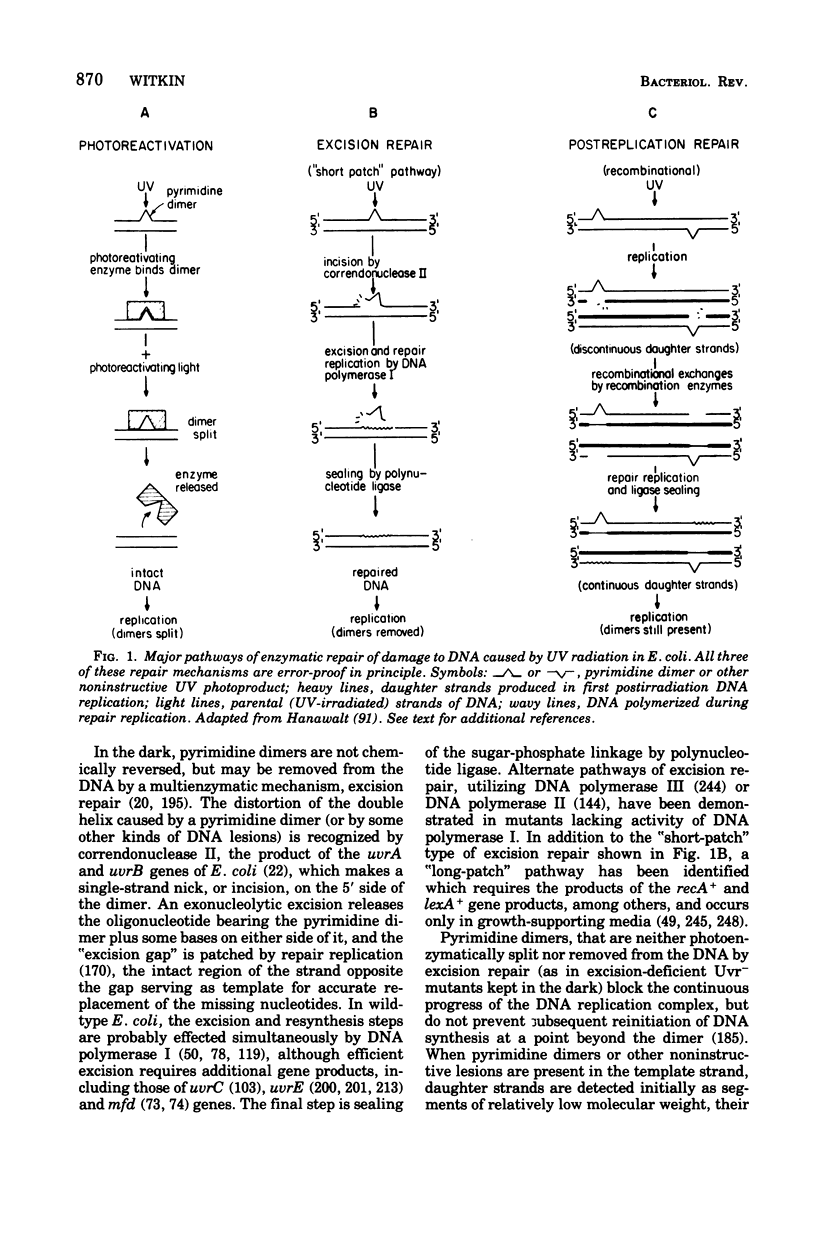
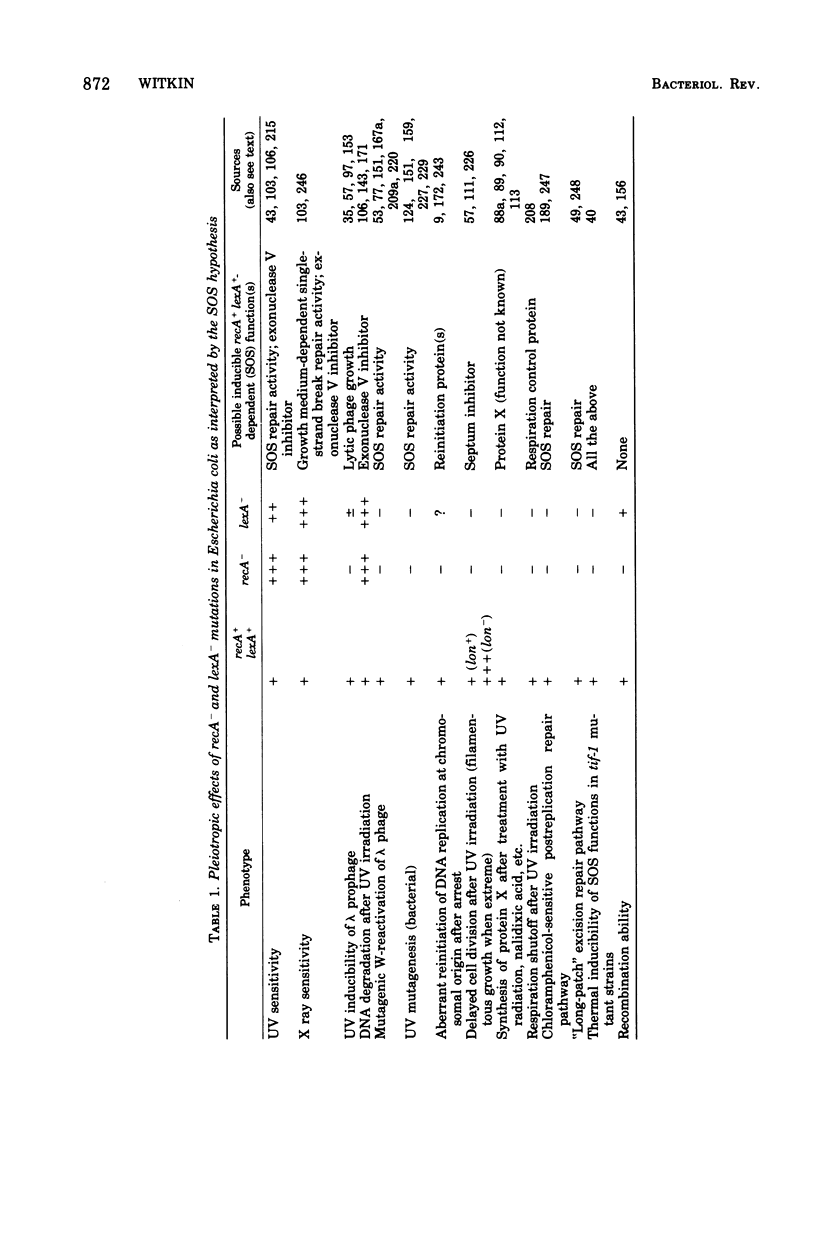
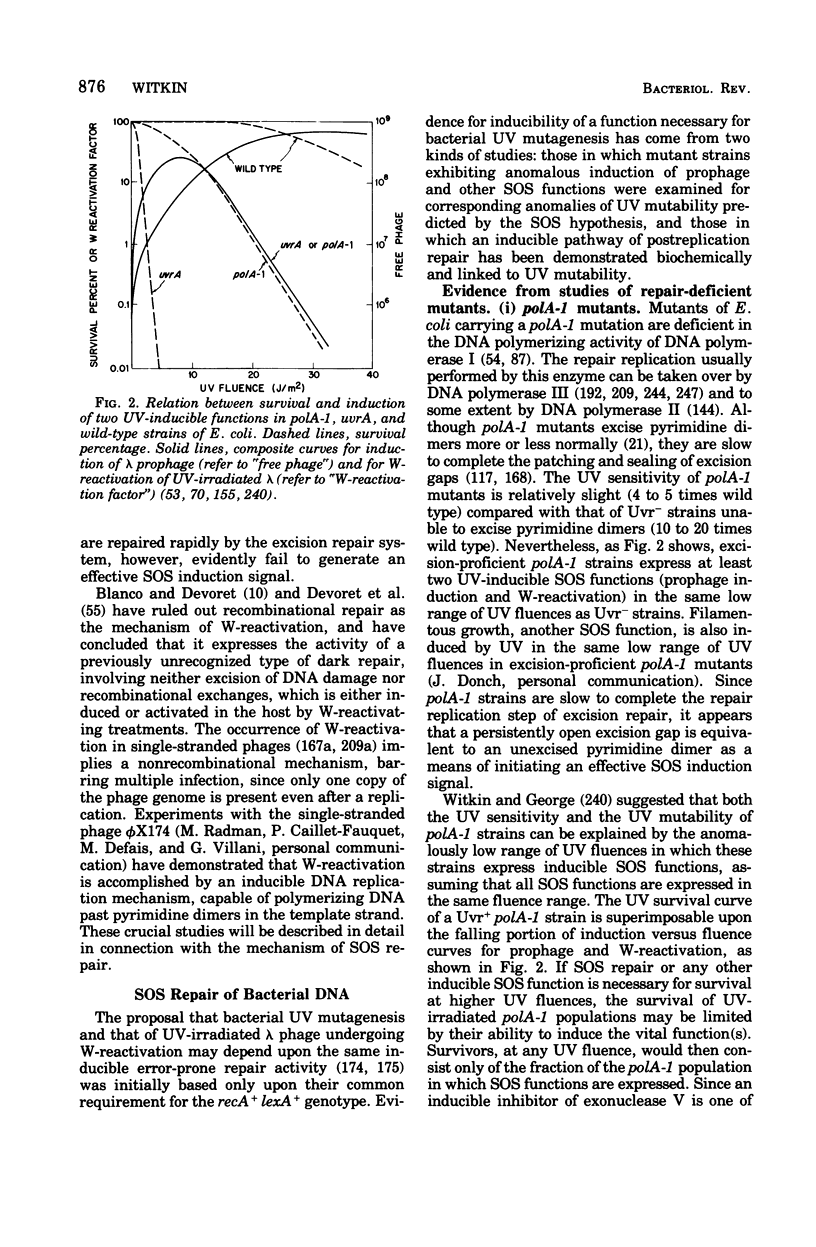
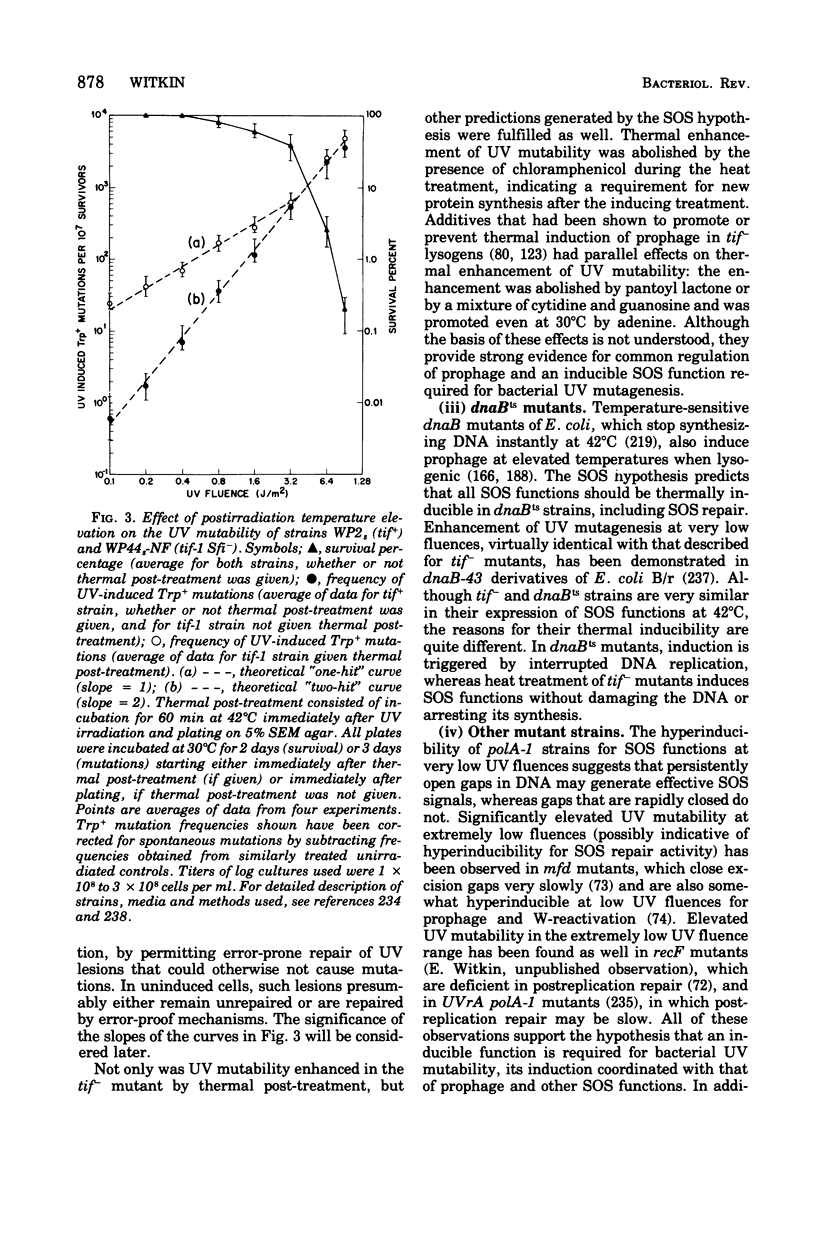
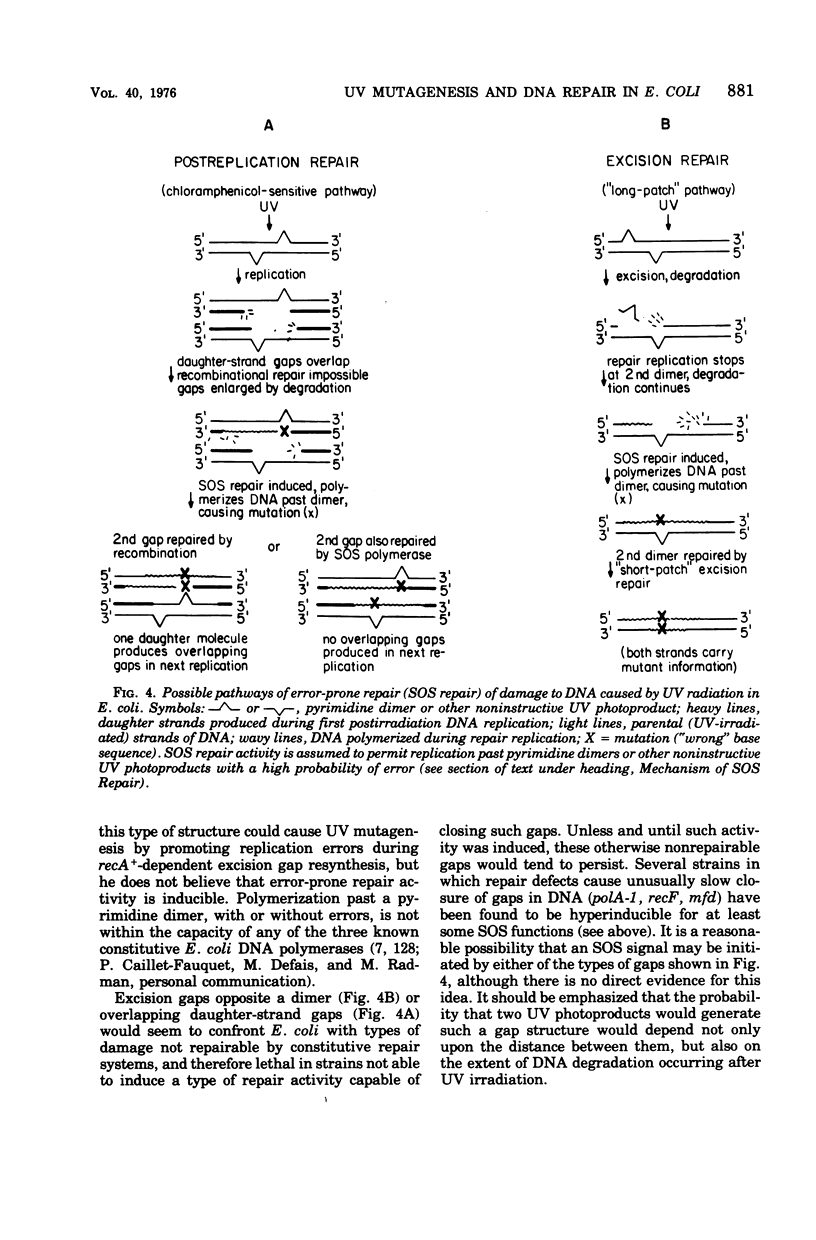
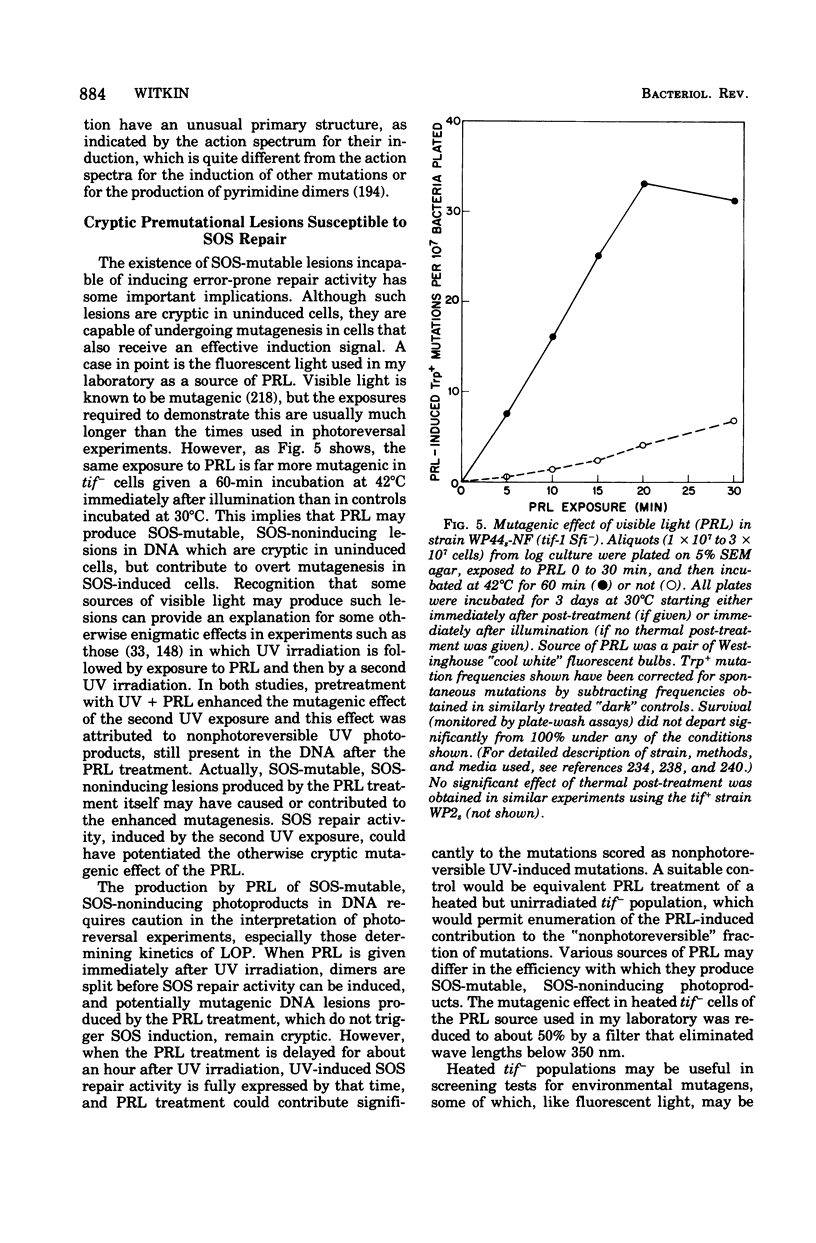
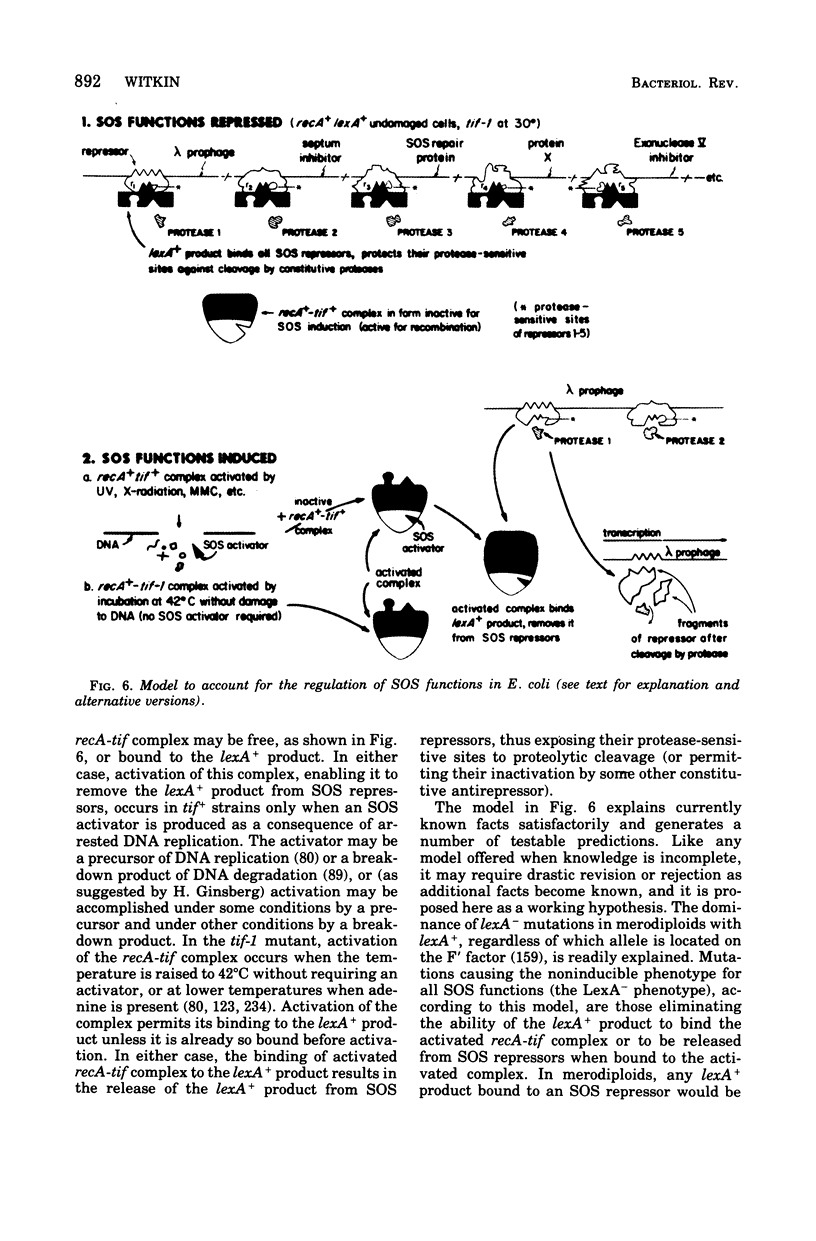
Full text
PDF































Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLER H. I., HARDIGREE A. A. POSTIRRADIATION GROWTH, DIVISION, AND RECOVERY IN BACTERIA. Radiat Res. 1965 May;25:92–102. [PubMed] [Google Scholar]
- ALPER T., GILLIES N. E. Restoration of Escherichia coli strain B after irradiation: its dependence on suboptimal growth conditions. J Gen Microbiol. 1958 Apr;18(2):461–472. doi: 10.1099/00221287-18-2-461. [DOI] [PubMed] [Google Scholar]
- ANDERSON E. H. Heat reactivation of ultraviolet-inactivated bacteria. J Bacteriol. 1951 Apr;61(4):389–394. doi: 10.1128/jb.61.4.389-394.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler H. I., Fisher W. D., Hardigree A. A., Stapleton G. E. Repair of radiation-induced damage to the cell division mechanism of Escherichia coli. J Bacteriol. 1966 Feb;91(2):737–742. doi: 10.1128/jb.91.2.737-742.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N., Durston W. E., Yamasaki E., Lee F. D. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2281–2285. doi: 10.1073/pnas.70.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte B. N., Rhodes H., Zipser D. Mutation blocking the specific degradation of reinitiation polypeptides in E. coli. Nature. 1975 Sep 25;257(5524):329–331. doi: 10.1038/257329a0. [DOI] [PubMed] [Google Scholar]
- BEUKERS R., BERENDS W. Isolation and identification of the irradiation product of thymine. Biochim Biophys Acta. 1960 Jul 15;41:550–551. doi: 10.1016/0006-3002(60)90063-9. [DOI] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Is terminal deoxynucleotidyl transferase a somatic mutagen in lymphocytes? Nature. 1974 Mar 29;248(447):409–411. doi: 10.1038/248409a0. [DOI] [PubMed] [Google Scholar]
- Beckwith J., Rossow P. Analysis of genetic regulatory mechanisms. Annu Rev Genet. 1974;8:1–13. doi: 10.1146/annurev.ge.08.120174.000245. [DOI] [PubMed] [Google Scholar]
- Benbow R. M., Zuccarelli A. J., Sinsheimer R. L. A role for single-strand breaks in bacteriophage phi-X174 genetic recombination. J Mol Biol. 1974 Sep 25;88(3):629–651. doi: 10.1016/0022-2836(74)90414-8. [DOI] [PubMed] [Google Scholar]
- Billen D. Replication of the bacterial chromosome: location of new initiation sites after irradiation. J Bacteriol. 1969 Mar;97(3):1169–1175. doi: 10.1128/jb.97.3.1169-1175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco M., Devoret R. Repair mechanisms involved in prophage reactivation and UV reactivation of UV-irradiated phage lambda. Mutat Res. 1973 Mar;17(3):293–305. doi: 10.1016/0027-5107(73)90001-8. [DOI] [PubMed] [Google Scholar]
- Blanco M., Levine A., Devoret R. IexB: a new gene governing radiation sensitivity and lysogenic induction in Escherichia coli K12. Basic Life Sci. 1975;5A:379–382. doi: 10.1007/978-1-4684-2895-7_50. [DOI] [PubMed] [Google Scholar]
- Bockrath R., Cheung M. K. The role of nutrient broth supplementation in UV mutagenesis of E. coli. Mutat Res. 1973 Jul;19(1):23–32. doi: 10.1016/0027-5107(73)90109-7. [DOI] [PubMed] [Google Scholar]
- Bockstahler L. E., Lytle C. D., Stafford J. E., Haynes K. F. Ultraviolet enhanced reactivation of a human virus: effect of delayed infection. Mutat Res. 1976 Jun;35(2):189–198. doi: 10.1016/0027-5107(76)90184-6. [DOI] [PubMed] [Google Scholar]
- Bockstahler L. E., Lytle C. D. X-ray-enhanced reactivation of ultraviolet-irradiated human virus. J Virol. 1971 Oct;8(4):601–602. doi: 10.1128/jvi.8.4.601-602.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonura T., Smith K. C. Enzymatic production of deoxyribonucleic acid double-strand breaks after ultraviolet irradiation of Escherichia coli K-12. J Bacteriol. 1975 Feb;121(2):511–517. doi: 10.1128/jb.121.2.511-517.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonura T., Smith K. C. Quantitative evidence for enzymatically-indeced DNA double-strand breaks as lethal lesions in UV irradiated pol+ and polA1 strains of E. coli K-12. Photochem Photobiol. 1975 Dec;22(6):243–248. doi: 10.1111/j.1751-1097.1975.tb06743.x. [DOI] [PubMed] [Google Scholar]
- Borek E., Ryan A. THE TRANSFER OF IRRADIATION-ELICITED INDUCTION IN A LYSOGENIC ORGANISM. Proc Natl Acad Sci U S A. 1958 May;44(5):374–377. doi: 10.1073/pnas.44.5.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. M., Paterson M. C., Setlow R. B. Excision-repair properties of an Escherichia coli mutant deficient in DNA polymerase. Nature. 1970 May 23;226(5247):708–710. doi: 10.1038/226708a0. [DOI] [PubMed] [Google Scholar]
- Braun A., Grossman L. An endonuclease from Escherichia coli that acts preferentially on UV-irradiated DNA and is absent from the uvrA and uvrB mutants. Proc Natl Acad Sci U S A. 1974 May;71(5):1838–1842. doi: 10.1073/pnas.71.5.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresler S. E., Mosevitsky M. I., Vyacheslavov L. G. Mutations as possible replication errors in bacteria growing under conditions of thymine deficiency. Mutat Res. 1973 Sep;19(3):281–293. doi: 10.1016/0027-5107(73)90228-5. [DOI] [PubMed] [Google Scholar]
- Bridges B. A. A note on the mechanism of UV mutagenesis in Escherichia coli. Mutat Res. 1966 Aug;3(4):273–279. doi: 10.1016/0027-5107(66)90034-0. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Dennis R. E., Munson R. J. Differential induction and repair of ultraviolet damage leading to true revesions and external suppressor mutations of an ochre codon in Escherichia coli B-r WP2. Genetics. 1967 Dec;57(4):897–908. doi: 10.1093/genetics/57.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges B. A. Genetic and physiological separation of the repair and mutagenic functions of the exrA gene in Escherichia coli. Genetics. 1973 Apr;73(Suppl):123–129. [PubMed] [Google Scholar]
- Bridges B. A., Law J., Munson R. J. Mutagenesis in Escherichia coli. II. Evidence for a common pathway for mutagenesis by ultraviolet light, ionizing radiation and thymine deprivation. Mol Gen Genet. 1968;103(3):266–273. doi: 10.1007/BF00273698. [DOI] [PubMed] [Google Scholar]
- Bridges B. A. Mechanisms of radiation mutagenesis in cellular and subcellular systems. Annu Rev Nucl Sci. 1969;19:139–178. doi: 10.1146/annurev.ns.19.120169.001035. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. P. Mutagenic DNA repair in Escherichia coli. III. Requirement for a function of DNA polymerase III in ultraviolet-light mutagenesis. Mol Gen Genet. 1976 Feb 27;144(1):53–58. doi: 10.1007/BF00277304. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. RecA + -dependent mutagenesis occurring before DNA replication in UV- and -irradiated Escherichia coli. Mutat Res. 1971 Sep;13(1):1–8. doi: 10.1016/0027-5107(71)90120-5. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Munson R. J. Excision-repair of DNA damage in an auxotrophic strain of Escherichia coli. Biochem Biophys Res Commun. 1966 Feb 3;22(3):268–273. doi: 10.1016/0006-291x(66)90476-1. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Rothwell M. A., Green M. H. Repair processes and dose-response curves in ultraviolet mutagenesis of bacteria. An Acad Bras Cienc. 1973;45(Suppl):203–209. [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Gene regulation for higher cells: a theory. Science. 1969 Jul 25;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Brooks K., Clark A. J. Behavior of lambda bacteriophage in a recombination deficienct strain of Escherichia coli. J Virol. 1967 Apr;1(2):283–293. doi: 10.1128/jvi.1.2.283-293.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl S. N., Setlow R. B., Regan J. D. Recovery of the ability to synthesize DNA in segments of normal size at long times after ultraviolet irradiation of human cells. Biophys J. 1973 Dec;13(12):1265–1275. doi: 10.1016/S0006-3495(73)86061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush J. W., Markovitz A. The Genetic Basis for Mucoidy and Radiation Sensitivity in capR (lon) Mutants of E. coli K-12. Genetics. 1973 Jun;74(2):215–225. doi: 10.1093/genetics/74.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillet-Fauquet P., Defais M. UV reactivation of phage lambda in a polA mutant of E. coli. Mutat Res. 1972 Jul;15(3):353–355. doi: 10.1016/0027-5107(72)90081-4. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. [Prophage induction and cell division in E. coli. II. Linked (recA, zab) and unlinked (lex) suppressors of tif-1-mediated induction and filamentation]. Mol Gen Genet. 1972;119(2):153–174. doi: 10.1007/BF00269134. [DOI] [PubMed] [Google Scholar]
- Cheung M. K., Bockrath R. C. On the specificity of UV mutagenesis in E. coli. Mutat Res. 1970 Nov;10(5):521–523. doi: 10.1016/0027-5107(70)90015-1. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Toward a metabolic interpretation of genetic recombination of E. coli and its phages. Annu Rev Microbiol. 1971;25:437–464. doi: 10.1146/annurev.mi.25.100171.002253. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Bootsma D. Xeroderma pigmentosum: biochemical and genetic characteristics. Annu Rev Genet. 1975;9:19–38. doi: 10.1146/annurev.ge.09.120175.000315. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968 May 18;218(5142):652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Xeroderma pigmentosum: a human disease in which an initial stage of DNA repair is defective. Proc Natl Acad Sci U S A. 1969 Jun;63(2):428–435. doi: 10.1073/pnas.63.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. S., Hutton J. J., De Simone P., Bollum F. J. Terminal deoxyribonucleotidyl transferase in human leukemia. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4404–4408. doi: 10.1073/pnas.71.11.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P. K., Hanawalt P. C. Heterogeneity of patch size in repair replicated DNA in Escherichia coli. J Mol Biol. 1972 Jun 14;67(1):1–10. doi: 10.1016/0022-2836(72)90381-6. [DOI] [PubMed] [Google Scholar]
- Cooper P. K., Hanawalt P. C. Role of DNA polymerase I and the rec system in excision-repair in Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1156–1160. doi: 10.1073/pnas.69.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio S. M., Setlow R. B. Enhancement of postreplication repair in Chinese hamster cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2396–2400. doi: 10.1073/pnas.73.7.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELAPORTE B. La restauration par voisinage chez des bactéries irradiées par des rayons X. Ann Inst Pasteur (Paris) 1956 Nov;91(5):727–735. [PubMed] [Google Scholar]
- DEMEREC M., CAHN E. Studies of mutability in nutritionally deficient strains of Escherichia coli. I. Genetic analysis of five auxotrophic strains. J Bacteriol. 1953 Jan;65(1):27–36. doi: 10.1128/jb.65.1.27-36.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Defais M., Caillet-Fauquet P., Fox M. S., Radman M. Induction kinetics of mutagenic DNA repair activity in E. coli following ultraviolet irradiation. Mol Gen Genet. 1976 Oct 18;148(2):125–130. doi: 10.1007/BF00268375. [DOI] [PubMed] [Google Scholar]
- Defais M., Fauquet P., Radman M., Errera M. Ultraviolet reactivation and ultraviolet mutagenesis of lambda in different genetic systems. Virology. 1971 Feb;43(2):495–503. doi: 10.1016/0042-6822(71)90321-7. [DOI] [PubMed] [Google Scholar]
- Devoret R., Blanco M., George J., Radman M. Recovery of phage lambda from ultraviolet damage. Basic Life Sci. 1975;5A:155–171. doi: 10.1007/978-1-4684-2895-7_20. [DOI] [PubMed] [Google Scholar]
- Devoret R., George J. Induction indirecte du prophage lambda par le rayonnement ultraviolet. Mutat Res. 1967 Nov-Dec;4(6):713–734. doi: 10.1016/0027-5107(67)90081-4. [DOI] [PubMed] [Google Scholar]
- Donch J. J., Greenberg J. Suppression of filamentation in a new lex mutant by a linked (lexA) mutation in Escherichia coli. Mutat Res. 1976 Mar;34(3):533–538. doi: 10.1016/0027-5107(76)90228-1. [DOI] [PubMed] [Google Scholar]
- Donch J. J., Greenberg J. The effect of lex on UV sensitivity, filament formation and lambda induction in ion mutants of Escherichia coli. Mol Gen Genet. 1974;128(4):277–281. doi: 10.1007/BF00268515. [DOI] [PubMed] [Google Scholar]
- Donch J., Greenberg J., Green M. H. Repression of induction by U.V. of lambda phage by exrA mutations in Escherichia coli. Genet Res. 1970 Feb;15(1):87–97. doi: 10.1017/s0016672300001397. [DOI] [PubMed] [Google Scholar]
- Doubleday O. P., Bridges B. A., Green M. H. Mutagenic DNA repair in Escherichia coli. II. Factors affecting loss of photoreversibility of UV induced mutations. Mol Gen Genet. 1975 Oct 3;140(3):221–230. doi: 10.1007/BF00334267. [DOI] [PubMed] [Google Scholar]
- Doudney C O, Young C S. Ultraviolet Light Induced Mutation and Deoxyribonucleic Acid Replication in Bacteria. Genetics. 1962 Sep;47(9):1125–1138. doi: 10.1093/genetics/47.9.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudney C. O. Complexity of the ultraviolet mutation frequency response curve in Escherichia coli B/r: SOS induction, one-lesion and two-lesion mutagenesis. J Bacteriol. 1976 Dec;128(3):815–826. doi: 10.1128/jb.128.3.815-826.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudney C. O. The two-lesion hypothesis for UV-induced mutation in relation to recovery of capacity for DNA replication. Basic Life Sci. 1975;5A:389–392. doi: 10.1007/978-1-4684-2895-7_52. [DOI] [PubMed] [Google Scholar]
- Drabble W. T., Stocker B. A. R (transmissible drug-resistance) factors in Salmonella typhimurium: pattern of transduction by phage P22 and ultraviolet-protection effect. J Gen Microbiol. 1968 Aug;53(1):109–123. doi: 10.1099/00221287-53-1-109. [DOI] [PubMed] [Google Scholar]
- Drake J. W., Baltz R. H. The biochemistry of mutagenesis. Annu Rev Biochem. 1976;45:11–37. doi: 10.1146/annurev.bi.45.070176.000303. [DOI] [PubMed] [Google Scholar]
- Emmerson P. T. Recombination deficient mutants of Escherichia coli K12 that map between thy A and argA. Genetics. 1968 Sep;60(1):19–30. doi: 10.1093/genetics/60.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyfjörd J. E., Green M. H., Bridges B. A. Mutagenic DNA repair in Escherichia coli: conditions for error-free filling of daughter strand gaps. J Gen Microbiol. 1975 Dec;91(2):369–375. doi: 10.1099/00221287-91-2-369. [DOI] [PubMed] [Google Scholar]
- GOLDTHWAIT D., JACOB F. SUR LE M'ECANISME DE L'INDUCTION DU D'EVELOPPEMENT DU PROPHAGE CHEZ LES BACT'ERIES LYSOG'ENES. C R Hebd Seances Acad Sci. 1964 Jul 20;259:661–664. [PubMed] [Google Scholar]
- Ganesan A. K. Persistence of pyrimidine dimers during post-replication repair in ultraviolet light-irradiated Escherichia coli K12. J Mol Biol. 1974 Jul 25;87(1):103–119. doi: 10.1016/0022-2836(74)90563-4. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K., Seawell P. C. The effect of lexA and recF mutations on post-replication repair and DNA synthesis in Escherichia coli K-12. Mol Gen Genet. 1975 Dec 1;141(3):189–205. doi: 10.1007/BF00341799. [DOI] [PubMed] [Google Scholar]
- Gellert M., Bullock M. L. DNA ligase mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1580–1587. doi: 10.1073/pnas.67.3.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D. L., Witkin E. M. Slow excision repair in an mfd mutant of Escherichia coli B/r. Mol Gen Genet. 1974;133(4):283–291. doi: 10.1007/BF00332704. [DOI] [PubMed] [Google Scholar]
- George D. L., Witkin E. M. Ultraviolet light-induced responses of an mfd mutant of Escherichia coli B/r having a slow rate of dimer excision. Mutat Res. 1975 Jun;28(3):347–354. doi: 10.1016/0027-5107(75)90229-8. [DOI] [PubMed] [Google Scholar]
- George J., Castellazzi M., Buttin G. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and lon strains and permit the expression of mutator properties of tif. Mol Gen Genet. 1975 Oct 22;140(4):309–332. [PubMed] [Google Scholar]
- George J., Devoret R. Conjugal transfer of UV-damaged F-prime sex factors and indirect induction of prophage- . Mol Gen Genet. 1971;111(2):103–119. doi: 10.1007/BF00267786. [DOI] [PubMed] [Google Scholar]
- George J., Devoret R., Radman M. Indirect ultraviolet-reactivation of phage lambda. Proc Natl Acad Sci U S A. 1974 Jan;71(1):144–147. doi: 10.1073/pnas.71.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Hicks M. L., Gellert M. Genetics and function of DNA ligase in Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):531–547. doi: 10.1016/0022-2836(73)90221-0. [DOI] [PubMed] [Google Scholar]
- Goze A., Sarsin A., Moulé Y., Devoret R. Induction and mutagenesis of prophage lambda in Escherichia coli K12 by metabolites of aflatoxin B1. Mutat Res. 1975 Apr;28(1):1–7. [PubMed] [Google Scholar]
- Green M. H., Greenberg J., Donch J. Effect of a recA gene on cell division and capsular polysaccharide production in a lon strain of Escherichia coli. Genet Res. 1969 Oct;14(2):159–162. doi: 10.1017/s0016672300001993. [DOI] [PubMed] [Google Scholar]
- Greenberg J., Berends L. J., Donch J., Green M. H. exrB: a malB-linked gene in Escherichia coli B involved in sensitivity to radiation and filament formation. Genet Res. 1974 Apr;23(2):175–184. doi: 10.1017/s0016672300014798. [DOI] [PubMed] [Google Scholar]
- Greenberg J., Berends L., Donch J., Johnson B. Reversion studies with exrB in Escherichia coli. Genet Res. 1975 Apr;25(2):109–117. doi: 10.1017/s0016672300015512. [DOI] [PubMed] [Google Scholar]
- Greenberg J., Donch J., Berends L. The dominance of exrB over exrB+ in heterodiploids of Escherichia coli. Genet Res. 1975 Feb;25(1):39–44. doi: 10.1017/s001667230001541x. [DOI] [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- Grossman L., Braun A., Feldberg R., Mahler I. Enzymatic repair of DNA. Annu Rev Biochem. 1975;44:19–43. doi: 10.1146/annurev.bi.44.070175.000315. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. DNA synthesis inhibition and the induction of protein X in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):459–477. doi: 10.1016/0022-2836(76)90240-0. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. Model for regulation of Escherichia coli DNA repair functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2330–2334. doi: 10.1073/pnas.72.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J. The induction of protein X in DNA repair and cell division mutants of Escherichia coli. J Mol Biol. 1976 Jul 5;104(3):567–587. doi: 10.1016/0022-2836(76)90121-2. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD B. D., TESSMAN I. IDENTIFICATION OF THE ALTERED BASES IN MUTATED SINGLE-STRANDED DNA. 3. MUTAGENESIS BY ULTRAVIOLET LIGHT. J Mol Biol. 1964 Aug;9:372–375. doi: 10.1016/s0022-2836(64)80214-x. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C. Molecular mechanisms involved in DNA repair. Genetics. 1975 Jun;79 (Suppl):179–197. [PubMed] [Google Scholar]
- Hanawalt P. Repair processes in diverse systems: overview. Basic Life Sci. 1975;5B:503–506. doi: 10.1007/978-1-4684-2898-8_12. [DOI] [PubMed] [Google Scholar]
- Hart R. W., Setlow R. B. Direct evidence that pyrimidine dimers in DNA result in neoplastic transformation. Basic Life Sci. 1975;5B:719–724. doi: 10.1007/978-1-4684-2898-8_47. [DOI] [PubMed] [Google Scholar]
- Hellman K. B., Haynes K. F., Bockstahler L. E. Radiation-enhanced survival of a human virus in normal and malignant rat cells. Proc Soc Exp Biol Med. 1974 Jan;145(1):255–262. doi: 10.3181/00379727-145-37788. [DOI] [PubMed] [Google Scholar]
- Hertman I., Luria S. E. Transduction studies on the role of a rec+ gene in the ultraviolet induction of prophage lambda. J Mol Biol. 1967 Jan 28;23(2):117–133. doi: 10.1016/s0022-2836(67)80021-4. [DOI] [PubMed] [Google Scholar]
- Hill R. F., Nestmann E. R. Effect of the recC gene in Escherichia coli on frequencies of ultraviolet-induced mutants. Mutat Res. 1973 Jan;17(1):27–36. doi: 10.1016/0027-5107(73)90250-9. [DOI] [PubMed] [Google Scholar]
- Hill R. F. Ultraviolet-induced lethality and reversion to prototrophy in Escherichia coli strains with normal and reduced dark repair ability. Photochem Photobiol. 1965 Jun;4(3):563–568. doi: 10.1111/j.1751-1097.1965.tb09774.x. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Howard-Flanders P. Repair by genetic recombination in bacteria: overview. Basic Life Sci. 1975;5A:265–274. doi: 10.1007/978-1-4684-2895-7_35. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Rupp W. D., Wilkins B. M., Cole R. S. DNA replication and recombination after UV irradiation. Cold Spring Harb Symp Quant Biol. 1968;33:195–207. doi: 10.1101/sqb.1968.033.01.023. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth S. Increase in frequency of ultraviolet-induced mutation brought about by the colicine factor, col-I in Salmonella typhimurium. Mutat Res. 1966 Apr;3(2):129–134. doi: 10.1016/0027-5107(66)90026-1. [DOI] [PubMed] [Google Scholar]
- Hull R. A. Effect of tsl mutations on Col E1 expression in a recA strain of Escherichia coli K-12. J Bacteriol. 1975 Aug;123(2):775–776. doi: 10.1128/jb.123.2.775-776.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa-Ryo H., Kondo S. Indirect mutagenesis in phage lambda by ultraviolet preirradiation of host bacteria. J Mol Biol. 1975 Sep 5;97(1):77–92. doi: 10.1016/s0022-2836(75)80023-4. [DOI] [PubMed] [Google Scholar]
- Ikenaga M., Ichikawa-Ryo H., Kondo S. The major cause of inactivation and mutation by 4-nitroquinoline 1-oixde in Escherichia coli: excisable 4NQO-purine adducts. J Mol Biol. 1975 Feb 25;92(2):341–356. doi: 10.1016/0022-2836(75)90233-8. [DOI] [PubMed] [Google Scholar]
- Inouye M., Guthrie J. P. A mutation which changes a membrane protein of E. coli. Proc Natl Acad Sci U S A. 1969 Nov;64(3):957–961. doi: 10.1073/pnas.64.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Pardee A. B. Changes of membrane proteins and their relation to deoxyribonucleic acid synthesis and cell division of Escherichia coli. J Biol Chem. 1970 Nov 10;245(21):5813–5819. [PubMed] [Google Scholar]
- Inouye M. Pleiotropic effect of the rec A gene of Escherichia coli: uncoupling of cell division from deoxyribonucleic acid replication. J Bacteriol. 1971 May;106(2):539–542. doi: 10.1128/jb.106.2.539-542.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer V. N., Rupp W. D. Usefulness of benzoylated naphthoylated DEAE-cellulose to distinguish and fractionate double-stranded DNA bearing different extents of single-stranded regions. Biochim Biophys Acta. 1971 Jan 1;228(1):117–126. doi: 10.1016/0005-2787(71)90551-x. [DOI] [PubMed] [Google Scholar]
- JACOB F. Mutation d'un bactériophage induite par l'irradiation des seules bactéries-hotes avant l'infection. C R Hebd Seances Acad Sci. 1954 Feb 8;238(6):732–734. [PubMed] [Google Scholar]
- KANAZIR D. The apparent mutagenicity of thymine deficiency. Biochim Biophys Acta. 1958 Oct;30(1):20–23. doi: 10.1016/0006-3002(58)90235-x. [DOI] [PubMed] [Google Scholar]
- Kanner L., Hanawalt P. Repair deficiency in a bacterial mutant defective in DNA polymerase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):149–155. doi: 10.1016/0006-291x(70)90770-9. [DOI] [PubMed] [Google Scholar]
- Kantor G. J., Deering R. A. Ultraviolet radiation studies of filamentous Escherichia coli B. J Bacteriol. 1966 Oct;92(4):1062–1069. doi: 10.1128/jb.92.4.1062-1069.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner A. Effect of Visible Light on the Recovery of Streptomyces Griseus Conidia from Ultra-violet Irradiation Injury. Proc Natl Acad Sci U S A. 1949 Feb;35(2):73–79. doi: 10.1073/pnas.35.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner A. PHOTOREACTIVATION OF ULTRAVIOLET-IRRADIATED ESCHERICHIA COLI, WITH SPECIAL REFERENCE TO THE DOSE-REDUCTION PRINCIPLE AND TO ULTRAVIOLET-INDUCED MUTATION. J Bacteriol. 1949 Oct;58(4):511–522. doi: 10.1128/jb.58.4.511-522.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby E. P., Jacob F., Goldthwait D. A. Prophage induction and filament formation in a mutant strain of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1903–1910. doi: 10.1073/pnas.58.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby E. P., Ruff W. L., Goldthwait D. A. Cell division and prophage induction in Escherichia coli: effects of pantoyl lactone and various furan derivatives. J Bacteriol. 1972 Aug;111(2):447–453. doi: 10.1128/jb.111.2.447-453.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S. Evidence that mutations are induced by errors in repair and replication. Genetics. 1973 Apr;73(Suppl):109–102. [PubMed] [Google Scholar]
- Kondo S., Ichikawa H. Evidence that pretreatment of Escherichia coli cells with N-methyl-N'-nitro-N-nitrosoguanidine enhances mutability of subsequently infecting phage lambda. Mol Gen Genet. 1973 Nov 22;126(4):319–324. doi: 10.1007/BF00269441. [DOI] [PubMed] [Google Scholar]
- Kondo S., Ichikawa H., Iwo K., Kato T. Base-change mutagenesis and prophage induction in strains of Escherichia coli with different DNA repair capacities. Genetics. 1970 Oct;66(2):187–217. doi: 10.1093/genetics/66.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATARJET R. Induction, par les rayons X, de la production d'un bactériophage chez B. megatherium lysogène. Ann Inst Pasteur (Paris) 1951 Oct;81(4):389–393. [PubMed] [Google Scholar]
- LWOFF A., SIMINOVITCH L., KJELDGAARD N. Induction de la production de bacteriophages chez une bactérie lysogène. Ann Inst Pasteur (Paris) 1950 Dec;79(6):815–859. [PubMed] [Google Scholar]
- Lawrence C. W., Christensen R. UV mutagenesis in radiation-sensitive strains of yeast. Genetics. 1976 Feb;82(2):207–232. doi: 10.1093/genetics/82.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A. R. Post-replication repair of DNA in ultraviolet-irradiated mammalian cells. No gaps in DNA synthesized late after ultraviolet irradiation. Eur J Biochem. 1972 Dec 18;31(3):438–445. doi: 10.1111/j.1432-1033.1972.tb02550.x. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972 May 28;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Springgate C. F., Battula N. Errors in DNA replication as a basis of malignant changes. Cancer Res. 1974 Sep;34(9):2311–2321. [PubMed] [Google Scholar]
- Lytle C. D., Benane S. G., Bockstahler L. E. Ultraviolet-enhanced reactivation of Herpes virus in human tumor cells. Photochem Photobiol. 1974 Aug;20(2):91–94. doi: 10.1111/j.1751-1097.1974.tb06554.x. [DOI] [PubMed] [Google Scholar]
- MARCOVICH H. Etude de l'action des rayons ultraviolets sur le système lysogène Escherichia coli K 12 (lambda), K 12 S, lambda. Ann Inst Pasteur (Paris) 1956 Oct;91(4):511–522. [PubMed] [Google Scholar]
- MARCOVICH H. Etude radiobiologique du système lysogène d'Escherichia coli K12. I. Rayons X. Ann Inst Pasteur (Paris) 1956 Mar;90(3):303–319. [PubMed] [Google Scholar]
- MELECHEN N. E., SKAAR P. D. The provocation of an early step of induction by thymine deprivation. Virology. 1962 Jan;16:21–29. doi: 10.1016/0042-6822(62)90198-8. [DOI] [PubMed] [Google Scholar]
- MacPhee D. G. Effect of rec mutations on the ultraviolet protecting and mutation-enhancing properties of the plasmid R-Utrecht in Salmonella typhimurium. Mutat Res. 1973 Sep;19(3):357–359. doi: 10.1016/0027-5107(73)90237-6. [DOI] [PubMed] [Google Scholar]
- MacPhee D. G. Effects of an R factor and caffeine on ultraviolet mutability in Salmonella typhimurium. Mutat Res. 1973 Jun;18(3):367–370. doi: 10.1016/0027-5107(73)90221-2. [DOI] [PubMed] [Google Scholar]
- Mackie G., Wilson D. B. Regulation of the gal operon of Escherichia coli by the capR gene. J Biol Chem. 1972 May 25;247(10):2973–2978. [PubMed] [Google Scholar]
- Maher V. M., Ouellette L. M., Curren R. D., McCormick J. J. Frequency of ultraviolet light-induced mutations is higher in xeroderma pigmentosum variant cells than in normal human cells. Nature. 1976 Jun 17;261(5561):593–595. doi: 10.1038/261593a0. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Pleiotropic effects of a DNA adenine methylation mutation (dam-3) in Escherichia coli K12. Mutat Res. 1975 Apr;28(1):15–26. doi: 10.1016/0027-5107(75)90309-7. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Pollard E. C., Ginoza W., Randall E. P. Involvement of recA and exr genes in the in vivo inhibition of the recBC nuclease. J Bacteriol. 1974 May;118(2):465–470. doi: 10.1128/jb.118.2.465-470.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey R., Smoler D. F., Baltimore D. Terminal deoxynucleotidyl transferase in a case of childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 1973 Feb;70(2):521–525. doi: 10.1073/pnas.70.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J., Spingarn N. E., Kobori J., Ames B. N. Detection of carcinogens as mutagens: bacterial tester strains with R factor plasmids. Proc Natl Acad Sci U S A. 1975 Mar;72(3):979–983. doi: 10.1073/pnas.72.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennigmann H. D. Pyrimidine dimers as pre-mutational lesions in Escherichia coli WP2 Hcr. Mol Gen Genet. 1972;117(2):167–186. doi: 10.1007/BF00267613. [DOI] [PubMed] [Google Scholar]
- Meyn R. E., Humphrey R. M. Deoxyribonucleic acid synthesis in ultraviolet-light-irradiated Chinese hamster cells. Biophys J. 1971 Mar;11(3):295–301. doi: 10.1016/S0006-3495(71)86215-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G. Peptidases and proteases of Escherichia coli and Salmonella typhimurium. Annu Rev Microbiol. 1975;29:485–504. doi: 10.1146/annurev.mi.29.100175.002413. [DOI] [PubMed] [Google Scholar]
- Monk M., Gross J. Induction of prophage lambda in a mutant of E. coli K12 defective in initiation of DNA replication at high temperature. Mol Gen Genet. 1971;110(4):299–306. doi: 10.1007/BF00438272. [DOI] [PubMed] [Google Scholar]
- Monk M. Induction of phage lambda by transferred irradiated colI DNA. Mol Gen Genet. 1969;106(1):14–24. doi: 10.1007/BF00332817. [DOI] [PubMed] [Google Scholar]
- Monk M. Observations on the mechanism of indirect induction by mating with ultraviolet-irradiated col I donors. Mol Gen Genet. 1967;100(3):264–274. doi: 10.1007/BF00381822. [DOI] [PubMed] [Google Scholar]
- Monk M., Peacey M., Gross J. D. Repair of damage induced by ultraviolet light in DNA polymerase-defective Escherichia coli cells. J Mol Biol. 1971 Jun 14;58(2):623–630. doi: 10.1016/0022-2836(71)90376-7. [DOI] [PubMed] [Google Scholar]
- Moreau P., Bailone A., Devoret R. Prophage lambda induction of Escherichia coli K12 envA uvrB: a highly sensitive test for potential carcinogens. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3700–3704. doi: 10.1073/pnas.73.10.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Pauling C. Induction of error-prone repair as a consequence of DNA ligase deficiency in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4645–4649. doi: 10.1073/pnas.72.11.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mound D. W., Walker A. C., Kosel C. Suppression of lex mutations affecting deoxyribonucleic acid repair in Escherichia coli K-12 by closely linked thermosensitive mutations. J Bacteriol. 1973 Nov;116(2):950–956. doi: 10.1128/jb.116.2.950-956.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W., Kosel C. K., Walker A. Inducible, error-free DNA Repair in tsl recA mutants of E. coli. Mol Gen Genet. 1976 Jul 5;146(1):37–41. doi: 10.1007/BF00267980. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Kosel C. Ultraviolet light-induced mutation in UV-resistant, thermosensitive derivatives of lexA-strains of Escherichia coli K-12. Mol Gen Genet. 1975;136(2):95–106. doi: 10.1007/BF00272033. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Low K. B., Edmiston S. J. Dominant mutations (lex) in Escherichia coli K-12 which affect radiation sensitivity and frequency of ultraviolet lght-induced mutations. J Bacteriol. 1972 Nov;112(2):886–893. doi: 10.1128/jb.112.2.886-893.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W., Walker A. C., Kosel C. Effect of tsl mutations in decreasing radiation sensitivity of a recA- strain of Escherichia coli K-12. J Bacteriol. 1975 Mar;121(3):1203–1207. doi: 10.1128/jb.121.3.1203-1207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W., Walker A. C., Kosel C. Indirect suppression of radiation sensitivity of a recA- strain of Escherichia coli K12. Basic Life Sci. 1975;5A:383–388. doi: 10.1007/978-1-4684-2895-7_51. [DOI] [PubMed] [Google Scholar]
- NOVICK A., SZILARD L. Experiments on light-reactivation of ultra-violet inactivated bacteria. Proc Natl Acad Sci U S A. 1949 Oct;35(10):591–600. doi: 10.1073/pnas.35.10.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. L., Mason H. S. An explicit hypothesis for chemical carcinogenesis. J Theor Biol. 1972 Oct;37(1):197–200. doi: 10.1016/0022-5193(72)90127-0. [DOI] [PubMed] [Google Scholar]
- Nishioka H., Doudney C. O. Different modes of loss of photoreversibility of mutation and lethal damage in ultraviolet-light resistant and sensitive bacteria. Mutat Res. 1969 Sep-Oct;8(2):215–228. doi: 10.1016/0027-5107(69)90001-3. [DOI] [PubMed] [Google Scholar]
- Nishioka H., Doudney C. O. Different modes of loss of photoreversibility of ultraviolet light-induced true and suppressor mutations to tryptophan independence in an auxotrophic strain of Escherichia coli. Mutat Res. 1970 Apr;9(4):349–358. doi: 10.1016/0027-5107(70)90017-5. [DOI] [PubMed] [Google Scholar]
- Noack D., Klaus S. Inactivation kinetics of lambda phage repressors in a mutant of E. coli temperature sensitive in DNA replication. Mol Gen Genet. 1972;115(3):216–224. doi: 10.1007/BF00268885. [DOI] [PubMed] [Google Scholar]
- OTSUJI N., SEKIGUCHI M., IIJIMA T., TAKAGI Y. Induction of phage formation in the lysogenic Escherichia coli K-12 by mitomycin C. Nature. 1959 Oct 3;184(Suppl 14):1079–1080. doi: 10.1038/1841079b0. [DOI] [PubMed] [Google Scholar]
- Ono J., Shimazu Y. Ultraviolet reactivation of a bacteriophage containing a single-stranded deoxyribonucleic acid as a genetic element. Virology. 1966 Jun;29(2):295–302. doi: 10.1016/0042-6822(66)90036-5. [DOI] [PubMed] [Google Scholar]
- Osborn M., Person S., Phillips S., Funk F. A determination of mutagen specificity in bacteria using nonsense mutants of bacteriophage T4. J Mol Biol. 1967 Jun 28;26(3):437–447. doi: 10.1016/0022-2836(67)90314-2. [DOI] [PubMed] [Google Scholar]
- PETTIJOHN D., HANAWALT P. EVIDENCE FOR REPAIR-REPLICATION OF ULTRAVIOLET DAMAGED DNA IN BACTERIA. J Mol Biol. 1964 Aug;9:395–410. doi: 10.1016/s0022-2836(64)80216-3. [DOI] [PubMed] [Google Scholar]
- PRITCHARD R. H., LARK K. G. INDUCTION OF REPLICATION BY THYMINE STARVATION AT THE CHROMOSOME ORIGIN IN ESCHERICHIA COLI. J Mol Biol. 1964 Aug;9:288–307. doi: 10.1016/s0022-2836(64)80208-4. [DOI] [PubMed] [Google Scholar]
- Paterson M. C., Boyle J. M., Setlow R. B. Ultraviolet- and X-ray-induced responses of a deoxyribonucleic acid polymerase-deficient mutant of Escherichia coli. J Bacteriol. 1971 Jul;107(1):61–67. doi: 10.1128/jb.107.1.61-67.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard E. C., Randall E. P. Studies on the inducible inhibitor of radiation-induced DNA degradation of Escherichia coli. Radiat Res. 1973 Aug;55(2):265–279. [PubMed] [Google Scholar]
- Ptashne M. Specific binding of the lambda phage repressor to lambda DNA. Nature. 1967 Apr 15;214(5085):232–234. doi: 10.1038/214232a0. [DOI] [PubMed] [Google Scholar]
- Radman M., Devoret R. UV-reactivation of bacteriophage lambda in excision repair-deficient hosts: independence of red functions and attachment regions. Virology. 1971 Feb;43(2):504–506. doi: 10.1016/0042-6822(71)90322-9. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B. Two forms of repair in the DNA of human cells damaged by chemical carcinogens and mutagens. Cancer Res. 1974 Dec;34(12):3318–3325. [PubMed] [Google Scholar]
- Regan J. D., Trosko J. E., Carrier W. L. Evidence for excision of ultraviolet-induced pyrimidine dimers from the DNA of human cells in vitro. Biophys J. 1968 Mar;8(3):319–325. doi: 10.1016/S0006-3495(68)86490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff W. S. The operon revisited. Annu Rev Genet. 1972;6:133–156. doi: 10.1146/annurev.ge.06.120172.001025. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L., Kass L. R., Yarmolinsky M. B. Parallel behavior of F and Pl in causing indirect induction of lysogenic bacteria. Cold Spring Harb Symp Quant Biol. 1968;33:785–789. doi: 10.1101/sqb.1968.033.01.090. [DOI] [PubMed] [Google Scholar]
- Rothman R. H., Kato T., Clark A. J. The beginning of an investigation of the role of recF in the pathways of metabolism of ultraviolet-irradiated DNA in Escherichia coli. Basic Life Sci. 1975;5A:283–291. doi: 10.1007/978-1-4684-2895-7_37. [DOI] [PubMed] [Google Scholar]
- Rupert C. S. Enzymatic photoreactivation: overview. Basic Life Sci. 1975;5A:73–87. doi: 10.1007/978-1-4684-2895-7_11. [DOI] [PubMed] [Google Scholar]
- Rupert C. S., Harm H., To K. The anatomy of direct repair. An Acad Bras Cienc. 1973;45(Suppl):151–159. [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SICARD N., DEVORET R. [Effects of thymine dificiency on the K 12 T lysogenic and 15 T colicinogenic strains of Escherichia coli]. C R Hebd Seances Acad Sci. 1962 Sep 10;255:1417–1419. [PubMed] [Google Scholar]
- Sahai Srivastava B. I., Minowada J. Terminal deoxynucleotidyl transferase activity in a cell line (molt-4) derived from the peripheral blood of a patient with acute lymphoblastic leukemia. Biochem Biophys Res Commun. 1973 Apr 2;51(3):529–535. doi: 10.1016/0006-291x(73)91346-6. [DOI] [PubMed] [Google Scholar]
- Sarin P. S., Gallo R. C. Terminal deoxynucleotidyltransferase in chronic myelogenous leukemia. J Biol Chem. 1974 Dec 25;249(24):8051–8053. [PubMed] [Google Scholar]
- Schuster H., Beyersmann D., Mikolajczyk M., Schlicht M. Prophage induction by high temperature in thermosensitive dna mutants lysogenic for bacteriophage lambda. J Virol. 1973 Jun;11(6):879–885. doi: 10.1128/jvi.11.6.879-885.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick S. G., Bridges B. A. Requirement for either DNA polymerase I or DNA polymerase 3 in post-replication repair in excision-proficient Escherichia coli. Nature. 1974 May 24;249(455):348–349. doi: 10.1038/249348a0. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G., Bridges B. A. Survival, mutation and capacity to repair single-strand DNA breaks after gamma irradiation in different Exr - strains of Escherichia coli. Mol Gen Genet. 1972;119(2):93–102. doi: 10.1007/BF00269129. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G. Genetic and kinetic evidence for different types of postreplication repair in Escherichia coli B. J Bacteriol. 1975 Jul;123(1):154–161. doi: 10.1128/jb.123.1.154-161.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick S. G. Inducible error-prone repair in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2753–2757. doi: 10.1073/pnas.72.7.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E. Ultraviolet action spectra for mutation in Escherichia coli. Mutat Res. 1970 May;9(5):437–442. doi: 10.1016/0027-5107(70)90027-8. [DOI] [PubMed] [Google Scholar]
- Setlow J. K. Photoreactivation. Radiat Res. 1966;(Suppl):141+–141+. [PubMed] [Google Scholar]
- Setlow R. B., Regan J. D., German J., Carrier W. L. Evidence that xeroderma pigmentosum cells do not perform the first step in the repair of ultraviolet damage to their DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1035–1041. doi: 10.1073/pnas.64.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B., Setlow J. K. Effects of radiation on polynucleotides. Annu Rev Biophys Bioeng. 1972;1:293–346. doi: 10.1146/annurev.bb.01.060172.001453. [DOI] [PubMed] [Google Scholar]
- Shinagawa H., Ito T. Inactivation of DNA-binding activity of repressor in extracts of lambda-lysogen treated with mitomycin C. Mol Gen Genet. 1973 Nov 2;126(2):103–110. doi: 10.1007/BF00330987. [DOI] [PubMed] [Google Scholar]
- Shineberg B., Zipser D. The ion gene and degradation of beta-galactosidase nonsense fragments. J Bacteriol. 1973 Dec;116(3):1469–1471. doi: 10.1128/jb.116.3.1469-1471.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E. C. Ultraviolet-sensitive mutator strain of Escherichia coli K-12. J Bacteriol. 1973 Jan;113(1):145–160. doi: 10.1128/jb.113.1.145-160.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinzinis B. I., Smirnov G. B., Saenko A. A. Repair deficiency in Escherichia coli UV-sensitive mutator strain uvr502. Biochem Biophys Res Commun. 1973 Jul 2;53(1):309–316. doi: 10.1016/0006-291x(73)91435-6. [DOI] [PubMed] [Google Scholar]
- Smith K. C., Meun D. H. Repair of radiation-induced damage in Escherichia coli. I. Effect of rec mutations on post-replication repair of damage due to ultraviolet radiation. J Mol Biol. 1970 Aug;51(3):459–472. doi: 10.1016/0022-2836(70)90001-x. [DOI] [PubMed] [Google Scholar]
- Springgate C. F., Loeb L. A. Mutagenic DNA polymerase in human leukemic cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):245–249. doi: 10.1073/pnas.70.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman R., Zeev H. B. Proposed mechanism of bacteriophage lambda induction: acquisition of binding sites for lambda repressor by DNA of the host. Proc Natl Acad Sci U S A. 1975 May;72(5):1973–1976. doi: 10.1073/pnas.72.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton H. E., Wagner R. P. Mutation and enzyme function in humans. Annu Rev Genet. 1975;9:187–212. doi: 10.1146/annurev.ge.09.120175.001155. [DOI] [PubMed] [Google Scholar]
- Swenson P. A., Schenley R. L. Respiration, growth and viability of repair-deficient mutants of Escherichia coli after ultraviolet irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1974 Jan;25(1):51–60. doi: 10.1080/09553007414550051. [DOI] [PubMed] [Google Scholar]
- TESSMAN E. S., OZAKI T. The interaction of phage S13 with ultraviolet-irradiated host cells and properties of the ultraviolet-irradiated phage. Virology. 1960 Nov;12:431–449. doi: 10.1016/0042-6822(60)90165-3. [DOI] [PubMed] [Google Scholar]
- TROSKO J. E., CHU E. H., CARRIER W. L. THE INDUCTION OF THYMINE DIMERS IN ULTRAVIOLET-IRRADIATED MAMMALIAN CELLS. Radiat Res. 1965 Apr;24:667–672. [PubMed] [Google Scholar]
- Tait R. C., Harris A. L., Smith D. W. DNA repair in Escherichia coli mutants deficient in DNA polymerases I, II and-or 3. Proc Natl Acad Sci U S A. 1974 Mar;71(3):675–679. doi: 10.1073/pnas.71.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa H. Structural genes of ATP-dependent deoxyribonuclease of Escherichia coli. Nat New Biol. 1972 Sep 6;239(88):14–16. doi: 10.1038/newbio239014a0. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa T. Effect of ultraviolet irradiation on bacteriophage lambda immunity. J Mol Biol. 1967 Jan 28;23(2):247–263. doi: 10.1016/s0022-2836(67)80031-7. [DOI] [PubMed] [Google Scholar]
- Van Sluis C. A., Mattern I. E., Paterson M. C. Properties of uvrE mutants of Escherichia coli K12. I. Effects of UV irradiation on DNA metabolism. Mutat Res. 1974 Dec;25(3):273–279. doi: 10.1016/0027-5107(74)90055-4. [DOI] [PubMed] [Google Scholar]
- Volkert M. R., George D. L., Witkin E. M. Partial suppression of the LexA phenotype by mutations (rnm) which restore ultraviolet resistance but not ultraviolet mutability to Escherichia coli B/r uvr A lexA. Mutat Res. 1976 Jul;36(1):17–28. doi: 10.1016/0027-5107(76)90017-8. [DOI] [PubMed] [Google Scholar]
- WACKER A., DELLWEG H., JACHERTS D. [Thymine dimerization and survival of bacteria]. J Mol Biol. 1962 May;4:410–412. doi: 10.1016/s0022-2836(62)80022-9. [DOI] [PubMed] [Google Scholar]
- WITKIN E. M. Time, temperature, and protein synthesis: a study of ultraviolet-induced mutation in bacteria. Cold Spring Harb Symp Quant Biol. 1956;21:123–140. doi: 10.1101/sqb.1956.021.01.011. [DOI] [PubMed] [Google Scholar]
- Walker J. R., Ussery C. L., Allen J. S. Bacterial cell division regulation: lysogenization of conditional cell division lon - mutants of Escherichia coli by bacteriophage. J Bacteriol. 1973 Mar;113(3):1326–1332. doi: 10.1128/jb.113.3.1326-1332.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb R. B., Malina M. M. Mutagenesis in Escherichia coli by visible light. Science. 1967 May 26;156(3778):1104–1105. doi: 10.1126/science.156.3778.1104. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Weigle J. J. Induction of Mutations in a Bacterial Virus. Proc Natl Acad Sci U S A. 1953 Jul;39(7):628–636. doi: 10.1073/pnas.39.7.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Elevated mutability of polA derivatives of Escherichia coli B/r at sublethal doses of ultraviolet light: evidence for an inducible error-prone repair system ("SOS repair") and its anomalous expression in these strains. Genetics. 1975 Jun;79 (Suppl):199–213. [PubMed] [Google Scholar]
- Witkin E. M. Genetics of Resistance to Radiation in ESCHERICHIA COLI. Genetics. 1947 May;32(3):221–248. doi: 10.1093/genetics/32.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M., George D. L. Ultraviolet mutagenesis in polA and UvrA polA derivatives of Escherichia coli B-R: evidence for an inducible error-prone repair system. Genetics. 1973 Apr;73(Suppl):91–10. [PubMed] [Google Scholar]
- Witkin E. M., Parisi E. C. Bromouracil mutagenesis: mispairing or misrepair? Mutat Res. 1974 Dec;25(3):407–409. doi: 10.1016/0027-5107(74)90071-2. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Persistence and decay of thermoinducible error-prone repair activity in nonfilamentous derivatives of tif-1, Escherichia coli B/r: the timing of some critical events in ultraviolet mutagenesis. Mol Gen Genet. 1975 Dec 29;142(2):87–103. doi: 10.1007/BF00266092. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Radiation-induced mutations and their repair. Science. 1966 Jun 3;152(3727):1345–1353. doi: 10.1126/science.152.3727.1345. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Relationships among repair, mutagenesis, and survival: overview. Basic Life Sci. 1975;5A:347–353. doi: 10.1007/978-1-4684-2895-7_47. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. The mutability toward ultraviolet light of recombination-deficient strains of Escherichia coli. Mutat Res. 1969 Jul-Aug;8(1):9–14. doi: 10.1016/0027-5107(69)90135-3. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. The radiation sensitivity of Escherichia coli B: a hypothesis relating filament formation and prophage induction. Proc Natl Acad Sci U S A. 1967 May;57(5):1275–1279. doi: 10.1073/pnas.57.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a dnaB uvrA derivative of Escherichia coli B/r: evidence for inducible error-prone repair. Basic Life Sci. 1975;5A:369–378. doi: 10.1007/978-1-4684-2895-7_49. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a tif-1 uvrA derivative of Escherichia coli B-r: evidence that ultraviolet mutagenesis depends upon an inducible function. Proc Natl Acad Sci U S A. 1974 May;71(5):1930–1934. doi: 10.1073/pnas.71.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis in repair-deficient derivatives of Escherichia coli B-r: uvrA recB and uvrA recC strains. Mutat Res. 1972 Nov;16(3):235–242. doi: 10.1016/0027-5107(72)90154-6. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis in strains of E. coli deficient in DNA polymerase. Nat New Biol. 1971 Jan 20;229(3):81–82. doi: 10.1038/newbio229081a0. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet-induced mutation and DNA repair. Annu Rev Microbiol. 1969;23:487–514. doi: 10.1146/annurev.mi.23.100169.002415. [DOI] [PubMed] [Google Scholar]
- Witkin E. M., Wermundsen I. E. Do ultraviolet-induced mutations to streptomycin resistance exhibit susceptibility to mutation frequency decline? Mutat Res. 1973 Aug;19(2):261–264. doi: 10.1016/0027-5107(73)90085-7. [DOI] [PubMed] [Google Scholar]
- Worcel A. Induction of chromosome re-initiations in a thermosensitive DNA mutant of Escherichiacoli. J Mol Biol. 1970 Sep 14;52(2):371–386. doi: 10.1016/0022-2836(70)90037-9. [DOI] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Evidence for the control by exrA and polA genes of two branches of the uvr gene-dependent excision repair pathway in Escherichia coli K-12. J Bacteriol. 1973 Oct;116(1):175–182. doi: 10.1128/jb.116.1.175-182.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Genetic control of multiple pathways of post-replicational repair in uvrB strains of Escherichia coli K-12. J Bacteriol. 1976 Jan;125(1):102–110. doi: 10.1128/jb.125.1.102-110.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Involvement of DNA polymerase 3 in excision repair after ultraviolet irradiation. Nat New Biol. 1973 Aug 22;244(138):240–241. doi: 10.1038/newbio244240a0. [DOI] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. X-ray sensitivity and repair capacity of a polA1 exrA strain of Escherichia coli K-12. J Bacteriol. 1973 Apr;114(1):121–127. doi: 10.1128/jb.114.1.121-127.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngs D. A., Van der Schueren E., Smith K. C. Separate branches of the uvr gene-dependent excision repair process in ultraviolet-irradiated Escherichia coli K-12 cells; their dependence upon growth medium and the polA, recA, recB, and exrA genes. J Bacteriol. 1974 Feb;117(2):717–725. doi: 10.1128/jb.117.2.717-725.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELLE M. R., OGG J. E., HOLLAENDER A. Photoreactivation of induced mutation and inactivation of Escherichia coli exposed to various wave lengths of monochromatic ultraviolet radiation. J Bacteriol. 1958 Feb;75(2):190–198. doi: 10.1128/jb.75.2.190-198.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]