Abstract
A series of new heterometallic gold(I) thiolates containing ferrocenyl-phoshines were synthesized. Their antimicrobial properties were studied and compared to that of FDA-approved drug, auranofin (Ridaura), prescribed for the treatment of rheumatoid arthritis. MIC in the order of one digit micromolar were found for most of the compounds against Gram-positive bacteria S. aureus and CA MRSA strains US300 and US400. Remarkably, auranofin inhibited S. aureus, US300 and US400 in the order of 150–300 nM. This is the first time that the potent inhibitory effect of auranofin on MRSA strains has been described. The effects of a selected heterometallic compound and auranofin were also studied in a non-tumorigenic human embryonic kidney cell line (HEK-293).
Keywords: Antimicrobial, auranofin, heterometallic gold-thiolates, MRSA
1. Introduction
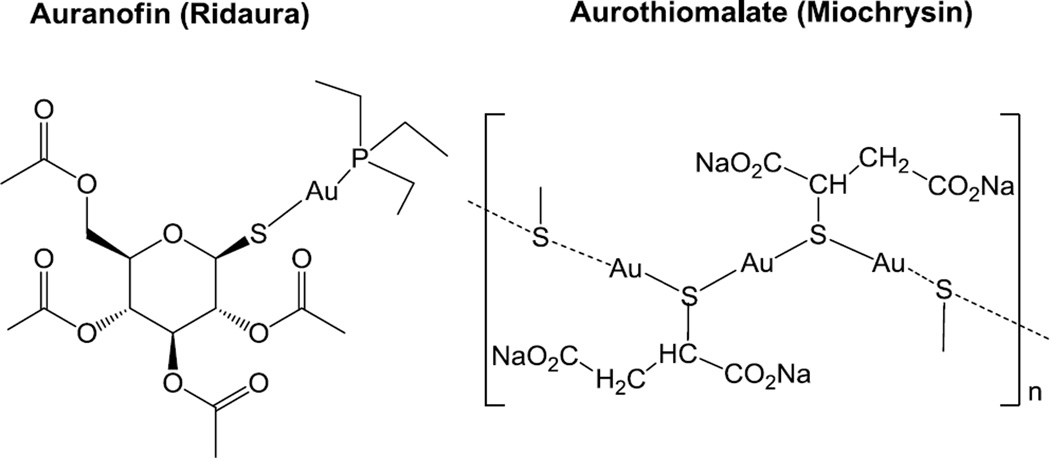
During the past two decades there has been a renewed interest in the study of the biological activities and potential medicinal applications of gold compounds [1]. In particular, gold(I) compounds have been thoroughly explored as cytotoxic agents [2]. Many of the compounds reported contain thiolates and/or phosphines as ligands [3,4] and their mode of action seems to be based on thioredoxin reductase inhibition [5]. Some of these gold(I) thiolates (e.g. aurothiomalate and auranofin (AF) in Figure 1) are used therapeutically [6] and belong to the group of disease-modifying antirheumatic drugs (DMARDs) used to slow down or stop the progression of this rheumatic disorder. Auranofin (approved by the FDA in 1985 and commercialized under the brand name of Ridaura) has been granted orphan-drug status and its potential medicinal applications have been reviewed recently [7].
Figure 1.
Auranofin and aurothiomalate used therapeutically in the treatment of rheumatoid arthritis.
Recent reports indicate that AF is able to effectively kill the parasites Schistosoma mansoni [8], Trypanosoma crucei [9], Echinococcus granulosusm [10], Plasmodium falciparum [11], Leishmania infantinum [12] and Giardia lamblia [13]. Last year, the potential of AF for the treatment of amebiasis was described [14]. In this report it was suggested that AF targets Entamoeba histolytica thioredoxin reductase activity [14]. In comparison, the antibacterial activities of gold(I) derivatives have been less explored [7]. Some reports on the effect of AF on Clostridium difficile and Treponema denticola in vitro have shown that disruption of the selenium metabolism is implied in the growth inhibition observed [15,16]. Both C. difficile and T. denticola utilize selenoproteins for energy. The growth inhibition effect of AF on Staphylococcus aureus had been reported before [17], but surprisingly this article has not been cited in any of these recent articles and reviews.
While S. aureus infections (staph) can be effectively treated with penicillinase-resistant β-lactam antibiotics (methicillin, oxacillin), resistant strains of S. aureus, such as the Methicillin-resistant Staphylococcus aureus (MRSA) strains, are very common. Hospital associated infections caused by MRSA (HA MRSA) are a major problem in hospitals, prisons and nursing homes around the world. Patients with open wounds, invasive devices, and weakened immune systems are at great risk of infection. However, in the 1990s community associated MRSA (CA MRSA) infections emerged in persons in which the healthcare associated risk factors were generally absent. Two of the best described CA MRSA strains are US300 and US400. The first line treatment for MRSA infections is the glycopeptide antibiotic vancomycin, although vancomycin-resistant strains of S. aureus (VRSA) exist and reports of these strains are on the rise globally. Additional effective antibiotics for both HA and CA MSRA bacteria, which are currently considered a serious public health threat in the US [18], are urgently needed.
In our group we have explored the antimicrobial properties of different gold(I)-phosphine compounds, including some heterometallic gold(I)-silver(I) and gold(I)-copper(I) derivatives [19,20]. The heterometallic compounds Au2-Ag were more active than the parent monometallic fragments against Gram-negative (S. typhimurium and E. coli) and Gram-positive bacteria (B. cereus and S. aureus) with MIC ranging from 1 to 10 µg/mL [20]. More recently, we have described the potential of gold(III) and palladium(II) heterometallic complexes (FeAu2 and FePd2) based on ferrocenyl iminophosphorane ligands as anticancer agents through zinc finger protein poly-(adenosine diphosphate (ADP)-ribose) polymerase 1 (PARP-1) inhibition. [21] Ferrocene has special properties such as low toxicity, high lipophilicity and unique electrochemical behavior which makes it an attractive motif in drug design [22].
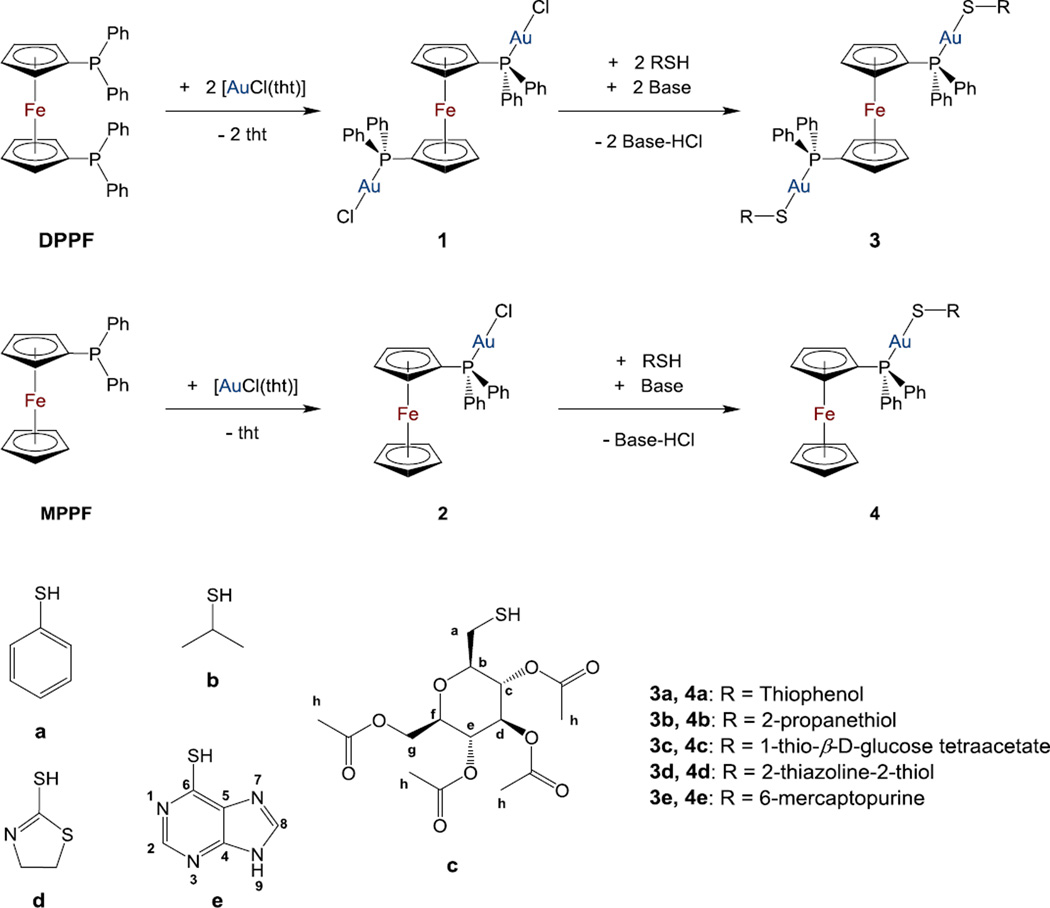
In this context, we report here on the synthesis, characterization and antimicrobial properties of heterometallic FeAu2 and FeAu thiolate gold(I) complexes containing ferrocenyl phosphines (scheme 1). Their antimicrobial properties have been studied on Gram-negative bacteria (E. coli and P. aeruginosa), Gram-positive bacteria (including S. aureus and MRSA US300 and US400 strains) and yeast (S. cerevisiae) and compared to that of AF.
Scheme 1.
Synthesis of heterometallic gold(I) thiolate complexes based on DPPF (3) or MPPF (4) ferrocenyl phosphines. Base = K2CO3 in CH2Cl2 or KOH/MeOH.
2. Experimental
2.1. Materials and methods
All manipulations involving air-free syntheses were performed using standard Schlenk-line techniques under a nitrogen atmosphere or in a glove-box MBraun MOD System. Solvents were purified by use of a PureSolv purification unit from Innovative Technology, Inc. Auranofin was purchased from Enzo LifeSciences, thiophenol (a) was purchased from Alfa Aesar, 2-propanethiol (b) and 2-mercaptothiazoline (d) were purchased from Acros Organic, 1-thio-beta-D-glucose tetraacetate (c) was purchased from Sigma-Aldrich and 6-mercaptopurine monohydrate (e) was purchased from MP Biochemicals. The compound [(Cp-PPh2)2Fe] 1,1'-Bis-diphenylphosphino-ferrocene (DPPF) was purchased from Strem chemicals and used without further purification. [AuCl(tht)] [23], [{AuCl}2(µ-DPPF)] (1) [24] and [(Cp-PPh2)Fe(Cp)] diphenylphosphino-ferrocene (MPPF) [25] were prepared by reported methods. [{AuSPh}2(µ-DPPF)] (3a) was prepared by a modification of a previously reported procedure [26], more detailed spectroscopic and crystallographic data for this published compound are included here. Purity of the compounds is based on elemental analysis and in all cases is ≥99.5%. Elemental analyses were performed on a Perkin Elmer 2400 CHNS/O Analyzer, Series II. NMR spectra were recorded in a Bruker AV400 (1H NMR at 400MHz, 13C NMR at 100.6 MHz, 31P NMR at 161.9 MHz). Chemical shifts (δ) are given in ppm using CDCl3 or DMSO-d6 as solvent, unless otherwise stated. 1H and 13C chemical shifts were measured relative to solvent peaks considering TMS (tetramethylsilane) = 0 ppm; 31P{1H} was externally referenced to H3PO4 (85%). MNova was the report software package used for NMR spectra analysis. Infrared spectra (4000-250 cm−1) were recorded on a Nicolet 6700 FT-IR spectrophotometer on KBr pellets. Mass spectra (ESI-MS and HR-ESI MS) were performed on an Agilent Analyzer or a Bruker Analyzer. X-ray collection was performed at room temperature on a Kappa CCD diffractometer using graphite monochromated Mo-Kα radiation (λ=0.71073 Å).
2.2. Synthesis and characterization of the new compounds
[{AuCl}(MPPF)] (2)
MPPF (0.738 g, 2 mmol) was added to a solution of [AuCl(tht)] (0.641 g, 2 mmol) in 40 mL of dichloromethane at 0°C. The mixture was stirred for 20 minutes at room temperature and then the solvent was removed to ca. 3 mL. The addition of 5 mL of cold diethyl ether afforded a yellow-brown precipitate that was isolated by filtration. Yield: 1.142 g, 95%. Anal. Calc. for C22H19AuClFeP (602.63): C, 43.85; H, 3.18. Found: C, 44.19; H, 3.28. MS(ESI+) [m/z]: 567.0 [M − Cl]+. 31P{1H} NMR (CDCl3): δ 28.46. 1H NMR (CDCl3): δ 7.60 (4H, m, C6H5-orto), δ 7.48 (6H, m, C6H5-meta/para), δ 4.60 (2H, s, C5H4), δ 4.38 (2H, s, C5H4), δ 4.22 (5H, s, C5H5). 13C{1H} NMR (CDCl3): δ 133.5 (d, JPC = 13.5 Hz, C6H5), δ 131.6 (s, C6H5), δ 130.9 (d, JPC = 63.5 Hz, C6H5), δ 128.8 (d, JPC = 11.8 Hz, C6H5), δ 73.4 (d, JPC = 14.07 Hz, C5H4), δ 72.5 (d, JPC = 9.6 Hz, C5H4), δ 70.2 (s, C5H5). IR (cm−1): v335 (Au−Cl).
[{AuSR}2(DPPF)] (3a-3e) and [{AuSR}(MPPF)] (4a-4e)
A mixture of the thiols (a-e) (0.2 mmol) and of K2CO3 (2.0 mmol) in 30 mL of dichloromethane was stirred for 10 minutes at room temperature. The corresponding gold complex, either [{AuCl}2(DPPF)] (1) (0.1 mmol) or [AuCl(MPPF)] (2) (0.2 mmol), was added to the solution. The reaction mixture was stirred during 2 h at room temperature. The solution was subsequently filtered through celite to remove any excess reagents and undesired side-products. The solvent was removed to dryness to afford the expected gold thiolate complex. 3a (ref 4): Yield: 0.132 g, 57%. 31P{1H} NMR (DMSO-d6): δ 33.0. 3b: Yield: 0.146 g, 66%. Anal. Calc. for C40H42Au2FeP2S2 (1,098.62): C, 43.73; H, 3.85; S, 5.84. Found: C, 43.29; H, 3.78, S, 5.91. MS(ESI+) [m/z]: 1024.1 [M - SiPr]+. 31P{1H} NMR (DMSO-d6): δ 32.21. 31P{1H} NMR (CDCl3): δ 33.58. 1H NMR (plus COSY, plus NOESY-2D, CDCl3): δ 7.48 (20H, m, C6H5), δ 4.78 (4H, s, C5H4), δ 4.25 (4H, s, C5H4), δ 3.73 (2H, s, CH(CH3)2), δ 1.51 (12H, d, 3JHH = 6.4 Hz, CH(CH3)2). 13C{1H} NMR (plus HSQC, CDCl3): δ 133.5 (d, JPC = 14.0 Hz, C6H5), δ 131.4 (d, JPC = 56.4 Hz, C6H5), δ 131.4 (s, C6H5), δ 128.9 (d, Jpc = 11.3 Hz, C6H5), δ 75.1 (d, JPC = 8.1 Hz, C5H4), 74.5 (d, JPC = 13.1 Hz, C5H4) 72.1 (d, JPC = 63.3 Hz, C5H4), δ 34.4 (s, CH(CH3)2),), δ 30.9 (s, CH(CH3)2).
3c: Yield: 0.206 g, 61%. Anal. Calc. for C62H66Au2FeO18P2S2 (1,6743.57): C, 44.46; H, 3.97; S, 3.83. Found: C, 44.93; H, 4.20, S, 3.30. MS(ESI+) [m/z]: 1311.1 [M − S(β-D-glucose tetraacetate)]+. 31P{1H} NMR (DMSO-d6): δ 32.52. 31P{1H} NMR (CDCl3): δ 32.94. 1H NMR (plus COSY, plus NOESY-2D, CDCl3): δ 7.50 (20H, m, C6H5), δ 5.20 (8H, m, Hb,c,d,e-β-D-glucose tetraacetate), δ 4.93 (2H, s, C5H4), δ 4.87 (2H, s, C5H4), δ 4.36 (2H, s, C5H4), δ 4.30 (2H, m, Hg-β-D-glucose tetraacetate), δ 4.28 (2H, s, C5H4), δ 4.13 (2H, m, Hg-β-D-glucose tetraacetate), δ 3.81 (2H, m, Hf-β-D-glucose tetraacetate), δ 2.10 (6H, s, Hh-β-D-glucose tetraacetate), δ 2.04 (6H, s, Hh-β-D-glucose tetraacetate), δ 1.98 (6H, s, Hh-β-D-glucose tetraacetate), δ 1.89 (6H, s, Hh-β-D-glucose tetraacetate). 13C{1H} NMR (plus HSQC, plus HMBC, CDCl3): δ 170.7, 170.2, 169.8, 169.5 (s, C=O), δ 133.6 (m, C6H5), δ 131.5 (m, C6H5), δ 131.0 (m, C6H5), δ 129.0 (m, C6H5), δ 83.4, 77.9, 74.2, 69.0 (s, Cb,c,d,e-β-D-glucose tetraacetate), δ 75.8 (s, Cf-β-D-glucose tetraacetate), δ 75.1 (m, C5H4), δ 71.7 (d, JPC = 65.05 Hz, C5H4), δ 62.9 (s, Cg-β-D-glucose tetraacetate), δ 21.2 (s, Ch-β-D-glucose tetraacetate), δ 20.8 (s, Ch-β-D-glucose tetraacetate), δ 20.7 (s, Ch-β-D-glucose tetraacetate), δ 20.6 (s, Ch-β-D-glucose tetraacetate).
3d: Yield: 0.184 g, 61%. Anal. Calc. for C40H36Au2FeN2P2S4 (1,184.71): C, 40.55; H, 3.06; N, 2.36; S, 10.82. Found: C, 40.59; H, 3.21, N, 2.45; S, 10.56. MS(ESI+) [m/z]: 751.1 [DPPF + Au]+, 1066.1 [M − S(thiazoline)]+. 31P{1H} NMR (DMSO-d6): δ 32.21. 31P{1H} NMR (CDCl3): δ 32.15. 1H NMR (plus COSY, plus NOESY-2D, CDCl3): δ 7.52 (20H, m, C6H5), δ 4.81 (4H, s, C5H4), δ 4.37 (4H, s, C5H4), δ 4.29 (4H, m, CH2N), δ 3.45 (4H, m, CH2S. 13C{1H} NMR (plus HSQC, CDCl3): δ 173.1 (s, CS(S)(N)). δ 133.6 (d, JPC = 13.9 Hz, C6H5), δ 131.7 (s, C6H5), δ 130.7 (d, JPC = 59.1 Hz, C6H5), δ 129.1 (d, JPC = 11.6 Hz, C6H5), δ 75.4 (d, JPC = 8.3 Hz, C5H4), 74.9 (d, JPC = 13.3 Hz, C5H4) 71.6 (d, JPC = 66.8 Hz, C5H4), δ 64.6 (s, CH2N), δ 37.5 (s, CH2S).
3e: Yield: 0.128 g, 52%. Anal. Calc. for C44H34Au2FeN8P2S2 (1,250.66): C, 42.26; H, 2.74; N, 8.96; S, 5.13. Found: C, 41.89; H, 3.06, N, 8.65; S, 5.37. MS(ESI+) [m/z]: 751.1 [DPPF + Au]+, 1099.1 [M − S(6-mercaptopurine)]+. 31P{1H} NMR (DMSO-d6): δ 27.17. 31P{1H} NMR (CDCl3): δ 27.67. 1H NMR (plus COSY, plus NOESY-2D, CDCl3): δ 8.81 (2H, s, 2-purine), δ 8.23 (2H, s, 8-purine), δ 7.52 (8H, m, C6H5), δ 7.46 (12H, m, C6H5), δ 4.68 (4H, s, C5H4), δ 4.35 (4H, s, C5H4), δ 1.62 (2H, s, 9-purine). 13C{1H} NMR (plus HSQC, CDCl3): δ 154.1 (s, 4-purine), δ 150.8 (s, 8-purine), δ 133.4 (d, JPC = 14.0 Hz, C6H5), δ 132.2 (d, JPC = 77.6 Hz, C6H5), δ 131.9 (s, C6H5), δ 129.1 (d, JPC = 11.9 Hz, C6H5), δ 128.4 (s, 2-purine), δ 126.3 (s, 5-purine), δ 77.2 (s, C5H4), 75.3 (s, C5H4), C6-purine not observed.
4a: Yield: 0.078 g, 58%. Anal. Calc. for C28H24AuFePS (676.35): C, 49.72; H, 3.58; S, 4.74. Found: C, 49.62; H, 3.55, S, 4.88. MS(ESI+) [m/z]: 567.16 [M − SPh]+, 677.04 [M + H],. 31P{1H} NMR (DMSO-d6): δ 32.40. 31P{1H} NMR (CDCl3): δ 33.87. 1H NMR (plus COSY, plus NOESY-2D, CDCl3): δ 7.70 (2H, d, 3JHH = 7.4 Hz, P(C6H5)), δ 7.63 (4H, dd, 3JHH = 7.4 Hz, 3JHH= 12.5 Hz P(C6H5)), δ 7.48 (4H, m, P(C6H5)), δ 7.26 (2H, m, S(C6H5)), δ 7.16 (2H, m, S(C6H5)), δ 7.03 (1H, m, S(C6H5)), δ 4.58 (2H, s, C5H4), δ 4.39 (2H, s, C5H4), δ 4.17 (5H, s, C5H5). 13C{1H} NMR (plus HSQC, CDCl3): δ 133.6 (d, JPC = 13.8 Hz, P(C6H5)), δ 132.8 (s, S(C6H5)), δ 132.0 (s, S(C6H5)), δ 131.3 (m, P(C6H5)), δ 131.1 (d, JPC = 68.8 Hz, P(C6H5)), δ 128.8 (d, Jpc = 11.3 Hz, P(C6H5)), δ 128.1 (s, S(C6H5)), δ 123.5 (s, S(C6H5)), δ 73.5 (d, JPC = 13.8 Hz, C5H4), 72.4 (d, JPC = 8.5 Hz, C5H4) 70.0 (s, C5H4).
4b: Yield: 0.130 g, 76%. Anal. Calc. for C25H26AuFePS (642.33): C, 46.75; H, 4.08; S, 4.99. Found: C, 47.14; H, 4.05, S, 4.79. MS(ESI+) [m/z]: 567.15 [M − SiPr]+, 643.05 [M + H]. 31P{1H} NMR (DMSO-d6): δ 34.32. 31P{1H} NMR (CDCl3): δ 34.50. 1H NMR (plus COSY, plus NOESY-2D, CDCl3): δ 7.61 (4H, m, C6H5), δ 7.45 (6H, m, C6H5), δ 4.56 (2H, s, C5H4), δ
4.37 (2H, s, C5H4), δ 4.20 (5H, s, C5H5), δ 3.76 (1H, sept, 3JHH = 6.5 Hz, CH(CH3)2), δ 1.55 (6H, d, 3JHH = 6.5 Hz, CH(CH3)2). 13C{1H} NMR (plus HSQC, CDCl3): δ 133.6 (d, JPC = 13.9 Hz, C6H5), δ 131.4 (d, JPC = 57.5 Hz, C6H5), δ 131.0 (s, C6H5), δ 128.7 (d, JPC = 11.0 Hz, C6H5), δ 73.4 (d, JPC = 13.6 Hz, C5H4), 72.2 (d, JPC = 8.3 Hz, C5H4) 69.9 (s, C5H4), δ 34.2 (s, CH(CH3)2),), δ 32.5 (s, CH(CH3)2).
4c: Yield: 0.106 g, 55%. Anal. Calc. for C36H38AuFeO9PS (930.54): C, 46.47; H, 4.12; S, 3.45. Found: C, 45.97; H, 4.23, S, 3.29. MS(ESI+) [m/z]: 567.1 [M − S(β-D-glucose tetraacetate)]+, 931.1 [M + H]. 31P{1H} NMR (DMSO-d6): δ 33.56. 31P{1H} NMR (CDCl3): δ 34.13. 1H NMR (plus COSY, plus NOESY-2D, CDCl3): δ 7.64 (4H, m, C6H5), δ 7.49 (6H, m, C6H5), δ 5.21 (4H, m, Hb,c,d,e-β-D-glucose tetraacetate), δ 4.59 (2H, s, C5H4), δ 4.45 (1H, m, Hg-β-D-glucose tetraacetate), δ 4.27 (7H, m, C5H4, C5H5), δ 4.16 (1H, m, Hg-β-D-glucose tetraacetate), δ 3.82 (1H, m, Hfβ-D-glucose tetraacetate), δ 2.06 (12H, m, Hh-β-D-glucose tetraacetate). 13C{1H} NMR (plus HSQC, plus HMBC, CDCl3): δ 170.9, 170.8, 170.3, 169.5 (s, C=O), δ 133.7 (d, JPC = 13.9 Hz, C6H5), 131.2 (s, C6H5), δ 130.7 (d, JPC = 58.1 Hz, C6H5), δ 128.8 (d, JPC = 11.5 Hz, C6H5), δ 83.4, 77.8, 73.6, 69.0 (s, Cb,c,d,e-β-D-glucose tetraacetate), δ 77.8 (s, Cf-β-D-glucose tetraacetate), δ 72.4 (s, C5H4), δ 70.1 (s, C5H4), δ 62.8 (s, Cg-β-D-glucose tetraacetate), δ 21.2 (s, Ch-β-D-glucose tetraacetate), δ 20.8 (s, Ch-β-D-glucose tetraacetate), δ 20.7 (s, Ch-β-D-glucose tetraacetate), δ 20.6 (s, Ch-β-D-glucose tetraacetate).
4d: Yield: 0.100 g, 72%. Anal. Calc. for C25H23AuFeNPS2 (685.37): C, 43.81; H, 3.38; N, 2.04; S, 9.36. Found: C, 43.46; H, 3.76, N, 2.08; S, 9.87. MS(ESI+) [m/z]: 567.15 [M − S(thiazoline)]+, 686.0 [M + H]. 31P{1H} NMR (DMSO-d6): δ 32.36. 31P{1H} NMR (CDCl3): δ 33.1,. 1H NMR (plus COSY, plus NOESY-2D, CDCl3): δ 7.64 (4H, m, C6H5), δ 7.48 (6H, m, C6H5), δ 4.59 (2H, s, C5H4), δ 4.42 (2H, s, C5H4), δ 4.32 (2H, d, 3JHH = 8.0, CH2N), δ 4. 24 (5H, s, C5H5), δ 3.47 (2H, d, 3JHH = 7.9, CH2S). 13C{1H} NMR (plus HSQC, CDCl3): δ 173.3 (s, CS(S)(N)). δ 133.7 (d, JPC = 13.8 Hz, C6H5), δ 131.3 (s, C6H5), δ 130.5 (d, JPC = 56.6 Hz, C6H5), δ 128.8 (d, JPC = 11.8 Hz, C6H5), δ 73.6 (d, JPC = 8.5 Hz, C5H4), 72.4 (d, JPC = 13.5 Hz, C5H4) 70.17 (s, C5H4), δ 68.1 (s, CH2N), δ 30.4 (s, CH2S).
4e: Yield: 0.079 g, 55%. Anal. Calc. for C27H22AuFeN4PS (718.34): C, 45.14; H, 3.09; N, 7.80; S, 4.46. Found: C, 45.35; H, 3.13, N, 7.71; S, 4.59. MS(ESI+) [m/z]: 567.0 [M − S(6-mercaptopurine)]+, 719.0 [M + H]. 31P{1H} NMR (DMSO-d6): δ 29.27. 31P{1H} NMR (CDCl3): δ 28.32,. 1H NMR (plus COSY, plus NOESY-2D, CDCl3): δ 8.74 (2H, s, 2-purine), δ 8.25 (2H, s, 8-purine), δ 7.66 (4H, m, C6H5), δ 7.41 (6H, m, C6H5), δ 4.53 (2H, s, C5H4), δ 4.39 (2H, s, C5H4), δ 4.16 (5H, s, C5H5), δ 1.95 (1H, s, purine). 13C{1H} NMR (plus HSQC, CDCl3): δ 152.1 (s, 4-purine), δ 150.7 (s, 8-purine), δ 133.7 (d, JPC = 13.9 Hz, C6H5), δ 133.5 (s, 2-purine), δ 131.9 (d, JPC = 56.8 Hz, C6H5), δ 131.2 (s, C6H5), δ 129.1 (s, 5-purine), δ 128.8 (d, JPC = 11.2 Hz, C6H5), δ 73.51 (d, JPC = 14.0 Hz, C5H4), δ 72.3 (d, JPC = 8.55 Hz, C5H4), 70.1 (s, C5H4), C6-purine not observed.
2.3.Crystallographic Data for Compounds 3a and 3d
Single crystals of 3a and 3d (see details below) were mounted on a glass fiber in a random orientation. Data collection was performed at room temperature on a Kappa CCD diffractometer using graphite monochromated Mo-Ka radiation (l=0.71073 Å). Space group assignments were based on systematic absences, E statistics and successful refinement of the structures. The structures were solved by direct methods with the aid of successive difference Fourier maps and were refined using the SHELXTL 6.1 software package. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were assigned to ideal positions and refined using a riding model. Details of the crystallographic data are given in Table S1 (Supporting information section). These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif. (CCDC 977988 for compound 3a, and 977989 for compound 3d). 3a: Crystals of 3a (yellow prisms with approximate dimensions 0.25 × 0.23 × 0.23 mm) were obtained from a solution of 3a in CH2Cl2 by slow diffusion of Et2O at RT. 3d: Crystals of 3d (yellow prisms with approximate dimensions 0.25 × 0.24 × 0.22 mm) were obtained from a solution of 3d in CH2Cl2 by slow diffusion of Et2O at RT.
2.4. DFT Studies for compounds 4a and 4d
The calculations have been performed using the hybrid density functional method B3LYP,[27,28] as implemented in Gaussian09.[29] Geometries were optimized with the 6–31G(d,p) basis set for the C, N, P, S, and H elements and the SDD pseudopotential for the iron and gold centers.[30,31] Frequency calculations have been done at the same level of theory as the geometry optimizations to confirm the nature of the stationary points.
2.5.Microbial Toxicity Assays
Bacteria and yeast were stored as glycerol stocks at −80 °C and streaked onto Meuller-Hinton or YEPD plates prior to each set of experiments. Colonies from newly prepared plates were inoculated into 5 mL of media (tryptic soy broth for E. coli, P. aeruginosa and S. aureus strains and YPD broth for S. cerevisiae) and grown overnight at 37 °C (30 °C for S. cerevisiae). The overnight cultures were diluted to an OD600 <0.01 (ThermoSpectronic, Genesys 8 spectrophotometer) in 2 mL of fresh media in sterile culture tubes. The compounds (auranofin, 3a-3e and 4a-4e) were brought up in DMSO to a concentration of 1 mg/mL and then diluted into the cell culture tubes at the specified concentrations. Control tubes for each cell type were inoculated with an equivalent concentration of DMSO alone. The cultures were then transferred as 200 µL volumes into a 96-well plate (typically 8 replicates were performed for each condition). The plate was incubated at the appropriate temperature and shaken in a Biotek ELx808 plate reader. Growth measurements (OD600) were automatically taken every hour for 24 hours. The minimal inhibitory concentration (MIC) was determined to be the concentration at which there was negligible increase in the OD600 value from the initial reading after 24 hours. All samples were independently tested two or three times as described here.
2.6. Toxicity of compounds on Human Embryonic Kidney Cells (HEK-293)
The Human Embryonic Kidney 293 cells (HEK-293), a non-tumoral immortalized human cell line (obtained from American Type Culture Collection, Manassas, Virginia, USA) were cultured in DMEM (Dulbecco’s Modified Eagle Medium, F-12 50/50 Mix with L-Glutamine and phenol red) (from Corning Cellgro) supplemented with 10% FBS (from Gibco Sera, Life Technology) and 1% penicillin/streptomycin, at 37°C in a humidified atmosphere of 95% of air and 5% CO2 (University of Hawaii Cancer Center, Honolulu, Hawaii, USA). For evaluation of cell viability, cells were seeded at a concentration of 5×1^3 cells/well in 90 µL DMEM complete medium without phenol red, into tissue culture grade 96-wellflat bottom microplates (Thermo Scientific BioLite Microwell Plate, Fisher Scientific, Waltham, Massachusetts, USA) and grown for 24 h. Solutions of the compounds were prepared by diluting a freshly prepared stock solution (in DMSO) of the corresponding compound in DMEM complete medium without phenol red. Afterwards, the intermediate dilutions of the compounds were added to the wells (10 µL) to obtain a final concentration ranging from 0.1 to 200 µM, and the cells were incubated for 24 h. DMSO at comparable concentrations did not show any effects on cell cytotoxicity. Following 24 h drug exposure, 50 µL of 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H–tetrazolium-5-carboxanilide (XTT) (Roche Diagnostics, Indianapolis, Indiana, USA) labeling mixture per well was added to the cells at a final concentration of 0.3 mg/mL and incubated for 4h at at 37°C in a humidified atmosphere of 95% of air and 5% CO2. The optical density of each well (96-well plates) was quantified using EnVision Multilabel Plate Readers (Perkin Elmer, Waltham Massachusetts,USA) at 450 nm wavelength to measure absorbance. The percentage of surviving cells was calculated from the ratio of absorbance of treated to untreated cells. The IC50 value was calculated as the concentration reducing the proliferation of the cells by 50% and is presented as a mean (± SE) of at least two independent experiments each with triplicates.
3. Results and Discussion
3.1. Synthesis and characterization of the heterometallic compounds
The synthesis and partial characterization of compound 3a had been described before [26]. The standard method employed for the synthesis of all the heterometallic complexes described here is based on the deprotonation of thiols with a base and subsequent reaction with [{AuCl}2(µ DPPF)] (1) [24] or [AuCl(MPPF)] (2) to obtain compounds 3a–e or 4a–e respectively (Scheme 1). The synthesis of the gold(I)-chlorophosphino starting materials with DPPF (1) and MPPF (2) is by displacement of the labile ligand tetrahydrothiophene (tht) in [AuCl(tht)] [23] by the ferrocenyl phosphines. Compound 1 has been reported previously and 2 was synthesized in a similar manner (Scheme 1) by reaction of [AuCl(tht)] and MPPF [26].
All new compounds are air-stable, yellow solids that are obtained in moderate yields. The structures proposed are based on NMR and IR spectroscopy, elemental analysis, and mass spectrometry (see spectra in supporting information). The heterometallic derivatives are soluble in DMSO and in mixtures DMSO:H2O (1:99) at micromolar concentrations. Compound 3c is more hydrophilic and is soluble in mixtures of 50:50 at milimolar concentrations. All complexes are stable for at least 8 days at RT in DMSO solutions as determined by 31P{1H} and 1H NMR spectroscopy (no more than 2–3% phosphine oxide generated, see selected spectra in DMSO-d6 overtime in the SI). DMSO solutions of the compounds can be kept for several weeks or months in the freezer without noticeable decomposition. The spectroscopic data 31P{1H} NMR in CDCl3 and DMSO-d6 is collected in Table 1. The main peaks observed in the MS spectra (ESI in CH2Cl2) are also collected in table 1. It has been reported that compounds of the type [Au(X)(PR3)] can give rise to species [Au(PR3)2][Au(X)2] (e.g. X = SR−, SeR−, CN−) by ligand scrambling and dissociation [32,33]. This is relevant in order to understand the metabolism of drugs like AF [34]. AF is known to bind an SH fragment from cysteine 34 to form AlbSAuPEt3 in vivo and subsequently displacing PEt3 which oxidizes to PEt3O (a process favored by increasing the affinity of the anion to bind gold(I) or retarded by the bulk and basicity of the phosphine) [34]. We have found by NMR spectroscopy and MS (HR ESI) spectrometry that the trimetallic FeAu2 species [{Au(SR)}2(DPPF)] (3a-3e) do not seem to undergo this type of ligand scrambling easily. No noticeable decomposition is noticed at RT in DMSO-d6 after 8 days (figures S36–39 in SI) and sharp peaks were found in the 31P{1H} NMR spectra in CDCl3 and DMSO-d6 solution (SI). The ESI MS spectra for 3a-3e (Table 1 and figures S44–56 in the SI) do not show peaks that can be assigned to species [Au(DPPF)2]+.
Table 1.
31P{1H} NMR data (CDCl3 and DMSO-d6) and main peaks in MS spectra (HR ESI) for the new gold compounds. 31P{1H} δ in DMSO-d6 remain the same after 8 days at RT. All spectra are collected in the SI section.
| Complex | 31P{1H} NMR (δ) | HR ESI-MS | |
|---|---|---|---|
| 2 | 28.46 (b) | 29.31 (b) | [M−Cl]+ or [MPPF + Au]+ m/z = 567.01 (100%) |
| 3a | 32.94 (s) | 33.00 (s) | [DPPF − Ph]+ m/z = 477.17 (80%) [DPPF + Au]+ m/z = 751.24 (100%) [M − SR]+ m/z = 1057.05 (1 %) |
| 3b | 33.58 (s) | 32.21 (s) | [M − SR]+ m/z = 1024.05 (100 %) [M + Au]+ m/z = 1295.08 (10%) |
| 3c | 32.94 (s) | 32.52 (s) | [DPPF + Au]+ m/z =751.10 (10%) [DPPF + 2Au]2+ m/z = 981.10 (35%) [M − SR]+ m/z = 1311.10 (100%) [M + Au]+ m/z = 1871.10 (5%) |
| 3d | 32.15 (s) | 32.21 (s) | [DPPF + Au]+ m/z = 751.10 (100%) [M −SR]+ m/z = 1066.10 (57%) |
| 3e | 27.67 (s) | 27.17 (s) | [DPPF + Au]+ m/z = 751.10 (58%) [M −SR]+ m/z = 1099.10 (100%) |
| 4a | 33.87 (s) | 32.40 (s) | [MPPF + Au]+ or [M− SR]+ m/z = 567.01 (75%) [M + H]+ m/z = 677.04 (0.1%) |
| 4b | 34.50 (b) | 34.32 (b) | [MPPF + Au]+ or [M− SR]+ m/z = 567.01 (75%) [M + H]+ m/z = 643.05 (0.5%) |
| 4c | 34.13 (b) | 33.56 (b) | [MPPF + Au]+ or [M− SR]+ m/z = 567.01 (95%) [M + H] + m/z = 931.10 (2%) |
| 4d | 33.17 (b) | 32.36 (b) | [MPPF + Au]+ or [M− SR]+ m/z = 567.01 (67%) [M + H]+ m/z = 686.17 (50%) |
| 4e | 28.32 (b) | 29.27 (b) | [MPPF + Au]+ or [M− SR]+ m/z = 567.01 (10%) [M + H]+ m/z = 719.03 (2%) |
The bimetallic FeAu species [Au(SR)(MMPF)] 4b-4e give rise to broader signals in the 31 P{1H} NMR spectra (see figures S20, S23, S26, S29, S32, S41 and S43 in SI). It is worth noting that these compounds are less soluble in DMSO than compounds 3a-3e or 4a (completely soluble) and the solutions prepared to get the 31P{1H} are almost saturated. As for compounds with DPPF their MS spectra (ESI+, figures S57–S65 in SI) do not show peaks which could be assigned to species [Au(MPPF)2]+ indicative of ligand scrambling. Dissociation of the SR- is evident by the peaks observed that correspond to [Au(MPPF)]+ (table 1) but this is common for the ESI+ or FAB+ MS spectra of gold complexes of the type [AuX(PR3)]. However with the data we have so far we cannot exclude the possibility of ligand scrambling in solution for compounds 4b-4e.
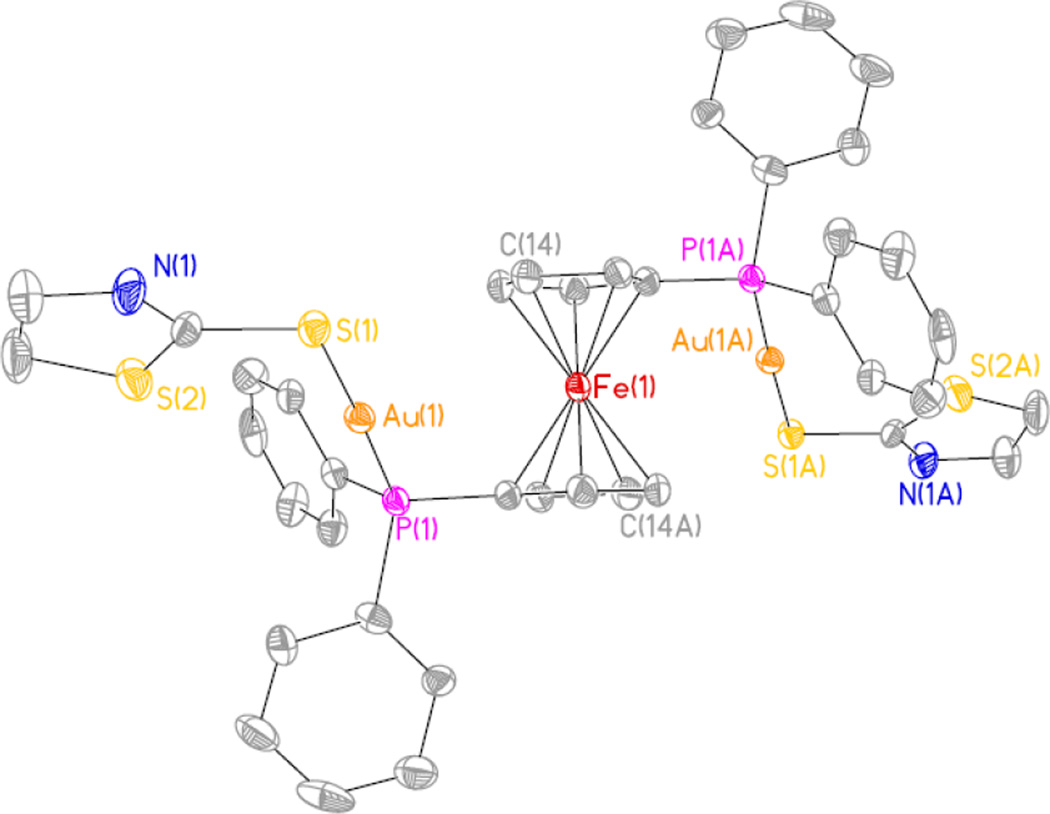
The structures of the trimetallic FeAu2 compounds 3a and 3d were further confirmed by X-ray single crystal diffraction (structure of 3d shown in Figure 2). Selected bond lengths and angles for both compounds are collected in Table 2 while the drawing for the crystal structure of 3a and crystallographic tables for 3a and 3d (Table S1) are in the supplementary material. A more complete table including more values for bond lengths and angles for complexes 3a and 3d is also included in the SI (Table S2).
Figure 2.
Ortep view of the crystal structure of 3d. Structure of 3a in supplementary material.
Table 2.
Selected bond lengths [Å] and angles [°] for complexes 3a and 3d.
| 3a | 3b | ||
|---|---|---|---|
| Au(1)-P(1) | 2.2546(14) | Au(1)-P(1) | 2.259(3) |
| Au(1)- S(1) | 2.3000(16) | Au(1)- S(1) | 2.307(3) |
| Fe-C | 2.032(4)-2.063(5) | Fe-C | 2.025(15) -2.050(15) |
| S-C(41) | 1.783(6) | S-C(1) | 1.761(14) |
| P(1)-Au(1)-S(1) | 171.33(5) | P(1)-Au(1)-S(1) | 177.75(11) |
| C(14)-Fe(1)-C(14A) | 180.0(3) | C(14)-Fe(1)-(C14A) | 179.999(2) |
| C(41)-S(1)-Au(1) | 100.93(19) | C(1)-S(1)-Au(1) | 104.0(5) |
Both crystal structures are very similar. The cyclopentadienyl rings are staggered by 36.09° (3a) and 36.88° (3d) around the Cp…Cp axis (Cp = center of the cyclopentadienyl ring) defined by the torsion angle C11-Cp-Cp-C13. The phosphorus atoms are located at the 1 and 3’ carbon atoms of the ferrocene rings. The molecular structures display a typical linear geometry around the gold centers with a P1-Au1-S1 angle of 171.33(5) (3a) and 177.75(11) (3d). the distances Au-S and Au-P are within the usual range of other gold(I) thiolates containing phosphine ligands [17,35]. Since we could not obtain crystals of the bimetallic complexes 4a–e of enough quality for a single crystal X-ray determination, good estimates for the structural parameters of 4a and 4d were obtained from density functional calculations (see figures in SI and Table S3). The distances Au-S and Au-P are similar to those in 3d and 3d and the main difference is the eclipsed disposition of the two Cp rings (one containing the phosphine group attached to the gold-thiolate group) for the ferrocene moiety as well as the smaller size of the overall molecule.
3.2. Biological Activity
The antimicrobial activities of the gold(I) trimetallic Fe-Au2 (3a–e) and bimetallic (4a–e) compounds were evaluated against Gram-negative (Escherichia coli and Pseudonomas aeruginosa), Gram-positive (Staphyloccocus aureus) bacteria and yeast (Saccharomyces cerevisiae) (Table 2). A number of the compounds analyzed in this work exhibited minimum inhibitory concentration (MIC) values in the 1 µg/mL - 100 µg/mL range (Table 3). For selected compounds the antimicrobial activities against MRSA strains US300 and US400 and two S. cerevisiae yeast mutants lacking thioredoxin reductase TR1 or TR2 were also evaluated (Table 3 and data not shown). The antimicrobial activity of AF was also studied against all the above mentioned microbes (Table 3). MIC values for AF against Gram-negative (Escherichia coli and P. aeruginosa), and Gram-positive (Staphyloccocus aureus) are similar to those reported before [17] (see Table 3). To the best of our knowledge the antimicrobial activity of AF against MRSA strains US300 and US400 and S. cerevisiae has not been reported before.
Table 3.
Minimum inhibitory concentration MIC (in µg/mL, +/−0.1) of compounds 3a–e, 4a–e and auranofin against microbial organisms.a
| Compound | Gram-negative | Gram-Positive | Yeast | |||
|---|---|---|---|---|---|---|
| P. aeruginosa | E. coli | S. aureus | US300 | US400 | S. cerevisiae | |
| 3a | >100 | >100 | >10 (>9)b |
---- | --- | >100 |
| 3b | >100 | >100 | 4 (3.6) b |
4 (3.6) b |
4 (3.6) b |
>100 |
| 3c | >100 | >100 | 4 (2.4) b |
6 (3.6) b |
6 (3.6) b |
>100 |
| 3d | >100 | >100 | >10 (>8) b |
---- | --- | >100 |
| 3e | >100 | >100 | >10 (>8) b |
---- | --- | >100 |
| 4a | >100 | >100 | 2 (3) b |
4 (6) b |
4 (6) b |
>100 |
| 4b | >100 | >100 | 2 (3.2) b |
4 (6.4) b |
2 (3.2) b |
>100 |
| 4c | >100 | >100 | 2 (2.2) b |
6 (6.6) b |
6 (6.6) b |
>100 |
| 4d | >100 | >100 | 2 (3) b |
4 (6) b |
4 (6) b |
100 |
| 4e | >100 | >100 | 2 (2.8) b |
2 (2.8) b |
2 (2.8) b |
>100 |
| Auranofin | >100 | >100 | 0.1 (0.15) b |
0.2 (0.3) b |
0.2 (0.3) b |
100 |
Auranofin and the heterometallic compounds were dissolved in DMSO. When compounds were not active in S. aureus below 10 µM they were not tested on US300 or US400.
MIC (micromolar units) in parentheses.
Some of the new heterometallic compounds, specially the bimetallic FeAu derivatives incorporating the MPPF phosphine (4b–e) and trimetallic FeAu2 compounds with DDPF (4b and 4c) have shown activity against the Gram-positive bacterium S. aureus with MIC in the low micromolar range (2.4–6.6 µM or 2–6 µg/mL). None of the new heterometallic complexes or AF[17] have a MIC below the 100 µg/mL range for E. coli or P. aeruginosa. Remarkably, AF inhibits the growth of S. aureus and the MRSA strains USA300 and USA400 in the nanomolar range (150–300 nM or 0.1 to 0.20 µg/mL). The most active heterometallic compound 4e inhibits the growth of S. aureus and the MRSA strains USA300 and USA400 at 2.8 µM. In addition, compound 4d containing 2-thiazoline and AF were equally effective against yeast S. cerevisiae with MIC of 100 µg/mL.
Recent studies have reported that the mechanism of AF toxicity against the eukaryotic microbial pathogens Entamoeba histolytica [14], and Giardia lamblia [13] is linked to the inhibition of thioredoxin reductase enzyme activity. We sought to test if there were a role for AF toxicity in yeast through a similar mechanism by testing mutants of S. cerevisiae lacking thioredoxin reductase genes, TRR1 or TRR2. The expectation being that these mutants lacking the target of AF would be less sensitive to the drug. However, upon testing AF we found no significant difference in activity between the wild type yeast and the thioredoxin reductase mutants. It could be that the TRR1 and TRR2 genes complement one another and that a double mutant in these two genes would need to be tested to see any decreased sensitivity to AF. Or it could be simply that the effects of AF work on yeast through a different pathway unrelated to thioredoxin reductase. Additional testing in the future will be required to characterize the mechanism of activity against yeast.
The activity is not solely correlated to the gold content. The bimetallic compounds have in general lower content of gold than the trimetallic derivatives (range 21.2–30.6 % 4a–e versus 23.5–35.8 % 3a–e) but display in general a better activity against the MSRA strains than three of the trimetallic compounds (3a, 3d and 3e). Effects such as ligand dissociation or ligand scrambling and subsequent formation of different metabolites in vivo will play a more important role for the biological activity [34]. The most active compounds are 3b, 3c and 4e (3.6 µM (3b, 3c) and 2.8 µM (4e) against MSRA strains). It is well established that MRSA strains carry the MecA gene, often in combination with other resistance genes, which inactivates β-lactam antibiotics such as methicillin. In the CA MRSA strains tested, it is unlikely that the presence of the MecA gene would influence the activity of AF on these cells, so that any differences in toxicity seen with AF, or AF-related compounds, is likely to be linked to differences in the genetic background of the CA MRSA strains and the routine laboratory S. aureus strains.
The MIC values obtained for the new heterometallic complexes on Gram-positive S. aureus are similar, or below, those described for some bimetallic aminothiol gold compounds containing phosphines [16]. Monometallic aminothiolates containing the triethyl phosphine ligand (like auranofin) and aminothiols had a MIC below 1 µg/mL (like AF) on this Gram-positive bacterium [17]. It is clear that the subtle interplay of the ligands coordinated to gold may be responsible for the biological activity observed on this type of compounds [17,19]. In this context, although the new heterometallic compounds described here are very potent they have not been as efficient as AF against S. aureus and the CA MRSA strains. We believe that the design of complexes with ferrocenyl-based alkyl phosphines may render new heterometallic complexes with improved antimicrobial and solubility properties.
An explanation for the behavior displayed by these new thiolate gold complexes and AF could be as follows: if these compounds interfere with respiration (a mitochondrial function), then the fungi should be the most resistant, because electron transport is shielded inside internal organelles, whereas in bacteria electron transport occurs at the plasma membrane. Gram-negative bacteria have a protective outer membrane; therefore the compounds may not have ready access to the electron-transport chain. These compounds should be more active in general against Gram-positive bacteria such as S. aureus which is what we have observed here. The testing of these compounds against selected mutant strains of Gram-negative bacteria that have a compromised outer membrane function may help to elucidate the mechanism of these gold-based compounds and the apparent reduced activity against Gram-negative bacteria relative to Gram-positive bacteria.
Studies of the toxicity of selected compounds (AF and compound 4e) against a non-tumorigenic human embryonic kidney cell line (HEK-293) were performed to assess the toxicity on human “normal” or healthy cells. The IC50 values obtained after 24 hours of incubation with these compounds (XTT assay) were 0.549 ± 0.022 µM (AF) and 0.403 ± 0.004 µM 4e. Auranofin was slightly less toxic than compound 4e to the immortalized healthy cell lines but it was 5 times or 2.5 times less toxic to the human cell line than to S. aureus or the resistant strains US300 and US400 respectively.
4. Conclusion
In conclusion, we have prepared a number of new heterometallic gold-thiolate complexes highly active against Gram-positive bacteria S. aureus and CA MRSA strains US300 and US400 (MIC in the order of one digit micromolar). Remarkably, auranofin inhibited S. aureus, US300 and US400 in the order of 150–300 nM or 0.1–0.2 µg/mL.
Therapeutic treatment rheumatoid arthritis with AF results in stable serum concentrations of 0.5–0.7 µg/mL [36,37]. The fact that an oral, FDA approved, drug is able to inhibit CA MRSA strains in nanomolar concentrations that are less (3.5 to 5 times) than those known to be well tolerated by patients suggests that treatment of MRSA with AF, and related gold-based compounds, could represent an important and novel option for patients.
The in vitro MIC for AF on S. aureus and methicillin resistant strains are in the order or below of those for recently reported oxazolidinone molecules (modifications of antibiotic linexolid) [37].This study supports the potential of the FDA approved drug AF on the treatment of diseases other than rheumatoid arthritis [14]. AF and related gold thiolates may offer an opportunity to increase the palette of inexpensive methicillin -resistant antibiotics. In this context, further modification of ferrocenyl-phosphine ligands by substitution of aryl groups by alkyl groups may allow for the preparation of compounds with some improved biological properties in terms of antimicrobial activity and solubility.
Studies on the mechanism of the growth inhibition of AF and some closely related gold(I) thiolates in S. aureus and several MRSA strains are ongoing at our laboratories.
Supplementary Material
Highlights.
-
-
Synthesis of new heterometallic gold(I) compounds containing ferrocenyl-phosphines
-
-
High antibacterial activity (one digit µM) against S. aureus and CA MRSA bacterial strains
-
-
First report on the activity of auranofin (AF) against CA MSRA bacterial strains (nM levels)
-
-
Activity of AF well below those tolerated by patients in the treatment of arthritis with Ridaura
Acknowledgment
Research at Brooklyn College was supported by a grant from the National Institute of General Medical Sciences (NIGMS), SC2GM082307 and a grant from the National Cancer Institute (NCI) 1SC1CA182844 (M.C.). We thank the New York Louis Stokes Alliance for Minority Participation (Fellowship to Masters’ student Y.H.) for funding. This research was supported, in part, by a grant of computer time from the City University of New York High Performance Computing Center under NSF Grants CNS-0855217, CNS-0958379 and ACI-1126113. We thank Prof. Joe W. Ramos (University of Hawaii Cancer Center) for the evaluation of the toxicity of auranofin and compound 4e on HEK-293 cells. We also thank research assistants Benelita T. Elie and Pierpaolo Cordone (Contel’s lab at Brooklyn College) for their assistance with some experiments.
Abbreviations
- AF
auranofin
- COSY
correlation spectroscopy
- DPPF
1,1'-Bis-diphenylphosphino-ferrocene
- DFT
Density functional theory
- ESI-MS
electrospray ionization- mass spectrometry
- FDA
Food and Drug Administration or USFDA, an agency of the United States Department of Health and Human Services
- HEK-293
inmortalized human embryonic kidney cells
- HMBC
heteronuclear multiple-bond correlation spectroscopy
- HSQC
heteronuclear single-quantum correlation spectroscopy
- MIC
minimum inhibitory concentration
- MPPF
diphenylphosphino-ferrocene
- MRSA
Methicillin-resistant Staphylococcus aureus
- NOESY
nuclear Overhauser effect spectroscopy
- SDD
Stuttgart–Dresden pseudopotential
- tht
tetrahydrothiophene
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary Material
Crystallographic tables for compounds 3a and 3d, drawing for the crystal structure of 3a, DFT calculations on molecules 4a and 4d1H, 31P{1H} and 13C{1H} NMR (CDCl3) spectra for all new compounds, 1H and 31P{1H} NMR (DMSO-d6) spectra overtime for selected compounds (4a, 3b, 3c, 4d and 4e), and MS spectra for all new compounds. DOI: …….
References
- 1.Berners-Price SJ. Gold-Based therapeutic agents: a new perspective. In: Alessio E, editor. Bioinorganic Medicinal Chemistry. Wiley-VCH: Weinheim; 2011. pp. 197–223. Ch 7. [Google Scholar]
- 2.Nobili S, Mini E, Landini I, Gabbiani C, Casini A, Messori L. Med. Res. Rev. 2010;30:550–580. doi: 10.1002/med.20168. [DOI] [PubMed] [Google Scholar]
- 3.Schuh E, Pluger C, Citta A, Folda A, Rigobello MP, Bindoli A, Casini A, Mohr F. J. Med. Chem. 2012;55:5518–5528. doi: 10.1021/jm300428v. [DOI] [PubMed] [Google Scholar]
- 4.Meyer A, Bagowski CP, Kokoschka M, Stefanopoulou M, Alborzinia H, Can S, Vleken DH, Sheldrick WS, Wölfl S, Ott I. Angew. Chem. Int. Ed. 2012;51:8895–8899. doi: 10.1002/anie.201202939. [DOI] [PubMed] [Google Scholar]
- 5.Bindoli R, Rigobello MP, Scutari G, Gabbiani C, Casini A, Messori L. Coord. Chem. Rev. 2009;253:1692–1707. [Google Scholar]
- 6.Eisler R. Inflammation Res. 2003;52:487–501. doi: 10.1007/s00011-003-1208-2. [DOI] [PubMed] [Google Scholar]
- 7.Madeira JM, Gibson DL, Kean WF, Klegeris A. Inflammopharmacol. 2012;20:297–306. doi: 10.1007/s10787-012-0149-1. [DOI] [PubMed] [Google Scholar]
- 8.Kuntz AN, Davioud-Charvet E, Sayed AA, Califf LL, Dessolin J, Amer ESJ, D.L. Williams DL. PLoS Med. 2007;4:e206. doi: 10.1371/journal.pmed.0040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lobanov AV, Gromer S, Salinas G, Gladyshev VN. Nucleic Acids Res. 2006;34:4012–4024. doi: 10.1093/nar/gkl541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonilla M, Denicola A, Novoselov SV, Turanov AA, Protasio A, Izmendi D, Gladyshev VN, Salinas G. J. Biol. Chem. 2008;283:17898–17907. doi: 10.1074/jbc.M710609200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sannella AR, Casini A, Gabbiani C, Messori L, Bilia AR, Vincieri FF, Majori G, Severini C. FEBS Lett. 2008;582:844–847. doi: 10.1016/j.febslet.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Ilari A, Baiocco P, Messori L, Fiorillo A, Boffi A, Gramiccia M, Di Muccio T, Colotti G. Amino acids. 2012;42:803–811. doi: 10.1007/s00726-011-0997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tejman-Yarden N, Miyamoto Y, Leitsch D, Santini J, Debnath A, Gut J, McKerrow JH, Reed SL, Eckman L. Antimicrob. Agents. Chemother. 2013;57:2029–2035. doi: 10.1128/AAC.01675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debnath A, Parsonage D, Andrade RM, He C, Cobo ER, Hirata K, Chen S, Garcia-Rivera G, Orozco E, Martinez MB, Gunatilleke SS, Barrios AM, Arkin MR, Poole LB, McKerrow JH, Reed SL. Nature Med. 2012;18:956–962. doi: 10.1038/nm.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson-Rosario J, Cowart D, Myers A, Tarrien R, Levine RL, Scott RA, Self WT. J. Biol. Inorg. Chem. 2009;14:507–519. doi: 10.1007/s00775-009-0466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson-Rosario S, Self WT. J. Bacteriol. 2009:4035–4040. doi: 10.1128/JB.00164-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novelli F, Recine M, Sparatore F, Juliano C. Il Farmaco. 1999;54:232–236. doi: 10.1016/s0014-827x(99)00019-1. [DOI] [PubMed] [Google Scholar]
- 18.Antibiotic resistant threats in the United States. U.S. Department of Health and Human Services. Centers for Disease, Control and Prevention. 2013 Web site: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf.
- 19.Ellie BT, Levine C, Ubarretxena-Belandia I, Varela-Ramírez A, Aguilera R, Ovalle R, Contel M. Eur. J. Inorg. Chem. 2009:3421–3430. doi: 10.1002/ejic.200900279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frik M, Jimenez J, Gracia I, Falvello LR, Abi-Habib S, Suriel K, Muth TR, Contel M. Chem. A Eur. J. 2012;18:3659–3674. doi: 10.1002/chem.201103145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lease N, Vasilevski V, Carreira M, de Almeida A, Sanaú M, Hirva P, Casini A, Contel M. J. Med. Chem. 2013;56:5806–5818. doi: 10.1021/jm4007615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Recent relevant example: Plazuk D, Wieczorek A, Blauz A, Rychlik B. Med.Chem.Commun. 2012;3:498–501.
- 23.Uson R, Laguna A, Laguna M. Inorg. Synth. 1989;26:85–91. [Google Scholar]
- 24.Gimeno MC, Laguna A, Sarroca C, Jones PG. Inorg. Chem. 1993;32:5926–5932. [Google Scholar]
- 25.Sollott GP, Mertwoy HE, Portnoy S, Snead JL. J. Org. Chem. 1963;28:1090–1092. [Google Scholar]
- 26.Viotte M, Gautheron B, Nifantev I, Kuzmina LG. Inorg. Chim. Acta. 1996;253:71–76. [Google Scholar]
- 27.Becke AD. Phys. Rev. 1988;A38:3098–3100. doi: 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]
- 28.Becke AD. J. Chem. Phys. 1993;98:5648–5652. [Google Scholar]
- 29.Frisch MJT, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa R, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Jr, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JN, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Ö Farkas, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, Revision A.1. Wallingford CT: Gaussian, Inc; 2009. [Google Scholar]
- 30.Dolg M, Wedig U, Stoll H, Preuss H. J. Chem. Phys. 1987;86:866–872. [Google Scholar]
- 31.Andrae D, Haussermann U, Dolg M, Stoll H, Preuss H. Theor. Chim. Acta. 1990;77:123–141. [Google Scholar]
- 32.Hormann AL, Shaw CF, III, Bennett DW, Reiff WM. Inorg. Chem. 1986;25:3953–3957. [Google Scholar]
- 33.Hill DT, Isab AA, Griswold DE, DiMartino MJ, Matz ED, Figueroa AL, Wawro JE, DeBrosee C, Reiff WM, Elder RC, Jones B, Webb JW, Shaw CF., III Inorg. Chem. 2010;49:7663–7675. doi: 10.1021/ic902335z. [DOI] [PubMed] [Google Scholar]
- 34.Isab AA, Shaw CF, III, Locke J. Inorg. Chem. 1988;27:3406–3409. [Google Scholar]
- 35.Vergara E, Cerrada E, Clavel C, Casini A, Laguna M. Dalton Trans. 2011:10927–10935. doi: 10.1039/c1dt10892a. and refs therein. [DOI] [PubMed] [Google Scholar]
- 36.Blocka K. Am. J. Med. 1983;75:114–122. doi: 10.1016/0002-9343(83)90483-7. 75. [DOI] [PubMed] [Google Scholar]
- 37.Gottlieb NL. Scand. J. Rheumatol. 1986;63(Suppl):19–28. [PubMed] [Google Scholar]
- 38.Suzuki H, Utsinomiya I, Shudo K, Fujimura T, Tsuji M, Kato I, Aoki T, Ino A, Iwaki T. ACS Med. Chem. Lett. 2013;4:1074–1078. doi: 10.1021/ml400280z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.