Full text
PDF








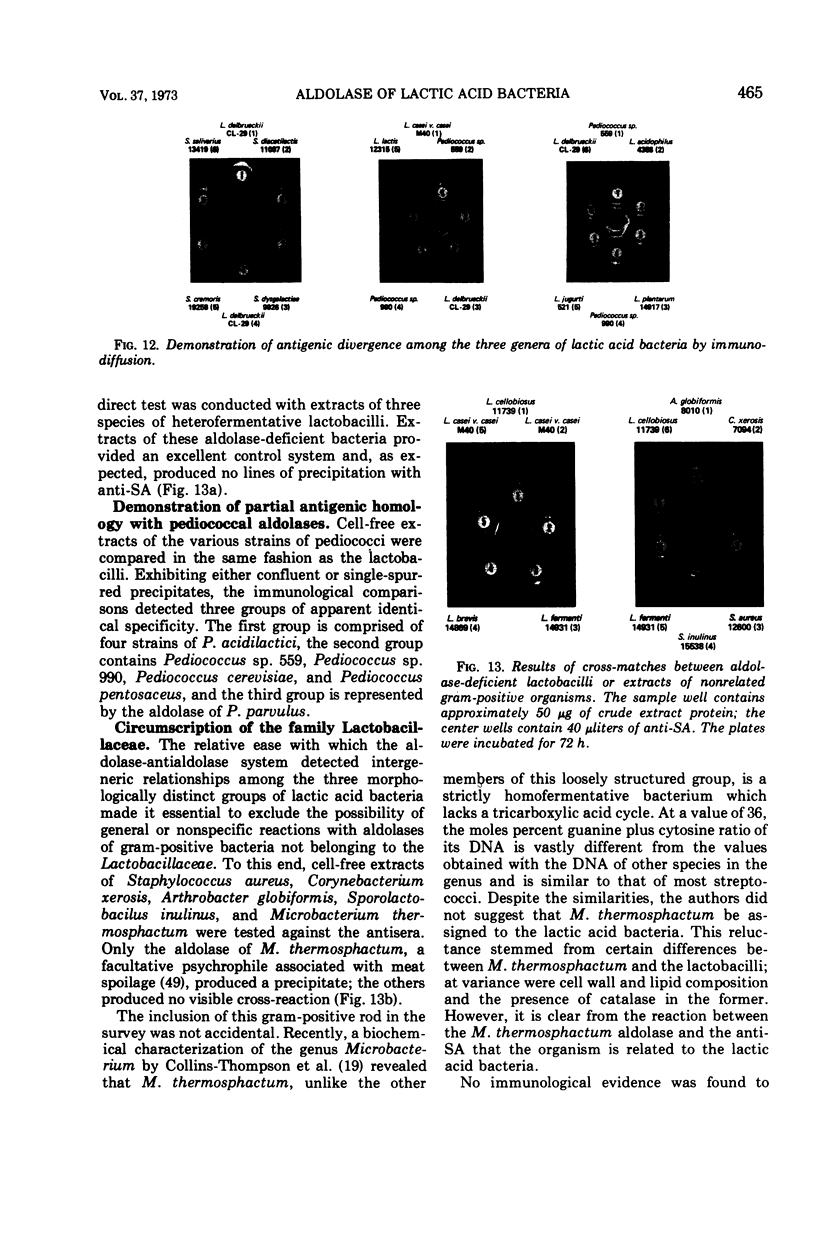





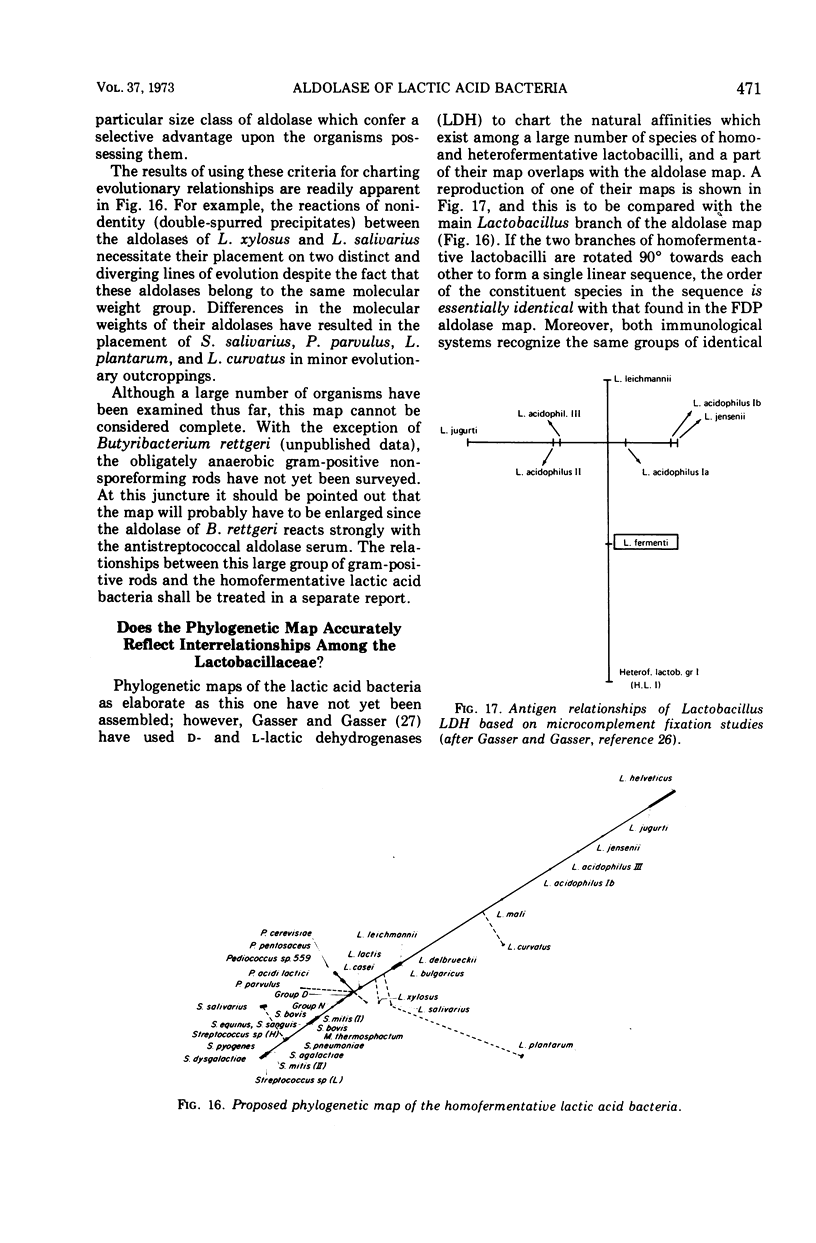



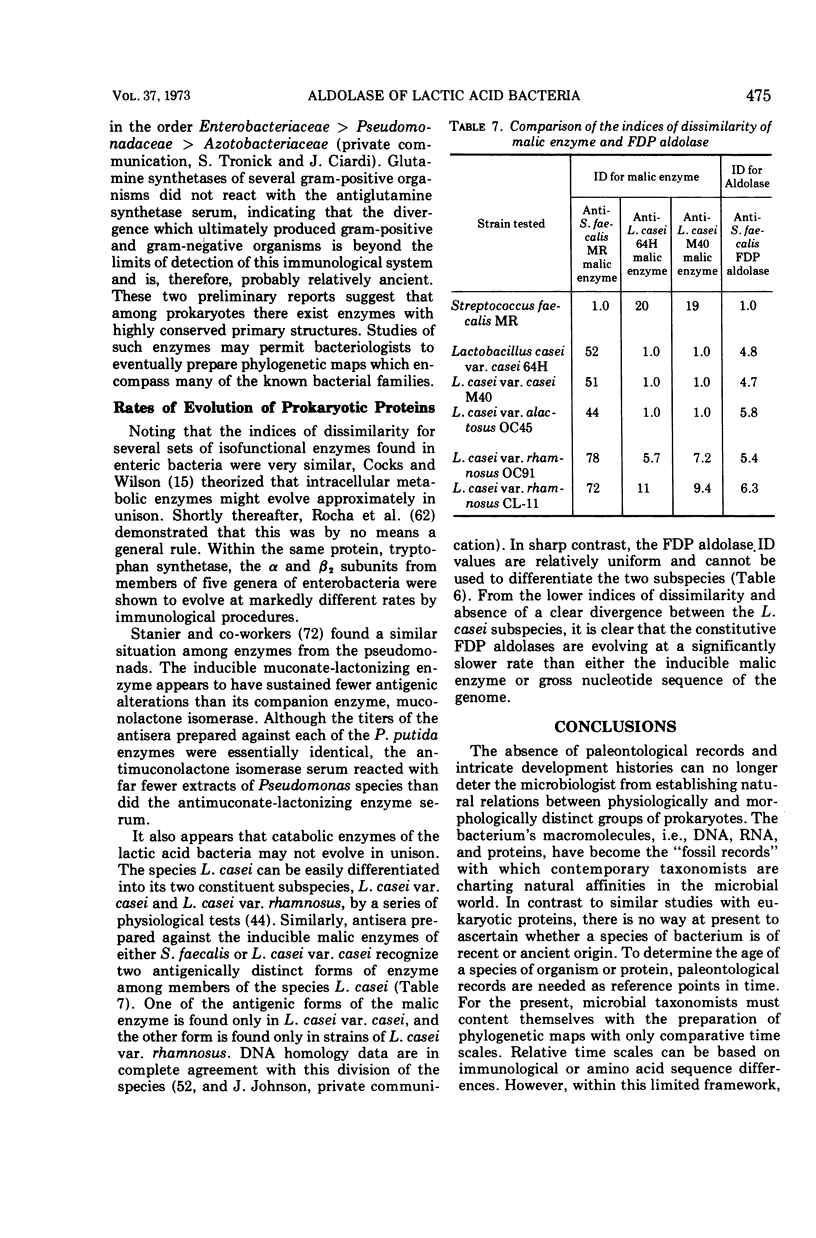



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANFINSEN C. B., AQVIST S. E., COOKE J. P., JONSSON B. A comparative study of the structures of bovine and ovine pancreatic ribonucleases. J Biol Chem. 1959 May;234(5):1118–1123. [PubMed] [Google Scholar]
- Abo-Elnaga I. G., Kandler O. Zur Taxonomie der Gattung Lactobacillus Beijerinck. I. Das Subgenus Streptobacterium Orla Jensen. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1965 Feb 25;119(1):1–36. [PubMed] [Google Scholar]
- Air G. M., Thompson E. O., Richardson B. J., Sharman G. B. Amino-acid sequences of kangaroo myoglobin and haemoglobin and the date of marsupial-eutherian divergence. Nature. 1971 Feb 5;229(5284):391–394. doi: 10.1038/229391a0. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnheim N., Prager E. M., Wilson A. C. Immunological prediction of sequence differences among proteins. Chemical comparison of chicken, quail, and phesant lysozymes. J Biol Chem. 1969 Apr 25;244(8):2085–2094. [PubMed] [Google Scholar]
- Baptist J. N., Shaw C. R., Mandel M. Comparative zone electrophoresis of enzymes of Pseudomonas solanacearum and Pseudomonas cepacia. J Bacteriol. 1971 Nov;108(2):799–803. doi: 10.1128/jb.108.2.799-803.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptist J. N., Shaw C. R., Mandel M. Zone electrophoresis of enzymes in bacterial taxonomy. J Bacteriol. 1969 Jul;99(1):180–188. doi: 10.1128/jb.99.1.180-188.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Johnson K. E., Citarella R. V., Falkow S. Polynucleotide sequence relationships among members of Enterobacteriaceae. J Bacteriol. 1969 May;98(2):637–650. doi: 10.1128/jb.98.2.637-650.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M., TORRIANI A. M. The relationships in biosynthesis of the beta-galactosidase- and Pz-proteins in Escherichia coli. Biochim Biophys Acta. 1953 Feb;10(2):280–289. doi: 10.1016/0006-3002(53)90251-0. [DOI] [PubMed] [Google Scholar]
- Canale-Parola E. Biology of the sugar-fermenting Sarcinae. Bacteriol Rev. 1970 Mar;34(1):82–97. doi: 10.1128/br.34.1.82-97.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrambach A., Rodbard D. Polyacrylamide gel electrophoresis. Science. 1971 Apr 30;172(3982):440–451. doi: 10.1126/science.172.3982.440. [DOI] [PubMed] [Google Scholar]
- Cocks G. T., Wilson A. C. Enzyme evolution in the Enterobacteriaceae. J Bacteriol. 1972 Jun;110(3):793–802. doi: 10.1128/jb.110.3.793-802.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks G. T., Wilson A. C. Immunological detection of single amino acid substitutions in alkaline phosphatase. Science. 1969 Apr 11;164(3876):188–189. doi: 10.1126/science.164.3876.188. [DOI] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Crick F. H. The origin of the genetic code. J Mol Biol. 1968 Dec;38(3):367–379. doi: 10.1016/0022-2836(68)90392-6. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Smith I., Morell P., Marmur J. Gene conservation in Bacillus species. I. Conserved genetic and nucleic acid base sequence homologies. Proc Natl Acad Sci U S A. 1965 Aug;54(2):491–498. doi: 10.1073/pnas.54.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON R. E., MIHM J. M. A comparative study of some strains received as nocardiae. J Bacteriol. 1957 Jan;73(1):15–27. doi: 10.1128/jb.73.1.15-27.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser F. Electrophoretic characterization of lactic dehydrogenases in the genus Lactobacillus. J Gen Microbiol. 1970 Aug;62(2):223–239. doi: 10.1099/00221287-62-2-223. [DOI] [PubMed] [Google Scholar]
- Gasser F., Gasser C. Immunological relationships among lactic dehydrogenases in the genera Lactobacillus and Leuconostoc. J Bacteriol. 1971 Apr;106(1):113–125. doi: 10.1128/jb.106.1.113-125.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser F., Mandel M., Rogosa M. Lactobacillus jensenii sp.nov., a new representative of the subgenus Thermobacterium. J Gen Microbiol. 1970 Aug;62(2):219–222. doi: 10.1099/00221287-62-2-219. [DOI] [PubMed] [Google Scholar]
- Gordon J., Schweiger M., Krisko I., Williams C. A. Specificity and evolutionary divergence of the antigenic structure of the polypeptide chain elongation factors. J Bacteriol. 1969 Oct;100(1):1–4. doi: 10.1128/jb.100.1.1-4.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOOK W. A., MUSCHEL L. H. ANTICOMPLEMENTARY EFFECTS AND COMPLEMENT ACTIVITY OF HUMAN SERA. Proc Soc Exp Biol Med. 1964 Oct;117:292–297. doi: 10.3181/00379727-117-29561. [DOI] [PubMed] [Google Scholar]
- Jensen R. A. Taxonomic implications of temperature dependence of the allosteric inhibition of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase in Bacillus. J Bacteriol. 1970 May;102(2):489–497. doi: 10.1128/jb.102.2.489-497.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keudell K. C., Goldberg H. S. Dehydrogenase patterns in the study of Bacteroidaceae. Appl Microbiol. 1970 Mar;19(3):505–511. doi: 10.1128/am.19.3.505-511.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. L., Jukes T. H. Non-Darwinian evolution. Science. 1969 May 16;164(3881):788–798. doi: 10.1126/science.164.3881.788. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Fanning G. R., Johnson K. E., Brenner D. J. Thermal stability of interspecies Neisseria DNA duplexes. J Gen Microbiol. 1969 Feb;55(2):201–208. doi: 10.1099/00221287-55-2-201. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. I. Cell wall composition and polysaccharides of the peptidoglycan. J Bacteriol. 1967 Sep;94(3):734–740. doi: 10.1128/jb.94.3.734-740.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Meyer E. Y., Kulczyk S. R. Detection of relationships between Streptococcus faecalis and Lactobacillus casei by immunological studies with two forms of malic enzyme. J Bacteriol. 1971 Oct;108(1):196–201. doi: 10.1128/jb.108.1.196-201.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Meyer E. Y., Kulczyk S. Comparative biochemical and immunological study of malic enzyme from two species of lactic acid bacteria: evolutionary implications. J Bacteriol. 1971 Apr;106(1):126–137. doi: 10.1128/jb.106.1.126-137.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J. Regulation and function of lactate oxidation in Streptococcus faecium. J Bacteriol. 1968 Apr;95(4):1380–1387. doi: 10.1128/jb.95.4.1380-1387.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LéJohn H. B. D(-)-lactate dehydrogenases in fungi. Kinetics and allosteric inhibition by guanosine triphosphate. J Biol Chem. 1971 Apr 10;246(7):2116–2126. [PubMed] [Google Scholar]
- MCLEAN R. A., SULZBACHER W. L. Microbacterium thermosphactum, spec: nov.; a nonheat resistant bacterium from fresh pork sausage. J Bacteriol. 1953 Apr;65(4):428–433. doi: 10.1128/jb.65.4.428-433.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M. New approaches to bacterial taxonomy: perspective and prospects. Annu Rev Microbiol. 1969;23:239–274. doi: 10.1146/annurev.mi.23.100169.001323. [DOI] [PubMed] [Google Scholar]
- Margoliash E., Nisonoff A., Reichlin M. Immunological activity of cytochrome c. I. Precipitating antibodies to monomeric vertebrate cytochromes c. J Biol Chem. 1970 Mar 10;245(5):931–939. [PubMed] [Google Scholar]
- McFadden B. A., Denend A. R. Ribulose diphosphate carboxylase from autotrophic microorganisms. J Bacteriol. 1972 May;110(2):633–642. doi: 10.1128/jb.110.2.633-642.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., 3rd, Sandine W. E., Elliker P. R. Deoxyribonucleic acid homology in the genus Lactobacillus. Can J Microbiol. 1971 May;17(5):625–634. doi: 10.1139/m71-102. [DOI] [PubMed] [Google Scholar]
- Murphy T. M., Mills S. E. Immunochemical and enzymatic comparisons of the tryptophan synthase alpha subunits from five species of Enterobacteriaceae. J Bacteriol. 1969 Mar;97(3):1310–1320. doi: 10.1128/jb.97.3.1310-1320.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIRENBERG M. W., MATTHAEI J. H. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1588–1602. doi: 10.1073/pnas.47.10.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan C., Margoliash E. Comparative aspects of primary structures of proteins. Annu Rev Biochem. 1968;37:727–790. doi: 10.1146/annurev.bi.37.070168.003455. [DOI] [PubMed] [Google Scholar]
- Phillips A. W., Boyd J. W., Ferguson D. A., Jr, Marucci A. A. Immunochemical comparison of L-asparaginases from Serratia marcescens and Escherichia coli. J Bacteriol. 1971 Aug;107(2):461–467. doi: 10.1128/jb.107.2.461-467.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager E. M., Wilson A. C. The dependence of immunological cross-reactivity upon sequence resemblance among lysozymes. II. Comparison of precipitin and micro-complement fixation results. J Biol Chem. 1971 Nov 25;246(22):7010–7017. [PubMed] [Google Scholar]
- REICHLIN M., HAY M., LEVINE L. ANTIBODIES TO HUMAN A1 HEMOGLOBIN AND THEIR REACTION WITH A2, S, C, AND H HEMOGLOBINS. Immunochemistry. 1964 Apr;1:21–30. doi: 10.1016/0019-2791(64)90052-7. [DOI] [PubMed] [Google Scholar]
- RUTTER W. J. EVOLUTION OF ALDOLASE. Fed Proc. 1964 Nov-Dec;23:1248–1257. [PubMed] [Google Scholar]
- Rocha V., Crawford I. P., Mills S. E. Comparative immunological and enzymatic study of the tryptophan synthetase beta 2 subunit in the Enterobacteriaceae. J Bacteriol. 1972 Jul;111(1):163–168. doi: 10.1128/jb.111.1.163-168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing S. R., Crawford I. P. Purification and characterization of the beta-galactosidase of Aeromonas formicans. J Bacteriol. 1966 Mar;91(3):1085–1097. doi: 10.1128/jb.91.3.1085-1097.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff H. L., Hitchins A. D., Celikkol E. Properties of fructose 1,6-diphosphate aldolases from spores and vegetative cells of Bacillus cereus. J Bacteriol. 1969 Jun;98(3):1208–1218. doi: 10.1128/jb.98.3.1208-1218.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarich V. M., Wilson A. C. Quantitative immunochemistry and the evolution of primate albumins: micro-complement fixation. Science. 1966 Dec 23;154(3756):1563–1566. doi: 10.1126/science.154.3756.1563. [DOI] [PubMed] [Google Scholar]
- Simonds J., Hansen P. A., Lakshmanan S. Deoxyribonucleic acid hybridization among strains of lactobacilli. J Bacteriol. 1971 Jul;107(1):382–384. doi: 10.1128/jb.107.1.382-384.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer C. E., Ames B. N. Sunlight ultraviolet and bacterial DNA base ratios. Science. 1970 Nov 20;170(3960):822–825. doi: 10.1126/science.170.3960.822. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Wachter D., Gasser C., Wilson A. C. Comparative immunological studies of two Pseudomonas enzymes. J Bacteriol. 1970 May;102(2):351–362. doi: 10.1128/jb.102.2.351-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen D. L., Cocks G. T., Wilson A. C. Micro-complement fixation in Klebsiella classification. J Bacteriol. 1972 Jun;110(3):803–808. doi: 10.1128/jb.110.3.803-808.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson R. M., LéJohn H. B. Glutamic dehydrogenases of Oomycetes. Kinetic mechanism and possible vvolutionary history. J Biol Chem. 1971 Apr 10;246(7):2127–2135. [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- WATSON J. D., CRICK F. H. Genetical implications of the structure of deoxyribonucleic acid. Nature. 1953 May 30;171(4361):964–967. doi: 10.1038/171964b0. [DOI] [PubMed] [Google Scholar]
- WILSON A. C., KAPLAN N. O., LEVINE L., PESCE A., REICHLIN M., ALLISON W. S. EVOLUTION OF LACTIC DEHYDROGENASES. Fed Proc. 1964 Nov-Dec;23:1258–1266. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Whiteside T. L., De Siervo A. J., Salton M. R. Use of antibody to membrane adenosine triphosphatase in the study of bacterial relatioships. J Bacteriol. 1971 Mar;105(3):957–967. doi: 10.1128/jb.105.3.957-967.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. A., Sadler S. A. Electrophoresis of glucose-6-phosphate dehydrogenase, cell wall composition and the taxonomy of heterofermentative lactobacilli. J Gen Microbiol. 1971 Mar;65(3):351–358. doi: 10.1099/00221287-65-3-351. [DOI] [PubMed] [Google Scholar]