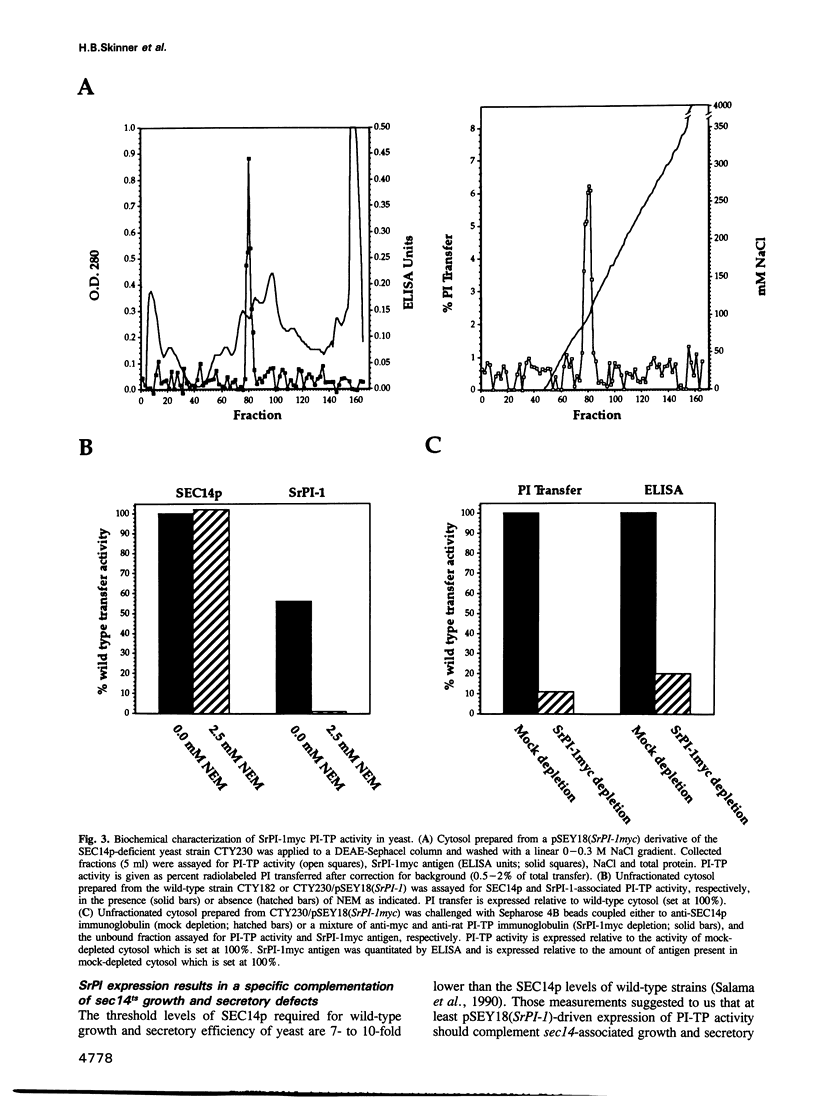
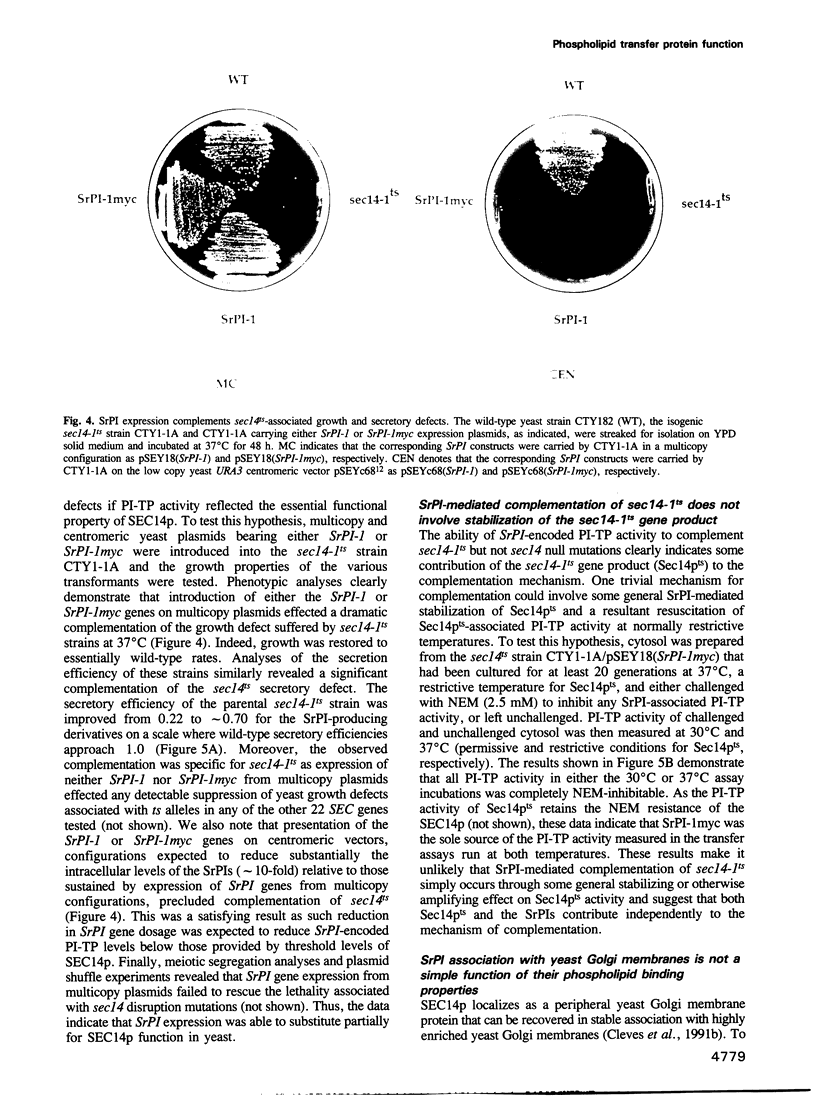
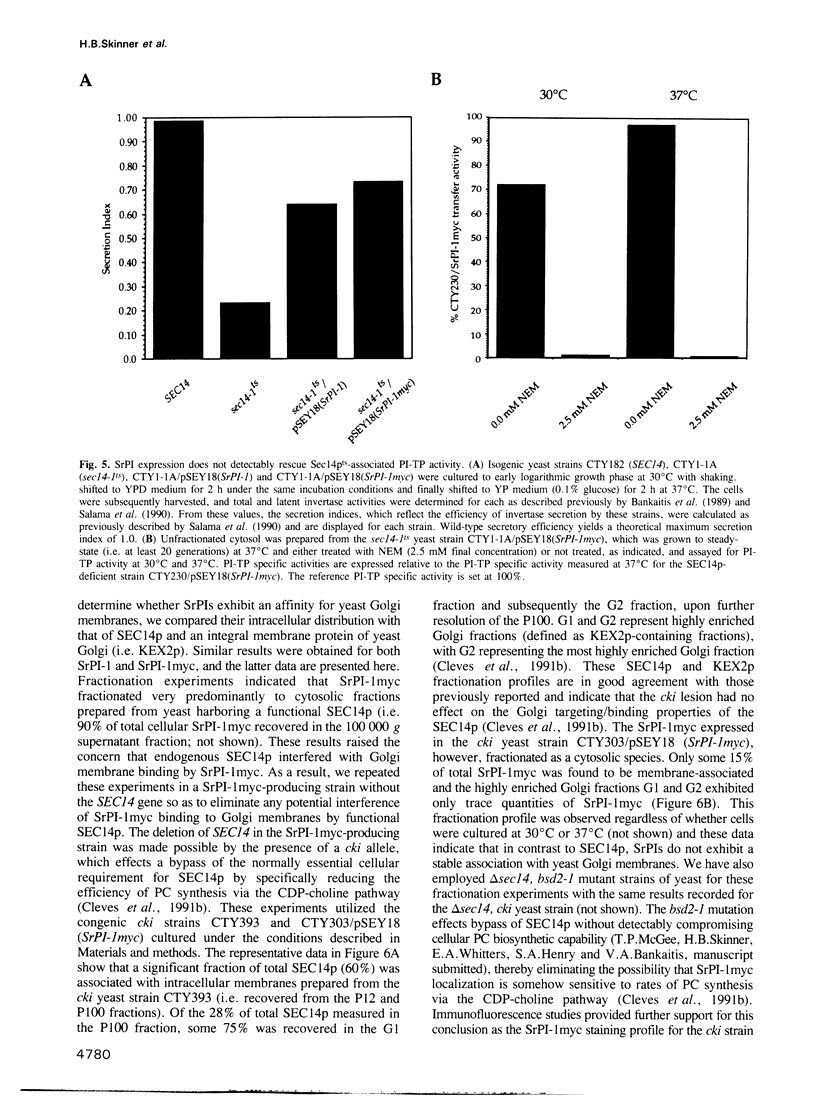
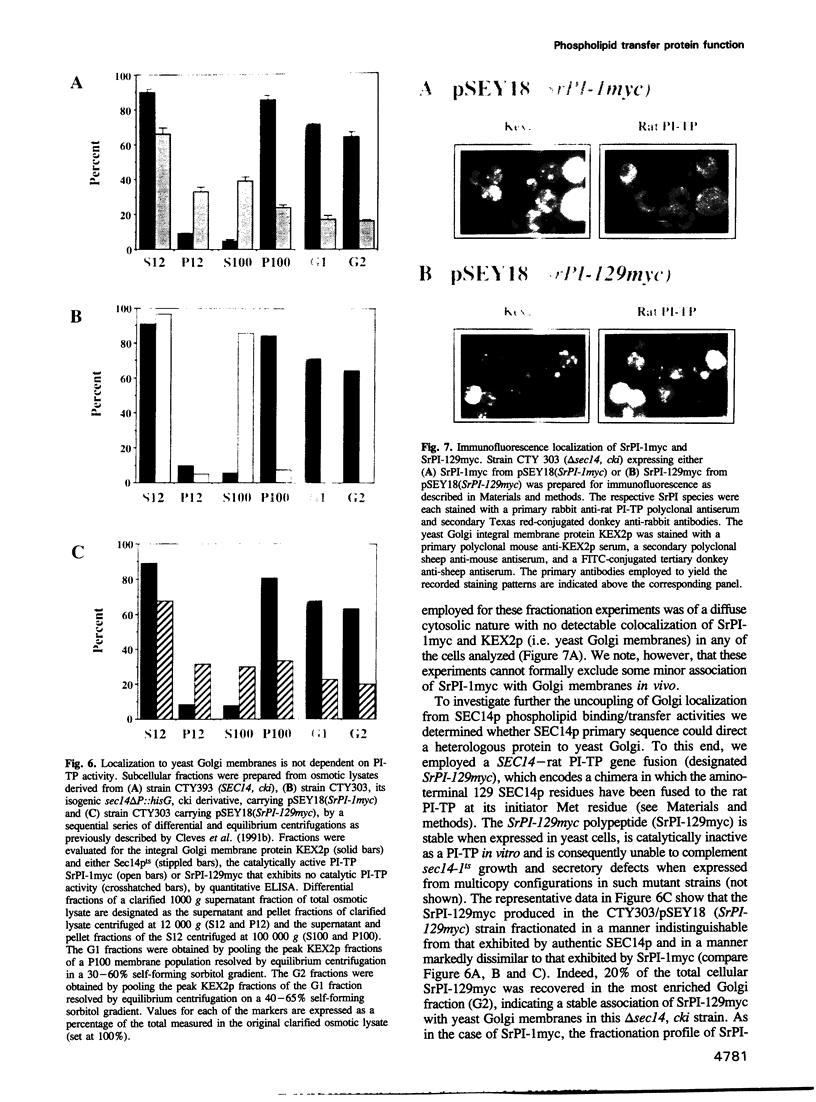
Abstract
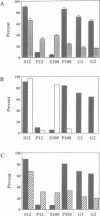
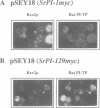
To investigate several key aspects of phosphatidylinositol transfer protein (PI-TP) function in eukaryotic cells, rat PI-TP was expressed in yeast strains carrying lesions in SEC14, the structural gene for yeast PI-TP (SEC14p), whose activity is essential for Golgi secretory function in vivo. Rat PI-TP expression effected a specific complementation of sec14ts growth and secretory defects. Complementation of sec14 mutations was not absolute as rat PI-TP expression failed to rescue sec14 null mutations. This partial complementation of sec14 lesions by rat PI-TP correlated with inability of the mammalian protein to stably associate with yeast Golgi membranes and was not a result of rat PI-TP stabilizing the endogenous sec14ts gene product. These collective data demonstrate that while the in vitro PI-TP activity of SEC14p clearly reflects some functional in vivo property of SEC14p, the PI-TP activity is not the sole essential activity of SEC14p. Those data further identify an efficient Golgi targeting capability as a likely essential feature of SEC14p function in vivo. Finally, the data suggest that stable association of SEC14p with yeast Golgi membranes is not a simple function of its lipid-binding properties, indicate that the amino-terminal 129 SEC14p residues are sufficient to direct a catalytically inactive form of rat PI-TP to the Golgi and provide the first evidence to indicate that a mammalian PI-TP can stimulate Golgi secretory function in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken J. F., van Heusden G. P., Temkin M., Dowhan W. The gene encoding the phosphatidylinositol transfer protein is essential for cell growth. J Biol Chem. 1990 Mar 15;265(8):4711–4717. [PubMed] [Google Scholar]
- Alani E., Cao L., Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987 Aug;116(4):541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis V. A., Aitken J. R., Cleves A. E., Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990 Oct 11;347(6293):561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- Bankaitis V. A., Malehorn D. E., Emr S. D., Greene R. The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J Cell Biol. 1989 Apr;108(4):1271–1281. doi: 10.1083/jcb.108.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta L. M., Vida T. A., Herman P. K., Emr S. D. Characterization of yeast Vps33p, a protein required for vacuolar protein sorting and vacuole biogenesis. Mol Cell Biol. 1990 Sep;10(9):4638–4649. doi: 10.1128/mcb.10.9.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Cleves A. E., McGee T. P., Whitters E. A., Champion K. M., Aitken J. R., Dowhan W., Goebl M., Bankaitis V. A. Mutations in the CDP-choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell. 1991 Feb 22;64(4):789–800. doi: 10.1016/0092-8674(91)90508-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleves A. E., Novick P. J., Bankaitis V. A. Mutations in the SAC1 gene suppress defects in yeast Golgi and yeast actin function. J Cell Biol. 1989 Dec;109(6 Pt 1):2939–2950. doi: 10.1083/jcb.109.6.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleves A., McGee T., Bankaitis V. Phospholipid transfer proteins: a biological debut. Trends Cell Biol. 1991 Jul;1(1):30–34. doi: 10.1016/0962-8924(91)90067-j. [DOI] [PubMed] [Google Scholar]
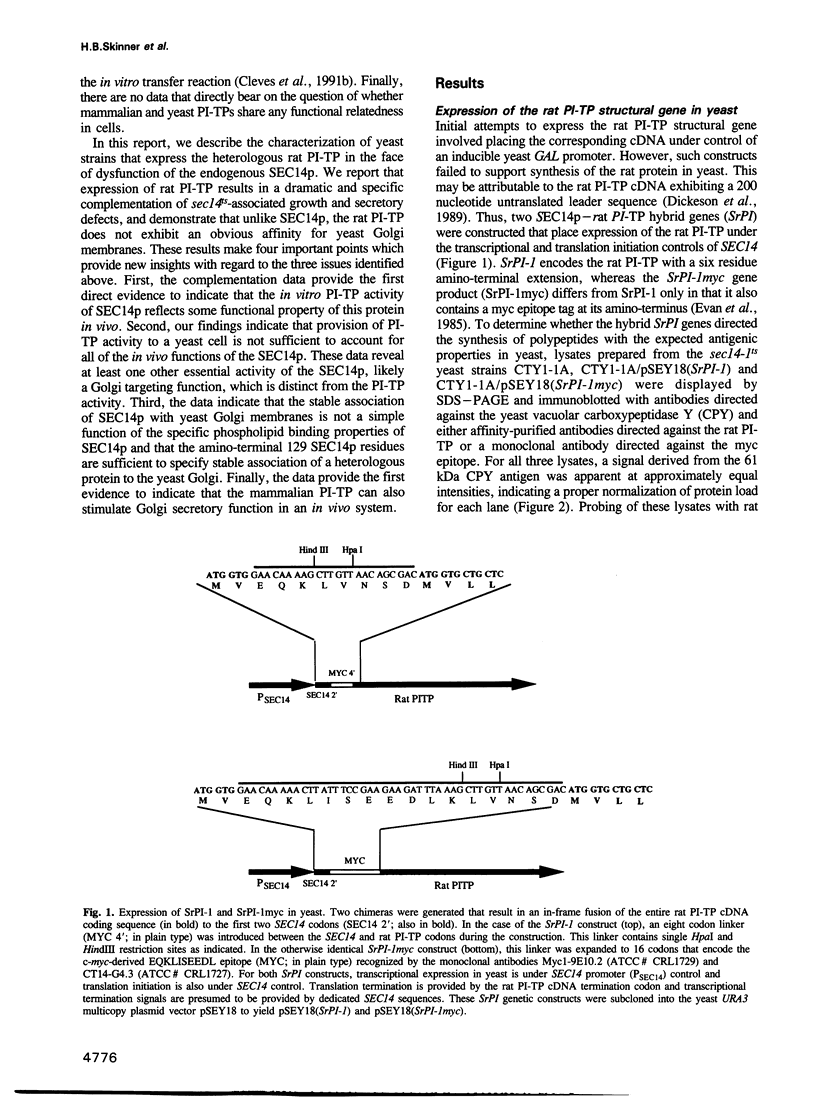
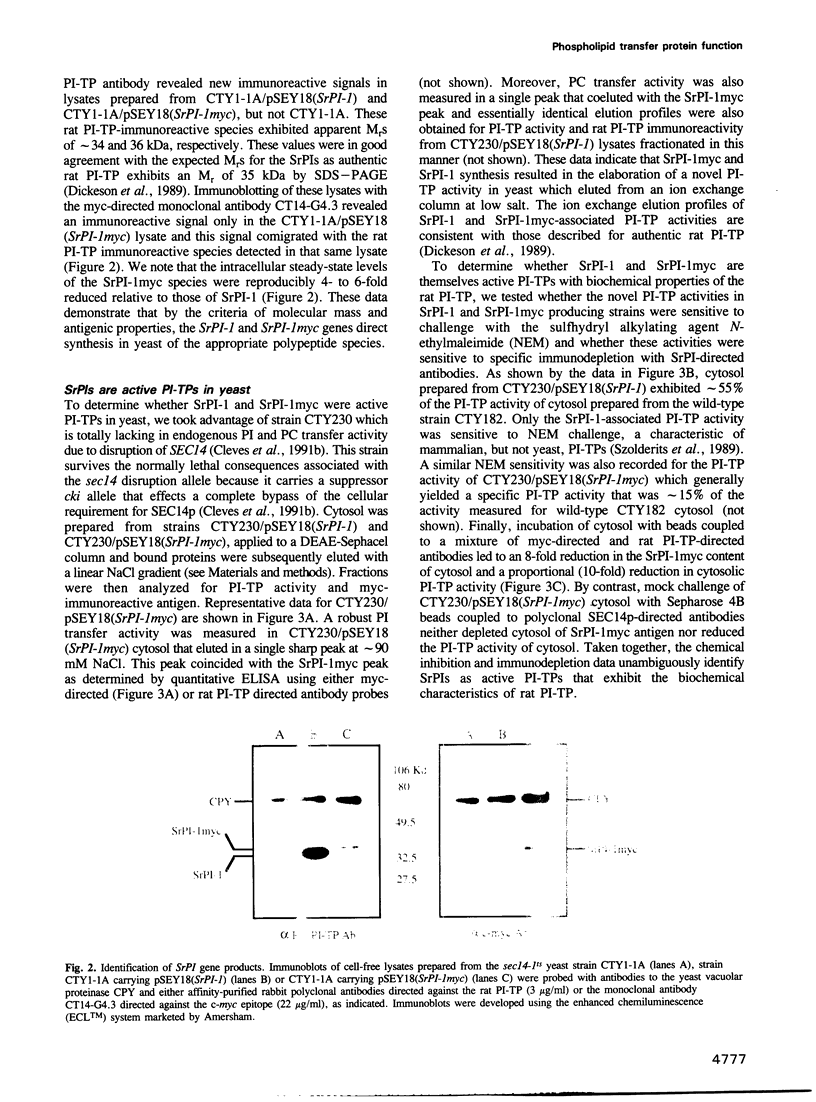
- Dickeson S. K., Lim C. N., Schuyler G. T., Dalton T. P., Helmkamp G. M., Jr, Yarbrough L. R. Isolation and sequence of cDNA clones encoding rat phosphatidylinositol transfer protein. J Biol Chem. 1989 Oct 5;264(28):16557–16564. [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmkamp G. M., Jr Phosphatidylinositol transfer proteins: structure, catalytic activity, and physiological function. Chem Phys Lipids. 1985 Aug 30;38(1-2):3–16. doi: 10.1016/0009-3084(85)90053-2. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- PAULUS H., KENNEDY E. P. The enzymatic synthesis of inositol monophosphatide. J Biol Chem. 1960 May;235:1303–1311. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Salama S. R., Cleves A. E., Malehorn D. E., Whitters E. A., Bankaitis V. A. Cloning and characterization of Kluyveromyces lactis SEC14, a gene whose product stimulates Golgi secretory function in Saccharomyces cerevisiae. J Bacteriol. 1990 Aug;172(8):4510–4521. doi: 10.1128/jb.172.8.4510-4521.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoek G. T., de Wit I. S., van Mourik J. H., Wirtz K. W. The phosphatidylinositol transfer protein in 3T3 mouse fibroblast cells is associated with the Golgi system. J Cell Biochem. 1992 Aug;49(4):339–348. doi: 10.1002/jcb.240490404. [DOI] [PubMed] [Google Scholar]
- Szolderits G., Hermetter A., Paltauf F., Daum G. Membrane properties modulate the activity of a phosphatidylinositol transfer protein from the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1989 Nov 27;986(2):301–309. doi: 10.1016/0005-2736(89)90481-1. [DOI] [PubMed] [Google Scholar]
- Wirtz K. W. Phospholipid transfer proteins. Annu Rev Biochem. 1991;60:73–99. doi: 10.1146/annurev.bi.60.070191.000445. [DOI] [PubMed] [Google Scholar]