Abstract
Background
Resistance exercise (RE) is also known as strength training, and it is performed to increase the strength and mass of muscles, bone strength and metabolism. RE has been increasingly prescribed for pain relief. However, the endogenous mechanisms underlying this antinociceptive effect are still largely unexplored. Thus, we investigated the involvement of the endocannabinoid system in RE-induced antinociception.
Methods
Male Wistar rats were submitted to acute RE in a weight-lifting model. The nociceptive threshold was measured by a mechanical nociceptive test (paw pressure) before and after exercise. To investigate the involvement of cannabinoid receptors and endocannabinoids in RE-induced antinociception, cannabinoid receptor inverse agonists, endocannabinoid metabolizing enzyme inhibitors and an anandamide reuptake inhibitor were injected before RE. After RE, CB1 cannabinoid receptors were quantified in rat brain tissue by Western blot and immunofluorescence. In addition, endocannabinoid plasma levels were measured by isotope dilution-liquid chromatography mass spectrometry.
Results
RE-induced antinociception was prevented by preinjection with CB1 and CB2 cannabinoid receptor inverse agonists. By contrast, preadministration of metabolizing enzyme inhibitors and the anandamide reuptake inhibitor prolonged and enhanced this effect. RE also produced an increase in the expression and activation of CB1 cannabinoid receptors in rat brain tissue and in the dorsolateral and ventrolateral periaqueductal regions and an increase of endocannabinoid plasma levels.
Conclusion
The present study suggests that a single session of RE activates the endocannabinoid system to induce antinociception.
Introduction
Worldwide, one in five people suffers from moderate to severe chronic pain, and one in three is unable or less able to conduct an independent lifestyle due to their pain.1 Thus, the annual cost of chronic pain in America is estimated to be more than $560 to 635 billion.1 Most of this cost is due to medications, which have not been as effective as one would have hoped. Therefore, nonpharmacological strategies such as resistance exercise (RE) have been widely used as a potent therapeutic approach for pain treatment, not only for providing relief from symptoms, but especially for reducing the financial burden and side effects associated with chronic use of analgesic and antiinflammatory medicines. In addition, some studies demonstrated that RE is effective at reducing pain and improving motor function in patients with osteoarthritis of the knee and rheumatoid arthritis.2–4 Furthermore, RE can potentially counteract the functional limitations and pain symptoms seen in patients with musculoskeletal disorders such as lateral epicondylosis, fibromyalgia and patellar tendinopathy.5,6 Harts et al. also demonstrated that a RE program was efficient in producing a better quality of life, with a consequent reduction of pain symptoms in patients with chronic low back pain.7
Although the analgesic effects induced by RE are well documented, the underlying mechanisms are not well understood. Recent studies published by our group demonstrated that endogenous opiates, nitric oxide and norepinephrine are involved in this effect,8–10 but other endogenous antinociceptive mediators may also participate. Among these, endocannabinoids have received great attention, and several studies have demonstrated their importance in the control of pain.11,12 Furthermore, studies have suggested that the endocannabinoid and endorphin systems are “linked” to promote synergistically several physiological effects.13
The endocannabinoid system is formed by cannabinoid receptors of type 1 (CB1) and type 2 (CB2); endogenous receptor ligands, such as anandamide (AEA) and 2 arachidonoylglycerol (2-AG), which are often accompanied in tissues by noncannabinoid receptor-active congeners, such as palmitoylethanolamide (PEA) and oleoylethanolamide (OEA); and endocannabinoid metabolizing enzymes, such as the enzyme fatty-acid amide hydrolase (FAAH), monoacylglycerol lipase (MGL), and a putative anandamide reuptake process.14 Importantly, some studies revealed that exercise increases endocannabinoid signaling and produces sensitization of cannabinoid receptors in mouse brain.15,16
Finally, Sparling et al17 demonstrated that acute exercise increases AEA plasma levels in humans. However, these previous works were conducted with aerobic exercise. Thus, the aim of present study was to investigate the hypothesis that the endocannabinoid system is involved in RE-induced antinociception.
Methods
Animals
The experiments were performed in accordance with the International Association for the Study of Pain (IASP) guidelines on use of laboratory animals,18 and the European Communities Council Directive of 24 November 1986 (86/609/EEC), and all experiments were approved by the Ethics Committee for Animal Experimentation of the Federal University of Minas Gerais (UFMG). The experiments were performed with male Wistar rats weighing 180–200 g obtained from UFMG Brazil. All animals were housed in individual cages under controlled light and temperature conditions, with water and rat chow ad libitum until the experiment and taken to the testing room at least 1 h before the experiments.
Drugs
The following drugs were used in this study: N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-car boxamide (AM251), a CB1 cannabinoid receptor inverse agonist; 6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)methanone (AM630), a CB2 cannabinoid receptor inverse agonist; (5Z,8Z,11Z,14Z)-5,8,11,14-eicosatetraenyl-methylester phosphonofluoridic acid (MAFP), an irreversible nonselective FAAH inhibitor; 4-[Bis(1,3-benzodioxol-5-yl)hydroxymethyl]-1-piperidinecarboxylic acid 4-nitrophenyl ester (JZL184), a potent and selective monoacylglycerol lipase inhibitor; (5Z,8Z,11Z,14Z)N(4Hydroxy2methylphenyl)5,8,11,14eicosatetraenamide (VDM11), a selective inhibitor of AEA cellular reuptake. All drugs were purchased from Tocris Bioscience (Ellisville, MO, USA). AM251, AM630 and JZL184 were dissolved in a physiological saline and dimethyl sulphoxide (20%, Sigma-Aldrich, St. Louis, MO, USA) vehicle. MAFP was dissolved in a physiological saline and ethanol (20%, Merck, New Jersey, NJ, USA) vehicle, and VDM11 was dissolved in a Tocrisolve (Tocris, Ellisville, MO, USA) vehicle. All drugs were administered in volumes of 1 mL/kg subcutaneous (s.c.), 10 μL intrathecal (i.t.) and 5 μL intracerebroventricular (i.c.v.). Each one of the substances, vehicles or diluents was tested alone and did not alter the nociceptive threshold.
Injections
Subcutaneous injection
The s.c. injections were given into the dorsal nuchal area of the rats in a volume of 1 ml/kg, 10 min before the start of RE in the exercise groups.
Intrathecal injection
The i.t. injections were given in a volume of 10 μL in the subarachnoid space between L5 and L6 using a 30 G X 1/2-inch needle and a 50 μL Hamilton syringe.19 An i.t. injection is stressful for rats and, according to IASP recommendation, requires anesthesia. Thus, before injection, rats were slightly anesthetized with isoflurane (3.5%) and recovered 5 min after the removal from the anesthesia chamber. Correct i.t. positioning of the needle tip was confirmed by a characteristic tail-flick response. Lidocaine 4% (10 μL) was administered to a group of test animals, using temporary paralysis of the hindlimbs as an end point to confirm the effectiveness of the injection technique. The i.t. injections were administered immediately before RE.
Intracerebroventricular injection
Initially, before i.c.v. injections, each rat was anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg) injected intraperitoneally, and then placed in a stereotaxic apparatus (Stoelting Co, Wood Dale, IL, USA). A 22-gauge, 12-mm stainless-steel guide cannula was inserted into the right lateral ventricle of the brain. The cannula was aimed at the following coordinates: 1.5 mm posterior to the bregma, 2.5 mm lateral to the midline, and 3.3 mm below the top of the skull.20 Animals were allowed 5-day recovery periods before experiments were initiated. The volume of the solutions to be injected into the lateral ventricle was 5 μl, and the injection was made over a period of 120 s, with the animals restrained. The i.c.v. injections also were administered immediately before RE.
Intra- dlPAG and vlPAG injection
The animals were anesthetized with ketamine (80 mg/kg) and xylazine (10 mg/kg) intraperitoneally and fixed to a stereotaxic apparatus. A stainless steel guide cannula (0.6 mm OD) was implanted unilaterally on the right side aimed at the dorsolateral periaqueductal grey (dlPAG) (coordinates: anterior-posterior (AP) = 0 from lambda, L = 1.9 mm at an angle of 16°, D = 4.0 mm) and the ventrolateral periaqueductal grey (vlPAG) (coordinates: AP = 0.7 mm, L = 1.7 mm, V = 6.1 mm) according to the atlas published by Paxinos and Watson.20 The cannula was attached to the bones with stainless steel screws and acrylic cement. An obturator inside the guide cannula to prevent obstruction was used.21 The injection was performed by gently holding the animal to remove the obturator and introduce the guide cannula (12 mm). A volume of 0.2 μL (1 μL/min) was injected in 30 s using a microsyringe (Hamilton, USA) connected to an infusion pump (Insight, Brazil) through a polyethylene catheter (PE10) that was interposed between the upper end of the dental needle and the syringe. In order to prevent reflux, the needle was left in place for 30 s after the end of each injection. Animals were allowed 5-day recovery periods before experiments were initiated. Intra-periaqueductal grey (PAG) injections were administered immediately before the RE protocol.
Resistance exercise
Acute RE was performed using a weight-lifting exercise model.22 Rats were fitted with a canvas jacket that allowed them to regulate the twisting and flexing of their torsos. They were then suspended in a standard position on their hindlimbs. An electrical stimulation (20 V, 0.3 sec duration at 3 sec intervals) was applied to the rat's tail through a surface electrode, which caused the rats to flex their legs repeatedly, which lifted the weight-arm of the exercise apparatus. Animals were exercised for 15 sets of 15 repetitions, with a 120-s rest between each set. The total resistance exercise time was 7.5 min. In each group, RE started after 2 days of adaptation (rats were fastened to the apparatus for 10 minutes per day to familiarize them with exercise, reducing the effects of stress). After measurement of the maximum weight lifted (1 repetition maximum; 1RM), the exercise load was set at 70% of 1RM.
The groups were divided into the following groups (n = 6 animals per group): control (Co): animals that did not perform exercise and received saline; acute RE (RE rats that exercised and received saline); RE+AM251: animals pretreated with CB1 receptor inverse agonist and exercised; RE+AM630: animals pretreated with CB2 receptor inverse agonist and exercised; RE+MAFP: animals pretreated with irreversible nonselective FAAH inhibitor and exercised; RE+JZL184: animals pretreated with the selective MGL inhibitor and exercised; RE+VDM11: animals pretreated with an inhibitor of AEA cellular reuptake and exercised. Each one of the substances, vehicles or diluents was tested alone and did not produce antinociception. A control group received the same numbers of electrical stimulus applied during the RE protocol, and there was no change in the nociceptive threshold (data no shown).
Nociceptive test
The mechanical nociceptive threshold was assessed by response to a paw pressure test.23 In a quiet room, 1h before the testing began rats were placed in acrylic cages (12 × 20 × 17 cm) that had wire grid floors. An analgesimeter (Ugo Basile, Comerio, Italy) with a cone-shaped paw-presser that had a rounded tip (9 mm base diameter) was used to apply a linearly increasing force to the hindpaw. The pressure intensity in grams (g) that caused an escape reaction was defined as the nociceptive threshold. A maximum intensity of 300 g was used to reduce the possibility of damage to the paws. The nociceptive threshold was measured in the right paw and determined as the average of three consecutive measurements.
Western blot analysis
A group of nonexercised animals (control, n = 5) and a group of exercised animals (n = 5) were killed by decapitation and immediately afterwards brains were homogenized in RIPA buffer and in a cocktail of protease inhibitors (Sigma-Aldrich, St. Louis, MO, USA). Two hundred mg brain tissue for each 500 μL lysis buffer was used. The samples were centrifuged at 10.000 rpm for 10 min and the amount of protein was measured using the method of Lowry et al.24 Proteins were separated by 12% SDS–PAGE and transferred onto polyvinylidene difluoride nylon membranes. The blots were probed with rabbit polyclonal antibody against CB1 cannabinoid receptors (1:200; AbCam, Cambridge, MA, USA) and then with horseradish peroxidase-conjugated secondary antibody (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and using an ECL detection kit (Amersham Pharmacia Biotech, Little Chalfont, UK). ECL films were scanned densitometrically, and the optical density of bands was quantified using Image J software, version 1.44 (http://rsb.info.nih.gov/ij/; accessed November 10, 2012).
Cannabinoid receptor and c-FOS double- immunofluorescence
To show the presence of co-localization of CB1 receptors and c-FOS in the PAG, we performed a double label immunofluorescence in sections from the dlPAG and vlPAG as previously described by Casarotto et al.25 Briefly, another group of exercised rats (n = 5) and a group of nonexercised rats (n = 5) were anesthetized two hours after RE with deep urethane anesthesia and perfused transcardially with saline followed by 4% paraformaldehyde (PFA 4%) in 0.1 M phosphate buffer (PBS, pH 7.4). The brains were removed and post-fixed over 2 h in PFA 4% and stored for at least 30 h in 30% sucrose for cryoprotection. Coronal sections (25μm) were obtained in a cryostat and were washed in PBS 0.01 M. After this, the sections were incubated in glycine 0.1M, washed again and incubated for 1h in 5% bovine serum (BSA, Sigma, USA) in PBS 0.01 M, pH 7.4 containing 0.3%Triton X-100. The sections were then incubated for 2 days at room temperature in a mixture of anti-CB1 receptor N-terminus (diluted 1:200, Santa Cruz raised in goat) to c-FOS N-terminus (diluted 1:2000, Santa Cruz raised in rabbit) diluted in PBS 0.01 M containing BSA 5% and 0.3% Triton X-100. After incubation in the primary antiserum, the tissue sections were washed in PBS and sequentially incubated for 1 hour in a mixture of Alexa 594 donkey anti-rabbit IgGs (1:1000; Molecular Probes, Eugene, OR, USA) and Alexa 488 donkey anti-goat IgGs (1:1000; Molecular Probes, Eugene, OR, USA). Slides were then rinsed in PBS and cover-slipped with Fluormount-G (Electron Microscopy Sciences, Hatfield, PA, USA). Images from sections were first obtained using an Olympus BX50 microscope through a computerized image system (Image Pro-Plus 4.0, Media Cybernetics). For a more detailed analysis of the double-stained cells, the slides were re-examined using an Olympus FV300 confocal system in an upright configuration (BX61WI microscope) and an UPlan FLN10X/0.3 objective. The immunostained slides (Alexa Fluor 488-coupled anti-CB1 cannabinoid receptors; Alexa Fluor 546-coupled anti-c-FOS) were excited by an argon laser at the wavelength of 488 nm and a He-Ne laser at 543 nm, respectively. The fluorescence emission was separated by a dichroic mirror at 570 nm and collected using band pass filters at 510–540 nm for the Alexa 488 (green channel) and at 560–600 nm for the Alexa 546 (red channel). The green and red channels were merged digitally by the Olympus Fluoview software and the images were exported in TIFF format.
Because immunofluorescence controls for nonspecific labeling, the brain sections were incubated without primary antibodies.
Analysis of immunofluorescence data
The identification method of double-stained cells was similar to that described in previous works.21,26 The number of c-FOS-like immunoreactive cells, CB1 immunoreactive cells (CB1IR) and double-stained cells were manually counted with the help of a computerized image analysis system Image J 1.42q (Wayne Rasband, National Institutes of Health, USA). An observer blind to group assignment performed the analysis. For each group one section from each animal was evaluated. All stained cells in the whole area of each brain region-of-interest were recorded. Neuroanatomical sites were identified with the help of the Paxinos and Watson's atlas.20 The AP localization from bregma of the analyzed regions was as follows: rostral portion containing the dlPAG and vlPAG was represented by level −7.4 mm from bregma.
Plasma collection
Exercised and nonexercised rats were anesthetized with ketamine and xylazine (n = 5 animals per group). Blood collection was performed by cardiac punction using a pre-heparinized needle (80 × 10 inches) and centrifuged for 10 min (2000 rpm, 313.6 G force). Plasma was collected and stored in polyethylene tubes in a −80 °C freezer for future doses of endocannabinoids. In the RE group plasma was collected immediately after exercise and after anesthesia (about 3 min after the end of exercise).
Liquid chromatography mass spectrometry analysis
Plasma was treated with 5 vol of chloroform:methanol (2:1) containing 10 pmol of d8-AEA, 2-AG, PEA and OEA and sonicated. The extract was centrifuged at 13000 × g for 16 min (4°C), the aqueous phase plus debris were collected and extracted twice again with 1 vol of chloroform. The organic phases from the three extractions were pooled and the organic solvents evaporated in a rotating evaporator. Lyophilized extracts were resuspended in chloroform:methanol (99:1, v v-1). The solutions were then purified by open bed chromatography on silica.27 Fractions eluted with chloroform:methanol (9:1, v v–1) and containing AEA, PEA, 2-AG and OEA were collected. The excess solvent was evaporated with a rotating evaporator, and aliquots were analyzed by isotope dilution-liquid chromatography/atmospheric pressure chemical ionization/mass spectrometry (MS) performed under conditions described by Marsicano et al,28 allowing the separation of 2-AG, PEA, AEA and OEA. Results are expressed as pmol per ml of plasma.
Research design
First the baseline latency (BL) of the nociceptive threshold of rats was measured in the paw pressure test. In exercised groups it was measured before beginning exercise. After 1, 15 and 30 minutes of cessation of exercise new measurements of the nociceptive threshold were made. In the control group (nonexercise rats) nociceptive threshold measurements were made at the same time intervals as in exercised animals. The drugs were always injected after measuring the nociceptive threshold and before the onset of exercise.
Statistical analysis
Values from each variable were measured before (BL) and after exercise (1, 15, 30 or 45 min) in the same animal of each group (n = 6) (Figs. 1, 2 and 3; Tables 1, 2 and 3), and these values were used for further calculations. BL data were compared with those of later time points after the end of exercise using one-way analysis of variance (ANOVA). In other groups, drugs were given 10 min (s.c.) or immediately before BL and data points were measured immediately and at 15 min intervals at the end of exercise (Figs. 1, 2 and 3; Tables 1, 2 and 3).
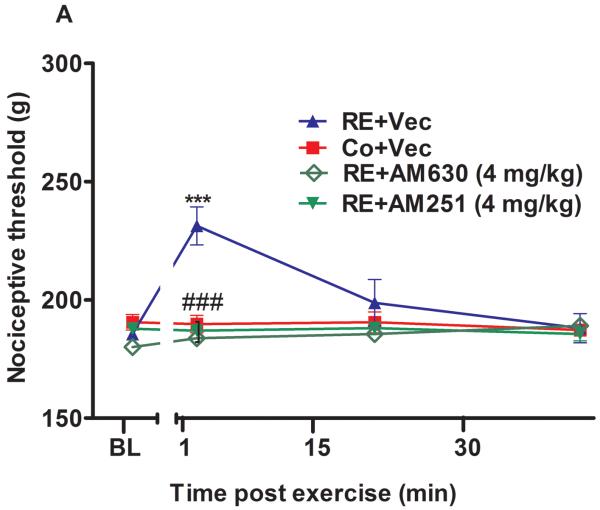
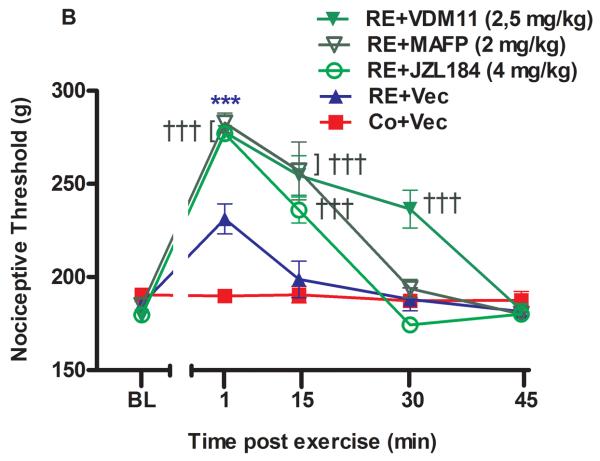
Figure 1.
The systemic involvement of cannabinoid receptors and endocannabinoids in the antinociception induced by acute resistance exercise (RE). (A) Rats were pretreated with subcutaneous injections of AM251 (4 mg/kg) or AM630 (4 mg/kg) 10 min before to onset of exercise. (B) Rats were pretreated with subcutaneous injections of VDM11 (2.5 mg/kg), methylester phosphonofluoridic acid (MAFP) (2 mg/kg) or JZL184 (4 mg/kg) 10 min before onset of exercise. Mechanical nociceptive thresholds were measured before and after 1, 15 and 30 min of RE. Data are expressed as the mean + S.E.M. of 6 animals per group. ***P < .01, indicates statistical significance compared to the control group (Co), immediately after RE (post hoc pairwise comparisons with Bonferroni correction). ###P < .01, indicates statistical significance of RE+AM251 and RE+AM630 groups compared to RE group, immediately after exercise. †††P < .01, indicates statistical significance of RE+VDM11, RE+MAFP and RE+JZL184 groups when compared to RE group, immediately, after 15 and 30 min of exercise. One-way ANOVA followed Student's t-test with equal variances. BL: pre-exercise baseline latency; Vec: vehicle.
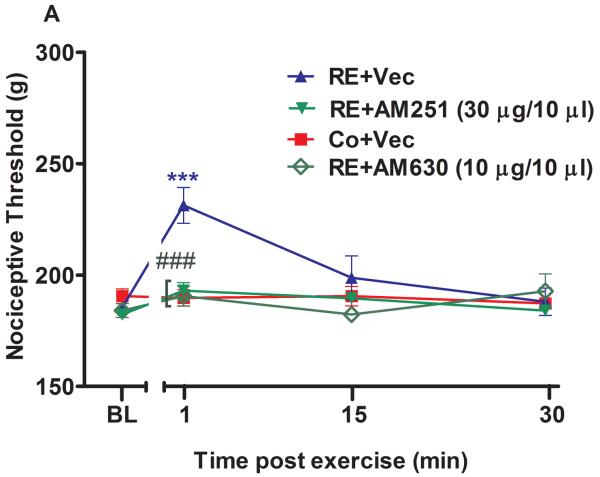
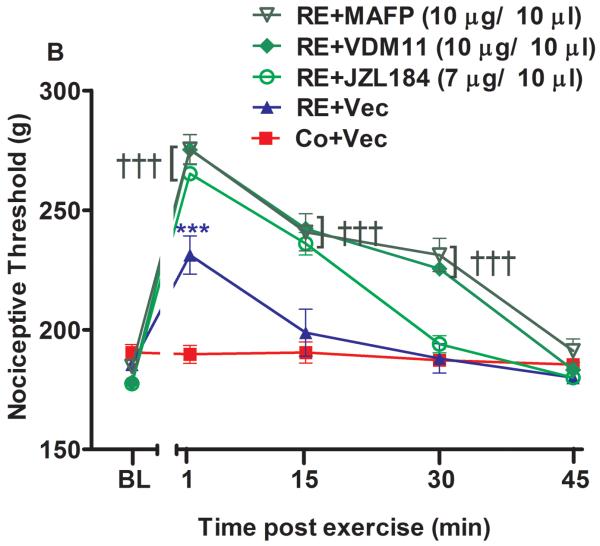
Figure 2.
The spinal involvement of cannabinoid receptors and endocannabinoids in the antinociception induced by acute resistance exercise (RE). (A) Rats were pretreated with intrathecal injections of AM251 (30 μg/10 μL) and AM630 (10 μg/10 μL) immediately before the onset of exercise. (B) Rats were pretreated with intrathecal injections of VDM11 (10 μg/10 μL), methylester phosphonofluoridic acid (MAFP) (10 μg/10 μL) or JZL184 (7 μg/10 μL) immediately before the onset of exercise. Mechanical nociceptive thresholds were measured before and after 1, 15 and 30 min of RE. Data are expressed as the mean + S.E.M. of 6 animals per group. ***P < .01, indicates statistical significance compared to the control group (Co), immediately after RE. ###P < .01, indicates statistical significance of RE+AM251 and RE+AM630 groups compared to RE group, immediately after exercise.†††P < .01, indicates statistical significance of RE+VDM11, RE+MAFP and RE+JZL184 groups compared to the RE group, immediately, 15 and 30 min after exercise. One-way ANOVA followed Student's t-test with equal variances. BL: pre-exercise baseline latency; Vec: vehicle.
Figure 3.
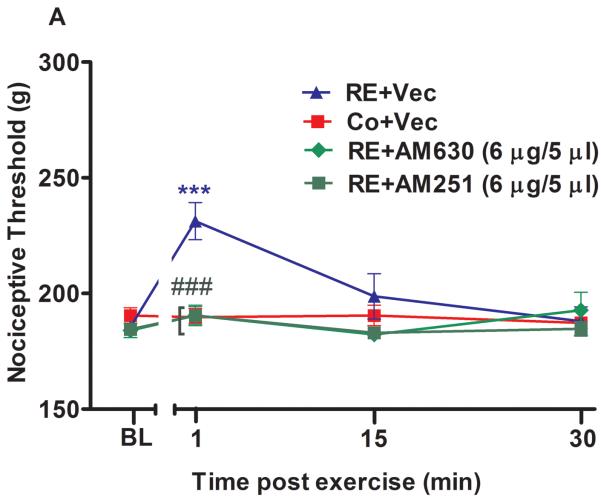
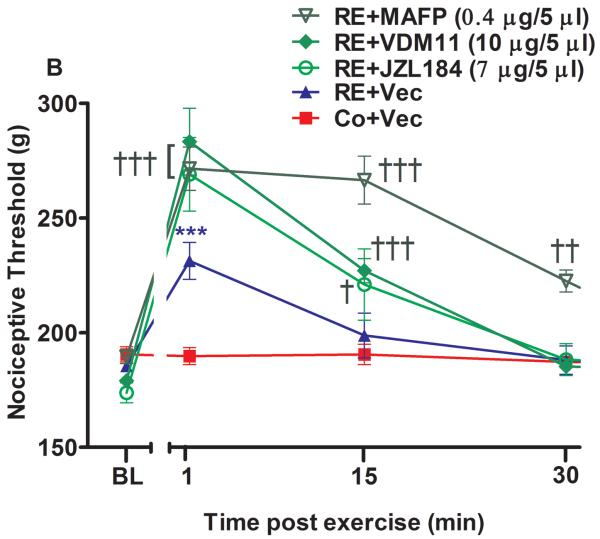
Supraspinal involvement of cannabinoid receptors and endocannabinoids in the antinociception induced by acute resistance exercise (RE). (A) Rats were pretreated with intracerebroventricular injections of AM251 (6 μg/5 μL) or AM630 (10 μg/5 μL) immediately before the onset of exercise. (B) Rats were pretreated with intracerebroventricular injections of VDM11 (10 μg/5 μL), methylester phosphonofluoridic acid (MAFP) (0.4 μg/5 μL) or JZL184 (7 μg/5 μL) immediately before the onset of exercise. Mechanical nociceptive thresholds were measured before and after 1, 15 and 30 min of RE. Data are expressed as the mean + S.E.M. of 6 animals per group. *** P < .01, indicates statistical significance compared to the control group (Co), immediately after RE. ### P < .01, indicates statistical significance of RE+AM251 and RE+AM630 groups compared to RE group, immediately after exercise † Indicates statistical significance of RE+VDM11, RE+MAFP and RE+JZL184 groups compared to RE group P < .01, immediately after, 15 and 30 min of exercise. One-way ANOVA followed Student's t-test with equal variances. BL: pre-exercise baseline latency; Vec: vehicle.
Table 1.
Mean and standard derivation of mechanical nociceptive threshold in nonexercise animals (Co+Vec), exercised animals (resistance exercise (RE)+Vec), exercised animals after subcutaneous (s.c.) pretreatment of CB1 cannabinoid receptor inverse agonist (RE+AM251), CB2 cannabinoid receptor inverse agonist (RE+AM630), selective inhibitor of anandamide cellular reuptake (RE+VDM11), irreversible nonselective fatty-acid amide hydrolase (FAAH) inhibitor (RE+methylester phosphonofluoridic acid (MAFP)) and selective monoacylglycerol lipase (MGL) inhibitor (RE+JZL184). The mean and standard derivation were compared among the different groups in each measured time of the mechanical nociceptive threshold. a, indicates without statistically significant difference when compared to Co+Vec; b, indicates statistically significant difference when compared to Co+Vec; c, indicates statistically significant difference when compared to RE+Vec. BL: baseline latency. Same letters in columns indicate no statistically significant difference in the Student's test protected by Bonferroni post hoc.
| Mechanical Nociceptive Threshold (g) | ||||
|---|---|---|---|---|
| Groups | Time (min) | |||
| Subcutaneous injection | BL | 1 | 15 | 30 |
| Co+Vec | 185.5±4.1a | 189.7±7.4a | 190.5±8.8a | 187.25±3.4a |
| RE+Vec | 188.0±2.3ab | 231.2±6.0b | 198.7±19.6ab | 188.0±12.3ab |
| RE+AM251 (4 mg/kg) | 187.5±3.5b | 187.0±4.2c | 188.0±4.3b | 184.7±5.6b |
| RE+AM630 (4 mg/kg) | 180.0±0.1b | 183.7±1.5c | 184.0±4.8b | 189.0±2.0b |
| RE+VDM11 (2.5 mg/kg) | 179.0±2.0b | 277.7±5.6c | 254.5±11.0c | 182.2±2.8b |
| RE+MAFP (2 mg/kg) | 184.7±6.1b | 282.7±9.0c | 257.0±15.0c | 193.7±9.7b |
| RE+JZL184 (4 mg/kg) | 179.6±6.5b | 277.0±2.6c | 236.0±12.1c | 174.3±5.1b |
Table 2.
Mean and standard derivation of mechanical nociceptive threshold in nonexercise animals (Co+Vec), exercised animals (resistance exercise (RE)+Vec), exercised animals after intrathecal (i.t.) pretreatment of CB1 cannabinoid receptor inverse agonist (RE+AM251), CB2 cannabinoid receptor inverse agonist (RE+AM630), selective inhibitor of anandamide cellular reuptake (RE+VDM11), irreversible nonselective fatty-acid amide hydrolase (FAAH) inhibitor (RE+methylester phosphonofluoridic acid (MAFP)) and selective monoacylglycerol lipase (MGL) inhibitor (RE+JZL184). The mean and standard derivation were compared among the different groups in each measured time of the mechanical nociceptive threshold. a, indicates without statistically significant difference when compared to Co+Vec; b, indicates statistically significant difference when compared to Co+Vec; c, indicates statistically significant difference when compared to RE+Vec. BL: baseline latency. Same letters in columns indicate no statistically significant difference in the Student's test protected by Bonferroni post hoc.
| Mechanical Nociceptive Threshold (g) | |||||
|---|---|---|---|---|---|
| Groups | Time (min) | ||||
| Intrathecal injection | BL | 1 | 15 | 30 | 45 |
| Co+Vec | 185.5±4.1a | 189.7±7.4a | 190.5±8.8a | 187.2±3.4a | 183.6±5.4a |
| RE+Vec | 188.0±2.3ab | 231.2±6.0ab | 198.7±9.6ab | 188.0±6.1ab | - |
| RE+AM251 (30 μg/10 μL) | 182.2±2.8b | 188.7±9.9b | 187.2±7.1b | 188.0±4.3b | 183.3±6.3ab |
| RE+AM630 (10 μg/10 μL) | 184.0±6.1b | 190.5±8.8b | 182.2±2.8b | 192.7±15.5b | - |
| RE+VDM11 (10 μg/10 μL) | 178.0±2.3b | 275.2±7.2c | 242.2±1.5c | 225.5±3.3c | 183.2±4.7b |
| RE+MAFP (10 μg/10 μL) | 184.7±4.2b | 275.5±12.2c | 240.7±7.7c | 229.7±6.9c | 191.2±9.9b |
| RE+JZL184(7 μg/10 μL) | 177.5±5.0b | 265.2±1.5c | 236.0±9.4c | 194.0±7.1b | - |
Table 3.
Mean and standard derivation of mechanical nociceptive threshold in nonexercise animals (Co+Vec), exercised animals (resistance exercise (RE)+Vec), exercised animals after intracerebroventricular (i.c.v.) pre-treatment of CB1 cannabinoid receptor inverse agonist (RE+AM251), CB2 cannabinoid receptor inverse agonist (RE+AM630), selective inhibitor of anandamide cellular reuptake (RE+VDM11), irreversible nonselective fatty-acid amide hydrolase (FAAH) inhibitor (RE+methylester phosphonofluoridic acid (MAFP)) and selective monoacylglycerol lipase (MGL) inhibitor (RE+JZL184). The mean and standard derivation were compared between the different groups in each measured time of the mechanical nociceptive threshold. a, indicates without statistically significant difference when compared to Co+Vec; b, indicates statistically significant difference when compared to Co+Vec; c, indicates statistically significant difference when compared to RE+Vec. BL: baseline latency. Same letters in columns indicate no statistically significant difference in the Student's test protected by Bonferroni post hoc.
| Mechanical Nociceptive Threshold (g) | |||||
|---|---|---|---|---|---|
| Groups | Time (min) | ||||
| Intracerebroventricular injection | BL | 1 | 15 | 30 | 45 |
| Co+Vec | 185.5±4.1a | 189.7±7.4a | 190.5±8.8a | 187.2±3.4a | 183.5±2.6a |
| RE+Vec | 188.0±2.3ab | 231.2±6.0ab | 198.7±9.6ab | 188.0±6.6ab | 178.6±3.2ab |
| RE+AM251 (6 μg/ 5 μL) | 182.2±2.8b | 188.7±9.9b | 187.2±7.1b | 188.0±4.3b | - |
| RE+AM630 (6 μg/ 5 μL) | 184.0±6.1b | 190.5±8.8b | 182.2±2.8b | 192.7±15.5b | - |
| RE+VDM11 (10 μg/ 5 μL) | 179.0±2.0b | 283.2±9.0c | 227.0±10.5c | 185.2±1.5b | - |
| RE+MAFP (0.4 μg/ 5 μL) | 189.5±6.0b | 271.5±8.5c | 266.5±10.8c | 222.5±9.5c | 179.7±2.8b |
| RE+JZL184 (6 μg/ 5 μL) | 173.7±8.6b | 269.0±12.8c | 221.0±11.0c | 188.2±13.8b | - |
All results are presented as means ± S.E.M of the evaluated parameter and were analyzed for statistical significance by ANOVA followed by Student's t-tests protected by Bonferroni post hoc. Statistical analysis was evaluated by comparison of mechanical nociceptive thresholds between groups in each time interval. In accord with aim of study, comparisons were not made between the mechanical nociceptive thresholds of the same group in each time interval. For all experiments, P < .01 was considered to represent a statistically significant difference and the data are reported as mean (99% two-tailed CI). The t test (comparisons between two groups) was used for the results obtained by Western blot analysis, immunofluorescence data and liquid chromatography mass spectrometry (n = 5) before and after exercise compared to the control group. Statistical analysis and preparation of figures were performed using GraphPad Prism Software, Version 5 (GraphPad Software, La Jolla, CA).
Results
The endocannabinoid system is involved systemically in resistance exercise-induced antinociception
The mechanical nociceptive threshold increased (P = .000, F3,20 = 7.74, CI 99%: 23.82 to 59.18, n = 6) immediately after 1 min of RE, returning to BL levels after 15 min. This effect was significantly prevented by preinjection of CB1 and CB2 cannabinoid receptor inverse agonists (AM251 4 mg/kg) (P = .000, F3,20 = 7.74, CI 99%: −61.93 to −26.57, n = 6) and AM630 (4 mg/kg) (P = .000, F3,20 = 7.74, CI 99%: −65.18 to −29.82, n = 6) respectively (Fig 1A). RE-induced antinociception was enhanced by systemic preinjection of MAFP (2 mg/kg) after 1 (P = .000, F4,25 = 15.19, CI 95%: 66.39 to 119.6, n = 6) and 15 min (P = .009, F4,25 = 15.19, CI 99%: 39.89 to 93.11) at the end of RE. The same effects were found with systemic pretreatment of JZL184 (4 mg/kg) after 1 (P = .000, F4,25 = 15.19, CI 99%: 58.51 to 116.0, n = 6) and 15 min (P = .007, F4,25 = 15.19, CI 99%: 16.76 to 74.24). Furthermore, these metabolizing enzyme inhibitors prolonged (P = .004, F4,25 = 15.19, CI 99%: 19.96 to 56.71) the antinociceptive effect from 1 min to 15 min (Fig. 1B). In addition, the AEA reuptake inhibitor VDM11 (2.5mg/kg) also significantly prolonged (P = .001, F4,25 = 15.19, CI 99%: 18.76 to 65.23, n = 6) the antinociception found after RE, for more than 30 min, and enhanced this effect after 1 (P = .000, F4,25 = 15.19, CI 99% 61.39 to 114.6), 15 (P = .009, F4,25 = 15.19, CI 99% 37.39 to 90.61) and 30 min (P = .006 F4,25 = 15.19, CI 99% 22.64 to 75.86) (Fig 1B). Each one of the substances, vehicles or diluents was also tested alone and did not produce antinociception nor did it change the performance of the exercised rats.
The endocannabinoid system is involved centrally in resistance exercise-induced antinociception
Administration of i.t. AM251 (30 μg/10 μL) (P = .001, F3,20 = 5.94, CI 99%: −58.63 to −17.87, n = 6) and AM630 (10 μg/10 μL) (P = .001, F3,20 = 5.94, CI 99%: −61.13 to −20.37, n = 6) immediately before RE significantly prevented the antinociceptive effect observed after exercise in the paw pressure test from 231.2 g (± 6.0) to 188.7 g (± 9.9) and 190.5 g (± 8.8) (Fig 2A). The preinjection of MAFP (10 μg/10 μL) enhanced the antinociception found immediately after RE from 231.2 g (± 6.0) to 275.5 g (± 12.2) (P = .001, F4,25 = 16.01, CI 99%: 24.09 to 64.41, n = 6). Furthermore, this effect was increased after 15 min from 198.7 g (± 9.6) to 240.7 g (± 7.78) (P = .004, F4,25 = 16.01, CI 99%: 21.84 to 62.16) and from 188.0 (± 6.16) g to 229.7 g (± 6.92) after (P = .000, F4,25 = 16.01, CI 99%: 23.09 to 63.41) 30 min of RE (Fig 2B). JZL184 (7 μg/10 μL) also enhanced the antinociceptive effect after 1 min of RE from 231.2 g (± 6.0) to 265.2 g (± 1.5) (P = .009, F4,25 = 16.01, CI 99%: 13.84 to 54.16, n = 6) and after 15 min from 198.7 g (± 9.6) to 236.00 g (± 9.4) (P = .009, F4,25 = 16.01, CI 99%: 17.09 to 57.41). Thus, JZL184 i.t. preinjection prolonged the duration of this effect from 1 min to 15 min (Fig 2B). In addition, VDM11 (10 μg/10 μL, i.t.) enhanced the antinociceptive effect induced by RE after 1 min from 231.2 g (± 6.0) to 275.2 g (± 7.2) (P = .001, F4,25 = 16.01, CI 99%: 23.84 to 64.16, n = 6), 15 min from 198.7 g (± 9.6) to 240.7 g (± 1.00) (P = .003, F4,25 = 16.01, CI 99%: 23.34 to 63.66), and 30 min from 188.0 g (± 6.16) to 225.5 g (± 3.3) (P = .001, F4,25 = 16.01, CI 99%: 17.34 to 57.66). This effect was also prolonged from 1 min to 30 min by VDM11 (P = .001) (Fig 2B).
When preadministered i.c.v. immediately before RE, both cannabinoid receptor inverse agonists also prevented the antinociception found after 1 min of RE. AM251 (6 μg/5 μL) prevented the increase of mechanical nociceptive threshold from 231.2 g (± 6.0) to 188.7 g (± 9.9) (P = .001, F3,20 = 6.41, CI 99%: −61.16 to −20.34, n = 6) and AM630 (10 μg/5 μL) from 231.2 g (± 8.0) to 190.5 g (± 8.8) (P = .001, F3,20 = 6.41, CI 99%: −62.61 to −21.36, n = 6) (Fig 3A). Furthermore, the antinociceptive effect produced by RE was prolonged from 1 to 30 min and enhanced after 1 min (P = .009, F4,25 = 7.48, CI 99%: 8.385 to 72.12, n = 6) from 231.2 g (± 6.0) to 271.5 g (± 8.5), 15 min (P = .004, F4,25 = 7.48, CI 99%: 35.88 to 99.62) from 198.7 g (± 9.6) to 266.5 g (± 10.8) and 30 min (P = .004, F4,25 = 7.48, CI 95%: 2.635 to 66.37) from 188.00 g (± 6.1) to 222.5 g (± 9.5) by i.c.v. preinjection of MAFP (0.4 μg/5 μL) (Fig 3B). Similar results were found by i.c.v. preinjection of JZL184 (7 μg/5 μL), which enhanced (P = .005, F4,25 = 7.48, CI 95%: 5.885 to 69.62, n = 6) the antinociceptive effect observed after the first minute of RE, from 231.25 g (± 6.0) to 269.00 g (± 12.8) (Fig 3B). An increase of this effect was also found after 1 min (P = .006, F4,25 = 7.48, CI 99%: 20.13 to 83.87, n = 6) from 231.2 g (± 6.0) to 283.2 g (± 9.0) by VDM11 (10 μg/5 μL, i.c.v.) (Fig 3B).
Tables 1, 2 and 3 demonstrate a brief review of the comparison of mechanical nociceptive threshold among the groups in each time interval.
Resistance exercise induces an increase of CB1 cannabinoid receptor expression in brain of rats
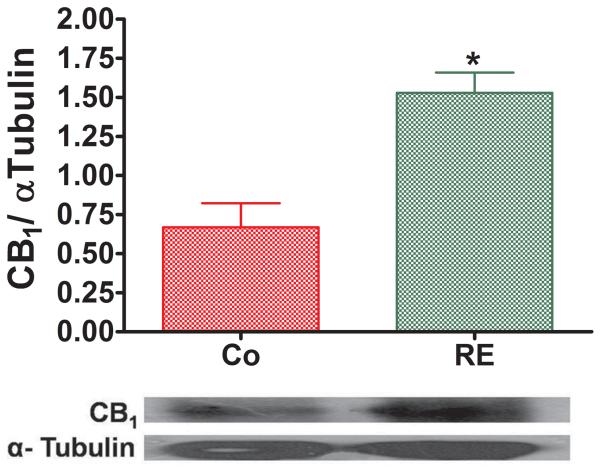
To confirm the hypothesis of the involvement of cannabinoid receptors in RE-induced antinociception, we used Western blot analysis. The results obtained after RE were compared with those obtained from nonexercised animals (control group) using an unpaired t test. Immediately after RE, a significant increase from 0.66 (± 0.15) to 1.53 (± 0.12) (P = .003, CI 99%: −1.42 to − 0.30, n = 5) of CB1 receptor expression in rat brain was observed as compared to the control group (nonexercised) (Fig 4).
Figure 4.
Resistance exercise (RE) increases CB1 cannabinoid receptor expression in the rat brain. Immediately after exercise or control conditions, brains samples were stained with antibodies against CB1, transblotted and analyzed by ImageJ (A). Representative immunoblots for CB1 cannabinoid receptor expression with corresponding α-Tubulin controls are shown (B). Data expressed as the mean + SE.M. of 5 animals per group.*Indicates statistical significance compared to control group (Co, rats nonexercised). P < .01, t test.
Resistance exercise increases endocannabinoid plasma levels
Figure 5 shows that AEA and 2-AG as well as the AEA congeners, PEA and OEA, were significantly increased in plasma immediately after RE compared to the control group (nonexercised animals). The following increases were found: AEA from 1.35 pmol/mL (± 0.10) to 2.07 pmol/mL (± 0.09) (P = .001, CI 99%: −1.600 to −0.1753, n = 5) (Fig 5 A); 2-AG from 14.64 pmol/mL (± 0.46) to 28.97 pmol/mL (± 4.03) (P = .009, CI 99%: −27.37 to −1.603, n = 5) (Fig 5 B); PEA from 13.36 pmol/mL (± 0.43) to 17.42 pmol/mL (± 0.10) (P = .005, CI 99%: −5.173 to −3.207, n = 5) (Fig 5C) and OEA from 20.18 pmol/mL (± 1.90) to 27.95 pmol/mL (± 1.83) (P = .004, CI 99%: −14.36 to −2.214, n = 5) (Fig 5D).
Figure 5.
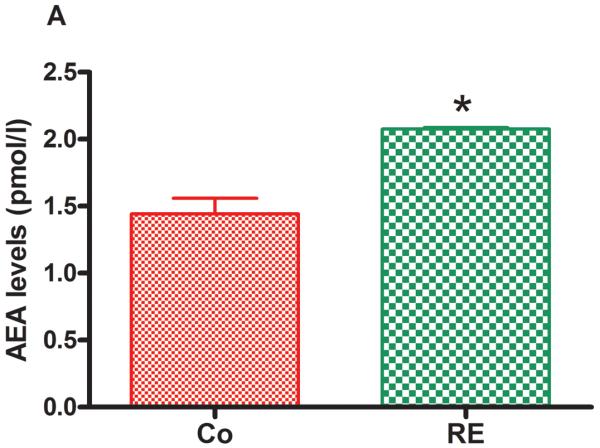
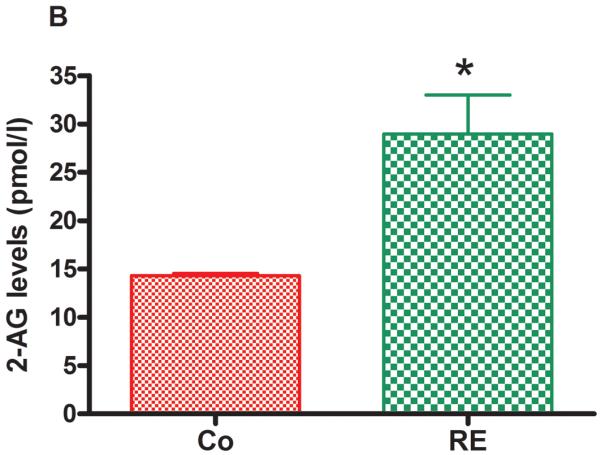
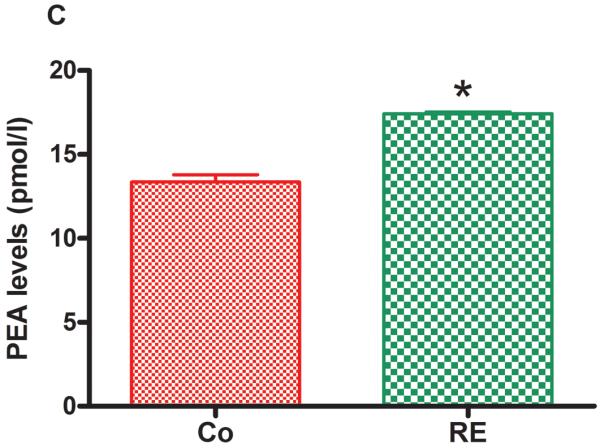
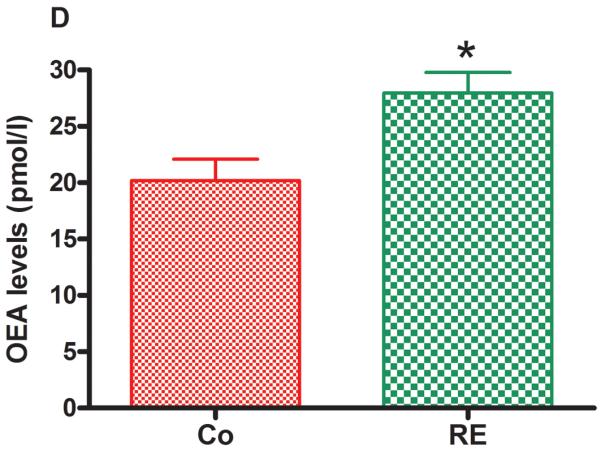
Resistance exercise produces an equivalent increase of AEA, anandamide (A); 2-AG, 2-arachidonoylglycerol (B); PEA, palmitoylethanolamide (C) and OEA, oleoylethanolamide (D) levels in rat plasma. Data are expressed as the mean + SE.M. of 5 animals per group. *Indicates statistical significance compared to control group (Co, rats non-exercised). P < .01, t test.
Resistance exercise activates CB1 cannabinoid receptors in PAG
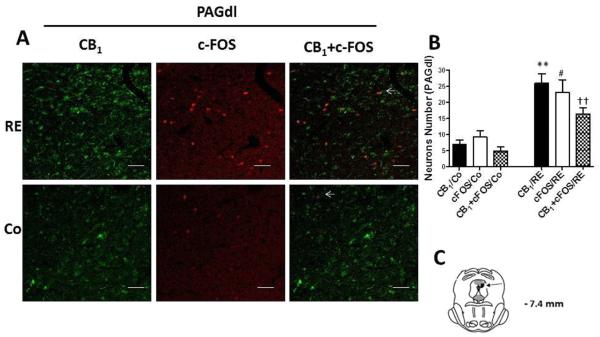
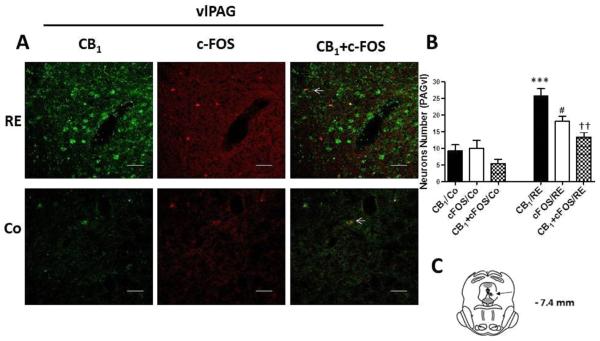
We evaluated the expression, activation and localization of CB1 cannabinoid receptors within the dlPAG and vlPAG regions (important regions in the modulation of pain) by double imunofluorescence staining with c-FOS (a marker of neuronal activity, red) and CB1 (green). After RE the expression of CB1 cannabinoid receptors in neurons of the PAG regions was significantly increased from 7.00 (± 1.22) to 26.00 (± 2.91) (P = .000, CI 99%: −27.13 to −10.87, n = 5) in the dlPAG and from 9.20 (± 1.82) to 25.80 (± 2.08) (P = .000, CI 99%: −23.15 to −10.05, n = 5) in the vlPAG compared to the control group (animals nonexercised) (Fig 6 and 7). Furthermore, c-FOS showed that more neurons were activated after RE within the PAG. This increase was from 9.20 (± 1.96) to 23.00 (± 3.96) (P = .006, CI 99%: −25.16 to −2.435, n = 5) in the dlPAG and from 10.00 (± 2.42) to 18.20 (± 1.42) (P = .004, CI 99%: −14.70 to −1.702, n = 5) in the vlPAG (Fig 6 and 7). Double immunofluorescence for CB1 and c-FOS also demonstrated that CB1 cannabinoid receptors were activated, upregulated and colocalized with c-FOS (arrowheads) after RE in the dlPAG from 4.80 (± 1.31) to 16.40 (± 1.77) (P = .003, CI 99%= −16.84 to −6.365, n = 5) and in the vlPAG from 5.40 (± 1.36) to 13.40 (± 1.47) (P = .004, CI 99% = −12.74 to −3.258, n = 5).
Figure 6.
CB1 cannabinoid receptor and c-FOS activation and co-expression in neurons of the dorsolateral periaqueductal gray region (dlPAG) after resistance exercise (RE). (A) Representative double immunofluorescence staining CB1 cannabinoid receptor (green) and c-FOS (red) in dlPAG. Scale bars, 50 μm. (B) Number of neurons in dlPAG after RE. Data are expressed as the mean + S.E.M. of 5 animals per group. *Indicates statistical significance of CB1 cannabinoid receptor activation and co-expression after RE (CB1/RE) compared to the control group (CB1/Co). #Indicates statistical significance of c-FOS activation and co-expression after RE (c-FOS/RE) compared to control group (c-FOS/Co). †Indicates statistical significance of double immunofluorescence for CB1 cannabinoid receptor and c-FOS activation and co-expression after RE (CB1+c-FOS/RE) compared to control group (CB1+c-FOS/Co). P < .01, t test. (C) Schematic representation of coronal section dlPAG (arrowheads on dark circle) of the rat brain (based on Paxinos and Watson atlas).
Figure 7.
CB1 cannabinoid receptors and c-FOS activation and co-expression in neurons of the ventrolateral periaqueductal gray region (vlPAG) after resistance exercise (RE). (A) Representative double immunofluorescence staining CB1 cannabinoid receptor (green) and c-FOS (red) in vlPAG. Scale bars, 50 μm. (B) Number of neurons in vlPAG after RE. Data are expressed as the mean + S.E.M. of 5 animals per group. *Indicates statistical significance of CB1 cannabinoid receptor activation and co-expression after RE (CB1/RE) compared to the control group (CB1/Co). #Indicates statistical significance of c-FOS activation and co-expression after RE (c-FOS/RE) compared to control group (c-FOS/Co). †Indicates statistical significance of double immunofluorescence for CB1 cannabinoid receptor and c-FOS activation and co-expression after RE (CB1+c-FOS/RE) compared to control group (CB1+c-FOS/Co). P < .01, t test. (C) Schematic representation of coronal section vlPAG (arrowheads on dark circle) of the rat brain (based on Paxinos and Watson atlas).
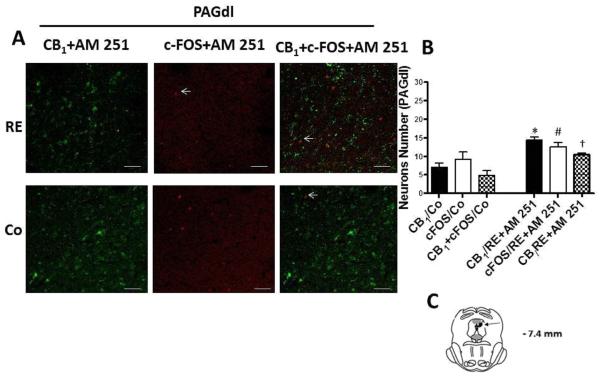
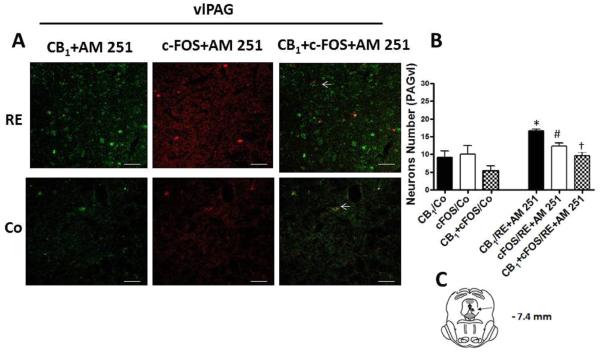
In addition, in order to confirm the increase of expression and activation of CB1 cannabinoid receptors by RE, we injected AM251 intra-vlPAG and dlPAG at a dose (100 pmol/0.2 μL) based on Moreira et al.'s study.29 The drug was administered immediately before exercise. Thus, the results showed that AM251 partially reduced CB1 expression after RE in both the dlPAG (P = .006, CI 99%= 3.198 to 20.00, n = 5) (Fig 8) and vlPAG (P = .007, CI 99%= 2.156 to 18.03, n = 5) (Fig 9) regions. Furthermore, the number of neurons activated, identified by c-FOS and the co-localization c-FOS/CB1were also partially reduced by AM251 pretreatment intra-dlPAG (P = .008, CI 99%= 2.130 to 20.67, n = 5, c-FOS; P = .007, CI 99%= 2.31 to 18.97, n = 5, c-FOS/CB1) and vlPAG (P = .007, CI 99% = 2.61 to 19.54, n = 5, c-FOS; P = .009, CI 99%= 2.09 to 19.97, n = 5, c-FOS/CB1) and the AM251 partially reduced, increased after RE, (Fig 8 and 9).
Figure 8.
Effects of the CB1 receptor inverse agonist AM251 (100 pmol) in the activation and co-expression of CB1 cannabinoid receptors (CB1/RE+AM 251) and c-FOS (c-FOS /RE+AM 251) in neurons of the dorsolateral periaqueductal gray region (dlPAG) after resistance exercise (RE). (A) Representative double immunofluorescence staining CB1 cannabinoid receptor (green) and c-FOS (red) in dlPAG. Scale bars, 50 μm. (B) Number of neurons in dlPAG after RE. Data are expressed as the mean + S.E.M. of 5 animals per group. *Indicates statistical significance of CB1 cannabinoid receptor activation and co-expression after RE followed by pretreatment with AM 251 (CB1/RE+AM251) compared to the control group (CB1/Co). P < .01, t test. (C) Schematic representation of coronal section dlPAG (arrowheads on dark circle) of the rat brain (based on Paxinos and Watson atlas).
Figure 9.
Effects of the CB1 receptor inverse agonist AM251 (100 pmol) in the activation and co-expression of CB1 cannabinoid receptors (CB1/RE+AM 251) and c-FOS (c-FOS /RE+AM 251) in neurons of the ventrolateral periaqueductal gray region (vlPAG) after resistance exercise (RE). (A) Representative double immunofluorescence staining CB1 cannabinoid receptor (green) and c-FOS (red) in vlPAG. Scale bars, 50 μm. (B) Number of neurons in vlPAG after RE. Data are expressed as the mean + S.E.M. of 5 animals per group. *Indicates statistical significance of CB1 cannabinoid receptor activation and co-expression after RE followed by pretreatment with AM251 (CB1/RE+AM 251) compared to the control group (CB1/Co). P < .01, t test. (C) Schematic representation of coronal section vlPAG (arrowheads on dark circle) of the rat brain (based on Paxinos and Watson atlas).
These results indicate that cannabinoid CB1 receptor expression within the dlPAG and vlPAG is increased by RE and that the neurons that express these receptors are activated, supporting the hypothesis that CB1 receptors participate in the antinociception induced by RE.
Discussion
We have confirmed that a single trial of RE produces antinociception. Focht and Koltyn also found antinociception 1 minute after RE at 75% in humans.30 Most importantly, we observed that the antinociceptive effect induced by acute RE in our study was prevented by AM251 and AM630, preinjected either systemically (s.c.) or centrally (i.t., i.c.v.), suggesting the participation of CB1 and CB2 cannabinoid receptors in this response. Both receptors, in fact, have been demonstrated to be widely involved in the modulation of nociception.31 In addition, some studies found that both cannabinoid receptor types participate in peripheral antinociception in rats.11,32 At the central level, studies verified that microinjection of AM251 reverses the suppressive effect of WIN55,212-2 (a cannabinoid agonist) on the spontaneous firing of spinal neurons evoked by neuropathic pain and the antinociception induced by epilepsy in different brain sites that participate in the descending control of pain in rats.33,34 However, at the central level, the involvement of CB2 cannabinoid receptors in pain control is not as well documented as that of CB1 receptors.35,36 Although CB2 receptors are found in immune cells,37 many studies have suggested their presence in the spinal cord and in some areas of the brain.38,39 Moreover, one study demonstrated that i.t. injection of JWH015, a CB2 cannabinoid receptor agonist, reduced hyperalgesia induced by bone cancer pain in mice and that this effect was prevented by i.t. preadministration of AM630.40 Others studies also confirmed the involvement of CB2 receptors in the control of nociception at the spinal level.41,42 However, at the supraspinal level only one study reported that noxious mechanically evoked responses in neurons of the ventral posterior nucleus of the thalamus,43 a site that modulates pain processing,44 was reduced in spinal nerve-ligated rats by intrathalamic administration of the selective CB2 cannabinoid receptor agonist JWH133. This effect was blocked by the CB2 cannabinoid receptor antagonist SR144528, suggesting the involvement of CB2 receptors in the control of noxious stimuli in this brain area. Thus, previous evidence supports our results that both CB1 and CB2 receptors may participate in the antinociception induced by exercise at both peripheral and central levels.
Previous studies have suggested the presence of CB2 receptors in the brain, which caused debate and controversy. Furthermore, the specificity of the antibody for this receptor has also been questioned because some could not detect the native, and in some cases, the transfected CB2 cannabinoid receptor antigen. Other problems with CB2 receptor brain expression are due to the low expression of the CB2A isoform in the brain regions and the low specificity of commercial antibodies against human hCB2 epitopes for rodent brain immunostaining. Some commercial antibodies may be interacting with a CB2B isoform, which is more strongly expressed in peripheral tissues than the CB2A isoform. For these reasons, we did not perform any immunostaining or Western blot with CB2 receptor antibodies to investigate CB2 expression or activation in the brain.
On the other hand, we demonstrated, by both Western blot and immunofluorescence analyses that after acute RE an up-regulation and, possibly, activation of CB1 cannabinoid receptors in the rat brain occurs, leading to neuronal activation within the dlPAG and vlPAG. The PAG has key functions in the descending control of pain.45 Some studies showed that electrical stimulation of dorsal and lateral PAG regions induces analgesia both in animals and humans.46,47 Moreover, cannabinoid receptor protein and mRNA are expressed in the PAG,48,49 and the activation of CB1 cannabinoid receptors in this site by endocannabinoids induces antinociception by reducing inhibitory GABAergic influences on output neurons that project from the PAG to OFF cells of the rostral ventromedial medulla.50
Additionally, the reversal of aerobic exercise-increased expression and activation of CB1 cannabinoid receptors in the dlPAG and vlPAG regions by the CB1 cannabinoid receptor inverse agonist AM251 in the present study confirmed the possible participation of these receptors in the descending control of pain activation by aerobic exercise. Indeed, functional studies have shown that the microinjection of cannabinoids into the PAG produces antinociception to thermoceptive stimuli prevented by selective cannabinoid CB1 receptor inverse agonist.45,51 The antinociceptive effect found after RE was enhanced and prolonged by inhibitors of endocannabinoid metabolizing enzymes. This finding supports the hypothesis that endocannabinoids are involved in the RE-induced antinociception. This was originally suggested by a study that found increased AEA plasma levels after aerobic exercise in humans, although in that study the nociceptive threshold was not measured.17 In addition, some studies have demonstrated that peripheral and central injection of AEA and peripheral injection of 2-AG and PEA produce antinociception in rats.52–55 Another study also found that systemic injection of OEA reduced pain produced in the writhing test in mice.56 Moreover, in support of our present results, it was reported that FAAH and MGL inhibitors, as well as AEA reuptake inhibitors, administrated peripherally or centrally, enhance endocannabinoid-mediated antinociception.57–59 These enzymes may produce antinociception via increase of endocannabinoids levels. Additionally, a study also found an increase of antinociceptive effect in AEA and 2-AG levels after administration of FAAH and MGL inhibitors.60 Furthermore, the present study demonstrated that VDM11 prolonged and enhanced the antinociceptive effect induced by RE. Although the molecular mechanism of inhibitors of AEA cellular uptake is undefined, studies have found an improvement of antinociception with VDM11. Hohmann61 demonstrated that VDM11 administered systemically enhanced stress-induced antinociception in rats.
In further support of the participation of endocannabinoids and related mediators in the antinociceptive effect induced by RE, in the present study we found increased plasma levels of AEA and 2-AG, as well as of the two AEA-related analgesic and anti-inflammatory mediators, PEA and OEA, immediately after the end of exercise. AEA has preferential affinity for CB1 receptors, whereas 2-AG exhibits affinity and functional activity for both CB1 and CB2 receptors.61 PEA and OEA do not activate cannabinoid receptors,62,63 although early studies reported that PEA may produce antinociception attenuated by the CB2 receptor antagonist SR144528, and that OEA also inhibits pain by unknown mechanisms.12,56 It has been proposed that both PEA and OEA can influence nociception by activating either peroxisome proliferator-activated receptor-α or by desensitizing transient receptor potential vanilloid-1 channels.64
Endocannabinoids are released from postsynaptic neurons to act retrogradely on presynaptic receptors and inhibit neurotransmitter release. This retrograde endocannabinoid signaling mediates two forms of short-term synaptic plasticity: depolarization-induced suppression of inhibition and depolarization-induced suppression of excitation.65 Thus, once released from postsynaptic neurons, endocannabinoids may suppress neurotransmitter release, such as gamma-aminobutyric acid and glutamate, which are involved in nociceptive transmission.
An important point of our study is that it is difficult to understand whether the antinociceptive effect was due to exercise or to electrical shock-induced stress. Early studies found stress-induced analgesia after electrical foot shock in rodents.66 Warren et al. found that electrical foot shock produced a stress-induced analgesia greater than that caused by swimming early in their experiment.67 In the present study, electrical shock applied in the control group (nonexercised animals) was the same intensity, frequency and number of electrical stimulus than in the exercised rats, and was not found to alter the mechanical nociceptive threshold.
However, physical exercise is a physiological stress, which is a defense mechanism produced by evolution. It is believed that stress-induced analgesia during exercise is the result of a phylogenetically ancient brain system whose function it is to modulate or inhibit pain from stressful stimuli. In this context, the exercise used in our study would be an aversive stimulus. However, when compared to others models, it has been suggested to be the best model of RE. Other studies have used swimming with load in rats as a RE model, which however induces greater stress.68
We suggest that during RE, an increase of actions potential derived from proprioceptors and nociceptors of muscle in contraction may occur, which will drive these imputs to the central nervous system with a consequent indirect increase of glutamatergic neurotransmission induced by endocannabinoids in the PAG, leading to enhancement of the descending control of pain.
The present study demonstrated that a single session of RE increased the mechanical nociceptive threshold of rats. Although this is a short-lasting effect, chronic training might be more efficient for the treatment of some types of chronic pain. However, some studies have found a reduction of exercise-induced analgesia after chronic training,69 which, based on our present results, might be interpreted with cannabinoid receptor down-regulation after chronic stimulation with increased endocannabinoid levels. On the other hand, the antinociceptive effect produced after a single session of exercise may be important to control acute pain during rehabilitation programs and may lead to feelings of effortlessness associated with the strict definition of the runner's high, thereby improving exercise performance by allowing individuals to run longer distances.70
In conclusion, the present study indicates that the endocannabinoid system in part mediates the antinociception induced by RE. Cannabinoid receptors have been found in key tissues that produce important responses during exercise, such as bronchodilatation, vasodilatation and euphoria.17 Moreover, studies have demonstrated the involvement of the endocannabinoid system in movement control,12,39 aerobic capacity and thermoregulation,66,67 supporting the hypothesis that exercise may activate it. Thus, the present findings open the way to further investigations of this system in exercise-induced therapies, whereas a single session of RE can provide pain relief and be useful for treatments of individuals or athletes undergoing rehabilitation programs. Furthermore, the present results warrant future investigations on the mechanisms responsible for reduction of pain during exercise in both athletes and nonathletes.
Acknowledgments
Funding: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerias (FAPEMIG), Brazil, and by the NIH, National Institute of Drug Abuse (DA-009789 to VD).
Footnotes
The authors declare no conflicts of interest.
DISCLOSURES:Name: Giovane Galdino, PhD
Contribution: This author helped with substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; final approval of the version to be published
Attestation: Dr. Giovane Galdino approved the final manuscript and is the archival author
Name: Thiago Romero, PhD
Contribution: This author helped with substantial contributions to conception and design, drafting the article or revising it critically for important intellectual content
Attestation: Dr. Thiago Romero approved the final manuscript
Name: José Felippe Pinho da Silva, PhD
Contribution: This author helped with substantial contributions to conception and design, drafting the article or revising it critically for important intellectual content
Attestation: Dr. José Felippe approved the final manuscript
Name: Daniele Aguiar, PhD
Contribution: This author helped with substantial contributions to conception and design, drafting the article or revising it critically for important intellectual content
Attestation: Dra. Daniele Aguiar approved the final manuscript
Name: Ana Maria de Paula, PhD
Contribution: This author helped with substantial contributions to conception and design, drafting the article or revising it critically for important intellectual content
Attestation: Dra. Ana Maria approved the final manuscript
Name: Jader cruz, PhD
Contribution: This author helped with substantial contributions to conception and design, drafting the article or revising it critically for important intellectual content
Attestation: Dr. Jader Cruz approved the final manuscript
Name: Cosimo Parrella, PhD
Contribution: This author helped with substantial contributions to conception and design, drafting the article or revising it critically for important intellectual content
Attestation: Dr. Cosimo Parrella approved the final manuscript
Name: Fabiana Piscitelli, PhD
Contribution: This author helped with substantial contributions to conception and design, drafting the article or revising it critically for important intellectual content
Attestation: Dra. Fabiana Piscitelli approved the final manuscript
Name: Igor Duarte, PhD
Contribution: This author helped with substantial contributions to conception and design, drafting the article or revising it critically for important intellectual content; final approval of the version to be submitted.
Attestation: Dr. Igor Duarte approved the final manuscript
Name: Vincenzo Di Marzo, PhD
Contribution: This author helped with substantial contributions to conception and design, drafting the article or revising it critically for important intellectual content; final approval of the version to be submitted.
Attestation: Dr. Vincenzo Di Marzo approved the final manuscript and attests to the integrity of the original data and the analysis reported in this manuscript
Name: Andrea Perez, PhD
Contribution: This author helped with substantial contributions to conception and design, drafting the article or revising it critically for important intellectual content; final approval of the version to be submitted.
Attestation: Dra. Andrea Perez approved the final manuscript and attests to the integrity of the original data and the analysis reported in this manuscript
References
- 1.Soyannwo OA. Obstacles to Pain Management. In: Kopf A, Patel NB, editors. Guide to Pain Management in Low-Resource Settings. IASP Press; Seatle: 2010. pp. 9–11. [Google Scholar]
- 2.Latham N, Liu CJ. Strength training in older adults: the benefits for osteoarthritis. Clin Geriatr Med. 2010;26:445–459. doi: 10.1016/j.cger.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jansen MJ, Viechtbauer W, Lenssen AF, Hendriks EJ, de Bie RA. Strength training alone, exercise therapy alone, and exercise therapy with passive manual mobilisation each reduce pain and disability in people with knee osteoarthritis: a systematic review. J Physiother. 2011;57:11–20. doi: 10.1016/S1836-9553(11)70002-9. [DOI] [PubMed] [Google Scholar]
- 4.Strasser B, Leeb G, Strehblow C, Schobersberger W, Haber P, Cauza E. The effects of strength and endurance training in patients with rheumatoid arthritis. Clin Rheumatol. 2011;30:623–632. doi: 10.1007/s10067-010-1584-2. [DOI] [PubMed] [Google Scholar]
- 5.Ayan C, Alvarez MJ, Alonso-Cortes B, Barrientos MJ, Valencia M, Martin V. Health education home-based program in females with fibromyalgia: a pilot study. J Back Musculoskelet Rehabil. 2009;22:99–105. doi: 10.3233/BMR-2009-0222. [DOI] [PubMed] [Google Scholar]
- 6.Raman J, Macdermid JC, Grewal R. Effectiveness of Different Methods of Resistance Exercises in Lateral Epicondylosis-A Systematic Review. J Hand Ther. 2012;25:2–25. doi: 10.1016/j.jht.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Harts CC, Helmhout PH, de Bie RA, Staal JB. A high-intensity lumbar extensor strengthening program is little better than a low-intensity program or a waiting list control group for chronic low back pain: a randomised clinical trial. Aust J Physiother. 2008;54:23–31. doi: 10.1016/s0004-9514(08)70062-x. [DOI] [PubMed] [Google Scholar]
- 8.Galdino GS, Duarte ID, Perez AC. Participation of endogenous opioids in the antinociception induced by resistance exercise in rats. Braz J Med Biol Res. 2010;43:906–909. doi: 10.1590/s0100-879x2010007500086. [DOI] [PubMed] [Google Scholar]
- 9.Galdino GS, Cortes SF, Duarte ID, Perez AC. Involvement of the nitric oxide/(C)GMP/K(ATP) pathway in antinociception induced by exercise in rats. Life Sci. 2010;86:505–509. doi: 10.1016/j.lfs.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 10.de Souza GG, Duarte ID, de Castro Perez A. Differential involvement of central and peripheral α2 adrenoreceptors in the antinociception induced by aerobic and resistance exercise. Anesth Analg. 2013;116:703–711. doi: 10.1213/ANE.0b013e31827ab6e4. [DOI] [PubMed] [Google Scholar]
- 11.Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 12.Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- 13.Manzanares J, Corchero J, Romero J, Fernández-Ruiz JJ, Ramos JA, Fuentes JA. Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol Sci. 1999;20:287–94. doi: 10.1016/s0165-6147(99)01339-5. [DOI] [PubMed] [Google Scholar]
- 14.Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- 15.De Chiara V, Errico F, Musella A, Rossi S, Mataluni G, Sacchetti L, Siracusano A, Castelli M, Cavasinni F, Bernardi G, Usiello A, Centonze D. Voluntary exercise and sucrose consumption enhance cannabinoid CB1 receptor sensitivity in the striatum. Neuropsychopharmacology. 2010;35:374–387. doi: 10.1038/npp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill MN, Titterness AK, Morrish AC, Carrier EJ, Lee TT, Gil-Mohapel J, Gorzalka BB, Hillard CJ, Christie BR. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus. 2010;20:513–523. doi: 10.1002/hipo.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sparling PB, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A. Exercise activates the endocannabinoid system. Neuroreport. 2003;14:2209–2211. doi: 10.1097/00001756-200312020-00015. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 19.Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed Academic Press; Toronto: 1986. [Google Scholar]
- 21.Aguiar DC, Guimaraes FS. Blockade of NMDA receptors and nitric oxide synthesis in the dorsolateral periaqueductal gray attenuates behavioral and cellular responses of rats exposed to a live predator. J Neurosci Res. 2009;87:2418–2429. doi: 10.1002/jnr.22082. [DOI] [PubMed] [Google Scholar]
- 22.Tamaki T, Uchiyama S, Nakano S. A weight-lifting exercise model for inducing hypertrophy in the hindlimb muscles of rats. Med Sci Sports Exerc. 1992;24:881–886. [PubMed] [Google Scholar]
- 23.Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111:409–419. [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Casarotto PC, Terzian AL, Aguiar DC, Zangrossi H, Guimaraes FS, Wotjak CT, Moreira FA. Opposing Roles for Cannabinoid Receptor Type-1 (CB(1)) and Transient Receptor Potential Vanilloid Type-1 Channel (TRPV1) on the Modulation of Panic-Like Responses in Rats. Neuropsychopharmacology. 2012;37:478–486. doi: 10.1038/npp.2011.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beijamini V, Guimaraes FS. c-Fos expression increase in NADPH-diaphorase positive neurons after exposure to a live cat. Behav Brain Res. 2006;170:52–61. doi: 10.1016/j.bbr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 27.Bisogno T, Maurelli S, Melck D, De Petrocellis L, Di Marzo V. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J Biol Chem. 1997;272:3315–3323. doi: 10.1074/jbc.272.6.3315. [DOI] [PubMed] [Google Scholar]
- 28.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 29.Moreira FA, Aguiar DC, Guimarães FS. Anxiolytic-like effect of cannabidiol in the rat Vogel conflict test. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:1466–1471. doi: 10.1016/j.pnpbp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Focht BC, Koltyn KF. Alterations in pain perception after resistance exercise performed in the morning and evening. JStrength Cond Res. 2009;23:891–897. doi: 10.1519/JSC.0b013e3181a05564. [DOI] [PubMed] [Google Scholar]
- 31.Hohmann AG, Suplita RL. Endocannabinoid mechanisms of pain modulation. AAPS J. 2006;8:E693–708. doi: 10.1208/aapsj080479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutierrez T, Farthing JN, Zvonok AM, Makriyannis A, Hohmann AG. Activation of peripheral cannabinoid CB1 and CB2 receptors suppresses the maintenance of inflammatory nociception: a comparative analysis. Br J Pharmacol. 2007;150:153–163. doi: 10.1038/sj.bjp.0706984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu C, Walker JM. Effects of a cannabinoid agonist on spinal nociceptive neurons in a rodent model of neuropathic pain. J Neurophysiol. 2006;96:2984–2994. doi: 10.1152/jn.00498.2006. [DOI] [PubMed] [Google Scholar]
- 34.Samineni VK, Premkumar LS, Faingold CL. Post-ictal analgesia in genetically epilepsy-prone rats is induced by audiogenic seizures and involves cannabinoid receptors in the periaqueductal gray. Brain Res. 2011;1389:177–182. doi: 10.1016/j.brainres.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohammadi-Farani A, Sahebgharani M, Sepehrizadeh Z, Jaberi E, Ghazi-Khansari M. Diabetic thermal hyperalgesia: role of TRPV1 and CB1 receptors of periaqueductal gray. Brain Res. 2010;1328:49–56. doi: 10.1016/j.brainres.2010.02.077. [DOI] [PubMed] [Google Scholar]
- 36.Hama A, Sagen J. Activation of spinal and supraspinal cannabinoid-1 receptors leads to antinociception in a rat model of neuropathic spinal cord injury pain. Brain Res. 2011;1412:44–54. doi: 10.1016/j.brainres.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Marzo V. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol. 2008;160:1–24. doi: 10.1007/112_0505. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O'Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- 39.Onaivi ES, Ishiguro H, Gu S, Liu QR. CNS effects of CB2 cannabinoid receptors: beyond neuro-immuno-cannabinoid activity. J Psychopharmacol. 2012;26:92–103. doi: 10.1177/0269881111400652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu X, Mei F, Liu Y, Zhang R, Zhang J, Ma Z. Intrathecal administration of the cannabinoid 2 receptor agonist JWH015 can attenuate cancer pain and decrease mRNA expression of the 2B subunit of N-methyl-D-aspartic acid. Anesth Analg. 2011;113:405–411. doi: 10.1213/ANE.0b013e31821d1062. [DOI] [PubMed] [Google Scholar]
- 41.McGaraughty S, Chu KL, Dart MJ, Yao BB, Meyer MD. A CB(2) receptor agonist, A-836339, modulates wide dynamic range neuronal activity in neuropathic rats: contributions of spinal and peripheral CB(2) receptors. Neuroscience. 2009;158:1652–1661. doi: 10.1016/j.neuroscience.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 42.Hsieh GC, Pai M, Chandran P, Hooker BA, Zhu CZ, Salyers AK, Wensink EJ, Zhan C, Carroll WA, Dart MJ, Yao BB, Honore P, Meyer MD. Central and peripheral sites of action for CB receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in rats. Br J Pharmacol. 2011;162:428–440. doi: 10.1111/j.1476-5381.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jhaveri MD, Elmes SJ, Richardson D, Barrett DA, Kendall DA, Mason R, Chapman V. Evidence for a novel functional role of cannabinoid CB(2) receptors in the thalamus of neuropathic rats. Eur J Neurosci. 2008;27:1722–1730. doi: 10.1111/j.1460-9568.2008.06162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 45.Martin WJ, Patrick SL, Coffin PO, Tsou K, Walker JM. An examination of the central sites of action of cannabinoid-induced antinociception in the rat. Life Sci. 1995;56:2103–2109. doi: 10.1016/0024-3205(95)00195-c. [DOI] [PubMed] [Google Scholar]
- 46.Cannon JT, Prieto GJ, Lee A, Liebeskind JC. Evidence for opioid and non-opioid forms of stimulation-produced analgesia in the rat. Brain Res. 1982;243:315–321. doi: 10.1016/0006-8993(82)90255-4. [DOI] [PubMed] [Google Scholar]
- 47.Mayer DJ. Analgesia produced by electrical stimulation of the brain. Prog. Neuropsychopharmacol Biol Psychiatry. 1984;8:557–564. doi: 10.1016/0278-5846(84)90015-0. [DOI] [PubMed] [Google Scholar]
- 48.Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- 49.Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 50.Vaughan CW, Connor M, Bagley EE, Christie MJ. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol Pharmacol. 2000;57:288–295. [PubMed] [Google Scholar]
- 51.Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid induced antinociception in rats: evidence supporting periaqueductal gray involvement. J Pharmacol Exp Ther. 1996;276:585–593. [PubMed] [Google Scholar]
- 52.Smith PB, Compton DR, Welch SP, Razdan RK, Mechoulam R, Martin BR. The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. J Pharmacol Exp Ther. 1994;270:219–227. [PubMed] [Google Scholar]
- 53.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin GE. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 54.Martin BR, Mechoulam R, Razdan RK. Discovery and characterization of endogenous cannabinoids. Life Sci. 1999;65:573–595. doi: 10.1016/s0024-3205(99)00281-7. [DOI] [PubMed] [Google Scholar]
- 55.Raffa RB, Stone DJ, Jr, Hipp SJ. Differential cholera-toxin sensitivity of supraspinal antinociception induced by the cannabinoid agonists delta9-THC, WIN 55,212-2 and anandamide in mice. Neurosci Lett. 1999;263:29–32. doi: 10.1016/s0304-3940(99)00096-8. [DOI] [PubMed] [Google Scholar]
- 56.Suardiaz M, Estivill-Torrus G, Goicoechea C, Bilbao A, Rodriguez de Fonseca F. Analgesic properties of oleoylethanolamide (OEA) in visceral and inflammatory pain. Pain. 2007;133:99–110. doi: 10.1016/j.pain.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 57.de Lago E, Petrosino S, Valenti M, Morera E, Ortega-Gutierrez S, Fernandez-Ruiz J, Di Marzo V. Effect of repeated systemic administration of selective inhibitors of endocannabinoid inactivation on rat brain endocannabinoid levels. Biochem Pharmacol. 2005;70:446–452. doi: 10.1016/j.bcp.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 58.Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- 59.Suplita RL, Farthing JN, Gutierrez T, Hohmann AG. Inhibition of fatty-acid amide hydrolase enhances cannabinoid stress-induced analgesia: sites of action in the dorsolateral periaqueductal gray and rostral ventromedial medulla. Neuropharmacology. 2005;49:1201–1209. doi: 10.1016/j.neuropharm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 60.Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol Disord Drug Targets. 2009;8:403–421. doi: 10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hohmann AG. Localization of cannabinoid CB1 receptor mRNA using ribonucleotide probes: methods for double- and single-label in situ hybridization. Methods Mol Med. 2006;123:71–89. [PubMed] [Google Scholar]
- 62.Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 63.Duncan RS, Chapman KD, Koulen P. The neuroprotective properties of palmitoylethanolamine against oxidative stress in a neuronal cell line. Mol Neurodegener. 2009;4:50. doi: 10.1186/1750-1326-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khanna IK, Alexander CW. Fatty acid amide hydrolase inhibitors--progress and potential. CNS Neurol Disord Drug Targets. 2011;10:545–558. doi: 10.2174/187152711796234989. [DOI] [PubMed] [Google Scholar]
- 65.Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007;13:127–137. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- 66.Terman GW, Penner ER, Liebeskind JC. Stimulation-produced and stress-induced analgesia: cross-tolerance between opioid forms. Brain Res. 1985;360:374–378. doi: 10.1016/0006-8993(85)91258-2. [DOI] [PubMed] [Google Scholar]
- 67.Warren DA, Castro CA, Rudy JW, Maier SF. No spatial learning impairment following exposure to inescapable shock. Psychobiology. 1991;19:127–134. [Google Scholar]
- 68.Jackson HC, Kitchen I. Swim-stress-induced antinociception in young rats. Br J Pharmacol. 1989;96:617–622. doi: 10.1111/j.1476-5381.1989.tb11860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ord P, Gijsbers K. Pain thresholds and tolerances of competitive rowers and their use of spontaneous self-generated pain-coping strategies. Percept Mot Skills. 2003;97:219–222. doi: 10.2466/pms.2003.97.3f.1219. [DOI] [PubMed] [Google Scholar]
- 70.Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sports Med. 2004;38:536–541. doi: 10.1136/bjsm.2004.011718. [DOI] [PMC free article] [PubMed] [Google Scholar]