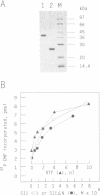
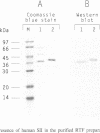
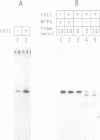
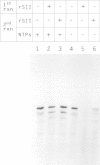
Abstract
Previous studies have revealed that the in vitro synthesis of reinitiated transcripts by RNA polymerase II requires an additional activity, designated reinitiation transcription factor (RTF), which is distinct from all of the general class II initiation factors. While further characterizing this activity, it was found that RTF displays properties indistinguishable from those of the RNA polymerase II elongation factor SII. In addition, Western blot analysis using SII-specific antibodies revealed that human SII is a major component in purified RTF preparations. The functional equivalence of the two proteins was established using recombinant SII, which proved fully capable of substituting for RTF in the reinitiation assay. In these reconstituted reactions, transcription complexes resulting from reinitiation events required SII to proceed through a 400 bp G-free cassette, while complexes resulting from the first round of initiations were SII-independent. Reinitiations can take place in the absence of SII; however, addition of the elongation factor is essential for full extension of the reinitiated transcripts. These results suggest that events taking place at the promoter (e.g. first-round initiations versus reinitiations) can create marked differences in the properties of RNA polymerase II elongation complexes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K., Baek K. H., Jeon C. J., Miyamoto K., Ueno A., Yoon H. S. Stimulation of transcript elongation requires both the zinc finger and RNA polymerase II binding domains of human TFIIS. Biochemistry. 1991 Aug 6;30(31):7842–7851. doi: 10.1021/bi00245a026. [DOI] [PubMed] [Google Scholar]
- Ahn B. Y., Gershon P. D., Jones E. V., Moss B. Identification of rpo30, a vaccinia virus RNA polymerase gene with structural similarity to a eucaryotic transcription elongation factor. Mol Cell Biol. 1990 Oct;10(10):5433–5441. doi: 10.1128/mcb.10.10.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chen H. C., England L., Kane C. M. Characterization of a HeLa cDNA clone encoding the human SII protein, an elongation factor for RNA polymerase II. Gene. 1992 Jul 15;116(2):253–258. doi: 10.1016/0378-1119(92)90522-q. [DOI] [PubMed] [Google Scholar]
- Clark A. B., Dykstra C. C., Sugino A. Isolation, DNA sequence, and regulation of a Saccharomyces cerevisiae gene that encodes DNA strand transfer protein alpha. Mol Cell Biol. 1991 May;11(5):2576–2582. doi: 10.1128/mcb.11.5.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway J. W., Conaway R. C. Initiation of eukaryotic messenger RNA synthesis. J Biol Chem. 1991 Sep 25;266(27):17721–17724. [PubMed] [Google Scholar]
- Cullen B. R. Does HIV-1 Tat induce a change in viral initiation rights? Cell. 1993 May 7;73(3):417–420. doi: 10.1016/0092-8674(93)90126-b. [DOI] [PubMed] [Google Scholar]
- Culotta V. C., Wides R. J., Sollner-Webb B. Eucaryotic transcription complexes are specifically associated in large sedimentable structures: rapid isolation of polymerase I, II, and III transcription factors. Mol Cell Biol. 1985 Jul;5(7):1582–1590. doi: 10.1128/mcb.5.7.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedrick R. L., Chamberlin M. J. Studies on transcription of 3'-extended templates by mammalian RNA polymerase II. Parameters that affect the initiation and elongation reactions. Biochemistry. 1985 Apr 23;24(9):2245–2253. doi: 10.1021/bi00330a019. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha I., Lane W. S., Reinberg D. Cloning of a human gene encoding the general transcription initiation factor IIB. Nature. 1991 Aug 22;352(6337):689–695. doi: 10.1038/352689a0. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., Roeder R. G. Functional steps in transcription initiation and reinitiation from the major late promoter in a HeLa nuclear extract. J Biol Chem. 1987 Mar 15;262(8):3452–3461. [PubMed] [Google Scholar]
- Hirashima S., Hirai H., Nakanishi Y., Natori S. Molecular cloning and characterization of cDNA for eukaryotic transcription factor S-II. J Biol Chem. 1988 Mar 15;263(8):3858–3863. [PubMed] [Google Scholar]
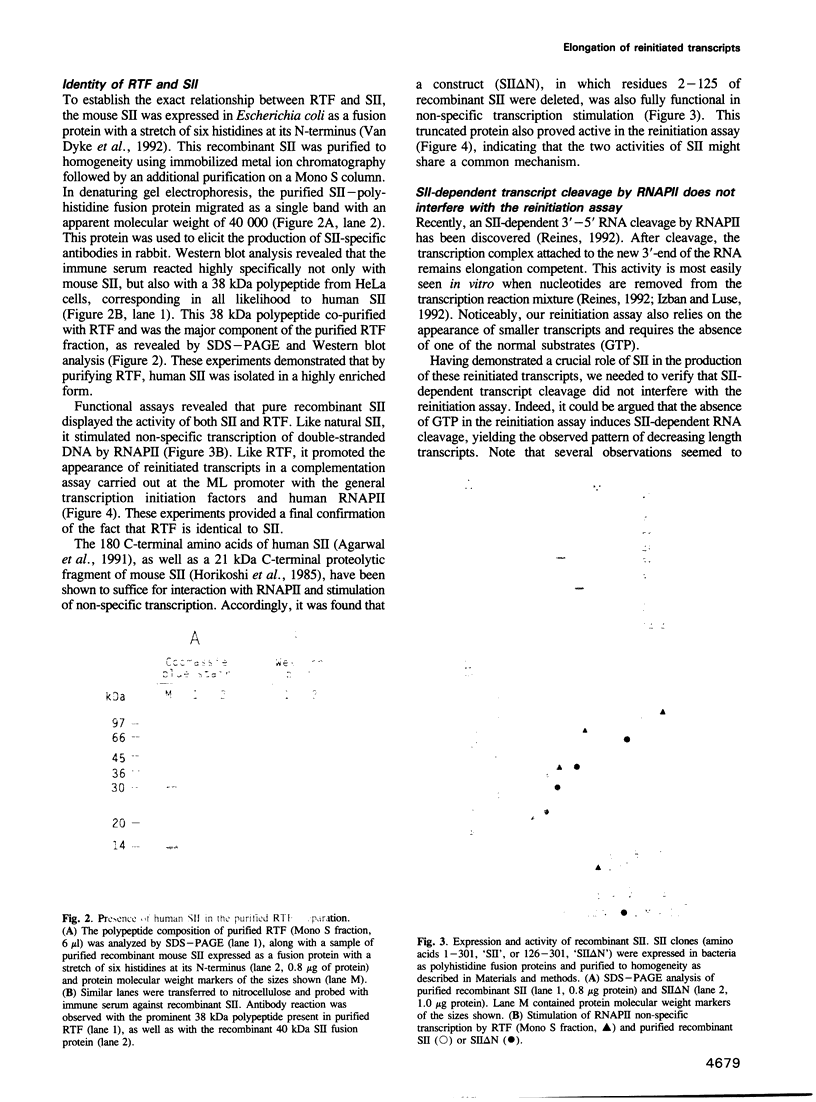
- Horikoshi M., Sekimizu K., Hirashima S., Mitsuhashi Y., Natori S. Structural relationships of the three stimulatory factors of RNA polymerase II from Ehrlich ascites tumor cells. J Biol Chem. 1985 May 10;260(9):5739–5744. [PubMed] [Google Scholar]
- Hubert J. C., Guyonvarch A., Kammerer B., Exinger F., Liljelund P., Lacroute F. Complete sequence of a eukaryotic regulatory gene. EMBO J. 1983;2(11):2071–2073. doi: 10.1002/j.1460-2075.1983.tb01702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izban M. G., Luse D. S. The RNA polymerase II ternary complex cleaves the nascent transcript in a 3'----5' direction in the presence of elongation factor SII. Genes Dev. 1992 Jul;6(7):1342–1356. doi: 10.1101/gad.6.7.1342. [DOI] [PubMed] [Google Scholar]
- Kadesch T. R., Chamberlin M. J. Studies of in vitro transcription by calf thymus RNA polymerase II using a novel duplex DNA template. J Biol Chem. 1982 May 10;257(9):5286–5295. [PubMed] [Google Scholar]
- Kanai A., Kuzuhara T., Sekimizu K., Natori S. Heterogeneity and tissue-specific expression of eukaryotic transcription factor S-II-related protein mRNA. J Biochem. 1991 May;109(5):674–677. doi: 10.1093/oxfordjournals.jbchem.a123439. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Geiduschek E. P. RNA polymerase marching backward. Science. 1993 Feb 12;259(5097):944–945. doi: 10.1126/science.7679800. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marciniak R. A., Sharp P. A. HIV-1 Tat protein promotes formation of more-processive elongation complexes. EMBO J. 1991 Dec;10(13):4189–4196. doi: 10.1002/j.1460-2075.1991.tb04997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall T. K., Guo H., Price D. H. Drosophila RNA polymerase II elongation factor DmS-II has homology to mouse S-II and sequence similarity to yeast PPR2. Nucleic Acids Res. 1990 Nov 11;18(21):6293–6298. doi: 10.1093/nar/18.21.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T., Nakano A., Nomura K., Sekimizu K., Natori S. Purification, gene cloning, and gene disruption of the transcription elongation factor S-II in Saccharomyces cerevisiae. J Biol Chem. 1992 Jul 5;267(19):13200–13204. [PubMed] [Google Scholar]
- Price D. H., Sluder A. E., Greenleaf A. L. Dynamic interaction between a Drosophila transcription factor and RNA polymerase II. Mol Cell Biol. 1989 Apr;9(4):1465–1475. doi: 10.1128/mcb.9.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport J., Cho K., Saltzman A., Prenger J., Golomb M., Weinmann R. Transcription elongation factor SII interacts with a domain of the large subunit of human RNA polymerase II. Mol Cell Biol. 1988 Aug;8(8):3136–3142. doi: 10.1128/mcb.8.8.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport J., Reinberg D., Zandomeni R., Weinmann R. Purification and functional characterization of transcription factor SII from calf thymus. Role in RNA polymerase II elongation. J Biol Chem. 1987 Apr 15;262(11):5227–5232. [PubMed] [Google Scholar]
- Reinberg D., Roeder R. G. Factors involved in specific transcription by mammalian RNA polymerase II. Transcription factor IIS stimulates elongation of RNA chains. J Biol Chem. 1987 Mar 5;262(7):3331–3337. [PubMed] [Google Scholar]
- Reines D., Chamberlin M. J., Kane C. M. Transcription elongation factor SII (TFIIS) enables RNA polymerase II to elongate through a block to transcription in a human gene in vitro. J Biol Chem. 1989 Jun 25;264(18):10799–10809. [PubMed] [Google Scholar]
- Reines D. Elongation factor-dependent transcript shortening by template-engaged RNA polymerase II. J Biol Chem. 1992 Feb 25;267(6):3795–3800. [PMC free article] [PubMed] [Google Scholar]
- Reines D., Ghanouni P., Li Q. Q., Mote J., Jr The RNA polymerase II elongation complex. Factor-dependent transcription elongation involves nascent RNA cleavage. J Biol Chem. 1992 Aug 5;267(22):15516–15522. [PMC free article] [PubMed] [Google Scholar]
- Reines D., Mote J., Jr Elongation factor SII-dependent transcription by RNA polymerase II through a sequence-specific DNA-binding protein. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1917–1921. doi: 10.1073/pnas.90.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougvie A. E., Lis J. T. Postinitiation transcriptional control in Drosophila melanogaster. Mol Cell Biol. 1990 Nov;10(11):6041–6045. doi: 10.1128/mcb.10.11.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougvie A. E., Lis J. T. The RNA polymerase II molecule at the 5' end of the uninduced hsp70 gene of D. melanogaster is transcriptionally engaged. Cell. 1988 Sep 9;54(6):795–804. doi: 10.1016/s0092-8674(88)91087-2. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Factors involved in specific transcription by human RNA polymerase II: analysis by a rapid and quantitative in vitro assay. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4394–4398. doi: 10.1073/pnas.82.13.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Sentenac A., Fromageot P. Interaction of a new polypeptide with yeast RNA polymerase B. J Biol Chem. 1980 Jan 10;255(1):12–15. [PubMed] [Google Scholar]
- Sawadogo M., Sentenac A. RNA polymerase B (II) and general transcription factors. Annu Rev Biochem. 1990;59:711–754. doi: 10.1146/annurev.bi.59.070190.003431. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Van Dyke M. W., Gregor P. D., Roeder R. G. Multiple forms of the human gene-specific transcription factor USF. I. Complete purification and identification of USF from HeLa cell nuclei. J Biol Chem. 1988 Aug 25;263(24):11985–11993. [PubMed] [Google Scholar]
- Sekimizu K., Nakanishi Y., Mizuno D., Natori S. Purification and preparation of antibody to RNA polymerase II stimulatory factors from Ehrlich ascites tumor cells. Biochemistry. 1979 Apr 17;18(8):1582–1588. doi: 10.1021/bi00575a031. [DOI] [PubMed] [Google Scholar]
- Sekimizu K., Yokoi H., Natori S. Evidence that stimulatory factor(s) of RNA polymerase II participates in accurate transcription in a HeLa cell lysate. J Biol Chem. 1982 Mar 25;257(6):2719–2721. [PubMed] [Google Scholar]
- SivaRaman L., Reines D., Kane C. M. Purified elongation factor SII is sufficient to promote read-through by purified RNA polymerase II at specific termination sites in the human histone H3.3 gene. J Biol Chem. 1990 Aug 25;265(24):14554–14560. [PubMed] [Google Scholar]
- Sluder A. E., Greenleaf A. L., Price D. H. Properties of a Drosophila RNA polymerase II elongation factor. J Biol Chem. 1989 May 25;264(15):8963–8969. [PubMed] [Google Scholar]
- Stein H., Hausen P. A factor from calf thymus stimulating DNA-dependent RNA polymerase isolated from this tissue. Eur J Biochem. 1970 Jun;14(2):270–277. doi: 10.1111/j.1432-1033.1970.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Szentirmay M. N., Sawadogo M. Transcription factor requirement for multiple rounds of initiation by human RNA polymerase II. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10691–10695. doi: 10.1073/pnas.88.23.10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke M. W., Roeder R. G., Sawadogo M. Physical analysis of transcription preinitiation complex assembly on a class II gene promoter. Science. 1988 Sep 9;241(4871):1335–1338. doi: 10.1126/science.3413495. [DOI] [PubMed] [Google Scholar]
- Van Dyke M. W., Sawadogo M. DNA-binding and transcriptional properties of human transcription factor TFIID after mild proteolysis. Mol Cell Biol. 1990 Jul;10(7):3415–3420. doi: 10.1128/mcb.10.7.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke M. W., Sirito M., Sawadogo M. Single-step purification of bacterially expressed polypeptides containing an oligo-histidine domain. Gene. 1992 Feb 1;111(1):99–104. doi: 10.1016/0378-1119(92)90608-r. [DOI] [PubMed] [Google Scholar]
- Wang D., Hawley D. K. Identification of a 3'-->5' exonuclease activity associated with human RNA polymerase II. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):843–847. doi: 10.1073/pnas.90.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo O. J., Yoon H. S., Baek K. H., Jeon C. J., Miyamoto K., Ueno A., Agarwal K. Cloning, expression and characterization of the human transcription elongation factor, TFIIS. Nucleic Acids Res. 1991 Mar 11;19(5):1073–1079. doi: 10.1093/nar/19.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]