Full text
PDF



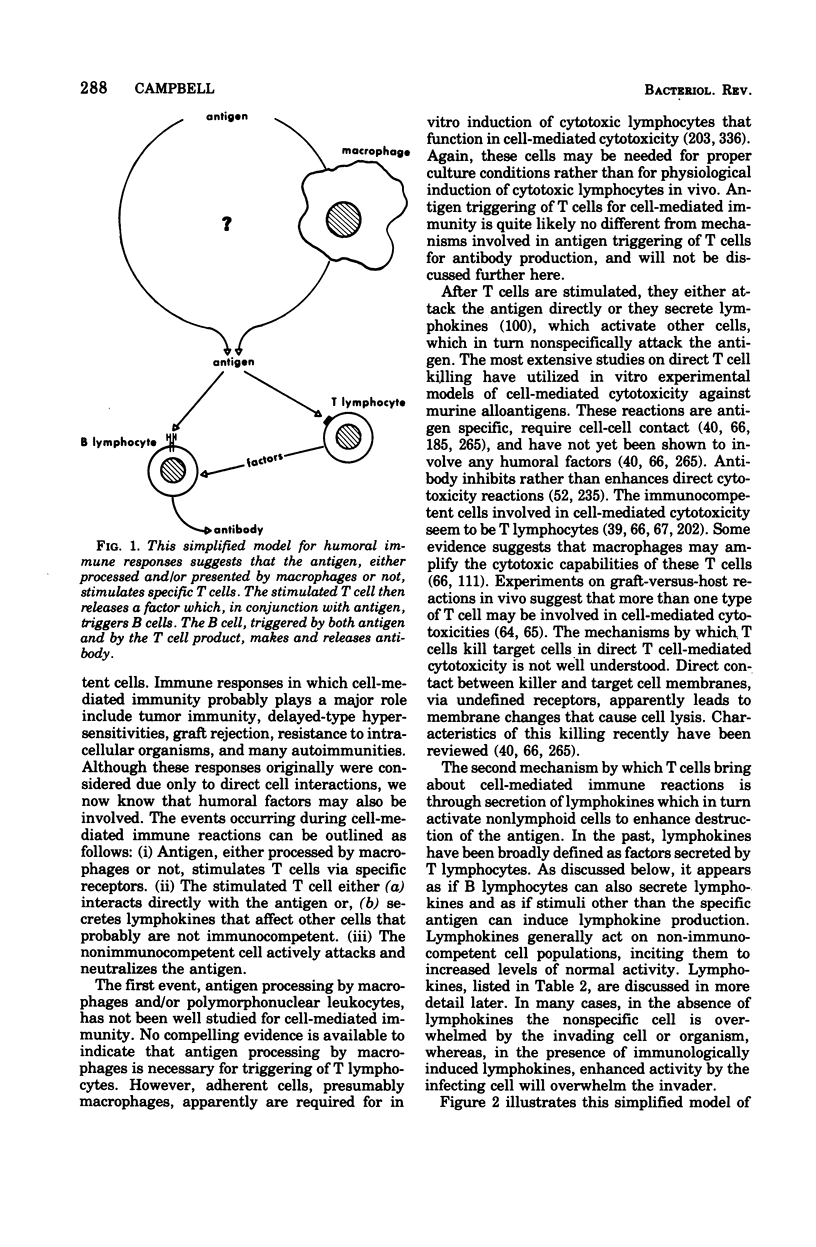
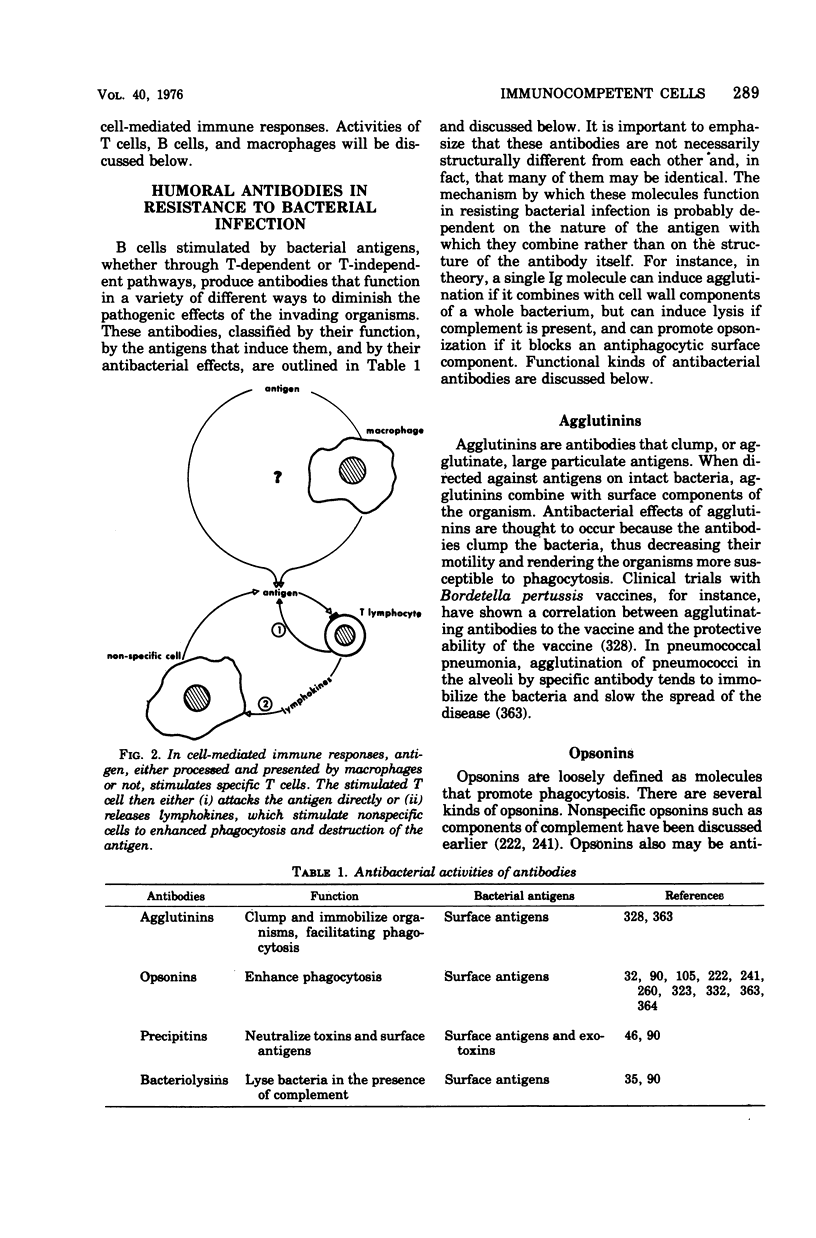
























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AL-ASKARI S., ZWEIMAN B., LAWRENCE H. S., THOMAS L. THE EFFECT OF ENDOTOXIN ON SKIN HOMOGRAFTS IN RABBITS. J Immunol. 1964 Nov;93:742–748. [PubMed] [Google Scholar]
- Ackerman A., Eidinger D. Further studies of thymus-bone marrow cell synergism in cutaneous manifestations of delayed hypersensitivity to methylated human serum albumin. The effect of cortisone acetate. Immunology. 1973 May;24(5):813–822. [PMC free article] [PubMed] [Google Scholar]
- Ada G. L., Byrt P. Specific inactivation of antigen-reactive cells with 125I-labelled antigen. Nature. 1969 Jun 28;222(5200):1291–1292. doi: 10.1038/2221291a0. [DOI] [PubMed] [Google Scholar]
- Adam A., Ciorbaru R., Petit J. F., Lederer E. Isolation and properties of a macromolecular, water-soluble, immuno-adjuvant fraction from the cell wall of Mycobacterium smegmatis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):851–854. doi: 10.1073/pnas.69.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlam C., Broughton E. S., Scott M. T. Enhanced resistance of mice to infection with bacteria following pre-treatment with Corynebacterium parvum. Nat New Biol. 1972 Feb 16;235(59):219–220. doi: 10.1038/newbio235219a0. [DOI] [PubMed] [Google Scholar]
- Alexander P., Evans R. Endotoxin and double stranded RNA render macrophages cytotoxic. Nat New Biol. 1971 Jul 21;232(29):76–78. doi: 10.1038/newbio232076a0. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Davies A. J. Requirement of thymus-dependent lymphocytes for potentiation by adjuvants of antibody formation. Nature. 1971 Oct 1;233(5318):330–332. doi: 10.1038/233330a0. [DOI] [PubMed] [Google Scholar]
- Alter B. J., Bach F. H. Lymphocyte reactivity in vitro. I. Cellular reconstitution of purified lymphocyte response. Cell Immunol. 1970 Jul;1(2):207–218. doi: 10.1016/0008-8749(70)90008-0. [DOI] [PubMed] [Google Scholar]
- Amos H. E., Lachmann P. J. The immunological specificity of a macrophage inhibition factor. Immunology. 1970 Feb;18(2):269–278. [PMC free article] [PubMed] [Google Scholar]
- Anderson C. L., Grey H. M. Receptors for aggregated IgG on mouse lymphocytes: their presence on thymocytes, thymus-derived, and bone marrow-derived lymphocytes. J Exp Med. 1974 May 1;139(5):1175–1188. doi: 10.1084/jem.139.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B., Blomgren H. Evidence for thymus-independent humoral antibody production in mice against polyvinylpyrrolidone and E. coli lipopolysaccharide. Cell Immunol. 1971 Oct;2(5):411–424. doi: 10.1016/0008-8749(71)90052-9. [DOI] [PubMed] [Google Scholar]
- Andersson J., Melchers F., Galanos C., Lüderitz O. The mitogenic effect of lipopolysaccharide on bone marrow-derived mouse lymphocytes. Lipid A as the mitogenic part of the molecule. J Exp Med. 1973 Apr 1;137(4):943–953. doi: 10.1084/jem.137.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Möller G., Sjöberg O. B lymphocytes can be stimulated by concanavalin A in the presence of humoral factors released by T cells. Eur J Immunol. 1972 Feb;2(1):99–101. doi: 10.1002/eji.1830020119. [DOI] [PubMed] [Google Scholar]
- Andersson J., Möller G., Sjöberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972 Aug;4(4):381–393. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Andersson J., Sjöberg O., Möller G. Mitogens as probes for immunocyte activation and cellular cooperation. Transplant Rev. 1972;11:131–177. doi: 10.1111/j.1600-065x.1972.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Armerding D., Katz D. H. Activation of T and B lymphocytes in vitro. I. Regulatory influence of bacterial lipopolysaccharide (LPS) on specific T-cell helper function. J Exp Med. 1974 Jan 1;139(1):24–43. doi: 10.1084/jem.139.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armerding D., Katz D. H. Activation of T and B lymphocytes in vitro. II. Biological and biochemical properties of an allogeneic effect factor (AEF) active in triggering specific B lymphocytes. J Exp Med. 1974 Jul 1;140(1):19–37. doi: 10.1084/jem.140.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975 Jul 1;142(1):1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong W. D., Diener E., Shellam G. R. Antigen-reactive cells in normal, immunized, and tolerant mice. J Exp Med. 1969 Feb 1;129(2):393–410. doi: 10.1084/jem.129.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M. Contact sensitivity in the mouse. IV. The role of lymphocytes and macrophages in passive transfer and the mechanism of their interaction. J Exp Med. 1970 Jul 1;132(1):1–15. doi: 10.1084/jem.132.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axline S. G., Mendenhall J. W., Remington J. S. Macrophage-mediated resistance to intracellular infection induced by poly-L-glutamic acid. J Immunol. 1973 Dec;111(6):1634–1638. [PubMed] [Google Scholar]
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach F. H., Alter B. J., Solliday S., Zoschke D. C., Janis M. Lymphocyte reactivity in vitro. II. Soluble reconstituting factor permitting response of purified lymphocyte. Cell Immunol. 1970 Jul;1(2):219–227. doi: 10.1016/0008-8749(70)90009-2. [DOI] [PubMed] [Google Scholar]
- Baker P. J. Homeostatic control of antibody responses: a model based on the recognition of cell-associated antibody by regulatory T cells. Transplant Rev. 1975;26:3–20. doi: 10.1111/j.1600-065x.1975.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Prescott B. Letter: The basis for conflicting results obtained in studies on the plaque-forming cell response to type III pneumococcal polysaccharide. J Immunol. 1975 Sep;115(3):891–892. [PubMed] [Google Scholar]
- Basch R. S. Immunologic competence after thymectomy. Int Arch Allergy Appl Immunol. 1966;30(2):105–119. doi: 10.1159/000229800. [DOI] [PubMed] [Google Scholar]
- Bast R. C., Jr, Cleveland R. P., Littman B. H., Zbar B., Rapp H. J. Acquired cellular immunity: extracellular killing of Listeria monocytogenes by a product of immunologically activated macrophages. Cell Immunol. 1974 Feb;10(2):248–259. doi: 10.1016/0008-8749(74)90116-6. [DOI] [PubMed] [Google Scholar]
- Basten A., Miller J. F., Abraham R. Relationship between Fc receptors, antigen-binding sites on T and B cells, and H-2 complex-associated determinants. J Exp Med. 1975 Mar 1;141(3):547–560. doi: 10.1084/jem.141.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten A., Miller J. F., Sprent J., Pye J. A receptor for antibody on B lymphocytes. I. Method of detection and functional significance. J Exp Med. 1972 Mar 1;135(3):610–626. doi: 10.1084/jem.135.3.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten A., Miller J. F., Warner N. L., Pye J. Specific inactivation of thymus-derived (T) and non-thymus-derived (B) lymphocytes by 125I-labelled antigen. Nat New Biol. 1971 May 26;231(21):104–106. doi: 10.1038/newbio231104a0. [DOI] [PubMed] [Google Scholar]
- Becker E. L., Henson P. M. In vitro studies of immunologically induced secretion of mediators from cells and related phenomena. Adv Immunol. 1973;17:93–193. doi: 10.1016/s0065-2776(08)60732-4. [DOI] [PubMed] [Google Scholar]
- Berken A., Benacerraf B. Properties of antibodies cytophilic for macrophages. J Exp Med. 1966 Jan 1;123(1):119–144. doi: 10.1084/jem.123.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthrong M. The macrophage in tuberculosis. Bibl Tuberc. 1970;26:1–27. [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen H. A., Evans R. T., Mergenhagen S. E. Lesions in Escherichia coli membranes after action of antibody and complement. J Bacteriol. 1966 Jun;91(6):2377–2381. doi: 10.1128/jb.91.6.2377-2381.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Langman R. E. Cell-mediated immunity to bacterial infection in the mouse. Thymus-derived cells as effectors of acquired resistance to Listeria monocytogenes. Scand J Immunol. 1972;1(4):379–391. doi: 10.1111/j.1365-3083.1972.tb03304.x. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. T cell response to viral and bacterial infection. Transplant Rev. 1974;19(0):56–88. doi: 10.1111/j.1600-065x.1974.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Blomgren H., Takasugi M., Friberg S., Jr Specific cytotoxicity by sensitized mouse thymus cells on tissue culture target cells. Cell Immunol. 1970 Dec;1(6):619–631. doi: 10.1016/0008-8749(70)90027-4. [DOI] [PubMed] [Google Scholar]
- Bloom B. R. Does transfer factor act specifically or as an immunologic adjuvant? N Engl J Med. 1973 Apr 26;288(17):908–909. doi: 10.1056/NEJM197304262881712. [DOI] [PubMed] [Google Scholar]
- Bloom B. R. In vitro approaches to the mechanism of cell-mediated immune reactions. Adv Immunol. 1971;13:101–208. doi: 10.1016/s0065-2776(08)60184-4. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Stoner G., Gaffney J., Shevach E., Green I. Production of migration inhibitory factor and lymphotoxin by non-T cells. Eur J Immunol. 1975 Mar;5(3):218–220. doi: 10.1002/eji.1830050314. [DOI] [PubMed] [Google Scholar]
- Bokisch V. A., Sobel A. T. Receptor for the fourth component of complement on human B lymphocytes and cultured human lymphoblastoid cells. J Exp Med. 1974 Nov 1;140(5):1336–1347. doi: 10.1084/jem.140.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges J. S., Johnson W. D., Jr Inhibition of multiplication of Toxoplasma gondii by human monocytes exposed to T-lymphocyte products. J Exp Med. 1975 Feb 1;141(2):483–496. doi: 10.1084/jem.141.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulos G. N., Rosenau W., Goldberg M. L. Comparison and yield of antigen- or mitogen-induced human lymphotoxins. J Immunol. 1974 Apr;112(4):1347–1353. [PubMed] [Google Scholar]
- Bretscher P. A., Cohn M. Minimal model for the mechanism of antibody induction and paralysis by antigen. Nature. 1968 Nov 2;220(5166):444–448. doi: 10.1038/220444a0. [DOI] [PubMed] [Google Scholar]
- Bretscher P. A. The two signal model for b cell induction. Transplant Rev. 1975;23:37–48. doi: 10.1111/j.1600-065x.1975.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Bretscher P., Cohn M. A theory of self-nonself discrimination. Science. 1970 Sep 11;169(3950):1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- Britton S. Unmasking and functional characterization of T-cell-replacing factors obtained from supernatants of allogeneic spleen cell mixtures. Scand J Immunol. 1974;3(4):433–444. doi: 10.1111/j.1365-3083.1974.tb01276.x. [DOI] [PubMed] [Google Scholar]
- Britton S. When allogeneic mouse spleen cells are mixed in vitro the T-cells secrete a product which guides the maturation of B-cells. Scand J Immunol. 1972;1(1):89–98. doi: 10.1111/j.1365-3083.1972.tb03738.x. [DOI] [PubMed] [Google Scholar]
- Bullock W. E., Fields J. P., Brandriss M. W. An evaluation of transfer factor as immunotherapy for patients with lepromatous leprosy. N Engl J Med. 1972 Nov 23;287(21):1053–1059. doi: 10.1056/NEJM197211232872101. [DOI] [PubMed] [Google Scholar]
- Bullock W. W., Katz D. H., Benacerraf B. Induction of T-lymphocyte responses to a small molecular weight antigen. II. specific tolerance induced in azebenzenearsonate (ABA)-specific T cells in Guniea pigs by administration of low doses of an ABA conjugate of chloroacetyl tyrosine in incomplete Freund's adjuvant. J Exp Med. 1975 Aug 1;142(2):261–274. doi: 10.1084/jem.142.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock W. W., Möller E. The continuing carrier problem. Transplant Rev. 1974;18:3–50. doi: 10.1111/j.1600-065x.1974.tb01586.x. [DOI] [PubMed] [Google Scholar]
- Byrt P., Ada G. L. An in vitro reaction between labelled flagellin or haemocyanin and lymphocyte-like cells from normal animals. Immunology. 1969 Oct;17(4):503–516. [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Functional and metabolic properties of polymorphonuclear leucocytes. II. The influence of a lipopolysaccharide endotoxin. J Exp Med. 1960 May 1;111:689–704. doi: 10.1084/jem.111.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A. The fate of bacteria within phagocytic cells. II. The modification of intracellular degradation. J Exp Med. 1963 Jan 1;117:43–53. doi: 10.1084/jem.117.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. A., Cooper H. R., Heinzerling R. H., Tengerdy R. P. Vitamin E enhances in vitro immune response by normal and nonadherent spleen cells. Proc Soc Exp Biol Med. 1974 Jun;146(2):465–469. doi: 10.3181/00379727-146-38127. [DOI] [PubMed] [Google Scholar]
- Campbell P. A. Heterogeneity of antibodies produced by single hemolytic foci. Cell Immunol. 1971 Jun;2(3):250–258. doi: 10.1016/0008-8749(71)90044-x. [DOI] [PubMed] [Google Scholar]
- Campbell P. A., Martens B. L., Cooper H. R., McClatchy J. K. Requirement for bone marrow-derived cells in resistance to Listeria. J Immunol. 1974 Apr;112(4):1407–1414. [PubMed] [Google Scholar]
- Campbell P. A., Rodriguez G. E., Schuffler C. Listeria cell wall fraction: adjuvant activity in vivo and in vitro. Cell Immunol. 1975 Jun;17(2):418–422. doi: 10.1016/s0008-8749(75)80045-1. [DOI] [PubMed] [Google Scholar]
- Campbell P. A., Schuffler C., Rodriguez G. E. Listeria cell wall fraction: a B cell adjuvant. J Immunol. 1976 Mar;116(3):590–594. [PubMed] [Google Scholar]
- Campbell P. A. Short communication. T cells. The limiting cells in the initiation of immune responses in normal mouse spleens. Cell Immunol. 1972 Oct;5(2):338–340. doi: 10.1016/0008-8749(72)90059-7. [DOI] [PubMed] [Google Scholar]
- Cantor H., Asofsky R. Synergy among lymphoid cells mediating the graft-versus-host response. II. Synergy in graft-versus-host reactions produced by Balb-c lymphoid cells of differing anatomic origin. J Exp Med. 1970 Feb;131(2):235–246. doi: 10.1084/jem.131.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Nordin A. A., Brunner K. T. In vitro cytotoxic activity of thymus cells sensitized to alloantigens. Nature. 1970 Jul 4;227(5253):72–73. doi: 10.1038/227072a0. [DOI] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Chen C., Hirsch J. G. The effects of mercaptoethanol and of peritoneal macrophages on the antibody-forming capacity of nonadherent mouse spleen cells in vitro. J Exp Med. 1972 Sep 1;136(3):604–617. doi: 10.1084/jem.136.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. I. Quantitative isolation of human T and B cells and response to mitogens. J Immunol. 1974 Oct;113(4):1113–1121. [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. II. Antigen triggering of T and B cells in vitro. J Immunol. 1974 Oct;113(4):1122–1127. [PubMed] [Google Scholar]
- Chiller J. M., Skidmore B. J., Morrison D. C., Weigle W. O. Relationship of the structure of bacterial lipopolysaccharides to its function in mitogenesis and adjuvanticity. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2129–2133. doi: 10.1073/pnas.70.7.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie G. H., Bomford R. Mechanisms of macrophage activation by Corynebacterium parvum. I. In vitro experiments. Cell Immunol. 1975 May;17(1):141–149. doi: 10.1016/s0008-8749(75)80014-1. [DOI] [PubMed] [Google Scholar]
- Ciorbaru R., Adam A., Petit J. F., Lederer E., Bona C., Chedid L. Isolation of mitogenic and adjuvant active fractions from various species of Nocardiae. Infect Immun. 1975 Feb;11(2):257–264. doi: 10.1128/iai.11.2.257-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A. Immunologic complementation between thymus and marrow cells--a model for the two-cell theory of immunocompetence. Transplant Rev. 1969;1:92–113. doi: 10.1111/j.1600-065x.1969.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Swett V. C. The interaction of human monocytes and lymphocytes. J Exp Med. 1968 Dec 1;128(6):1309–1325. doi: 10.1084/jem.128.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Rodriguez G. E., Kind P. D., Campbell P. A. Listeria cell wall fraction: a B cell mitogen. J Immunol. 1975 Mar;114(3):1132–1134. [PubMed] [Google Scholar]
- Collins F. M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974 Dec;38(4):371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. G., Ada G. L. Delayed-type hypersensitivity in the mouse. 3. Inactivation of thymus-derived effector cells and their precursors. Scand J Immunol. 1972;1(3):247–253. doi: 10.1111/j.1365-3083.1972.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Gronowicz E., Bullock W. W., Möller G. Mechanism of thymus-independent immunocyte triggering. Mitogenic activation of B cells results in specific immune responses. J Exp Med. 1974 Jan 1;139(1):74–92. doi: 10.1084/jem.139.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Möller G. Editorial: Immune activation of B cells: evidence for 'one nonspecific triggering signal' not delivered by the Ig receptors. Scand J Immunol. 1974;3(2):133–146. [PubMed] [Google Scholar]
- Coutinho A., Möller G. Thymus-independent B-cell induction and paralysis. Adv Immunol. 1975;21:113–236. doi: 10.1016/s0065-2776(08)60220-5. [DOI] [PubMed] [Google Scholar]
- Coutinho A. The theory of the 'one nonspecific signal' model for b cell activation. Transplant Rev. 1975;23:49–65. doi: 10.1111/j.1600-065x.1975.tb00148.x. [DOI] [PubMed] [Google Scholar]
- Crowle A. J. Delayed hypersensitivity in the mouse. Adv Immunol. 1975;20:197–264. doi: 10.1016/s0065-2776(08)60209-6. [DOI] [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEDLER R. W. Effects of cellular constituents of mycobacteria on the resistance of mice to heterologous infections I. Protective effects. J Exp Med. 1957 Nov 1;106(5):703–717. doi: 10.1084/jem.106.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUTTON R. W., EADY J. D. AN IN VITRO SYSTEM FOR THE STUDY OF THE MECHANISM OF ANTIGENIC STIMULATION IN THE SECONDARY RESPONSE. Immunology. 1964 Jan;7:40–53. [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriol Rev. 1968 Jun;32(2):85–102. doi: 10.1128/br.32.2.85-102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantstein T., Rühl H., Vogt W., Bochert G. Stimulation of B-cells by dextran sulphate in vitro. Immunology. 1973 Oct;25(4):743–747. [PMC free article] [PubMed] [Google Scholar]
- Diamantstein T., Wagner B., L'Age-Stehr J., Beyse I., Odenwald M. V., Schultz G. Stimulation of humoral antibody formation by polyanions. 3. Restoration of the immune response to sheep red blood cells by polyanions in thymectomized and lethally irradiated mice protected with bone marrow cells. Eur J Immunol. 1971 Aug;1(4):302–304. doi: 10.1002/eji.1830010418. [DOI] [PubMed] [Google Scholar]
- Dickler H. B., Kunkel H. G. Interaction of aggregated -globulin with B lymphocytes. J Exp Med. 1972 Jul 1;136(1):191–196. doi: 10.1084/jem.136.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria G., Agarossi G., Di Pietro S. Enhancing activity of thymocyte culture cell-free medium on the in vitro immune response of spleen cells from neonatally thymectomized mice to sheep RBC. J Immunol. 1972 Jan;108(1):268–270. [PubMed] [Google Scholar]
- Dresser D. W., Mitchison N. A. The mechanism of immunological paralysis. Adv Immunol. 1968;8:129–181. doi: 10.1016/s0065-2776(08)60466-6. [DOI] [PubMed] [Google Scholar]
- Dresser D. W., Wortis H. H., Anderson H. R. The effect of pertussis vaccine on the immune response of mice to sheep red blood cells. Clin Exp Immunol. 1970 Dec;7(6):817–831. [PMC free article] [PubMed] [Google Scholar]
- Dukor P., Bianco C., Nussenzweig V. Bone marrow origin of complement-receptor lymphocytes. Eur J Immunol. 1971 Dec;1(6):491–494. doi: 10.1002/eji.1830010617. [DOI] [PubMed] [Google Scholar]
- Dukor P., Hartmann K. U. Hypothesis. Bound C3 as the second signal for B-cell activation. Cell Immunol. 1973 Jun;7(3):349–356. doi: 10.1016/0008-8749(73)90199-8. [DOI] [PubMed] [Google Scholar]
- Dumonde D. C., Wolstencroft R. A., Panayi G. S., Matthew M., Morley J., Howson W. T. "Lymphokines": non-antibody mediators of cellular immunity generated by lymphocyte activation. Nature. 1969 Oct 4;224(5214):38–42. doi: 10.1038/224038a0. [DOI] [PubMed] [Google Scholar]
- Dutton R. W., Mishell R. I. Cell populations and cell proliferation in the in vitro response of normal mouse spleen to heterologous erythrocytes. Analysis by the hot pulse technique. J Exp Med. 1967 Sep 1;126(3):443–454. doi: 10.1084/jem.126.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton R. W. Separate signals for the initiation of proliferation and differentiation in the b cell response to antigen. Transplant Rev. 1975;23:66–77. doi: 10.1111/j.1600-065x.1975.tb00149.x. [DOI] [PubMed] [Google Scholar]
- ELBERG S. S., SCHNEIDER P., FONG J. Cross-immunity between Brucella melitensis and Mycobacterium tuberculosis; intracellular behavior of Brucella melitensis in monocytes from vaccinated animals. J Exp Med. 1957 Oct 1;106(4):545–554. doi: 10.1084/jem.106.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddie D. S., Schulkind M. L., Robbins J. B. The isolation and biologic activities of purified secretory IgA and IgG anti-Salmonella typhimurium "O" antibodies from rabbit intestinal fluid and colostrum. J Immunol. 1971 Jan;106(1):181–190. [PubMed] [Google Scholar]
- Eidinger D., Ackerman A. A cellular deficit in the reconstitutive capacity of immune populations of lymphoid cells demonstrable in studies of delayed hypersensitivity in mice. Evidence for thymus-bone marrow cell synergism. J Exp Med. 1971 May 1;133(5):1061–1073. doi: 10.1084/jem.133.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb P., Feldmann M. The role of macrophages in the generation of T-helper cells. I. The requirement for macrophages in helper cell induction and characteristics of the macrophage-T cell interaction. Cell Immunol. 1975 Oct;19(2):356–367. doi: 10.1016/0008-8749(75)90217-8. [DOI] [PubMed] [Google Scholar]
- Evans R., Alexander P. Cooperation of immune lymphoid cells with macrophages in tumour immunity. Nature. 1970 Nov 14;228(5272):620–622. doi: 10.1038/228620a0. [DOI] [PubMed] [Google Scholar]
- Evans R., Alexander P. Role of macrophages in tumour immunity. I. Co-operation between macrophages and lymphoid cells in syngeneic tumour immunity. Immunology. 1972 Oct;23(4):615–626. [PMC free article] [PubMed] [Google Scholar]
- Evans R., Grant C. K., Cox H., Steele K., Alexander P. Thymus-derived lymphocytes produce an immunologically specific macrophage-arming factor. J Exp Med. 1972 Nov 1;136(5):1318–1322. doi: 10.1084/jem.136.5.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLEY M. J., WOOD W. B., Jr Studies on the pathogenicity of group A streptococci. II. The antiphagocytic effects of the M protein and the capsular gel. J Exp Med. 1959 Oct 1;110:617–628. doi: 10.1084/jem.110.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FONG J., CHIN D., AKIYAMA H. J., ELBERG S. S. Studies on tubercle bacillus-monocyte relationship. III. Conditions affecting the action of serum and cells; modification of bacilli in an immune system. J Exp Med. 1959 Jun 1;109(6):523–543. doi: 10.1084/jem.109.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUND J. The mode of action of immunologic adjuvants. Bibl Tuberc. 1956;(10):130–148. [PubMed] [Google Scholar]
- Feldman J. D., Unanue E. R. Role of macrophages in delayed hypersensitivity. II. Effects of anti-macrophage antibody. Cell Immunol. 1971 Jun;2(3):275–282. doi: 10.1016/0008-8749(71)90047-5. [DOI] [PubMed] [Google Scholar]
- Feldmann M. Cell interactions in the immune response in vitro. V. Specific collaboration via complexes of antigen and thymus-derived cell immunoglobulin. J Exp Med. 1972 Oct 1;136(4):737–760. doi: 10.1084/jem.136.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Nossal G. J. Tolerance, enhancement and the regulation of interactions between T cells, B cells and macrophages. Transplant Rev. 1972;13:3–34. doi: 10.1111/j.1600-065x.1972.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Pepys M. B. Role of C3 in in vitro lymphocyte cooperation. Nature. 1974 May 10;249(453):159–161. doi: 10.1038/249159a0. [DOI] [PubMed] [Google Scholar]
- Finger H., Emmerling P. The influence of Bordetella pertussis on the kinetics of antibody production to sheep red blood cells in NMRI mice. Experientia. 1968 Aug 15;24(8):832–833. doi: 10.1007/BF02144900. [DOI] [PubMed] [Google Scholar]
- Florentin I., Bruley M., Belpomme D. Production of migration inhibition factor (MIF) by human established B type cell lines derived from normal and malignant tissues: studies of some factors affecting MIF release. Cell Immunol. 1975 May;17(1):285–294. doi: 10.1016/s0008-8749(75)80028-1. [DOI] [PubMed] [Google Scholar]
- Fowles R. E., Fajardo I. M., Leibowitch J. L., David J. R. The enhancement of macrophage bacteriostasis by products of activated lymphocytes. J Exp Med. 1973 Oct 1;138(4):952–964. doi: 10.1084/jem.138.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzl R. E., McMaster P. D. The primary immune response in mice. I. The enhancement and suppression of hemolysin production by a bacterial endotoxin. J Exp Med. 1968 Jun 1;127(6):1087–1107. doi: 10.1084/jem.127.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARD K. F., MURRAY E. G. The influence of a sustained monocytosis upon the antibody response in rabbits to various antigens. Can J Biochem Physiol. 1954 Jan;32(1):1–13. [PubMed] [Google Scholar]
- GLEDHILL A. W., REES R. J. Effect of a primary tuberculous infection on the resistance of male and female mice to Ectromelia. Nature. 1960 Aug 20;187:703–704. doi: 10.1038/187703b0. [DOI] [PubMed] [Google Scholar]
- Gallily R., Feldman M. The role of macrophages in the induction of antibody in x-irradiated animals. Immunology. 1967 Feb;12(2):197–206. [PMC free article] [PubMed] [Google Scholar]
- Gambrill M. R., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. 3. Influence of human immune serum and complement on the fate of Rickettsia mooseri within the human macrophages. Infect Immun. 1973 Oct;8(4):631–640. doi: 10.1128/iai.8.4.631-640.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gately M. K., Gately C. L., Henney C. S., Mayer M. M. Studies on lymphokines: the production of antibody to guinea pig lymphotoxin and its use to distinguish lymphotoxin from migration inhibitory factor and mitogenic factor. J Immunol. 1975 Sep;115(3):817–826. [PubMed] [Google Scholar]
- Geczy C. L., Friedrich W., de Weck A. L. Production and in vivo effect of antibodies against guinea pig lymphokines. Cell Immunol. 1975 Sep;19(1):65–77. doi: 10.1016/0008-8749(75)90292-0. [DOI] [PubMed] [Google Scholar]
- Gelfand M. C., Elfenbein G. J., Frank M. M., Paul W. E. Ontogeny of B lymphocytes. II. Relative rates of appearance of lymphocytes bearing surface immunoglobulin and complement receptors. J Exp Med. 1974 May 1;139(5):1125–1141. doi: 10.1084/jem.139.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery I., Krüger J., Spiesel S. Z. Stimulation of B-lymphocytes by endotoxin. Reactions of thymus-deprived mice and karyotypic analysis of dividing cells in mice bearing T 6 T 6 thymus grafts. J Immunol. 1972 Apr;108(4):1088–1091. [PubMed] [Google Scholar]
- Gisler R. H., Staber F., Rüde E., Dukor P. Soluble mediators of T-B interaction. Eur J Immunol. 1973 Oct;3(10):650–652. doi: 10.1002/eji.1830031012. [DOI] [PubMed] [Google Scholar]
- Godal T., Rees R. J., Lamvik J. O. Lymphocyte-mediated modification of blood-derived macrophage function in vitro; inhibition of growth of intracellular mycobacteria with lymphokines. Clin Exp Immunol. 1971 Apr;8(4):625–637. [PMC free article] [PubMed] [Google Scholar]
- Gorczynski R. M., Miller R. G., Phillips R. A. In vivo requirement for a radiation-resistant cells in the immune response to sheep erythrocytes. J Exp Med. 1971 Nov 1;134(5):1201–1221. doi: 10.1084/jem.134.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczynski R. M., Miller R. G., Phillips R. A. Initiation of antibody production to sheep erythrocytes in vitro: replacement of the requirement for T-cells with a cell-free factor isolated from cultures of lymphoid cells. J Immunol. 1972 Feb;108(2):547–551. [PubMed] [Google Scholar]
- Granger G. A., Moore G. E., White J. G., Matzinger P., Sundsmo J. S., Shupe S., Kolb W. P., Kramer J., Glade P. R. Production of lymphotoxin and migration inhibitory factor by established human lymphocytic cell lines. J Immunol. 1970 Jun;104(6):1476–1485. [PubMed] [Google Scholar]
- Greaves M. F., Bauminger S. Activation of T and B lymphocytes by insoluble phytomitogens. Nat New Biol. 1972 Jan 19;235(55):67–70. doi: 10.1038/newbio235067a0. [DOI] [PubMed] [Google Scholar]
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Rabellino E., Pirofsky B. Immunoglobulins on the surface of lymphocytes. IV. Distribution in hypogammaglobulinemia, cellular immune deficiency, and chronic lymphatic leukemia. J Clin Invest. 1971 Nov;50(11):2368–2375. doi: 10.1172/JCI106735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMPHREY J. H., PARROTT D. M., EAST J. STUDIES ON GLOBULIN AND ANTIBODY PRODUCTION IN MICE THYMECTOMIZED AT BIRTH. Immunology. 1964 Jul;7:419–439. [PMC free article] [PubMed] [Google Scholar]
- Hahn H. Effects of Dextran Sulfate 500 on Cell-Mediated Resistance to Infection with Listeria monocytogenes in Mice. Infect Immun. 1974 Nov;10(5):1105–1109. doi: 10.1128/iai.10.5.1105-1109.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H. Requirement for a bone marrow-derived component in the expression of cell-mediated antibacterial immunity. Infect Immun. 1975 May;11(5):949–954. doi: 10.1128/iai.11.5.949-954.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond M. E., Selvaggio S. S., Dvorak H. F. Antigen-enhanced glucosamine incorporation by peritoneal macrophages in cell-mediated hypersensitivity. I. Studies on biology and mechanism. J Immunol. 1975 Oct;115(4):914–921. [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann K. U. Induction of a hemolysin response in vitro. Interaction of cells of bone marrow origin and thymic origin. J Exp Med. 1970 Dec 1;132(6):1267–1278. doi: 10.1084/jem.132.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann K. U. Possible involvement of c3 during stimulation of b lymphocytes. Transplant Rev. 1975;23:98–104. doi: 10.1111/j.1600-065x.1975.tb00151.x. [DOI] [PubMed] [Google Scholar]
- Hartmann K., Dutton R. W., McCarthy M. M., Mishell R. I. Cell components in the immune response. II. Cell attachment separation of immune cells. Cell Immunol. 1970 Jul;1(2):182–189. doi: 10.1016/0008-8749(70)90005-5. [DOI] [PubMed] [Google Scholar]
- Heinzerling R. H., Tengerdy R. P., Wick L. L., Lueker D. C. Vitamin E protects mice against Diplococcus pneumoniae type I infection. Infect Immun. 1974 Dec;10(6):1292–1295. doi: 10.1128/iai.10.6.1292-1295.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise E. R., Weiser R. S. Factors in delayed sensitivity: lymphocyte and macrophage cytotoxins in the tuberculin reaction. J Immunol. 1969 Sep;103(3):570–576. [PubMed] [Google Scholar]
- Hersh E. M., Harris J. E. Macrophage-lymphocyte interaction in the antigen-induced blastogenic response of human peripheral blood leukocytes. J Immunol. 1968 Jun;100(6):1184–1194. [PubMed] [Google Scholar]
- Hilgard H. R. Synergism of thymus and bone marrow in the production of gra a5hilgard HR: Synergism of thymus and bone marrow in the production of graft-versus-host splenomegaly in x-irradiated hosts. J Exp Med. 1970 Aug 1;132(2):317–328. doi: 10.1084/jem.132.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiu I. J. Water-soluble and lipid-free fraction from BCG with adjuvant and antitumour activity. Nat New Biol. 1972 Aug 23;238(86):241–242. doi: 10.1038/newbio238241a0. [DOI] [PubMed] [Google Scholar]
- Hodes R. J., Hathcock K. S., Shearer G. M. Synergy between subpopulations of normal mouse spleen cells in the in vitro generation of cell-mediated cytotoxicity specific for "modified self" antigens. J Immunol. 1975 Oct;115(4):1122–1125. [PubMed] [Google Scholar]
- Hoffmann G. W. A theory of regulation and self-nonself discrimination in an immune network. Eur J Immunol. 1975 Sep;5(9):638–647. doi: 10.1002/eji.1830050912. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Dutton R. W. Immune response restoration with macrophage culture supernatants. Science. 1971 Jun 4;172(3987):1047–1048. doi: 10.1126/science.172.3987.1047. [DOI] [PubMed] [Google Scholar]
- Holder I. A., Sword C. P. Characterization and biological activity of the monocytosis-producing agent of Listeria monocytogenes. J Bacteriol. 1969 Feb;97(2):603–611. doi: 10.1128/jb.97.2.603-611.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat M., Havemann K., Sodomann C. P., Bürger S. Mitogenic factor and migration inhibitory factor in supernatants of serum-free human lymphocyte cultures stimulated with concanavalin A. Int Arch Allergy Appl Immunol. 1972;43(3):446–456. doi: 10.1159/000230861. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Scott M. T. Biological effects of Corynebacterium parvum. IV. Adjuvant and inhibitory activities on B lymphocytes. Cell Immunol. 1973 May;7(2):290–301. doi: 10.1016/0008-8749(73)90251-7. [DOI] [PubMed] [Google Scholar]
- Huber H., Fudenberg H. H. Receptor sites of human monocytes for IgG. Int Arch Allergy Appl Immunol. 1968;34(1):18–31. doi: 10.1159/000230091. [DOI] [PubMed] [Google Scholar]
- Huber H., Polley M. J., Linscott W. D., Fudenberg H. H., Müller-Eberhard H. J. Human monocytes: distinct receptor sites for the third component of complement and for immunoglobulin G. Science. 1968 Dec 13;162(3859):1281–1283. doi: 10.1126/science.162.3859.1281. [DOI] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D. BASIS FOR IMMUNITY TO TYPHOID IN MICE AND THE QUESTION OF "CELLULAR IMMUNITY". Bacteriol Rev. 1963 Dec;27:391–404. doi: 10.1128/br.27.4.391-404.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN C., BENACERRAF B. In vitro studies on the interaction between mouse peritoneal macrophages and strains of Salmonella and Escherichia coli. J Exp Med. 1960 Aug 1;112:403–417. doi: 10.1084/jem.112.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON A. G., GAINES S., LANDY M. Studies on the O antigen of Salmonella typhosa. V. Enhancement of antibody response to protein antigens by the purified lipopolysaccharide. J Exp Med. 1956 Feb 1;103(2):225–246. doi: 10.1084/jem.103.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D. M., Morrison D. C. Stimulation of a T-independent primary anti-hapten response in vitro by TNP-lipopolysaccharide (TNP-LPS). J Immunol. 1975 Jan;114(1 Pt 2):360–364. [PubMed] [Google Scholar]
- Jacobs M. D., Morrison D. C. Dissociation between mitogenicity and immunogenicity of TNP-lipopolysaccharide, a T-independent antigen. J Exp Med. 1975 Jun 1;141(6):1453–1458. doi: 10.1084/jem.141.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoniuk P., Borowski J. The influence of Listeria monocytogenes on the primary and secondary response in mice. Arch Immunol Ther Exp (Warsz) 1972;20(2):181–187. [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- John C., Sprătkova I., Morávková M., Patocka F., Mára M. Effect of Listeria-factor Ei on spleen cells migration in rabbits hypersensitive to Listeria monocytogenes. J Hyg Epidemiol Microbiol Immunol. 1974;18(3):369–372. [PubMed] [Google Scholar]
- Jones J. M., Kind P. D. Enhancing effect of bacterial endotoxins on bone marrow cells in the immune response to SRBC. J Immunol. 1972 May;108(5):1453–1455. [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Len L., Hirsch J. G. Assessment in vitro of immunity against Toxoplasma gondii. J Exp Med. 1975 Feb 1;141(2):466–482. doi: 10.1084/jem.141.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juy D., Chedid L. Comparison between macrophage activation and enhancement of nonspecific resistance to tumors by mycobacterial immunoadjuvants. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4105–4109. doi: 10.1073/pnas.72.10.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagnoff M. F., Billings P., Cohn M. Functional characteristics of Peyer's patch lymphoid cells. II. Lipopolysaccharide is thymus dependent. J Exp Med. 1974 Feb 1;139(2):407–413. doi: 10.1084/jem.139.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kateley J. R., Kasarov L., Friedman H. Modulation of in vivo antibody responses by cholera toxin. J Immunol. 1975 Jan;114(1 Pt 1):81–84. [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The function and interrelationships of T-cell receptors, Ir genes and other histocompatibility gene products. Transplant Rev. 1975;22:175–195. doi: 10.1111/j.1600-065x.1975.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- Keller H. U., Sorkin E. Chemotaxis of leucocytes. Experientia. 1968 Jul 15;24(7):641–652. doi: 10.1007/BF02138287. [DOI] [PubMed] [Google Scholar]
- Keller R. Major changes in lymphocyte proliferation evoked by activated macrophages. Cell Immunol. 1975 Jun;17(2):542–551. doi: 10.1016/s0008-8749(75)80058-x. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. H. Properties and activities of transfer factor. J Allergy Clin Immunol. 1975 Jun;55(6):411–421. doi: 10.1016/0091-6749(75)90080-9. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Rich R. R., Smith T. K. Effect of transfer factor on lymphocyte function in anergic patients. J Clin Invest. 1972 Nov;51(11):2948–2958. doi: 10.1172/JCI107119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T., Miyake T., Nishizawa Y., Watanabe T., Yamamura Y. Triggering mechanism of B lymphocytes. I. Effect of anti-immunoglobulin and enhancing soluble factor on differentiation and proliferation of B cells. J Immunol. 1975 Nov;115(5):1179–1184. [PubMed] [Google Scholar]
- Kong Y. C., Savage D. C., Kong L. N. Delayed dermal hypersensitivity in mice to spherule and mycelial extracts of Coccidioides immitis. J Bacteriol. 1966 Feb;91(2):876–883. doi: 10.1128/jb.91.2.876-883.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren H. S., Ax W., Freund-Moelbert E. Morphological observations on the contact-induced lysis of target cells. Eur J Immunol. 1973 Jan;3(1):32–37. doi: 10.1002/eji.1830030108. [DOI] [PubMed] [Google Scholar]
- Kotani S., Narita T., Stewart-Tull D. E., Shimono T., Watanabe Y. Immunoadjuvant activities of cell walls and their water-soluble fractions prepared from various gram-positive bacteria. Biken J. 1975 Jun;18(2):77–92. [PubMed] [Google Scholar]
- Krahenbuhl J. L., Remington J. S. In vitro induction of nonspecific resistance in macrophages by specifically sensitized lymphocytes. Infect Immun. 1971 Oct;4(4):337–343. doi: 10.1128/iai.4.4.337-343.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahenbuhl J. L., Rosenberg L. T., Remington J. S. The role of thymus-derived lymphocytes in the in vitro activation of macrophages to kill Listeria monocytogenes. J Immunol. 1973 Oct;111(4):992–995. [PubMed] [Google Scholar]
- Kubo R. T., Grey H. M., Pirofsky B. IgD: a major immunoglobulin on the surface of lymphocytes from patients with chronic lymphatic leukemia. J Immunol. 1974 May;112(5):1952–1954. [PubMed] [Google Scholar]
- Kurnick J. T., Grey H. M. Relationship between immunoglobulin-bearing lymphocytes and cells reactive with sensitized human erythrocytes. J Immunol. 1975 Jul;115(1):305–307. [PubMed] [Google Scholar]
- Kyminski J. W., Smith R. T. Evidence for a B-cell -like helper function in mixed lymphocyte culture between immunocompetent thymus cells. J Exp Med. 1975 Feb 1;141(2):360–373. doi: 10.1084/jem.141.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWRENCE H. S. The transfer in humans of delayed skin sensitivity to streptococcal M substance and to tuberculin with disrupted leucocytes. J Clin Invest. 1955 Feb;34(2):219–230. doi: 10.1172/JCI103075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Immunologic events during Listeria monocytogenes infection in mice: adjuvanticity and immunogenicity of macrophage-bound antigens. J Immunol. 1973 Mar;110(3):829–834. [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence H. S. Transfer factor. Adv Immunol. 1969;11:195–266. doi: 10.1016/s0065-2776(08)60480-0. [DOI] [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Ca++-dependent binding of antigen-19 S antibody complexes to macrophages. J Immunol. 1969 May;102(5):1172–1178. [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L., Ng H., Evans M. J., Krahenbuhl J. L. Susceptibility of thymectomized and irradiated mice to challenge with several organisms and the effect of dapsone on infection with Mycobacterium leprae. Infect Immun. 1975 May;11(5):1122–1132. doi: 10.1128/iai.11.5.1122-1132.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., Rosenthal A. S. Macrophage-lymphocyte interaction. I. Characteristics of the antigen-independent-binding of guinea pig thymocytes and lymphocytes to syngeneic macrophages. J Exp Med. 1973 Oct 1;138(4):900–924. doi: 10.1084/jem.138.4.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., Rosenthal A. S. Macrophage-lymphocyte interaction: antigen-independent binding of guinea pig lymph node lymphocytes by macrophages. J Immunol. 1975 Aug;115(2):440–445. [PubMed] [Google Scholar]
- LoBuglio A. F., Cotran R. S., Jandl J. H. Red cells coated with immunoglobulin G: binding and sphering by mononuclear cells in man. Science. 1967 Dec 22;158(3808):1582–1585. doi: 10.1126/science.158.3808.1582. [DOI] [PubMed] [Google Scholar]
- Lonai P., Clark W. R., Feldman M. Participation of theta-bearing cell in an in vitro assay of transplantation immunity. Nature. 1971 Feb 19;229(5286):566–567. doi: 10.1038/229566a0. [DOI] [PubMed] [Google Scholar]
- Lonai P., Feldman M. Studies on the effect of macrophages in an in vitro graft reaction system. Immunology. 1971 Nov;21(5):861–867. [PMC free article] [PubMed] [Google Scholar]
- Lubaroff D. M., Waksman B. H. Bone marrow as a source of cells in reactions of cellular hypersensitivity. I. Passive transfer of tuberculin sensitivity in syngeneic systems. J Exp Med. 1968 Dec 1;128(6):1425–1435. doi: 10.1084/jem.128.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubaroff D. M., Waksman B. H. Delayed hypersensitivity: bone marrow as the source of cells in delayed skin reactions. Science. 1967 Jul 21;157(3786):322–323. doi: 10.1126/science.157.3786.322. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCLUSKEY R. T., BENACERRAF B., MCCLUSKEY J. W. STUDIES ON THE SPECIFICITY OF THE CELLULAR INFILTRATE IN DELAYED HYPERSENSITIVITY REACTIONS. J Immunol. 1963 Mar;90:466–477. [PubMed] [Google Scholar]
- MERRITT K., JOHNSON A. G. STUDIES ON THE ADJUVANT ACTION OF BACTERIAL ENDOTOXINS ON ANTIBODY FORMATION. VI. ENHANCEMENT OF ANTIBODY FORMATION BY NUCLEIC ACIDS. J Immunol. 1965 Mar;94:416–422. [PubMed] [Google Scholar]
- MOELLER E. ANTAGONISTIC EFFECTS OF HUMORAL ISOANTIBODIES ON THE IN VITRO CYTOTOXICITY OF IMMUNE LYMPHOID CELLS. J Exp Med. 1965 Jul 1;122:11–23. doi: 10.1084/jem.122.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOUTON D., BIOZZI G., BOUTHILLIER Y., STIFFEL C. Phagocytosis of Salmonellae by reticuloendothelial cells of newborn piglets lacking natural antibody. Nature. 1963 Feb 16;197:706–706. doi: 10.1038/197706a0. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V. Cellular immunity. Prog Allergy. 1967;11:89–140. [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackler B. F., Altman L. C., Rosenstreich D. L., Oppenheim J. J. Induction of lymphokine production by EAC and of blastogenesis by soluble mitogens during human B-cell activation. Nature. 1974 Jun 28;249(460):834–837. doi: 10.1038/249834a0. [DOI] [PubMed] [Google Scholar]
- Mantovani B., Rabinovitch M., Nussenzweig V. Phagocytosis of immune complexes by macrophages. Different roles of the macrophage receptor sites for complement (C3) and for immunoglobulin (IgG). J Exp Med. 1972 Apr 1;135(4):780–792. doi: 10.1084/jem.135.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Hahn H. H., Mackaness G. B. The mediator of cellular immunity. V. Development of cellular resistance to infection in thymectomized irradiated rats. Cell Immunol. 1973 Feb;6(2):186–199. doi: 10.1016/0008-8749(73)90021-x. [DOI] [PubMed] [Google Scholar]
- Melchers F., Andersson J. Synthesis, surface deposition and secretion of immunoglobulin M in bone marrow-derived lymphocytes before and after mitogenic stimulation. Transplant Rev. 1973;14:76–130. doi: 10.1111/j.1600-065x.1973.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Melchers F., Braun V., Galanos C. The lipoprotein of the outer membrane of Escherichia coli: a B-lymphocyte mitogen. J Exp Med. 1975 Aug 1;142(2):473–482. doi: 10.1084/jem.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook G., Salmon B. J., Kreisberg J. I. Sterilization of Listeria monocytogenes by guinea pig peritoneal exudate cell cultures. Cell Immunol. 1974 Nov;14(2):270–283. doi: 10.1016/0008-8749(74)90211-1. [DOI] [PubMed] [Google Scholar]
- Miler I., Tlaskalová H., Mandel L., Trávnícek J. The phagocytic activity of reticuloendothelial system of newborn precolostral pigs to smooth and rough Escherichia coli. Folia Microbiol (Praha) 1968;13(6):472–481. doi: 10.1007/BF02874217. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Miller T. E., Mackaness G. B., Lagrange P. H. Immunopotentiation with BCG. II. Modulation of the response to sheep red blood cells. J Natl Cancer Inst. 1973 Nov;51(5):1669–1676. doi: 10.1093/jnci/51.5.1669. [DOI] [PubMed] [Google Scholar]
- Minden P., McClatchy J. K., Cooper R., Bardana E. J., Jr, Farr R. S. Shared antigens between Mycobacterium bovis (BCG) and other bacterial species. Science. 1972 Apr 7;176(4030):57–58. doi: 10.1126/science.176.4030.57. [DOI] [PubMed] [Google Scholar]
- Minden P., McClatchy J. K., Farr R. S. Shared antigens between heterologous bacterial species. Infect Immun. 1972 Oct;6(4):574–582. doi: 10.1128/iai.6.4.574-582.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden P., McClatchy J. K., Wainberg M., Weiss D. W. Shared antigens between Mycobacterium bovis (BCG) and neoplastic cells. J Natl Cancer Inst. 1974 Nov;53(5):1325–1331. doi: 10.1093/jnci/53.5.1325. [DOI] [PubMed] [Google Scholar]
- Miranda J. J. Studies on immunological paralysis. IX. The immunogenicity and tolerogenicity of levan (polyfructose) in mice. Immunology. 1972 Dec;23(6):829–842. [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F. A proposal that one in vivo function of t cells is to regulate the availability of antigen for b cells. Transplant Rev. 1975;23:119–125. doi: 10.1111/j.1600-065x.1975.tb00153.x. [DOI] [PubMed] [Google Scholar]
- Mitchison N. A. The immunogenic capacity of antigen taken up by peritoneal exudate cells. Immunology. 1969 Jan;16(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- Modolell M., Luckenbach G. A., Parant M., Munder P. G. The adjuvant activity of a mycobacterial water soluble adjuvant (WSA) in vitro. I. The requirement of macrophages. J Immunol. 1974 Jul;113(1):395–403. [PubMed] [Google Scholar]
- Mokyr M. B., Mitchell M. S. Activation of lymphoid cells by BCG in vitro. Cell Immunol. 1975 Feb;15(2):264–273. doi: 10.1016/0008-8749(75)90005-2. [DOI] [PubMed] [Google Scholar]
- Mooney J. J., Waksman B. H. Activation of normal rabbit macrophage monolayers by supernatants of antigen-stimulated lymphocytes. J Immunol. 1970 Nov;105(5):1138–1145. [PubMed] [Google Scholar]
- Mosier D. E. A requirement for two cell types for antibody formation in vitro. Science. 1967 Dec 22;158(3808):1573–1575. doi: 10.1126/science.158.3808.1573. [DOI] [PubMed] [Google Scholar]
- Mouton D., Bouthillier Y., Feingold N., Feingold J., Decreusefond C., Stiffel C., Biozzi G. Genetic control of macrophage functions. I. Polygenic regulation of phagocytosis stimulation produced by Glyceryl Trioleate. J Exp Med. 1975 Feb 1;141(2):306–321. doi: 10.1084/jem.141.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgo A. J., Athanassiades T. J. Studies on the adjuvant effect of Bordetella pertussis vaccine to sheep erythrocytes in the mouse. I. In vitro enhancement of antibody formation with normal spleen cells. J Immunol. 1975 Oct;115(4):928–931. [PubMed] [Google Scholar]
- Mäkelä O. Analogies between lymphocyte receptors and the resulting humoral antibodies. Transplant Rev. 1970;5:3–18. [PubMed] [Google Scholar]
- Möller G., Andersson J., Sjöberg O. Lipopolysaccharides can convert heterologous red cells into thymus-independent antigens. Cell Immunol. 1972 Aug;4(4):416–424. doi: 10.1016/0008-8749(72)90043-3. [DOI] [PubMed] [Google Scholar]
- NEVEU T., BRANELLEC A., BIOZZI G. PROPRI'ET'ES ADJUVANTES DE CORYNEBACTERIUM PARVUM SUR LA PRODUCTION D'ANTICORPS ET SUR L'INDUCTION DE L'HYPERSENSIBILIT'E RETARD'EE ENVERS LES PROT'EINES CONJUGU'EES. Ann Inst Pasteur (Paris) 1964 May;106:771–777. [PubMed] [Google Scholar]
- Nakano M., Shimamura T., Saito K. Cellular mechanisms of adjuvant action of bacterial lipopolysaccharide in anti-sheep red blood cell antibody response. Jpn J Microbiol. 1971 Mar;15(2):149–158. doi: 10.1111/j.1348-0421.1971.tb00564.x. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Karnovsky M. L., David J. R. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971 Jun 1;133(6):1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Remold H. G., David J. R. Characterization of a lymphocyte factor which alters macrophage functions. J Exp Med. 1973 Feb 1;137(2):275–290. doi: 10.1084/jem.137.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauciel C., Fleck J., Martin J. P., Mock M., Nguyen-Huy H. Adjuvant activity of bacterial peptidoglycans on the production of delayed hypersensitivity and on antibody response. Eur J Immunol. 1974 May;4(5):352–356. doi: 10.1002/eji.1830040509. [DOI] [PubMed] [Google Scholar]
- Neta R., Salvin S. B. Specific suppression of delayed hypersensitivity: the possible presence of a suppressor B cell in the regulation of delayed hypersensitivity. J Immunol. 1974 Dec;113(6):1716–1725. [PubMed] [Google Scholar]
- Neter E. Endotoxins and the immune response. Curr Top Microbiol Immunol. 1969;47:82–124. doi: 10.1007/978-3-642-46160-6_5. [DOI] [PubMed] [Google Scholar]
- Nilsson B. S., Sultzer B. M., Bullock W. W. Purified protein derivative of tuberculin induces immunoglobulin production in normal mouse spleen cells. J Exp Med. 1973 Jan 1;137(1):127–139. doi: 10.1084/jem.137.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- Nowotny A., Behling U. H., Chang H. L. Relation of structure to function in bacterial endotoxins. VIII. Biological activities in a polysaccharide-rich fraction. J Immunol. 1975 Jul;115(1):199–203. [PubMed] [Google Scholar]
- Nussenzweig V. Receptors for immune complexes on lymphocytes. Adv Immunol. 1974;19(0):217–258. doi: 10.1016/s0065-2776(08)60253-9. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., ZELEZNICK L. D., HORECKER B. L. LIPOPOLYSACCHARIDE OF THE GRAM-NEGATIVE CELL WALL. Science. 1964 Aug 21;145(3634):783–789. doi: 10.1126/science.145.3634.783. [DOI] [PubMed] [Google Scholar]
- Ortiz-Ortiz L., Jaroslow B. N. Enhancement by the adjuvant, endotoxin, of an immune response induced in vitro. Immunology. 1970 Sep;19(3):387–399. [PMC free article] [PubMed] [Google Scholar]
- POTTER E. V., STOLLERMAN G. H. The opsonization of bentonite particles by gamma-globulin. J Immunol. 1961 Jul;87:110–118. [PubMed] [Google Scholar]
- Papageorgiou P. S., Glade P. R. Migration inhibitory factor (MIF) production by skin fibroblast cultures from patients with severe combined immunodeficiency. Cell Immunol. 1974 May;12(2):326–330. doi: 10.1016/0008-8749(74)90086-0. [DOI] [PubMed] [Google Scholar]
- Papageorgiou P. S., Henley W. L., Glade P. R. Production and characterization of migration inhibitory factor(s) (MIF) of established lymphoid and non-lymphoid cell lines. J Immunol. 1972 Feb;108(2):494–504. [PubMed] [Google Scholar]
- Papageorgiou P. S., Sorokin C. F., Glade P. R. Similarity of migration inhibitory factor(s) produced by human lymphoid cell line and phytohemagglutinin and tuberculin-stimulated human peripheral lymphocytes. J Immunol. 1974 Feb;112(2):675–682. [PubMed] [Google Scholar]
- Parker J. W., Metcalf D. Production of colony-stimulating factor in mitogen-stimulated lymphocyte cultures. J Immunol. 1974 Feb;112(2):502–510. [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy D. L., Adler W. H., Smith R. T. The mitogenic effects of endotoxin and staphylococcal enterotoxin B on mouse spleen cells and human peripheral lymphocytes. J Immunol. 1970 Dec;105(6):1453–1458. [PubMed] [Google Scholar]
- Peavy D. L., Shands J. W., Jr, Adler W. H., Smith R. T. Mitogenicity of bacterial endotoxins: characterization of the mitogenic principle. J Immunol. 1973 Aug;111(2):352–357. [PubMed] [Google Scholar]
- Perlmann P., Holm G. Cytotoxic effects of lymphoid cells in vitro. Adv Immunol. 1969;11:117–193. doi: 10.1016/s0065-2776(08)60479-4. [DOI] [PubMed] [Google Scholar]
- Petit J. C., Unanue E. R. Effects of bacterial products on lymphocytes and macrophages: their possible role in natural resistance to listeria infetion in mice. J Immunol. 1974 Sep;113(3):984–992. [PubMed] [Google Scholar]
- Phanuphak P., Moorhead J. W., Claman H. N. Immunologic activities of pertussis-treated lymphocytes. Int Arch Allergy Appl Immunol. 1972;43(2):305–316. doi: 10.1159/000230844. [DOI] [PubMed] [Google Scholar]
- Pick E., Krejci J., Cech K., Turk J. L. Interaction between 'sensitized lymphocytes' and antigen in vitro. I. The release of a skin reactive factor. Immunology. 1969 Nov;17(5):741–767. [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H. The role of antibody in T-cell responses. Clin Exp Immunol. 1974 May;17(1):1–18. [PMC free article] [PubMed] [Google Scholar]
- ROWLANDS D. T., Jr, CLAMAN H. N., KIND P. D. THE EFFECT OF ENDOTOXIN ON THE THYMUS OF YOUNG MICE. Am J Pathol. 1965 Feb;46:165–176. [PMC free article] [PubMed] [Google Scholar]
- Ratzan K. R., Musher D. M., Keusch G. T., Weinstein L. Correlation of increased metabolic activity, resistance to infection, enhanced phagocytosis, and inhibition of bacterial growth by macrophages from Listeria- and BCG-infected mice. Infect Immun. 1972 Apr;5(4):499–504. doi: 10.1128/iai.5.4.499-504.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees R. J. Enhanced susceptibility of thymectomized and irradiated mice to infection with Mycobacterium leprae. Nature. 1966 Aug 6;211(5049):657–658. doi: 10.1038/211657a0. [DOI] [PubMed] [Google Scholar]
- Remington J. S., Merigan T. C. Synthetic polyanions protect mice against intracellular bacterial infection. Nature. 1970 Apr 25;226(5243):361–363. doi: 10.1038/226361a0. [DOI] [PubMed] [Google Scholar]
- Remold H. G., David R. A., David J. R. Characterization of migration inhibitory factor (MIF) from guinea pig lymphocytes stimulated with concanavalin A. J Immunol. 1972 Sep;109(3):578–586. [PubMed] [Google Scholar]
- Rocklin R. E., MacDermott R. P., Chess L., Schlossman S. F., David J. R. Studies on mediator production by highly purified human T and B lymphocytes. J Exp Med. 1974 Nov 1;140(5):1303–1316. doi: 10.1084/jem.140.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklin R. E. Production of migration inhibitory factor by non-dividing lymphocytes. J Immunol. 1973 Mar;110(3):674–678. [PubMed] [Google Scholar]
- Roelants G. E., Askonas B. A. Cell cooperation in antibody induction. The susceptibility of helper cells to specific lethal radioactive antigen. Eur J Immunol. 1971 Jun;1(3):151–157. doi: 10.1002/eji.1830010302. [DOI] [PubMed] [Google Scholar]
- Roseman J. X-ray resistant cell required for the induction of in vitro antibody formation. Science. 1969 Sep 12;165(3898):1125–1127. doi: 10.1126/science.165.3898.1125. [DOI] [PubMed] [Google Scholar]
- Rosenstreich D. L., Farrar J. J., Dougherty S. Absolute macrophage dependency of T lymphocyte activation by mitogens. J Immunol. 1976 Jan;116(1):131–139. [PubMed] [Google Scholar]
- Rosenstreich D. L., Nowotny A., Chused T., Mergenhagen S. E. In vitro transformation of mouse bone-marrow-derived (B) lymphocytes induced by the lipid component of endotoxin. Infect Immun. 1973 Sep;8(3):406–411. doi: 10.1128/iai.8.3.406-411.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. S., Lipsky P. E., Shevach E. M. Macrophage-lymphocyte interaction and antigen recognition. Fed Proc. 1975 Jul;34(8):1743–1748. [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Polley M. J., Rabellino E. M., Grey H. M. Two different complement receptors on human lymphocytes. One specific for C3b and one specific for C3b inactivator-cleaved C3b. J Exp Med. 1973 Oct 1;138(4):798–811. doi: 10.1084/jem.138.4.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. S., Hug K., Forni L., Pernis B. Immunoglobulin D as a lymphocyte receptor. J Exp Med. 1973 Oct 1;138(4):965–972. doi: 10.1084/jem.138.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAW C. M., ALVORD E. C., Jr, FAHLBERG W. J., KIES M. W. SUBSTITUTES FOR THE MYCOBACTERIA IN FREUND'S ADJUVANTS IN THE PRODUCTION OF EXPERIMENTAL "ALLERGIC" ENCEPHALOMYELITIS IN THE GUINEA PIG. J Immunol. 1964 Jan;92:28–40. [PubMed] [Google Scholar]
- STANLEY N. F. The augmenting action of lecithin and the lipoids of Aspergillus fumigatus and listeria monocytogenes in antibody production using Salmonella typhi-murium as an antigen. Aust J Exp Biol Med Sci. 1950 Jan;28(1):109–115. doi: 10.1038/icb.1950.10. [DOI] [PubMed] [Google Scholar]
- SUTER E. Interaction between phagocytes and pathogenic microorganisms. Bacteriol Rev. 1956 Jun;20(2):94–132. doi: 10.1128/br.20.2.94-132.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E., RAMSEIAR H. CELLULAR REACTIONS IN INFECTION. Adv Immunol. 1964;27:117–173. doi: 10.1016/s0065-2776(08)60707-5. [DOI] [PubMed] [Google Scholar]
- Salsano F., Froland S. S., Natvig J. B., Michaelsen T. E. Same idiotype of B-lymphocyte membrane IgD and IgM. Formal evidence for monoclonality of chronic lymphocytic leukemia cells. Scand J Immunol. 1974;3(6):841–846. doi: 10.1111/j.1365-3083.1974.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Schechter G. P. McFarland W,+MACFARLAND W: Interaction of lymphocytes and a radioresistant cell in PPD-stimulated human leukocyte cultures. J Immunol. 1970 Sep;105(3):661–669. [PubMed] [Google Scholar]
- Scheid M. P., Goldstein G., Hammerling U., Boyse E. A. Lymphocyte differentiation from precursor cells in vitro. Ann N Y Acad Sci. 1975 Feb 28;249:531–540. doi: 10.1111/j.1749-6632.1975.tb29102.x. [DOI] [PubMed] [Google Scholar]
- Schimpl A., Wecker E. A third signal in B cell activation given by TRF. Transplant Rev. 1975;23:176–188. doi: 10.1111/j.1600-065x.1975.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Schimpl A., Wecker E. Replacement of T-cell function by a T-cell product. Nat New Biol. 1972 May 3;237(70):15–17. doi: 10.1038/newbio237015a0. [DOI] [PubMed] [Google Scholar]
- Schmidt M. E., Douglas S. D., Rubin A. D. Human monocyte activation by supernatants from concanavalin A (con A) stimulated lymphocytes. Cell Immunol. 1973 Oct;9(1):45–59. doi: 10.1016/0008-8749(73)90166-4. [DOI] [PubMed] [Google Scholar]
- Schmidtke J. R., Dixon F. J. Immune response to a hapten coupled to a nonimmunogenic carrier. Influence of lipopolysaccharide. J Exp Med. 1972 Aug 1;136(2):392–397. doi: 10.1084/jem.136.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke J. R., Dixon F. J. The effect of in vivo irradiation on macrophage function. J Immunol. 1973 Mar;110(3):848–854. [PubMed] [Google Scholar]
- Schmidtke J., Unanue E. R. Interaction of macrophages and lymphocytes with surface immunoglobulin. Nat New Biol. 1971 Sep 15;233(37):84–86. doi: 10.1038/newbio233084a0. [DOI] [PubMed] [Google Scholar]
- Schrader J. W. Mechanism of activation of the bone marrow-derived lymphocyte. 3. A distinction between a macrophage-produced triggering signal and the amplifying effect on triggered B lymphocytes of allogeneic interactions. J Exp Med. 1973 Dec 1;138(6):1466–1480. doi: 10.1084/jem.138.6.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H. Suppression of the immune response by microorganisms. Bacteriol Rev. 1975 Jun;39(2):121–143. doi: 10.1128/br.39.2.121-143.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. T. Biological effects of the adjuvant Corynebacterium parvum. I. Inhibition of PHA, mixed lymphocyte and GVH reactivity. Cell Immunol. 1972 Nov;5(3):459–468. doi: 10.1016/0008-8749(72)90072-x. [DOI] [PubMed] [Google Scholar]
- Scott M. T. Biological effects of the adjuvant Corynebacterium parvum. II. Evidence for macrophage-T-cell interaction. Cell Immunol. 1972 Nov;5(3):469–479. doi: 10.1016/0008-8749(72)90073-1. [DOI] [PubMed] [Google Scholar]
- Seeger R. C., Oppenheim J. J. Synergistic interaction of macrophages and lymphocytes in antigen-induced transformation of lymphocytes. J Exp Med. 1970 Jul 1;132(1):44–65. doi: 10.1084/jem.132.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi K. K., Teschner M., Brandis H. In vitro antilisterial activity of soluble product(s) released from Listeria-immune murine peritoneal macrophages. Infect Immun. 1974 Oct;10(4):960–962. doi: 10.1128/iai.10.4.960-962.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon R., McMaster P. R., Kask A. M., Owens J. D., Paul W. E. DNP-Lys-ficoll: a T-independent antigen which elicits both IgM and IgG anti-DNP antibody-secreting cells. J Immunol. 1975 May;114(5):1585–1589. [PubMed] [Google Scholar]
- Shin H. S., Smith M. R., Wood W. B., Jr Heat labile opsonins to pneumococcus. II. Involvement of C3 and C5. J Exp Med. 1969 Dec 1;130(6):1229–1241. doi: 10.1084/jem.130.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel I. Autologous macrophage-thymocyte interactions. J Allergy. 1970 Sep;46(3):190–194. doi: 10.1016/0021-8707(70)90097-3. [DOI] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Enhancement of macrophage bactericidal capacity by antigenically stimulated immune lymphocytes. Cell Immunol. 1972 Jun;4(2):163–174. doi: 10.1016/0008-8749(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Sjöberg O., Andersson J., Möller G. Reconstitution of the antibody response in vitro of T cell-deprived spleen cells by supernatants from spleen cell cultures. J Immunol. 1972 Dec;109(6):1379–1385. [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Morrison D. C., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J Immunol. 1975 Feb;114(2 Pt 2):770–775. [PubMed] [Google Scholar]
- Stobo J. D. Phytohemagglutin and concanavalin A: probes for murine 'T' cell activivation and differentiation. Transplant Rev. 1972;11:60–86. doi: 10.1111/j.1600-065x.1972.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Stockman G. D., Gallagher M. T., Heim L. R., South M. A., Trentin J. J. Differential stimulation of mouse lymphoid cells by phytohemagglutinin and pokeweed mitogen. Proc Soc Exp Biol Med. 1971 Mar;136(3):980–982. doi: 10.3181/00379727-136-35410. [DOI] [PubMed] [Google Scholar]
- Stubbs M., Kühner A. V., Glass E. A., David J. R., Karnovsky M. L. Metabolic and functonal studies on activated mouse macrophages. J Exp Med. 1973 Feb 1;137(2):537–542. doi: 10.1084/jem.137.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M., Nilsson B. S. PPD tuberculin--a B-cell mitogen. Nat New Biol. 1972 Dec 13;240(102):198–200. doi: 10.1038/newbio240198a0. [DOI] [PubMed] [Google Scholar]
- THOMAS L. The physiological disturbances produced by endotoxins. Annu Rev Physiol. 1954;16:467–490. doi: 10.1146/annurev.ph.16.030154.002343. [DOI] [PubMed] [Google Scholar]
- Takeya K., Mori R., Nomoto K., Nakayama H. Experimental mycobacterial infections in neonatally thymectomized mice. Am Rev Respir Dis. 1967 Sep;96(3):469–477. doi: 10.1164/arrd.1967.96.3.469. [DOI] [PubMed] [Google Scholar]
- Takiguchi T., Adler W. H., Smith R. T. Cellular recognition in vitro by mouse lymphocytes. Effects of neonatal thymectomy and thymus graft restoration on alloantigen and PHA stimulation of whole and gradient-separated subpopulations of spleen cells. J Exp Med. 1971 Jan 1;133(1):63–80. doi: 10.1084/jem.133.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig M. J. T cell factor which can replace T cells in vivo. Nature. 1974 Mar 15;248(445):234–236. doi: 10.1038/248234a0. [DOI] [PubMed] [Google Scholar]
- Taylor M. M., Burman C. J., Fantes K. H. Problems encountered in the preparation of migration inhibitory factor (MIF) and mitogenic factor (MIT) from phytomitogen-stimulated human peripheral lymphocytes and in the preparation of MIF from human lymphoid cell lines (LCL). Cell Immunol. 1975 Sep;19(1):41–57. doi: 10.1016/0008-8749(75)90290-7. [DOI] [PubMed] [Google Scholar]
- Taylor R. B., Wortis H. H. Thymus dependence of antibody response: variation with dose of antigen and class of antibody. Nature. 1968 Nov 30;220(5170):927–928. doi: 10.1038/220927a0. [DOI] [PubMed] [Google Scholar]
- Tizard I. R. Macrophage cytophilic antibody in mice. The relationship between the adherence of antigen to macrophages mediated by macrophage-cytophilic antibodies and opsonic adherence antibodies. Int Arch Allergy Appl Immunol. 1970;39(2-3):201–209. [PubMed] [Google Scholar]
- Tubergen D. G., Feldman J. D., Pollock E. M., Lerner R. A. Production of macrophage migration inhibition factor by continuous cell lines. J Exp Med. 1972 Feb 1;135(2):255–266. doi: 10.1084/jem.135.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Askonas B. A., Allison A. C. A role of macrophages in the stimulation of immune responses by adjuvants. J Immunol. 1969 Jul;103(1):71–78. [PubMed] [Google Scholar]
- Unanue E. R., Askonas B. A. The immune response of mice to antigen in macrophages. Immunology. 1968 Aug;15(2):287–296. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]
- VOLKMAN A., GOWANS J. L. THE ORIGIN OF MACROPHAGES FROM BONE MARROW IN THE RAT. Br J Exp Pathol. 1965 Feb;46:62–70. [PMC free article] [PubMed] [Google Scholar]
- Van Boxel J. A., Rosenstreich D. L. Binding of aggregated gamma-globulin to activated T lymphocytes in the guinea pig. J Exp Med. 1974 Apr 1;139(4):1002–1012. doi: 10.1084/jem.139.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oss C. J., Stinson M. W. Immunoglobulins as aspecific opsonins. I. The influence of polyclonal and monoclonal immunoglobulins on the in vitro phagocytosis of latex particles and Staphylococci by human neutrophils. J Reticuloendothel Soc. 1970 Nov;8(5):397–406. [PubMed] [Google Scholar]
- Vann D. C., Campbell P. A. Plaque-forming cells of two different origins in single hemolytic foci. J Immunol. 1970 Dec;105(6):1584–1586. [PubMed] [Google Scholar]
- Volkman A., Collins F. M. The restorative effect of peritoneal macrophages on delayed hypersensitivity following ionizing radiation. Cell Immunol. 1971 Dec;2(6):552–566. doi: 10.1016/0008-8749(71)90004-9. [DOI] [PubMed] [Google Scholar]
- WARD P. A., JOHNSON A. G., ABELL M. R. Studies on the adjuvant action of bacterial endotoxins on antibody formation. III. Histologic response of the rabbit spleen to a single injection of a purified protein antigen. J Exp Med. 1959 May 1;109(5):463–474. doi: 10.1084/jem.109.5.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS D. W. Enhanced resistance of mice to infection with Pasteurella pestis following vaccination with fractions of phenol-killed tubercle bacilli. Nature. 1960 Jun 25;186:1060–1061. doi: 10.1038/1861060a0. [DOI] [PubMed] [Google Scholar]
- WHITE R. G., JOLLES P., SAMOUR D., LEDERER E. CORRELATION OF ADJUVANT ACTIVITY AND CHEMICAL STRUCTURE OF WAX D FRACTIONS OF MYCOBACTERIA. Immunology. 1964 Mar;7:158–171. [PMC free article] [PubMed] [Google Scholar]
- Wagner H., Feldmann M., Boyle W., Schrader J. W. Cell-mediated immune response in vitro. 3. The requirement for macrophages in cytotoxic reactions against cell-bound and subcellular alloantigens. J Exp Med. 1972 Aug 1;136(2):331–343. doi: 10.1084/jem.136.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Iverson G. M., Oppenheim J. J. Induction of guinea pig B-cell lymphokine synthesis by mitogenic and nonmitogenic signals to Fc, Ig, and C3 receptors. J Exp Med. 1974 Dec 1;140(6):1631–1645. doi: 10.1084/jem.140.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Wilton J. M., Rosenstreich D. L., Oppenheim J. J. The role of macrophages in the production of lymphokines by T and B lymphocytes. J Immunol. 1975 Apr;114(4):1296–1301. [PubMed] [Google Scholar]
- Waldron J. A., Jr, Horn R. G., Rosenthal A. S. Antigen-induced proliferation of guinea pig lymphocytes in vitro: obligatory role of macrophages in the recognition of antigen by immune T-lymphocytes. J Immunol. 1973 Jul;111(1):58–64. [PubMed] [Google Scholar]
- Ward P. A. Chemotoxis of mononuclear cells. J Exp Med. 1968 Nov 1;128(5):1201–1221. doi: 10.1084/jem.128.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Lepow I. H., Newman L. J. Bacterial factors chemotactic for polymorphonuclear leukocytes. Am J Pathol. 1968 Apr;52(4):725–736. [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Remold H. G., David J. R. The production by antigen-stimulated lymphocytes of a leukotactic factor distinct from migration inhibitory factor. Cell Immunol. 1970 Jul;1(2):162–174. doi: 10.1016/0008-8749(70)90003-1. [DOI] [PubMed] [Google Scholar]
- Warner N. L. Membrane immunoglobulins and antigen receptors on B and T lymphocytes. Adv Immunol. 1974;19(0):67–216. doi: 10.1016/s0065-2776(08)60252-7. [DOI] [PubMed] [Google Scholar]
- Warr G. W., Ghaffar A., James K. The response of mice to type III pneumococcal polysaccharide: failure to detect thymus-derived suppressor cells. Cell Immunol. 1975 Jun;17(2):366–373. doi: 10.1016/s0008-8749(75)80040-2. [DOI] [PubMed] [Google Scholar]
- Warr G. W., James K. Effect of Corynebacterium parvum on the class and subclass of antibody produced in the response of different strains of mice to sheep erythrocytes. Immunology. 1975 Mar;28(3):431–442. [PMC free article] [PubMed] [Google Scholar]
- Watson J. Cyclic nucleotides as intracellular mediators of B cell activation. Transplant Rev. 1975;23:223–249. doi: 10.1111/j.1600-065x.1975.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. I. Evidence for a single gene that influences mitogenic and immunogenic respones to lipopolysaccharides. J Exp Med. 1974 Nov 1;140(5):1147–1161. doi: 10.1084/jem.140.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. The influence of intracellular levels of cyclic nucleotides on cell proliferation and the induction of antibody synthesis. J Exp Med. 1975 Jan 1;141(1):97–111. doi: 10.1084/jem.141.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. The role of humoral factors in the initiation of in vitro primary immune responses. 3. Characterization of factors that replace thymus-derived cells. J Immunol. 1973 Nov;111(5):1301–1313. [PubMed] [Google Scholar]
- Webb S. R., Cooper M. D. T cells can bind antigen via cytophilic IgM antibody made by B cells. J Immunol. 1973 Jul;111(1):275–277. [PubMed] [Google Scholar]
- Wecker E., Schimpl A., Hünig T., Kühn L. A T-cell -produced mediator substance active in the humoral immune response. Ann N Y Acad Sci. 1975 Feb 28;249:258–263. doi: 10.1111/j.1749-6632.1975.tb29073.x. [DOI] [PubMed] [Google Scholar]
- Werdelin O., Braendstrup O., Pedersen E. Macrophage-lymphocyte clusters in the immune response to soluble protein antigen in vitro. J Exp Med. 1974 Nov 1;140(5):1245–1259. doi: 10.1084/jem.140.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb M. E., Rocklin R. E. Transfer factor therapy in a patient with progressive primary tuberculosis. Ann Intern Med. 1973 Aug;79(2):161–166. doi: 10.7326/0003-4819-79-2-161. [DOI] [PubMed] [Google Scholar]
- Wiener E., Bandieri A. Modifications in the handling in vitro of 125I-labelled keyhole limpet haemocyanin by peritoneal macrophages from mice pretreated with the adjuvant Corynebacterium parvum. Immunology. 1975 Aug;29(2):265–274. [PMC free article] [PubMed] [Google Scholar]
- Wiener E. The role of macrophages in the amplified in vitro response to sheep red blood cells by spleen cells from Corynebacterium parvum treated mice. Cell Immunol. 1975 Sep;19(1):1–7. doi: 10.1016/0008-8749(75)90286-5. [DOI] [PubMed] [Google Scholar]
- Wigzell H., Andersson B. Cell separation on antigen-coated columns. Elimination of high rate antibody-forming cells and immunological memory cells. J Exp Med. 1969 Jan 1;129(1):23–36. doi: 10.1084/jem.129.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigzell H. Specific fractionation of immunocompetent cells. Transplant Rev. 1970;5:76–104. doi: 10.1111/j.1600-065x.1970.tb00357.x. [DOI] [PubMed] [Google Scholar]
- Wilton J. M., Rosenstreich D. L., Oppenheim J. J. Activation of guinea pig macrophages by bacterial lipopolysaccharide requires bone marrow-derived lymphocytes. J Immunol. 1975 Jan;114(1 Pt 2):388–393. [PubMed] [Google Scholar]
- Yang C., Nowotny A. Effect of endotoxin on tumor resistance in mice. Infect Immun. 1974 Jan;9(1):95–100. doi: 10.1128/iai.9.1.95-100.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashphe D. J., Weiss D. W. Modulation of the immune response by a methanol-insoluble fraction of attenuated tubercle bacilli (BCG). I. Primary and secondary responses to sheep red blood cells and T2 phage. Clin Exp Immunol. 1970 Aug;7(2):269–281. [PMC free article] [PubMed] [Google Scholar]
- Yoshida T. O., Andersson B. Evidence for a receptor recognizing antigen complexed immunoglobulin on the surface of activated mouse thymus lymphocytes. Scand J Immunol. 1972;1(4):401–408. doi: 10.1111/j.1365-3083.1972.tb03306.x. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Sonozaki H., Cohen S. The production of migration inhibition factor by B and T cells of the guinea pig. J Exp Med. 1973 Oct 1;138(4):784–797. doi: 10.1084/jem.138.4.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim S., Stuntman O., Good R. A. Thymus dependency of cells involved in transfer of delayed hypersensitivity to Listeria monocytogenes in mice. Cell Immunol. 1973 Sep;8(3):395–402. doi: 10.1016/0008-8749(73)90129-9. [DOI] [PubMed] [Google Scholar]
- Youdim S., Stutman O., Good R. A. Studies of delayed hypersensitivity to L. Monocytogenes in mice: nature of cells involved in passive transfers. Cell Immunol. 1973 Jan;6(1):98–109. doi: 10.1016/0008-8749(73)90010-5. [DOI] [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. Recent studies on acquired immunity in tuberculosis. Curr Top Microbiol Immunol. 1969;48:129–178. doi: 10.1007/978-3-642-46163-7_6. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V., Langman R. E. Early appearance of sensitized lymphocytes in mice infected with Listeria monocytogenes. J Immunol. 1974 Feb;112(2):496–501. [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A., Hirsch J. G., Humphrey J. H., Spector W. G., Langevoort H. L. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46(6):845–852. [PMC free article] [PubMed] [Google Scholar]


