition of SOX-2 B cell epitopes using ELISA. Serum samples from 8 HD and 19 glioblastoma patients (A) and 19 prostate cancer and 5 lung cancer patients (B) were used to screen for recognition of SOX2 peptides predicted as B cell epitopes.
Verification of SOX2 peptide epitopes by Western blot
To determine that sera reacting with the 2 synthetic peptides also recognized the full-length SOX2 protein, ELISA and Western blot were conducted using the above-referenced 3 sero-positive samples as well as a previously known sero-positive sample (#KHA-71, small cell lung cancer patient). Both ELISA and Western blot experiments indicated that the 3 seropositive patients identified by screening against the peptides recognized the full-length SOX2 recombinant protein. The previously known seropositive patient (#KHA-71) served as a positive control; meanwhile a prostate cancer serum sample that did not react to these targets served as a negative control (Figure 3). None of the seropositive samples had significant recognition of the negative control proteins, which contained more than 6 μg HEK293T cell lysates in each lane. These data reconfirmed that the SOX2:52-87 and SOX2:98-124 peptides served as B-cell epitopes recognized by serum samples from various cancer patients.
Figure 3.
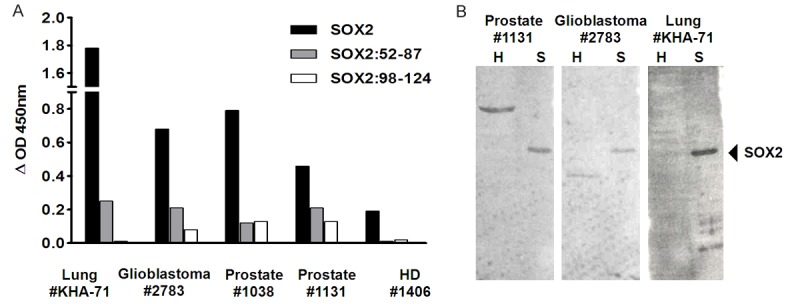
Validation of autoAb responses against B cell epitopes derived from SOX2 using a seropositive sample (#KHA-71) known to contain high titer autoAb against SOX2 as control, by ELISA (A) and by Western blot on the full-length SOX2 protein (B). In the Western blot, about 6 μg lysates from HEK293T cells, labeled as H, were used as negative controls side by side with 200 ng purified recombinant SOX2 protein, labeled as S.
Discussion
Recent success in the therapeutic arena using immune checkpoint blockers [24,25] has indicated the importance of tipping the balance of spontaneous anti-tumor immunity in vivo. Immune responses against TAA have also attractedsignificant attention as emerging cancer biomarkers [26]. Comparing to classic biomarkers, immune responses such as autoAb responses against TAA may be better surrogates for cancer-related inflammation, immunocompetence of the host, and the immunogenicity of the endogenously arising cancer. With the development of more sensitive and multiplex technologies, autoAb against TAA with significant roles in oncogenesis (such as SOX2) may open new doors for biomarkers in cancer detection, prognosis and responses to therapy. As a TAA shared by a couple of human cancers, autoAb responses against SOX2 peptide epitopes may find utilities in the above-referenced fields. Other members of the SOX family are also expressed in cancer tissues, including lung, brain, pancreas, stomach, and breast cancers [27]. At least one of them, SOX4 has been found to induce T cell and B cell responses in small cell lung cancer patients [27].
It is also noteworthy that autoAb responses against dominant peptide epitopes may overlook conformational epitopes preserved among full-length protein antigens. However, more sensitive technology platforms such as those using microspheres (Luminex Corp., Austin, TX) may achieve high sensitivity by compensating the loss of conformational epitopes based on our previous experience [28]. Even though autoAb responses against the currently described SOX2 epitopes only have limited sensitivities, multiplex technologies allow us to measure autoAb against a panel rather than individual targets and develop assays in conjunction with conventional cancer biomarkers such as prostate specific antigen (PSA) to improve sensitivities and specificities for certain cancer types [28].
SOX2 represents an excellent example of a protein that plays important roles in both oncogenesis and pluripotency. Interestingly, the same unique properties that make human pluripotent stem cells greatly promising, namely self-renewal and pluripotency, also directly contribute to their unbridled tumorigenicity when organs are transplanted into human hosts. Indeed, it is reasonable to predict that human pluripotent stem cells are the archetype of tumor-forming stem cells, and unless their tumorigenicity fully attenuated, it will represent a significant threat in the clinical application of stem cell-based therapies. In this regards, SOX2-derived B cell epitopes may also find great promise in monitoring tumorigenicity, such as teratoma formation of pluripotent stem cell-based therapies in the future.
Acknowledgements
This work is supported in part by the NIHR01CA164388 to G.Z. J.S. and M.R. are undergraduate students participating in the UCLA student research program. Other UCLA undergraduate students involved are Michael Mangubat, Gabriel Mendoza Jr., and Arianna Truong. S.H.L. is a recipient of the NIH diversity supplement postdoctoral fellowship. Maize Wang at UCLA and Sukru Atakan at Bilkent University provided excellent technical assistance.
Disclosure of conflict of interest
None to disclose.
References
- 1.Scaffidi P, Bianchi ME. Spatially precise DNA bending is an essential activity of the sox2 transcription factor. J Biol Chem. 2001;276:47296–47302. doi: 10.1074/jbc.M107619200. [DOI] [PubMed] [Google Scholar]
- 2.Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 1999;27:1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aruga J, Nagai T, Tokuyama T, Hayashizaki Y, Okazaki Y, Chapman VM, Mikoshiba K. The mouse zic gene family. Homologues of the Drosophila pair-rule gene odd-paired. J Biol Chem. 1996;271:1043–1047. doi: 10.1074/jbc.271.2.1043. [DOI] [PubMed] [Google Scholar]
- 4.Uwanogho D, Rex M, Cartwright EJ, Pearl G, Healy C, Scotting PJ, Sharpe PT. Embryonic expression of the chicken Sox2, Sox3 and Sox11 genes suggests an interactive role in neuronal development. Mech Dev. 1995;49:23–36. doi: 10.1016/0925-4773(94)00299-3. [DOI] [PubMed] [Google Scholar]
- 5.Gure AO, Stockert E, Scanlan MJ, Keresztes RS, Jager D, Altorki NK, Old LJ, Chen YT. Serological identification of embryonic neural proteins as highly immunogenic tumor antigens in small cell lung cancer. Proc Natl Acad Sci U S A. 2000;97:4198–4203. doi: 10.1073/pnas.97.8.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 9.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gangemi RM, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P, Ravetti GL, Zona GL, Daga A, Corte G. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27:40–48. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- 11.Cox JL, Wilder PJ, Desler M, Rizzino A. Elevating SOX2 levels deleteriously affects the growth of medulloblastoma and glioblastoma cells. PLoS One. 2012;7:e44087. doi: 10.1371/journal.pone.0044087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu G, Yuan J, Wills M, Kasper S. Prostate Cancer Cells with Stem Cell Characteristics Reconstitute the Original Human Tumor In vivo. Cancer Res. 2007;67:4807–4815. doi: 10.1158/0008-5472.CAN-06-4608. [DOI] [PubMed] [Google Scholar]
- 13.Nakatsugawa M, Takahashi A, Hirohashi Y, Torigoe T, Inoda S, Murase M, Asanuma H, Tamura Y, Morita R, Michifuri Y, Kondo T, Hasegawa T, Takahashi H, Sato N. SOX2 is overexpressed in stem-like cells of human lung adenocarcinoma and augments the tumorigenicity. Lab Invest. 2011;91:1796–1804. doi: 10.1038/labinvest.2011.140. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Shi L, Zhang L, Li R, Liang J, Yu W, Sun L, Yang X, Wang Y, Zhang Y, Shang Y. The molecular mechanism governing the oncogenic potential of sox2 in breast cancer. J Biol Chem. 2008;283:17969–78. doi: 10.1074/jbc.M802917200. [DOI] [PubMed] [Google Scholar]
- 15.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I, Ramos AH, Woo MS, Weir BA, Getz G, Beroukhim R, O’Kelly M, Dutt A, Rozenblatt-Rosen O, Dziunycz P, Komisarof J, Chirieac LR, Lafargue CJ, Scheble V, Wilbertz T, Ma C, Rao S, Nakagawa H, Stairs DB, Lin L, Giordano TJ, Wagner P, Minna JD, Gazdar AF, Zhu CQ, Brose MS, Cecconello I, Jr UR, Marie SK, Dahl O, Shivdasani RA, Tsao MS, Rubin MA, Wong KK, Regev A, Hahn WC, Beer DG, Rustgi AK, Meyerson M. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girouard SD, Laga AC, Mihm MC, Scolyer RA, Thompson JF, Zhan Q, Widlund HR, Lee CW, Murphy GF. SOX2 contributes to melanoma cell invasion. Lab Invest. 2012;92:362–370. doi: 10.1038/labinvest.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seiler D, Zheng J, Liu G, Wang S, Yamashiro J, Reiter RE, Huang J, Zeng G. Enrichment of putative prostate cancer stem cells after androgen deprivation: Upregulation of pluripotency transactivators concurs with resistance to androgen deprivation in LNCaP cell lines. Prostate. 2013;73:1378–90. doi: 10.1002/pros.22685. [DOI] [PubMed] [Google Scholar]
- 18.Vencio EF, Nelson AM, Cavanaugh C, Ware CB, Milller DG, Garcia JC, Vencio RZ, Loprieno MA, Liu AY. Reprogramming of prostate cancer-associated stromal cells to embryonic stem-like. Prostate. 2012;72:1453–1463. doi: 10.1002/pros.22497. [DOI] [PubMed] [Google Scholar]
- 19.Vural B, Chen LC, Saip P, Chen YT, Ustuner Z, Gonen M, Simpson AJ, Old LJ, Ozbek U, Gure AO. Frequency of SOX Group B (SOX1, 2, 3) and ZIC2 antibodies in Turkish patients with small cell lung carcinoma and their correlation with clinical parameters. Cancer. 2005;103:2575–2583. doi: 10.1002/cncr.21088. [DOI] [PubMed] [Google Scholar]
- 20.Spisek R, Kukreja A, Chen LC, Matthews P, Mazumder A, Vesole D, Jagannath S, Zebroski HA, Simpson AJ, Ritter G, Durie B, Crowley J, Shaughnessy JD Jr, Scanlan MJ, Gure AO, Barlogie B, Dhodapkar MV. Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med. 2007;204:831–840. doi: 10.1084/jem.20062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng G, Touloukian CE, Wang X, Restifo NP, Rosenberg SA, Wang RF. Identification of CD4+ T cell epitopes from NY-ESO-1 presented by HLA-DR molecules. J Immunol. 2000;165:1153–1159. doi: 10.4049/jimmunol.165.2.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyte J, Doolitle R. A simple method for displaying the hydrophobic character of a protein. J Mol Bio. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 23.Alix AJ. Predictive estimation of protein linear epitopes by using the program people. Vaccine. 1999;18:311–314. doi: 10.1016/s0264-410x(99)00329-1. [DOI] [PubMed] [Google Scholar]
- 24.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, Sedrak C, Jungbluth AA, Chua R, Yang AS, Roman RA, Rosner S, Benson B, Allison JP, Lesokhin AM, Gnjatic S, Wolchok JD. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finn OJ. Immune response as a biomarker for cancer detection and a lot more. N Engl J Med. 2005;353:1288–1290. doi: 10.1056/NEJMe058157. [DOI] [PubMed] [Google Scholar]
- 27.Friedman RS, Bangur CS, Zasloff EJ, Fan L, Wang T, Watanabe Y, Kalos M. Molecular and immunological evaluation of the transcription factor SOX-4 as a lung tumor vaccine antigen. J Immunol. 2004;172:3319–3327. doi: 10.4049/jimmunol.172.5.3319. [DOI] [PubMed] [Google Scholar]
- 28.Xie C, Kim HJ, Haw JG, Kalbasi A, Gardner BK, Li G, Rao JY, Chia D, Liong M, Punzalan RR, Marks LS, Pantuck AJ, de la Taille A, Wang G, Mukouyama H, Zeng G. A Novel Multiplex Assay Combining Autoantibodies Plus PSA Has Potential Implications for Classification of Prostate Cancer from Non-malignant Cases. J Transl Med. 2011;9:43. doi: 10.1186/1479-5876-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]