Abstract
The recent Deoni et al. (2013) manuscript proposed that breastfeeding was associated with increased cognitive ability and white-matter in older children (over 26 months), using ms-DESPOT MRI imaging to indirectly measure white matter in children who were either breastfed, formula fed, or combined breast + formula fed. In this response, we identify limitations in drawing causal inference among white matter, cognitive ability, and breastfeeding. We propose that the observed cognitive and neurodevelopmental differences between breastfed and formula-fed infants might actually be caused by the premature introduction of cow's milk in the second year of life, among other contributing factors.
The implication of a causal relationship between intelligence and white matter metrics, especially in a developmentally young population, is premature given the recency of this field. The original analyses did not control for important covariates; when comparing both white matter and test scores, mothers were not controlled for age and socio-economic status (SES) and their children were not controlled for gender. Raw test scores, instead of age-adjusted test scores, were used even though the children were of different ages. Mothers were not controlled for reason(s) not to breastfeed, even though many prenatal factors are known to predict this such as stress, parity, obesity, and smoking habits. The observed cognitive ability and white matter benefits identified primarily within the long-term breastfed children are at least partially attributable to other factors such as age, gender, and SES. We suggest methodological approaches to removing such ambiguity, and ways to dissociate cause from effect. The formula and breastfeeding groups didn't show differences until the “formula fed” children likely had been fed cow's milk for longer than they had been fed formula, at 2.2 years. The greatest cognitive differences however were observed within the high SES breastfed infants depending on breastfeeding duration; infants who were breastfed over 15 months showed increased cognitive ability compared to those breastfed less than months. This implicates the source of dairy during the second year of life, and not other SES factors or infant formula, as the most likely nutritional factor responsible for the observed differences within the breastfed children.
Given the known nutritional deficiencies of cow's milk, these findings imply infants who received cow's milk during the second year of life were at a disadvantage compared to those who were breastfed, independent of whether they were fed formula or breast milk during the first year of life. This evidence suggests that infants should receive formula in lieu of cow's milk when breast milk is unavailable as a dairy source, until roughly 2 years of age.
Keywords: Breastfeeding, Cognition, Brain development, Myelin maturation, White matter development, Infant imaging, Myelin, Myelin water fraction, Magnetic resonance imaging, Nutrition
Comment
Recently, Deoni et al. (2013) used mcDESPOT MRI imaging to link infant feeding modality (breastfeeding, formula feeding, and breastfeeding + formula feeding) to both cognitive ability (measured through the Mullen tests) and white-matter development. As scientists and as mothers who have nursed a total of 7 children, we believe that a strictly causal relationship among breastfeeding, white matter development, and cognitive ability is unsubstantiated by the data and the statistical models used to analyze it. In this response, we discuss the limitations of this study, including the contributing effects of infant age and gender, and the general inability to dissociate cause from correlation. We discuss methodological ways in which this ambiguity can be reduced and propose a separate conclusion from the original study: observed differences between breastfed and formula fed infants may be caused by the premature substitution of cow's milk into the child's diet, along with other contributing factors.
The Deoni study suggests a causal link between white matter and intelligence. White matter was not measured directly, but myelin-associated water pool (VFm), was used as a proxy for it. In the literature there is some evidence for a relationship between white matter integrity, as assessed by a measure of white matter microstructure, fractional anisotropy (FA), and IQ. Recent findings suggest that a positive correlation between FA values in the corpus callosum and IQ might underlie increased efficiency of information transfer between the two hemispheres (Navas-Sánchez et al., 2014). Although recent results point to age itself as mediating the association between white matter integrity and higher order abilities and processing speed (Borghesani et al., 2013), little is known about the relationship in the very earliest developmental stages of life. The article by Borghesani and colleagues showed that FA was associated with processing speed, but not reasoning or flexibility. To infer that greater FA values represent generally higher cognitive abilities as calculated by IQ testing is a field at the very earliest stages of scientific research. While the relationship between cognitive abilities such as flexibility, processing speed and reasoning and IQ is well-characterized in the neuropsychiatric literature (Gottfredson, 1997; Neisser et al., 1996; Snyderman and Rothman, 1987), only recent advances in neuroimaging techniques have allowed for a similar relationship to be explored between white matter integrity and IQ in vivo (Brancucci, 2012; Colom et al., 2010). To characterize the structure–function relationship between IQ and FA as sufficiently established to make causal inferences in a developmentally immature population is premature.
When comparing both white matter and test scores, mothers were not controlled for age and socio-economic status (SES) and their children were not controlled for gender. A 2-sample t-test found that the largest differences between formula fed and breastfed infants was not in any of the five cognitive domains compared (gross motor, fine motor, receptive language, expressive language, and visual reception), but rather in the SES of the children's mothers. Although a univariate test showed these groups not to be significantly different, this does not imply that the multivariate effect of age, SES, and gender was not significant. Their multivariate effect could have been tested directly, using a hierarchical regression or propensity scores to predict receptive language scores using feeding modality, maternal age, infant age, SES, and child gender as covariates. Given that these covariates are well-studied and known to affect infant development, they should have been controlled for explicitly.
It is known that the neurodevelopmental trajectories of infant males and females differ, and that males have more white-matter (Gur et al., 1999). The observed white-matter group differences were markedly inconsistent with an expected dose-dependent benefit from breastfeeding; this study found greater differences between the breastfed group and breastfed + formula group than between the breastfed and formula groups. Coincidentally, the breastfed + formula group had the lowest percentage of male subjects (58.8%), while the exclusively breastfed group had the largest (65.9%). This suggests that some of the observed white matter differences, originally attributed to feeding modality, are actually attributable to gender. Given that the breastfed group contained more males, this should have been controlled for explicitly in the regression models predicting, for example, mean VFm.
In addition, the Mullen test scores were not adjusted for age (Del Carmen-Wiggins et al., 2004); raw scores instead of age-normalized scores were used. Within the breastfed children, there is a clear linear relationship between age and test score as shown in Fig. 1. This was not accounted for explicitly when comparing subgroups (formula, breast milk, and breast milk + formula); instead the ages were compared using a 2-sample t-test and found not statistically significant. However, given that the breastfed children were older and received higher test scores than the other groups, this calls into question how much of the observed “dairy” effect is actually due to age. Using the summary statistics supplied in the original Deoni manuscript, we compare directly two subgroups with nearly identical ages: formula fed children aged 807.5 ± 369 days and extended breastfed children aged 807 ± 341 days. There were no statistically significant differences between any of the five domains tested using a 2-sample t-test, as shown in Table 1. Deoni found differences in receptive language between breastfed and formula fed older children using raw test scores, but the average age of the breastfed group, and the standard deviation, were both greater than the formula fed group. The older breastfed-group implies that some of these observed test differences between breastfed and formula fed children were actually due to the ages of the children, an artifact introduced by not correcting the test scores for the infants' ages.
Fig. 1.
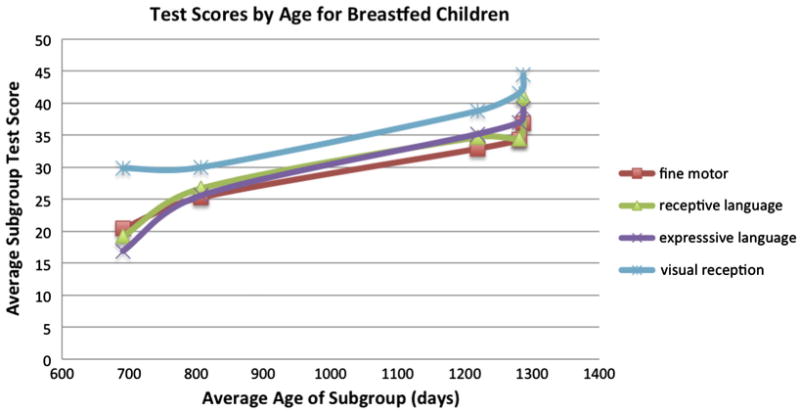
Within breastfed children, increasing age was associated with increasing raw test scores. The original manuscript compared subgroups (formula, breast milk, breast milk + formula) which were not identical in age, yet age was not explicitly adjusted for when comparing raw test scores. Summary statistics obtained from the Deoni et al. manuscript.
Table 1.
When comparing age-matched subgroups of extended-breastfed and formula fed infants, there were no statistically significant differences in any of the five cognitive domains compared. Summary statistics obtained from the original Deoni manuscript.
| Extended breastfed mean | SD | Formula fed mean | SD | Pooled SD | t-Statistic | df | p-Values | |
|---|---|---|---|---|---|---|---|---|
| n | 25.00 | 38.00 | ||||||
| Age (days) | 807.00 | 341.00 | 807.50 | 369.00 | 90.74 | 0.01 | 54.31 | 0.50 |
| Gross motor | 23.00 | 5.00 | 21.40 | 5.03 | 1.29 | −1.24 | 51.72 | 0.11 |
| Fine motor | 25.30 | 8.60 | 24.60 | 8.30 | 2.18 | −0.32 | 50.20 | 0.37 |
| Receptive language | 26.70 | 11.20 | 24.40 | 9.80 | 2.75 | −0.84 | 46.60 | 0.20 |
| Expressive language | 25.60 | 10.70 | 23.20 | 11.70 | 2.86 | −0.84 | 54.67 | 0.20 |
| Visual reception | 30.00 | 11.10 | 28.00 | 1.00 | 2.23 | −0.90 | 24.26 | 0.19 |
Mothers were not controlled for reason(s) not to breastfeed, and newer analyses which have implemented causal modeling (propensity score matching) to account for this have recently shown little to no effect of breastfeeding on early childhood outcomes (Jenkins and Foster, 2014). Many prenatal factors are known to predict breastfeeding duration, such as parity, obesity, smoking, stress, plans to return to work or school, method of delivery, and physical abuse (Insaf et al., 2011; Kendall-Tackett, 2007; O'Campo et al., 1992; Thulier and Mercer, 2009). This implies that the white matter differences, and the decision of mothers to breastfeed, could both be related to a latent factor present during the prenatal, and not postnatal, period. This could have been tested indirectly using the models presented in the article. The regression model predicted VFm based upon log(age), and included a coefficient, β, for the intercept which estimates VFm shortly after birth. A tested difference in β between groups would imply that even at birth the VFm was different. If so, later observed VFm differences may not be attributable to postnatal feeding choices, but rather prenatal influences.
Although breastfed infants showed advanced white-matter development in some regions, they showed lower rates of white-matter development in others. This, combined with the Mullen tests being statistically identical among groups (with or without age-matching), implies that the observed VFm (white-matter proxy measure) changes did not affect the infant's overall development. The data showed both increased and decreased white matter changes in breastfed infants which were regionally dependent. Averaged across all ages, these changes seemed inconsequential on infant development as measured by their expressive and receptive language, visual ability, and gross motor skills, although there were some differences in test scores within older children.
Differences in white matter and cognition among older children may be attributable to an entirely different dairy product altogether: cow's milk. It is advised that formula fed infants transition to cow's milk at 12 months, receiving 16 ounces per day thereafter (Committee on Nutrition, 1992; Maguire et al., 2013). White matter differences were not observed until children were over 26 months of age, when the “formula fed” groups would actually have been exposed to cow's milk longer than they had been exposed to formula. The most drastic cognitive test differences were found within the high-SES breastfed children, who had even less exposure to formula than the other groups. Children who were breastfed for shorter amounts of time (<12 months) compared to extended breastfeeding (>15 months) exhibited the greatest differences in Mullen test subscores. This suggests that a nutritional factor during the second year of life is responsible for cognitive and neurodevelopment differences in the third year. Given that the high-SES breastfed children likely received a uniformly high-quality table food diet, the source of dairy becomes the most likely nutritional factor responsible for differences between the extended breastfed and short-term breastfed infants.
Formula milk is more similar nutritionally to breast milk than cow's milk (Emmett and Rogers, 1997; Picciano, 2001). Cow's milk contains inordinate amounts of calcium, phosphorus, potassium and sodium, and insufficient amounts of iron, vitamin C, and linoleic acid, as well as different levels of fat needed for myelination in infants (Tunnessen and Oski, 1987; Wijndaele et al., 2009). Although certainly not identical, formula is engineered to be as similar as possible to breast milk and has been fortified with DHA and ARA since 2002, which has been shown to lead to developmental advantages (Willatts et al., 2013).
Given (1) the distinct nutritional profile of cow's milk, (2) the substantial cognitive gap within breastfed children who received limited formula yet had different exposure levels to cow's milk, and (3) the ages at which children showed neurodevelopment differences conditioned on feeding modality, we argue that the culprit underlying the observed development differences may be the premature substitution of cow's milk for infant formula. Differences within breastfed children, as well as differences between breastfed and formula fed children, appear to be partially caused during the second year of life, likely by cow's milk. This suggests that cow's milk is markedly inferior to both human breast milk and infant formula.
The “cow's milk” hypothesis is based upon our assumption that parents followed the recommended age to introduce solid food and cow's milk as determined by the American Academy of Pediatrics, however no data is provided in the original manuscript on the actual starting dates. In addition, we assume that the breastfed group was fairly homogenous based upon the SES statistics, and that the main difference between extended breastfed and short-term breastfed children is the time breastfed; however, there could be relevant contributing reasons for mothers terminating breastfeeding. For example, a mother might wean because of returning to work, which would change the home environment of an infant as well as the primary caretaker. Cow's milk may just be an indicator variable of other underlying environmental factors. In addition to nutrition, SES, gender and age, there may be several other causal factors responsible for observed differences in white matter development. The stimulation within the home environment and child sleep patterns are both linked to cognitive ability in children (Bradley and Caldwell, 1976; Tarullo et al., 2011). Dairy is only one, of many, factors which may impact a child's development.
Although we agree that there likely are developmental benefits to breastfeeding, the specific association with white-matter and cognitive ability presented in the Deoni manuscript does not imply a causal relationship between breast milk and improved cognitive functioning. Instead, we find that the data tell another compelling story which suggests that infants may be transitioning prematurely to cow's milk, which may lack adequate nutrition for their developing brains. The recommended 2 servings of dairy per day is roughly 25% of a child's caloric intake beginning at 12 months of age, a substantial nutritional contribution during an important developmental time. Given the extensive body of work on nutrition and brain development (Birch et al., 2000; Helland et al., 2003; Koletzko et al., 2008) and the recommendation to supplement infants with long-chain polyunsaturated fatty acids after 6 months of age (Koletzko et al., 2008), there may be advantages for extending the amount of time a child drinks infant formula when breastfeeding is not an option. Future studies are needed to confirm this hypothesis.
Acknowledgments
The authors gratefully acknowledge the support received from NIH K01DA034728-02.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- Birch Eileen E, Garfield Sharon, Hoffman Dennis R, Uauy Ricardo, Birch David G. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol. 2000;42(3):174–181. doi: 10.1017/s0012162200000311. [DOI] [PubMed] [Google Scholar]
- Borghesani Paul R, Madhyastha Tara M, Aylward Elizabeth H, Reiter Maya A, Swarny Bruce R, Schaie K Warner, Willis Sherry L. The association between higher order abilities, processing speed, and age are variably mediated by white matter integrity during typical aging. Neuropsychologia. 2013;51(8):1435–1444. doi: 10.1016/j.neuropsychologia.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley Robert H, Caldwell Bettye M. The relation of infants' home environments to mental test performance at fifty-four months: a follow-up study. Child Dev. 1976:1172–1174. doi: 10.1111/j.1467-8624.1984.tb03817.x. [DOI] [PubMed] [Google Scholar]
- Brancucci Alfredo. Neural correlates of cognitive ability. J Neurosci Res. 2012;90(7):1299–1309. doi: 10.1002/jnr.23045. [DOI] [PubMed] [Google Scholar]
- Colom Roberto, Karama Sherif, Jung Rex E, Haier Richard J. Human intelligence and brain networks. Dialogues Clin Neurosci. 2010;12(4):489. doi: 10.31887/DCNS.2010.12.4/rcolom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Nutrition. The use of whole cows milk in infancy. Pediatrics. 1992;89:1105–1109. [PubMed] [Google Scholar]
- Del Carmen-Wiggins Rebecca, et al. Handbook of Infant, Toddler, and Preschool Mental Health Assessment. Oxford University Press; 2004. [Google Scholar]
- Deoni Sean CL, Dean Douglas C, III, Piryatinksy Irene, OMuircheartaigh Jonathan, Waskiewicz Nicole, Lehman Katie, Han Michelle, Dirks Holly. Breastfeeding and early white matter development: a cross sectional study. NeuroImage. 2013;82:77–86. doi: 10.1016/j.neuroimage.2013.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett Pauline M, Rogers Imogen S. Properties of human milk and their relationship with maternal nutrition. Early Hum Dev. 1997;49:S7–S28. doi: 10.1016/s0378-3782(97)00051-0. [DOI] [PubMed] [Google Scholar]
- Gottfredson Linda S. Why g matters: the complexity of everyday life. Intelligence. 1997;24(1):79–132. [Google Scholar]
- Gur Ruben C, Turetsky Bruce I, Matsui Mie, Yan Michelle, Bilker Warren, Hughett Paul, Gur Raquel E. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19(10):4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helland Ingrid B, Smith Lars, Saarem Kristin, Saugstad Ola D, Drevon Christian A. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics. 2003;111(1):e39–e44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- Insaf Tabassum Z, Fortner Renée Turzanski, Pekow Penelope, Dole Nancy, Markenson Glenn, Chasan-Taber Lisa. Prenatal stress, anxiety, and depressive symptoms as predictors of intention to breastfeed among Hispanic women. J Women's Health. 2011;20(8):1183–1192. doi: 10.1089/jwh.2010.2276. [DOI] [PubMed] [Google Scholar]
- Jenkins Jade Marcus, Foster E Michael. The effects of breastfeeding exclusivity on early childhood outcomes. American Journal of Public Health. 2014;104(S1):S128–S135. doi: 10.2105/AJPH.2013.301713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall-Tackett Kathleen A. Violence against women and the perinatal period the impact of lifetime violence and abuse on pregnancy, postpartum, and breastfeeding. Trauma Violence Abuse. 2007;8(3):344–353. doi: 10.1177/1524838007304406. [DOI] [PubMed] [Google Scholar]
- Koletzko Berthold, Lien Eric, Agostoni Carlo, Böhles Hansjosef, Campoy Cristina, Cetin Irene, Decsi Tamas, Dudenhausen Joachim W, Dupont Cristophe, Forsyth Stewart, et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med. 2008;36(1):5–14. doi: 10.1515/JPM.2008.001. [DOI] [PubMed] [Google Scholar]
- Maguire Jonathon L, Lebovic Gerald, Kandasamy Sharmilaa, Khovratovich Marina, Mamdani Muhammad, Birken Catherine S, Parkin Patricia C, et al. The relationship between cow's milk and stores of vitamin D and iron in early childhood. Pediatrics. 2013;131(1):e144–e151. doi: 10.1542/peds.2012-1793. [DOI] [PubMed] [Google Scholar]
- Navas-Sánchez Francisco J, Alemán-Gómez Yasser, Sánchez-Gonzalez Javier, Guzmán-De-Villoria Juan A, Franco Carolina, Robles Olalla, Arango Celso, Desco Manuel. White matter microstructure correlates of mathematical giftedness and intelligence quotient. Hum Brain Mapp. 2014;35(6):2619–2631. doi: 10.1002/hbm.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisser Ulric, Boodoo Gwyneth, Bouchard Thomas J, Jr, Boykin A Wade, Brody Nathan, Ceci Stephen J, Halpern Diane F, Loehlin John C, Perloff Robert, Sternberg Robert J, et al. Intelligence: knowns and unknowns. Am Psychol. 1996;51(2):77. [Google Scholar]
- O'Campo Patricia, Faden Ruth R, Gielen Andrea C, Wang Mei Cheng. Prenatal factors associated with breastfeeding duration: recommendations for prenatal interventions. Birth. 1992;19(4):195–201. doi: 10.1111/j.1523-536x.1992.tb00402.x. [DOI] [PubMed] [Google Scholar]
- Picciano Mary Frances. Nutrient composition of human milk. Pediatr Clin N Am. 2001;48(1):53–67. doi: 10.1016/s0031-3955(05)70285-6. [DOI] [PubMed] [Google Scholar]
- Snyderman Mark, Rothman Stanley. Survey of expert opinion on intelligence and aptitude testing. Am Psychol. 1987;42(2):137. [Google Scholar]
- Tarullo Amanda R, Balsam Peter D, Fifer William P. Sleep and infant learning. Infant Child Dev. 2011;20(1):35–46. doi: 10.1002/icd.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulier Diane, Mercer Judith. Variables associated with breastfeeding duration. J Obstet Gynecol Neonatal Nurs. 2009;38(3):259–268. doi: 10.1111/j.1552-6909.2009.01021.x. [DOI] [PubMed] [Google Scholar]
- Tunnessen Walter W, Jr, Oski Frank A. Consequences of starting whole cow milk at 6 months of age. J Pediatr. 1987;111(6):813–816. doi: 10.1016/s0022-3476(87)80193-2. [DOI] [PubMed] [Google Scholar]
- Wijndaele Katrien, Lakshman Rajalakshmi, Landsbaugh Jill R, Ong Ken K, Ogilvie David. Determinants of early weaning and use of unmodified cow's milk in infants: a systematic review. J Am Diet Assoc. 2009;109(12):2017–2028. doi: 10.1016/j.jada.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Willatts Peter, Forsyth Stewart, Agostoni Carlo, Casaer Paul, Riva Enrica, Boehm Günther. Effects of long-chain PUFA supplementation in infant formula on cognitive function in later childhood. Am J Clin Nutr. 2013;98(2):536S–542S. doi: 10.3945/ajcn.112.038612. [DOI] [PubMed] [Google Scholar]


