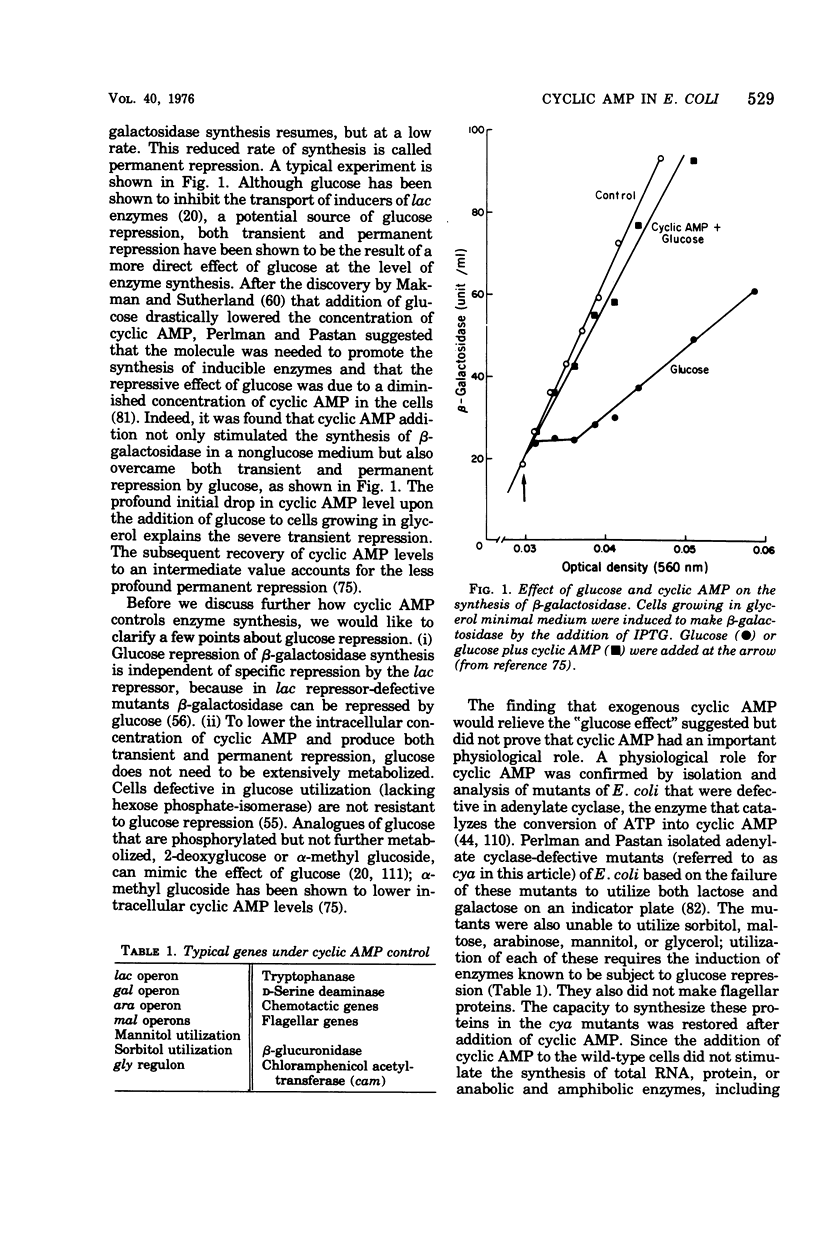
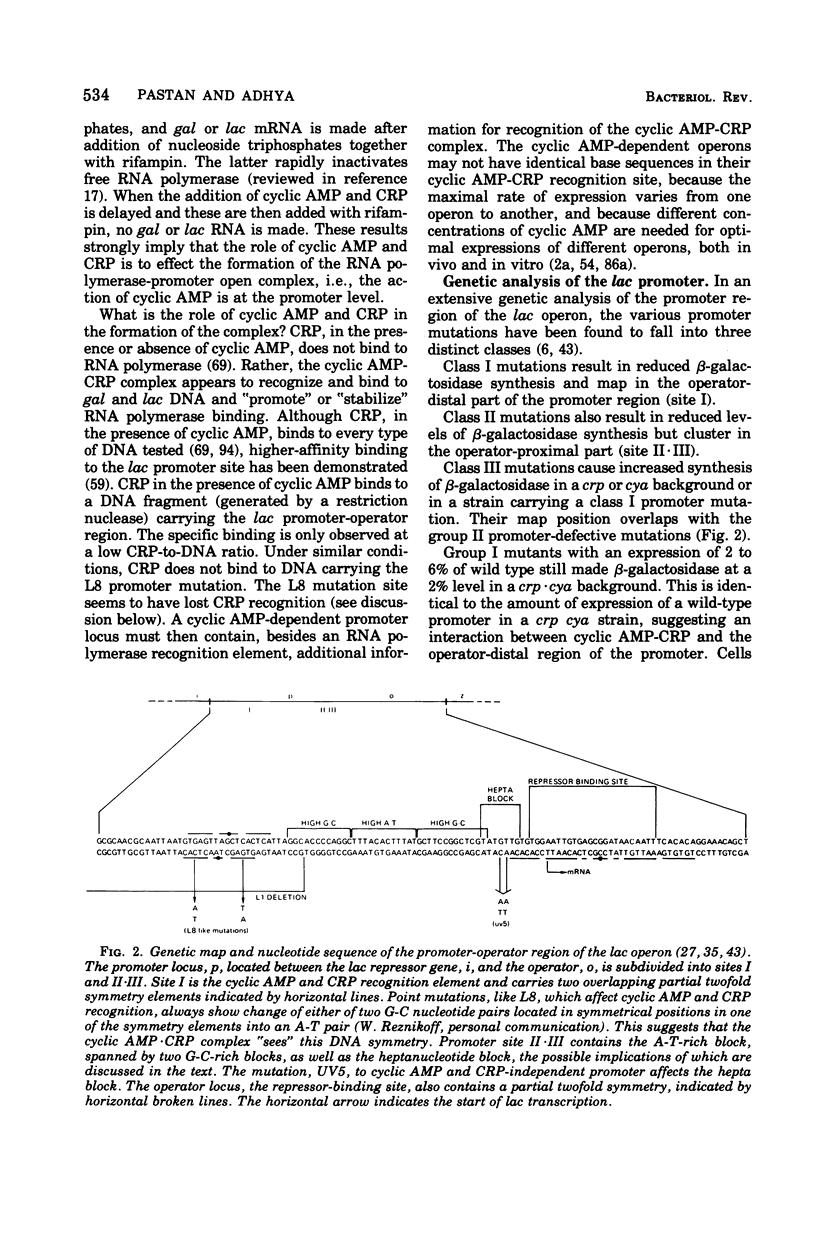
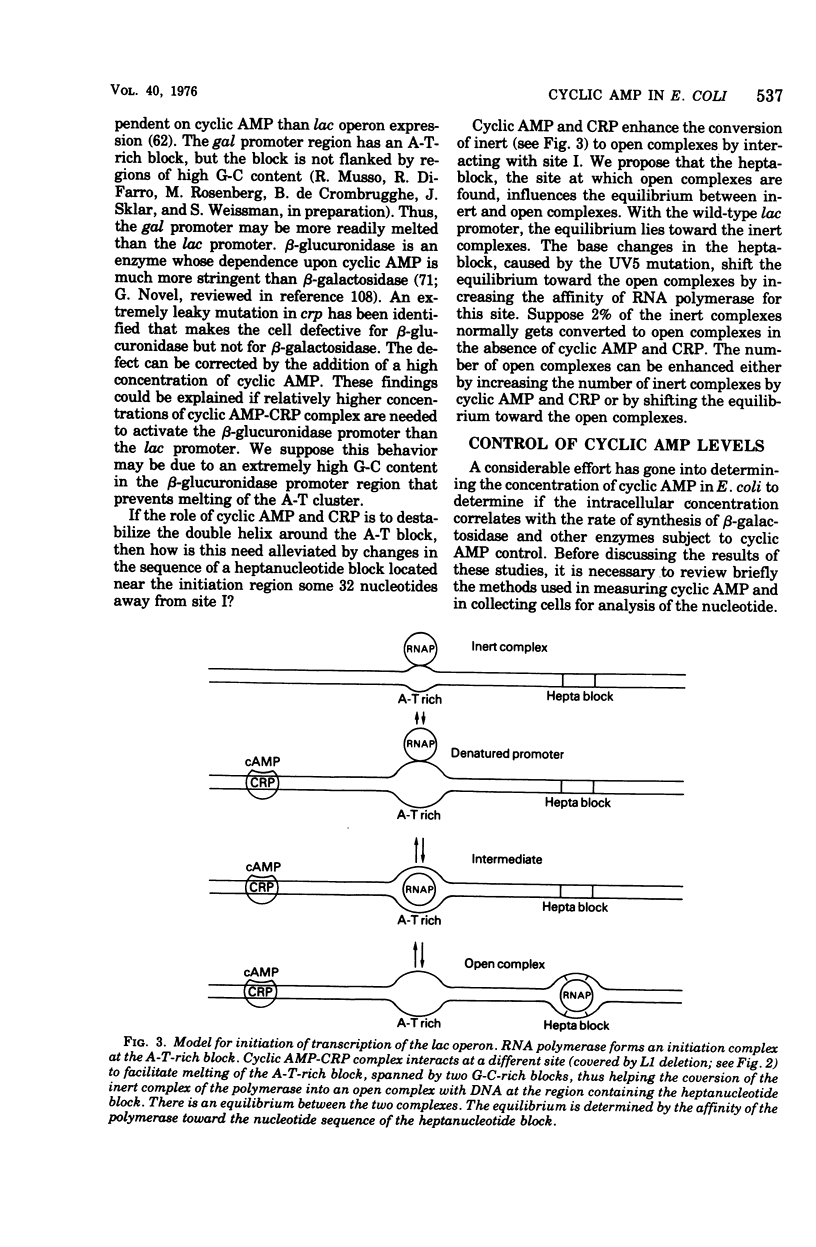
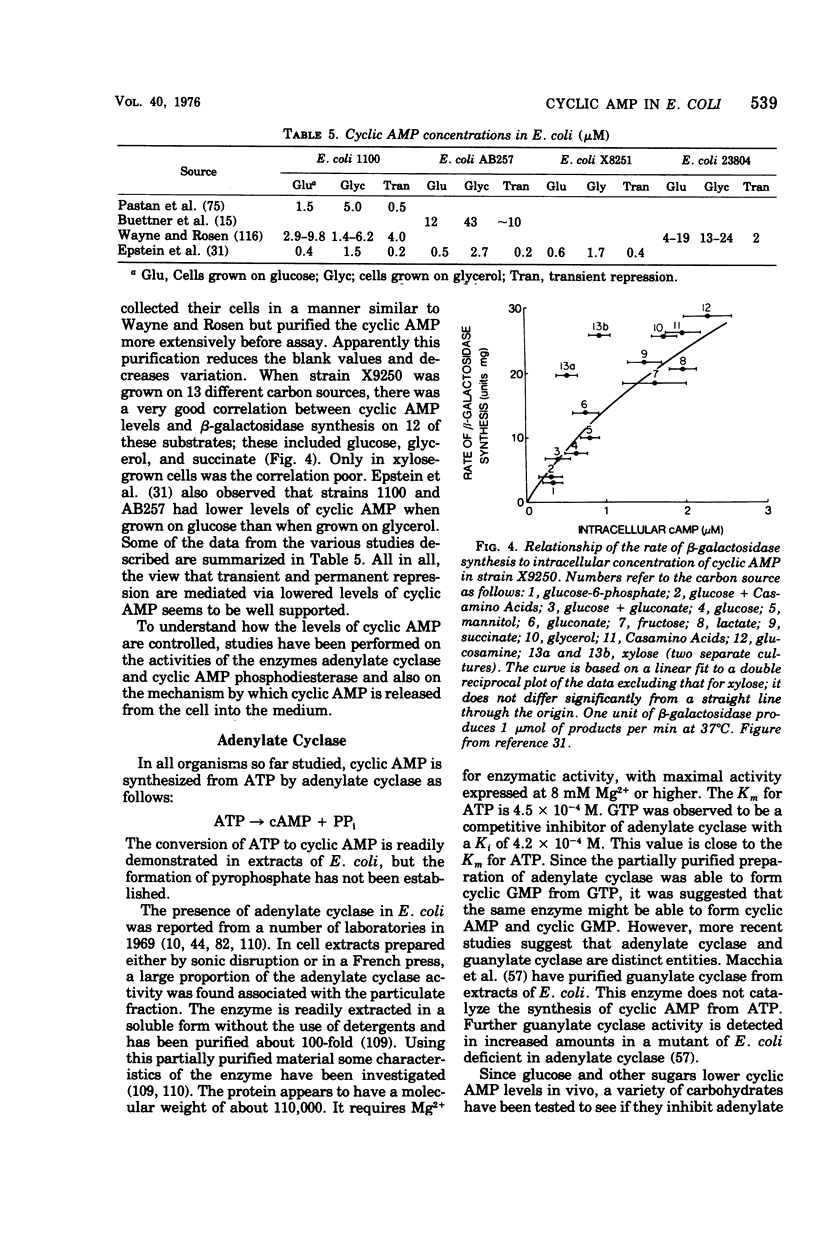
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboud M., Burger M. The effect of catabolite repression and of cyclic 3',5' adenosine monophosphate on the translation of the lactose messenger RNA in Escherichia coli. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1023–1032. doi: 10.1016/0006-291x(70)90342-6. [DOI] [PubMed] [Google Scholar]
- Aboud M., Pastan I. Activation of transcription by guanosine 5'-diphosphate,3'-diphosphate, transfer ribonucleic acid, and novel protein from Escherichia coli. J Biol Chem. 1975 Mar 25;250(6):2189–2195. [PubMed] [Google Scholar]
- Alper M. D., Ames B. N. Cyclic 3', 5'-adenosine monophosphate phosphodiesterase mutants of Salmonella typhimurium. J Bacteriol. 1975 Jun;122(3):1081–1090. doi: 10.1128/jb.122.3.1081-1090.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. B., Pastan I. The cyclic AMP receptor of Escherichia coli: immunological studies in extracts of Escherichia coli and other organisms. Biochim Biophys Acta. 1973 Oct 5;320(3):577–587. doi: 10.1016/0304-4165(73)90137-2. [DOI] [PubMed] [Google Scholar]
- Anderson W. B., Perlman R. L., Pastan I. Effect of adenosine 3',5'-monophosphate analogues on the activity of the cyclic adenosine 3',5'-monophosphate receptor in Escherichia coli. J Biol Chem. 1972 May 10;247(9):2717–2722. [PubMed] [Google Scholar]
- Beckwith J., Grodzicker T., Arditti R. Evidence for two sites in the lac promoter region. J Mol Biol. 1972 Aug 14;69(1):155–160. doi: 10.1016/0022-2836(72)90031-9. [DOI] [PubMed] [Google Scholar]
- Belfort M., Wulff D. The roles of the lambda c3 gene and the Escherichia coli catabolite gene activation system in the establishment of lysogeny by bacteriophage lambda. Proc Natl Acad Sci U S A. 1974 Mar;71(3):779–782. doi: 10.1073/pnas.71.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M., Zwaig N., Lin E. C. Suppression of a pleiotropic mutant affecting glycerol dissimilation. Biochem Biophys Res Commun. 1970 Jan 23;38(2):272–278. doi: 10.1016/0006-291x(70)90708-4. [DOI] [PubMed] [Google Scholar]
- Bernlohr R. W., Haddox M. K., Goldberg N. D. Cyclic guanosine 3':5'-monophosphate in Escherichia coli and Bacillus lichenformis. J Biol Chem. 1974 Jul 10;249(13):4329–4331. [PubMed] [Google Scholar]
- Braná H. Adenyl cyclase in cell-free extracts of Escherichia coli. Folia Microbiol (Praha) 1969;14(3):185–189. doi: 10.1007/BF02872776. [DOI] [PubMed] [Google Scholar]
- Braná H., Chytil F. Splitting of the cyclic 3',5'-adenosine monophosphate in cell--free system of Escherichia coli. Folia Microbiol (Praha) 1966;11(1):43–46. doi: 10.1007/BF02877154. [DOI] [PubMed] [Google Scholar]
- Braná H. Degradation of 5' adenosine monophosphate in a cell-free system of Escherichia coli. Folia Microbiol (Praha) 1967;12(1):1–5. doi: 10.1007/BF02895083. [DOI] [PubMed] [Google Scholar]
- Brickman E., Soll L., Beckwith J. Genetic characterization of mutations which affect catabolite-sensitive operons in Escherichia coli, including deletions of the gene for adenyl cyclase. J Bacteriol. 1973 Nov;116(2):582–587. doi: 10.1128/jb.116.2.582-587.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner M. J., Spitz E., Rickenberg H. V. Cyclic adenosine 3',5'-monophosphate in Escherichia coli. J Bacteriol. 1973 Jun;114(3):1068–1073. doi: 10.1128/jb.114.3.1068-1073.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd J. F., Wartell R. M., Dodgson J. B., Wells R. D. Transmission of stability (telestability) in deoxyribonucleic acid. Physical and enzymatic studies on the duplex block polymer d(C15A15) - d(T15G15). J Biol Chem. 1975 Jul 10;250(13):5109–5113. [PubMed] [Google Scholar]
- COHN M., HORIBATA K. Physiology of the inhibition by glucose of the induced synthesis of the beta-galactosideenzyme system of Escherichia coli. J Bacteriol. 1959 Nov;78:624–635. doi: 10.1128/jb.78.5.624-635.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Chambers D. A., Zubay G. The stimulatory effect of cyclic adenosine 3'5'-monophosphate on DNA-directed synthesis of beta-galactosidase in a cell-free system. Proc Natl Acad Sci U S A. 1969 May;63(1):118–122. doi: 10.1073/pnas.63.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., de Crombrugghe B., Anderson W. B., Gottesman M. E., Pastan I., Perlman R. L. On the mechanism of action of lac repressor. Nat New Biol. 1971 Sep 15;233(37):67–70. doi: 10.1038/newbio233067a0. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Magasanik B. Transcription of the lac operon of Escherichia coli. J Biol Chem. 1974 Oct 25;249(20):6556–6561. [PubMed] [Google Scholar]
- Crepin M., Cukier-Kahn R., Gros F. Effect of a low-molecular-weight DNA binding protein, H1 factor, on the in vitro transcription of the lactose operon in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jan;72(1):333–337. doi: 10.1073/pnas.72.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R., Morse H. G., Morse M. L. Carbohydrate transport and cyclic 3',5' adenosine monophosphate (cAMP) levels in a temperature sensitive phosphotransferase mutant of Escherichia coli. Mol Gen Genet. 1974 Mar 6;129(1):1–10. doi: 10.1007/BF00269261. [DOI] [PubMed] [Google Scholar]
- De Crombrugghe B., Chen B., Anderson W., Nissley P., Gottesman M., Pastan I., Perlman R. Lac DNA, RNA polymerase and cyclic AMP receptor protein, cyclic AMP, lac repressor and inducer are the essential elements for controlled lac transcription. Nat New Biol. 1971 Jun 2;231(22):139–142. doi: 10.1038/newbio231139a0. [DOI] [PubMed] [Google Scholar]
- De Crombrugghe B., Pastan I., Shaw W. V., Rosner J. L. Stimulation by cyclic AMP and ppGpp of chloramphenicol acetyl transferase synthesis. Nat New Biol. 1973 Feb 21;241(112):237–239. doi: 10.1038/newbio241237a0. [DOI] [PubMed] [Google Scholar]
- De Robertis E. M., Jr, Judewicz R. D., Torres H. N. On the control mechanism of bacterial growth by cyclic adenosine 3',5'-monophosphate. Biochem Biophys Res Commun. 1973 Dec 10;55(3):758–764. doi: 10.1016/0006-291x(73)91209-6. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Echols H., Green L. Establishment and maintenance of repression by bacteriophage lambda: the role of the cI, cII, and c3 proteins. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2190–2194. doi: 10.1073/pnas.68.9.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen H., Brachet P., Pereira da Silva L., Jacob F. Regulation of repressor expression in lambda. Proc Natl Acad Sci U S A. 1970 Jul;66(3):855–862. doi: 10.1073/pnas.66.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Rothman-Denes L. B., Hesse J. Adenosine 3':5'-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2300–2304. doi: 10.1073/pnas.72.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Maxam A. The nucleotide sequence of the lac operator. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3581–3584. doi: 10.1073/pnas.70.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. The lac operator is DNA. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2415–2421. doi: 10.1073/pnas.58.6.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J. E., Peterkofsky A. Diverse directional changes of cGMP relative to cAMP in E. coli. Biochem Biophys Res Commun. 1975 Nov 3;67(1):190–197. doi: 10.1016/0006-291x(75)90301-0. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Arditti R. R., Eisen H. Establishment of repression by lambdoid phage in catabolite activator protein and adenylate cyclase mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Feb;69(2):366–370. doi: 10.1073/pnas.69.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Hochman J., Insel P. A., Bourne H. R., Coffino P., Tomkins G. M. A structural gene mutation affecting the regulatory subunit of cyclic AMP-dependent protein kinase in mouse lymphoma cells. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5051–5055. doi: 10.1073/pnas.72.12.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofnung M. Divergent operons and the genetic structure of the maltose B region in Escherichia coli K12. Genetics. 1974 Feb;76(2):169–184. doi: 10.1093/genetics/76.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Smith G. R., Ames B. N. Adenosine 3':5'-cyclic monophosphate concentration in the bacterial host regulates the viral decision between lysogeny and lysis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2258–2262. doi: 10.1073/pnas.68.9.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins J. D. A new class of promoter mutations in the lactose operon of Escherichia coli. J Mol Biol. 1974 Aug 25;87(4):715–724. doi: 10.1016/0022-2836(74)90080-1. [DOI] [PubMed] [Google Scholar]
- Ide M. Adenyl cyclase of Escherichia coli. Biochem Biophys Res Commun. 1969 Jul 7;36(1):42–46. doi: 10.1016/0006-291x(69)90646-9. [DOI] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Kepes A. The step sensitive to catabolite repression and its reversal by 3'-5' cyclic AMP during induced synthesis of beta-galactosidase in E. coli. Biochem Biophys Res Commun. 1969 Jul 7;36(1):84–92. doi: 10.1016/0006-291x(69)90653-6. [DOI] [PubMed] [Google Scholar]
- Jordan E., Green L., Echols H. Establishment of repression by bacteriophage lambda: lack of a direct regulatory effect of cyclic AMP. Virology. 1973 Oct;55(2):521–523. doi: 10.1016/0042-6822(73)90194-3. [DOI] [PubMed] [Google Scholar]
- KAISER A. D. Mutations in a temperate bacteriophage affecting its ability to lysogenize Escherichia coli. Virology. 1957 Feb;3(1):42–61. doi: 10.1016/0042-6822(57)90022-3. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow J. S. Cyclic adenosine monophosphate receptor: effect of cyclic AMP analogues on DNA binding and proteolytic inactivation. Biochim Biophys Acta. 1975 Apr 2;383(4):345–350. doi: 10.1016/0005-2787(75)90303-2. [DOI] [PubMed] [Google Scholar]
- Krakow J. S., Pastan I. Cyclic adenosine monophosphate receptor: loss of cAMP-dependent DNA binding activity after proteolysis in the presence of cyclic adenosine monophosphate. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2529–2533. doi: 10.1073/pnas.70.9.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. An adenosine 3',5'-monophosphate-dependent protein kinase from Escherichia coli. J Biol Chem. 1969 Jun 25;244(12):3417–3419. [PubMed] [Google Scholar]
- LOOMIS W. F., Jr, MAGASANIK B. THE RELATION OF CATABOLITE REPRESSION TO THE INDUCTION SYSTEM FOR BETA-GALACTOSIDASE IN ESCHERICHIA COLI. J Mol Biol. 1964 Mar;8:417–426. doi: 10.1016/s0022-2836(64)80205-9. [DOI] [PubMed] [Google Scholar]
- Li H. C., Brown G. G. Orthophosphate and histone dependent polyphosphate kinase from E. coli. Biochem Biophys Res Commun. 1973 Aug 6;53(3):875–881. doi: 10.1016/0006-291x(73)90174-5. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Photochemical attachment of lac repressor to bromodeoxyuridine-substituted lac operator by ultraviolet radiation. Proc Natl Acad Sci U S A. 1974 Mar;71(3):947–951. doi: 10.1073/pnas.71.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis J. T., Schleif R. Different cyclic AMP requirements for induction of the arabinose and lactose operons of Escherichia coli. J Mol Biol. 1973 Sep 5;79(1):149–162. doi: 10.1016/0022-2836(73)90276-3. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Jr, Magasanik B. Nature of the effector of catabolite repression of beta-galactosidase in Escherichia coli. J Bacteriol. 1966 Jul;92(1):170–177. doi: 10.1128/jb.92.1.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- MARKOVITZ A. REGULATORY MECHANISMS FOR SYNTHESIS OF CAPSULAR POLYSACCHARIDE IN MUCOID MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1964 Feb;51:239–246. doi: 10.1073/pnas.51.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchia V., Varrone S., Weissbach H., Miller D. L. Guanylate cyclase in Escherichia coli. Purification and properties. J Biol Chem. 1975 Aug 25;250(16):6214–6217. [PubMed] [Google Scholar]
- Majors J. Specific binding of CAP factor to lac promoter DNA. Nature. 1975 Aug 21;256(5519):672–674. doi: 10.1038/256672a0. [DOI] [PubMed] [Google Scholar]
- Miller Z., Varmus H. E., Parks J. S., Perlman R. L., Pastan I. Regulation of gal messenger ribonucleic acid synthesis in Escherichia coli by 3',5'-cyclic adenosine monophosphate. J Biol Chem. 1971 May 10;246(9):2898–2903. [PubMed] [Google Scholar]
- Monard D., Janecek J., Rickenberg H. V. The enzymic degradation of 3',5' cyclic AMP in strains of E. Coli sensitive and resistant to catobolite repression. Biochem Biophys Res Commun. 1969 May 22;35(4):584–591. doi: 10.1016/0006-291x(69)90388-x. [DOI] [PubMed] [Google Scholar]
- NAKADA D., MAGASANIK B. THE ROLES OF INDUCER AND CATABOLITE REPRESSOR IN THE SYNTHESIS OF BETA-GALACTOSIDASE BY ESCHERICHIA COLI. J Mol Biol. 1964 Jan;8:105–127. doi: 10.1016/s0022-2836(64)80153-4. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Adhya S., Gottesman M., Pastan I. Activation of transcription at specific promoters by glycerol. J Biol Chem. 1974 Jul 10;249(13):4050–4056. [PubMed] [Google Scholar]
- Nakanishi S., Adhya S., Gottesman M., Pastan I. Selective effects of MgCl2 and temperature on the initiation of transcription at lac, gal, and lambda promoters. J Biol Chem. 1975 Oct 25;250(20):8202–8208. [PubMed] [Google Scholar]
- Nisseley S. P., Anderson W. B., Gottesman M. E., Perlman R. L., Pastan I. In vitro transcription of the gal operon requires cyclic adenosine monophosphate and cyclic adenosine monophosphate receptor protein. J Biol Chem. 1971 Aug 10;246(15):4671–4678. [PubMed] [Google Scholar]
- Nissley P., Anderson W. B., Gallo M., Pastan I., Perlman R. L. The binding of cyclic adenosine monophosphate receptor to deoxyribonucleic acid. J Biol Chem. 1972 Jul 10;247(13):4264–4269. [PubMed] [Google Scholar]
- Novel G., Novel M., Didier-Fichet M. L., Stoeber F. Etude génétique de mutants du système de dégradation des hexuronides chez Escherichia coli K 12. C R Acad Sci Hebd Seances Acad Sci D. 1970 Jul 27;271(4):457–460. [PubMed] [Google Scholar]
- Parks J. S., Gottesman M., Perlman R. L., Pastan I. Regulation of galactokinase synthesis by cyclic adenosine 3',5'-monophosphate in cell-free extracts of Escherichia coli. J Biol Chem. 1971 Apr 25;246(8):2419–2424. [PubMed] [Google Scholar]
- Pastan I., Gallo M., Anderson W. B. The purification and analysis of mechanism of action of a cyclic AMP-receptor protein from Escherichia coli. Methods Enzymol. 1974;38:367–376. doi: 10.1016/0076-6879(74)38053-6. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. Cyclic AMP in metabolism. Nat New Biol. 1971 Jan 6;229(1):5–7. doi: 10.1038/newbio229005a0. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. Regulation of gene transcription in Escherichia coli by cyclic AMP. Adv Cyclic Nucleotide Res. 1972;1:11–16. [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. Repression of beta-galactosidase synthesis by glucose in phosphotransferase mutants of Escherichia coli. Repression in the absence of glucose phosphorylation. J Biol Chem. 1969 Nov 10;244(21):5836–5842. [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Pearson M. L. The role of adenosine 3',5'-cyclic monophosphate in the growth of bacteriophage lambda. Virology. 1972 Aug;49(2):605–609. doi: 10.1016/0042-6822(72)90513-2. [DOI] [PubMed] [Google Scholar]
- Perlman R. L., De Crombrugghe B., Pastan I. Cyclic AMP regulates catabolite and transient repression in E. coli. Nature. 1969 Aug 23;223(5208):810–812. doi: 10.1038/223810a0. [DOI] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Pleiotropic deficiency of carbohydrate utilization in an adenyl cyclase deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1969 Sep 24;37(1):151–157. doi: 10.1016/0006-291x(69)90893-6. [DOI] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Regulation of beta-galactosidase synthesis in Escherichia coli by cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1968 Oct 25;243(20):5420–5427. [PubMed] [Google Scholar]
- Perlman R., Pastan I. Cyclic 3'5-AMP: stimulation of beta-galactosidase and tryptophanase induction in E. coli. Biochem Biophys Res Commun. 1968 Mar 27;30(6):656–664. doi: 10.1016/0006-291x(68)90563-9. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Interaction of enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system with adenylate cyclase of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2920–2924. doi: 10.1073/pnas.72.8.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Measurements of rates of adenosine 3':5'-cyclic monophosphate synthesis in intact Escherichia coli B. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2149–2152. doi: 10.1073/pnas.70.7.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piovant M., Lazdunski C. Different cyclic adenosine 3',5'-monophosphate requirements for induction of beta-galactosidase and tryptophanase. Effect of osmotic pressure on intracellular cyclic adenosine 3,5-monophosphate concentrations. Biochemistry. 1975 May 6;14(9):1821–1825. doi: 10.1021/bi00680a003. [DOI] [PubMed] [Google Scholar]
- Potter K., Chaloner-Larsson G., Yamazaki H. Abnormally high rate of cyclic AMP excretion from an Escherichia coli mutant deficient in cyclic AMP receptor protein. Biochem Biophys Res Commun. 1974 Mar 25;57(2):379–385. doi: 10.1016/0006-291x(74)90941-3. [DOI] [PubMed] [Google Scholar]
- Pribnow D. Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc Natl Acad Sci U S A. 1975 Mar;72(3):784–788. doi: 10.1073/pnas.72.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S., Miller R. E., Valentine R. C. Adenosine 3':5'-cyclic monophosphate control of the enzymes of glutamine metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2922–2926. doi: 10.1073/pnas.69.10.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R. N., Raj C. V. Salmonella typhimurium mutants affecting establishment of lysogeny. Mol Gen Genet. 1973 Sep 5;125(2):119–123. doi: 10.1007/BF00268864. [DOI] [PubMed] [Google Scholar]
- Reichardt L., Kaiser A. D. Control of lambda repressor synthesis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2185–2189. doi: 10.1073/pnas.68.9.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiness G., Zubay G. The messenger-directed synthesis of the alpha-fragment of the enzyme beta-galactosidase. Biochem Biophys Res Commun. 1973 Aug 6;53(3):967–974. doi: 10.1016/0006-291x(73)90186-1. [DOI] [PubMed] [Google Scholar]
- Rickenberg H. V. Cyclic AMP in prokaryotes. Annu Rev Microbiol. 1974;28(0):353–369. doi: 10.1146/annurev.mi.28.100174.002033. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Reiness G., Zubay G. Purification and DNA-binding properties of the catabolite gene activator protein. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1222–1225. doi: 10.1073/pnas.68.6.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U. Coordinate regulation of adenylate cyclase and carbohydrate permeases by the phosphoenolpyruvate:sugar phosphotransferase system in Salmonella typhimurium. J Biol Chem. 1975 Sep 10;250(17):7078–7080. [PubMed] [Google Scholar]
- Sanders R., McGeoch D. A mutant transcription factor that is activated by 3':5'-cyclic guanosine monophosphate. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1017–1021. doi: 10.1073/pnas.70.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran L., Pogell B. M. Differential inhibition of catabolite-sensitive enzyme induction by intercalating dyes. Nat New Biol. 1973 Oct 31;245(148):257–260. doi: 10.1038/newbio245257a0. [DOI] [PubMed] [Google Scholar]
- Scaife J., Beckwith J. R. Mutational alteration of the maximal level of Lac operon expression. Cold Spring Harb Symp Quant Biol. 1966;31:403–408. doi: 10.1101/sqb.1966.031.01.052. [DOI] [PubMed] [Google Scholar]
- Schaller H., Gray C., Herrmann K. Nucleotide sequence of an RNA polymerase binding site from the DNA of bacteriophage fd. Proc Natl Acad Sci U S A. 1975 Feb;72(2):737–741. doi: 10.1073/pnas.72.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher G., Ehring R. RNA-directed cell-free synthesis of the galactose enzymes of Escherichia coli. Mol Gen Genet. 1973 Aug 28;124(4):329–344. doi: 10.1007/BF00267662. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Sur l'existence chez Escherichia coli K 12 d'une régulation commune à la biosynthèse des récepteurs du bactériophage et au métabolisme du maltose. Ann Inst Pasteur (Paris) 1967 Nov;113(5):685–704. [PubMed] [Google Scholar]
- Silverstone A. E., Arditti R. R., Magasanik B. Catabolite-insensitive revertants of lac promoter mutants. Proc Natl Acad Sci U S A. 1970 Jul;66(3):773–779. doi: 10.1073/pnas.66.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone A. E., Magasanik B., Reznikoff W. S., Miller J. H., Beckwith J. R. Catabolite sensitive site of the lac operon. Nature. 1969 Mar 15;221(5185):1012–1014. doi: 10.1038/2211012b0. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Hays J. B., Nakazawa T., Roseman S. Sugar transport. VI. Phosphoryl transfer in the lactose phosphotransferase system of Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):957–965. [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Stoeber F., Lagarde A., Nemoz G., Novel G., Novel M., Portalier R., Pouyssegur J., Robert-Baudouy J. Le métabolisme des hexuronides et des hexuronates chez Escherichia coli K 12: aspects physiologiques et et génétiques de sa régulation. Biochimie. 1974;56(2):199–213. doi: 10.1016/s0300-9084(74)80379-2. [DOI] [PubMed] [Google Scholar]
- Tao M., Huberman A. Some properties of Escherichia coli adenyl cyclase. Arch Biochem Biophys. 1970 Nov;141(1):236–240. doi: 10.1016/0003-9861(70)90127-x. [DOI] [PubMed] [Google Scholar]
- Tao M., Lipmann F. Isolation of adenyl cyclase from Escherichia coli. Proc Natl Acad Sci U S A. 1969 May;63(1):86–92. doi: 10.1073/pnas.63.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B., Loomis W. F., Jr, Magasanik B. Transient repression of the lac operon. J Bacteriol. 1967 Dec;94(6):2001–2011. doi: 10.1128/jb.94.6.2001-2011.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Perlman R. L., Pastan I. Regulation of lac messenger ribonucleic acid synthesis by cyclic adenosine 3',5'-monophosphate and glucose. J Biol Chem. 1970 May 10;245(9):2259–2267. [PubMed] [Google Scholar]
- Varmus H. E., Perlman R. L., Pastan I. Regulation of lac transcription in Escherichia coli by cyclic adenosine 3',5'-monophosphate. Studies with deoxyribonucleic acid-ribonucleic acid hybridization and hybridization competition. J Biol Chem. 1970 Dec 10;245(23):6366–6372. [PubMed] [Google Scholar]
- WEISSBACH A., JACOB F. Effect of glucose on the formation of bacteriophage lambda. Nature. 1962 Jan 13;193:197–198. doi: 10.1038/193197a0. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Barkley M. D., Bourgeois S. Measurements of unwinding of lac operator by repressor. Nature. 1974 Sep 20;251(5472):247–249. doi: 10.1038/251247a0. [DOI] [PubMed] [Google Scholar]
- Wayne P. K., Fetell J., Rosen O. M. Measurement of cyclic 3',5'-adenosine monophosphate synthesis in growing Escherichia coli. Biochem Biophys Res Commun. 1975 May 5;64(1):81–88. doi: 10.1016/0006-291x(75)90222-3. [DOI] [PubMed] [Google Scholar]
- Wayne P. K., Rosen O. M. Cyclic 3':5'-adenosine monophosphate in Escherichia coli during transient and catabolite repression. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1436–1440. doi: 10.1073/pnas.71.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. W., Wu F. Y. Conformational transitions of cyclic adenosine monophosphate receptor protein of Escherichia coli. A temperature-jump study. Biochemistry. 1974 Jun 4;13(12):2573–2578. doi: 10.1021/bi00709a016. [DOI] [PubMed] [Google Scholar]
- Wu F. Y., Nath K., Wu C. W. Conformational transitions of cyclic adenosine monophosphate receptor protein of Escherichia coli. A fluorescent probe study. Biochemistry. 1974 Jun 4;13(12):2567–2572. doi: 10.1021/bi00709a015. [DOI] [PubMed] [Google Scholar]
- Yokota T., Gots J. S. Requirement of adenosine 3', 5'-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1970 Aug;103(2):513–516. doi: 10.1128/jb.103.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Kasuga T. Requirement of adenosine 3',5'-cyclic phosphate for formation of the phage lambda receptor in Escherichia coli. J Bacteriol. 1972 Mar;109(3):1304–1306. doi: 10.1128/jb.109.3.1304-1306.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G., Gielow L., Englesberg E. Cell-free studies on the regulation of the arabinose operon. Nat New Biol. 1971 Oct 6;233(40):164–165. doi: 10.1038/newbio233164a0. [DOI] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crombrugghe B., Varmus H. E., Perlman R. L., Pastan I. H. Stimulation of lac mRNA synthesis by cyclic AMP in cell free extracts of Escherichia coli. Biochem Biophys Res Commun. 1970 Mar 12;38(5):894–901. doi: 10.1016/0006-291x(70)90805-3. [DOI] [PubMed] [Google Scholar]



