Abstract
Caveolae, flask-like invaginations of the plasma membrane, were discovered nearly 60 years ago. Originally regarded as fixation artifacts of electron microscopy, the functional role for these structures has taken decades to unravel. The discovery of the caveolin protein in 1992 (by the late Richard G.W. Anderson) accelerated progress in defining the contribution of caveolae to cellular physiology and pathophysiology. The three isoforms of caveolin (caveolin-1, -2, and -3) are caveolae-resident structural and scaffolding proteins that are critical for the formation of caveolae and their localization of signaling entities. A PubMed search for “caveolae” reveals ∼280 publications from their discovery in the 1950s to the early 1990s, whereas a search for “caveolae or caveolin” after 1990, identifies ∼7000 entries. Most work on the regulation of biological responses by caveolae and caveolin since 1990 has focused on caveolae as plasma membrane microdomains and the function of caveolin proteins at the plasma membrane. By contrast, our recent work and that of others has explored the localization of caveolins in multiple cellular membrane compartments and in the regulation of intracellular signaling. Cellular organelles that contain caveolin include mitochondria, nuclei and the endoplasmic reticulum. Such intracellular localization allows for a complexity of responses to extracellular stimuli by caveolin and the possibility of novel organelle-targeted therapeutics. This review focuses on the impact of intracellular localization of caveolin on signal transduction and cell regulation.—Fridolfsson, H. N., Roth, D. M., Insel, P. A., Patel, H. H. Regulation of intracellular signaling and function by caveolin.
Keywords: cholesterol, cell physiology, cellular organelles
Caveolae are 50–100 nm invaginations of the plasma membrane that are enriched in cholesterol and sphingolipids (1). Caveolins, the structural proteins essential for caveolae formation, are present in three isoforms (2). Although the caveolin proteins are expressed ubiquitously, their level of expression varies among different tissues. Caveolin-1 (Cav-1) and caveolin-2 (Cav-2) are highly expressed in endothelial cells, adipocytes, and smooth muscle cells, while caveolin-3 (Cav-3) is predominantly found in striated (skeletal and cardiac) and smooth muscle (3). Caveolins were originally discovered as critical proteins for the formation of caveolae in cholesterol and phospholipid membrane (lipid) raft domains (1). Many studies have focused on the function of caveolins as signaling scaffolds within plasma membrane caveolae and lipid rafts (4, 5). However, evidence in recent years indicates that caveolin proteins can have roles independent of caveolae in the regulation of cellular activities, including lipid transport, gene expression, and mitochondrial function. Such caveolae-independent actions of caveolins may be facilitated by their presence in other cellular membranes, including: exocytic and endocytic vesicles (6), the endoplasmic reticulum (ER) (7), the Golgi complex (8), mitochondria (9, 10), the nucleus (11), endosomes (12), lysosomes (13), peroxisomes (14), and lipid droplets (15) (Fig. 1 and Table 1). Functions of caveolin at such cellular locations and the mechanisms involved in intracellular caveolin trafficking are the focus of this review.
Figure 1.
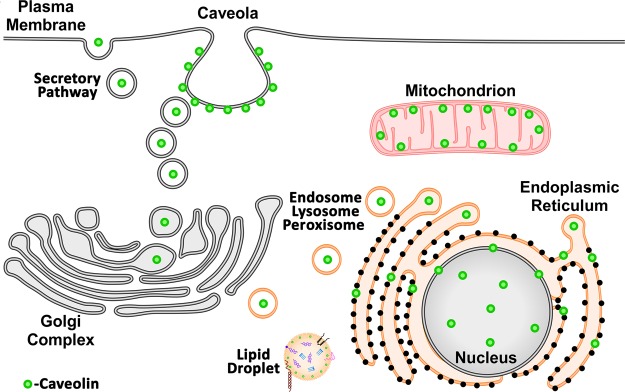
Caveolin localizes to multiple cellular compartments. A cartoon depicting localization of caveolin to various intracellular organelles to regulate a number of cellular processes.
Table 1.
Cellular localization of caveolin, role and specific original references
| Cellular location | Role of caveolin | References |
|---|---|---|
| Plasma membrane caveolae | Signal transduction | 24, 26, 35 |
| Secretory pathway vesicles | Endocytosis | 37, 39, 41, 42, 44, 46 |
| Exocytosis | ||
| Transcytosis | ||
| Endoplasmic reticulum | Lipid transport | 7, 56 |
| Golgi complex | Lipid transport | 58, 60 |
| Glycosyltransferase localization | ||
| Mitochondria | Lipid transport | 10, 53, 67, 68 |
| Metabolic regulation | ||
| Preserve function | ||
| Nucleus | Gene regulation | 11, 76, 78, 79, 86 |
| Tumor suppressor | ||
| Endosome | Signal transduction | 12 |
| Lysosome | Lipid transport | 13 |
| Peroxisome | Lipid transport | 14 |
| Lipid droplets | Lipid transport | 93, 94 |
Plasma membrane
Caveolin proteins are best known for their facilitation of the formation of plasma membrane caveolae. Caveolin is an integral ∼20 kDa membrane protein that is synthesized at the ER in a signal recognition particle (SRP)-dependent process (16). In the ER, newly synthesized caveolins undergo the first stage of oligomerization and these oligomers are stabilized by the binding of cholesterol (16, 17). Caveolin is then recruited to ER exit sites and is transported to the Golgi complex by coat protein II (COPII) vesicles (18). Transport through the Golgi complex is slower than in the ER; pools of caveolin protein are observed in the Golgi of many cell types (19). Within the Golgi, complex caveolins form high molecular weight oligomers (>400 kDa) that are necessary for incorporation into lipid rafts and exit to the plasma membrane (18). The association with cholesterol regulates the kinetics of caveolin transport from the Golgi complex (19). Caveolin protein continues through the secretory pathway and is incorporated into the plasma membrane where other proteins, in particular cavins, stabilize the formation of caveolae (20–22).
Caveolae can be organizing centers for cellular signal transduction. The caveolin signaling hypothesis proposes that caveolae bring downstream effectors in proximity to receptors through the binding of signaling molecules to a scaffolding domain of caveolin (CSD), which is comprised of ∼20 amino acids (23, 24). This hypothesis also suggests that caveolin maintains signaling proteins in an inactive form until a release cue is received. Caveolin proteins interact with a variety of signaling molecules, which include G-protein coupled receptors (GPCRs), Src family kinases, ion channels, endothelial nitric oxide synthase (eNOS), adenylyl cyclases, protein kinase A (PKA), and mitogen-activated kinases (MAPs) (5). A well-studied example of caveolin-mediated signaling is the inhibition of eNOS through its interaction with caveolin (25–27). Binding of eNOS to caveolin-1 inhibits its enzyme activity in vitro; infusion of a membrane-permeable caveolin-1 CSD peptide has a similar action in vivo (28, 29). Enhanced activation of eNOS in caveolin-1 knockout (KO) mice results in increased nitric oxide production, which contributes to cardiovascular defects in these mice (30). Additionally, caveolin enhances protective signaling in cardiac myocytes by organizing and regulating signaling molecules within caveolae (31). Caveolin interacts with components of the reperfusion injury salvage kinase (RISK) pathway, which provides cardiac protection through preconditioning and post-conditioning stimuli that include ischemia, opioids, and volatile anesthetics (32–34). For example, rapid activation of Src and phosphorylation of caveolin-1 is required for isofluorane-mediated cardioprotection (35). Caveolin thus has an important role in regulating signaling events that influence homeostasis and are essential to cell health.
Endocytosis, exocytosis, and transcytosis
Caveolae also have been implicated in cellular transport events that include endocytosis, exocytosis, and transcytosis but until recently there was little evidence that caveolin had a direct role in these processes. The activity of several signaling proteins can be altered by changing their expression on the cell surface and this expression can be regulated by caveolin-induced changes in membrane trafficking through endocytosis and exocytosis. For example, caveolin-1 negatively regulates transforming growth factor-β (TGF-β) signaling (36). In addition to a direct physical inhibition of TGF-β-induced Smad2 phosphorylation and signaling by caveolin, TGF-β can be endocytosed in a caveolae-dependent manner (37). TGF-β receptors are targeted to caveolae by interaction with Smad7 and Smad ubiquitin regulatory factor (Smurf) proteins, which mediate caveolin-dependent internalization of the receptor and attenuation of signaling (37). Caveolin-dependent endocytosis also regulates cell attachment through internalization of integrins and components of tight and adherens junctions (38–40). Caveolin-1 can also regulate the surface expression of the transient receptor potential cation channel, subfamily C, member 1 (TRPC1) through exocytic membrane trafficking (41).
Caveolin-mediated transcytosis is an important mechanism for the transport of albumin and delivery of albumin-conjugated nutrients, fatty acids, and hormones across the endothelium (42, 43). Inflammation-evoked pulmonary vascular hyperpermeability and protein-rich edema formation require caveolin-mediated transcytosis of macromolecules (44). This increase in transcellular permeability is triggered by the binding of neutrophils to endothelial cell surface intercellular adhesion molecule (ICAM)-1, which leads to Src activation and phosphorylation of caveolin-1 (44). Phosphorylation of caveolin-1 stimulates caveolae formation and trafficking, resulting in increased permeability (43, 45). Phosphorylation of caveolin-1 is also required for H2O2-induced stimulation of transcytosis and destabilization of cell–cell junctions and thus has an important role in the pathogenesis of oxidant-induced pulmonary vascular hyperpermeability (46).
Cholesterol and lipids in intracellular trafficking of caveolin
Cholesterol is a key component of caveolae and is required for the proper trafficking of caveolin to the plasma membrane. Caveolin binds cholesterol (47) and the role of caveolin in cholesterol trafficking and homeostasis has been studied by numerous investigators. For example, a dominant-negative truncation mutant of caveolin induces a cholesterol trafficking defect that results in depletion of cholesterol from the plasma membrane (48). The expression of caveolin-1 in cells that normally do not express caveolin (HEK 293) facilitates the uptake of fatty acids and increases the cellular levels of free cholesterol and cholesterol export (49, 50). Caveolin and the endocytosis of caveolae are required to maintain normal free cholesterol levels in lipid droplets of adipocytes (51). In support of this idea, caveolin-1 KO mice show decreased free cholesterol levels in adipocytes, decreased adiposity, and are resistant to diet-induced obesity (51). Caveolin not only regulates the import and export of cholesterol at the plasma membrane, but also controls cholesterol trafficking from the plasma membrane to other cellular sites. Whether caveolin's regulation of cholesterol trafficking is a critical element in creating lipid rafts or the caveolar structure is unknown; it is likely one component of a more complex mechanism that creates and maintains lipid raft and caveolae structure. It has been suggested that a soluble cytoplasmic form of caveolin may contribute to the intracellular transport of cholesterol. Soluble caveolin is embedded in a lipid particle and associates with cholesterol (19, 52). Caveolin can move from the plasma membrane to associate with lipid particles in response to the addition of lipid to cells and can return to the plasma membrane when the lipid source is removed (19). Thus, caveolin can act as a sensor of cellular lipid levels and function to maintain cholesterol homeostasis. This feature may be important for the localization and trafficking of caveolin to intracellular organelles in response to various stimuli (10, 53).
Endoplasmic reticulum and Golgi network in cholesterol transport: role of caveolin
The ER is the cellular site of cholesterol synthesis. Cholesterol is transported to the plasma membrane by a novel route that does not involve passing through the Golgi and reaches the cell surface within minutes of synthesis (54, 55). Newly synthesized cholesterol is transported to caveolae and then disperses to other regions of the plasma membrane and extracellular space (56). This rapid movement of cholesterol from the ER to the cell surface is dependent on caveolin (56) and soluble caveolin is likely responsible for the transport of plasma membrane cholesterol directly to the ER (57). The oxidation of plasma membrane cholesterol causes the rapid movement of caveolin to the ER (7), suggesting that caveolin has a role in transporting cholesterol between these two organelles. Thus, caveolin protein is not only synthesized in the ER but it also has a role in cholesterol transport and homeostasis within this organelle.
Oxidized or otherwise damaged cholesterol stimulates the movement of caveolin from the plasma membrane to the ER but caveolin then quickly moves from the ER to the Golgi apparatus (7). Caveolin collects in the Golgi in the presence of oxidized cholesterol but will return to the cell surface once cholesterol oxidase is removed. This is another example of the importance of cholesterol in the normal trafficking of caveolin. However, this trafficking pattern is not just a response to damaged cholesterol. Other evidence indicates that caveolin normally cycles between the plasma membrane, ER, and Golgi apparatus. Disruption of microtubules with nocodazole causes caveolin to redistribute from the plasma membrane to the ER (58). On removal of nocodazole, caveolin moves into the Golgi apparatus and then returns to the plasma membrane. Thus, microtubules are required for the transport of caveolin and help maintain its normal distribution and trafficking between the plasma membrane and ER/Golgi system. Caveolin may follow this trafficking pattern to shuttle cholesterol between the cell surface and the ER/Golgi membranes or to return Golgi resident proteins that moved to the plasma membrane.
An additional function of caveolin is within the Golgi apparatus to regulate protein glycosylation. Oligosaccharides on glycoproteins are assembled by the sequential action of glycosyltransferases during glycoprotein transport through the Golgi network (59). The sequential action of glycosyltransferases and their precise localization within the Golgi helps determine the final structure of oligosaccharides on proteins. Caveolin-1 regulates the localization of N-acetylglucosaminyltransferase III (GnT-III) in the intra-Golgi subcompartment and thereby modifies N-glycan biosynthesis (60). The expression of caveolin-1 decreases the extent of N-glycan branching because of the addition of GlcNAc, a product of GnT-III. Caveolin-1 therefore modifies the biosynthetic pathway of sugar chains by regulation of the subcompartment localization of this key glycosyltransferase in the Golgi.
Mitochondria
Caveolin-deficient or mutant mice display a myriad of disorders, including lipodystrophy, cancer, diabetes, muscular dystrophy, cardiovascular disease, and pulmonary fibrosis (61). Mitochondrial and metabolic dysfunction is postulated to be a primary cause of these disorders. Recent data regarding the effects of caveolin on mitochondria support the hypothesis that disruption of mitochondrial function and metabolism may be responsible for at least some of the abnormalities observed in caveolin-deficient mice. Some studies suggest that the role of caveolin in cholesterol transport and homeostasis affects mitochondrial function. The mitochondrion is a cholesterol-poor organelle; little is known regarding the regulation of cholesterol flux in mitochondria. One mechanism that has been proposed is that cholesterol enters mitochondria through specialized extensions of the ER, which contain caveolin and have been termed mitochondrial-associated membranes (MAM) (62, 63). Caveolin may indirectly regulate levels of mitochondrial cholesterol by promoting cholesterol efflux from the ER, which would reduce the availability of cholesterol in MAM and thus limit its entry into mitochondria (53). In the absence of caveolin-1, cholesterol accumulates in mitochondria and this causes dysfunction by reducing membrane fluidity, reducing efficiency of energy production by the respiratory chain, and increasing the production of reactive oxygen species (ROS) (53). Together, these factors impair cellular proliferation and induce apoptosis when glucose availability is limited. An alternative possibility is that caveolin may regulate mitochondrial cholesterol by directly transporting lipid/cholesterol to mitochondria (9).
Caveolin-1 KO mice are lean and resistant to diet-induced obesity. The metabolic phenotype in those mice includes lipodystrophy, elevated levels of triglycerides and free fatty acids, lower adiponectin levels, and impaired insulin signaling in adipose tissue (64–66). Such dysregulation in adipose tissue of caveolin-deficient mice causes metabolic inflexibility and an increase in hepatic glucose production (67). These metabolic alterations are likely caused by defects in mitochondrial function. Caveolin-1 deficiency is associated with an increased dependence on glucose and a higher mitochondrial membrane potential (67). Mitochondria from caveolin-1 KO adipose tissue display increased oxidative damage, increased susceptibility to high fat diet-induced apoptosis, and altered expression of mitochondrial and redox-sensitive genes (67). These mitochondrial defects may result from metabolic changes in caveolin-1 KO mice or loss of caveolin may directly alter mitochondrial function.
Evidence from studies of liver steatosis, which is caused by abnormal liver lipidogenesis creating an intrahepatic accumulation of triglycerides, suggests that caveolin regulates mitochondrial homeostasis and metabolism within this organelle. The accumulation of fat in the hepatocyte causes caveolin-1 to concentrate in lipid droplets and in the inner mitochondrial membrane near the site of lipid accumulation (68). This increase in mitochondrial caveolin-1 may be a mechanism to prevent damage to mitochondria from the oxidative stress that occurs with steatosis. Water, protein, and ion transport across the inner mitochondrial membrane are altered with hepatic stress; caveolin may regulate the transport of these molecules to preserve mitochondrial structure and function. Consistent with this idea, mitochondria in adipocytes from caveolin-1 KO mice have swelling of their matrix, which likely is a result of changes in the osmotic gradient (69).
In highly metabolic tissues such as the heart, brain, and liver, mitochondria play a key role in adaptation to cellular stress. Cardiac-specific overexpression of caveolin-3 protects the heart from ischemic injury and pressure overload-promoted failure (70, 71). Recent studies into the mechanism of this protection by caveolin-3 have revealed the trafficking of caveolin into mitochondria and further emphasize its importance in preserving the function of mitochondria (10). Mitochondria and plasma membrane caveolae are closely apposed in many cell types and can have a physical and functional connection that develops with cellular stress (Fig. 2). For example, cardiac myocytes that undergo sublethal ischemia have a physical connection between the plasma membrane and mitochondria and an increased amount of mitochondrial caveolin. Caveolin-3 may translocate to mitochondria in response to stress as a means to resist damage and maintain cellular function. Support for this idea is the evidence that mitochondria with increased caveolin protein have improved calcium tolerance, improved respiration, and reduced ROS production (10). Caveolin within the mitochondria may influence components of the electron transport chain and alter mitochondrial membranes (decreasing fluidity and ion permeability). Such effects could delay opening of the mitochondrial permeability transition pore (mPTP) and limit apoptosis. Indeed, by increasing caveolin-3 levels in mitochondria, one can reduce the size of a myocardial infarction following an ischemic insult (10).
Figure 2.
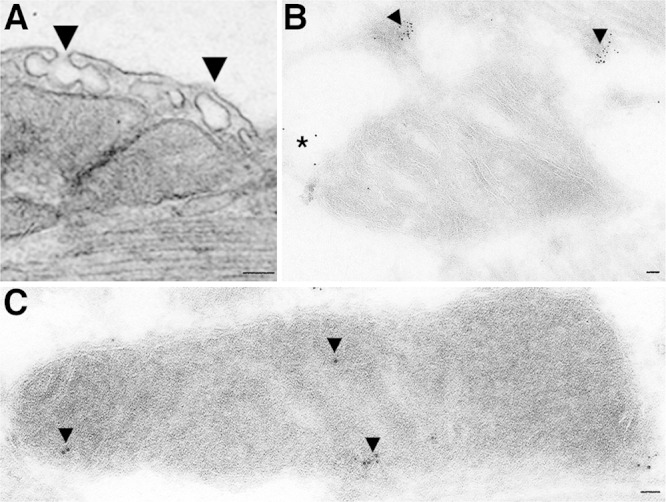
Electron microscopy of caveolae and immunogold localization of caveolin to mitochondria. A) Electron micrograph showing close apposition of membrane caveolae to subsarcolemmal mitochondria in adult mouse heart tissue. B) and (C) Perfusion fixed mouse hearts subjected to immunogold labeling of caveolin-3 show caveolin enrichment of caveolin in membrane regions consistent with caveolae, including some caveolin trafficking and localized to mitochondria (B) and to the inner mitochondrial membrane (C). Scale bar is 50 nm.
Nucleus
Nuclear localized caveolin is thought to function in gene regulation. In some instances this response in the nucleus may be an extension of the function of caveolin in plasma membrane caveolae. For example, membrane receptors for platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) localize to caveolae, but on activation, undergo endocytosis and translocation to the nucleus (72, 73). Vascular endothelial growth factor (VEGF) stimulates cell growth and migration by signaling through two receptor tyrosine kinases and activation of eNOS, which all colocalize with caveolin-1 in caveolae (74, 75). Activation induces nuclear translocation of VEGF receptor, eNOS, and caveolin-1 (76). As noted above, caveolin-1 regulates eNOS and restricts nitric oxide signaling to specific cellular sites (26). Translocation of eNOS and caveolin to the nucleus may thus be a mechanism to target nitric oxide production and control gene activation.
Alternatively, caveolin may traffic directly to the nucleus rather than from caveolae. Caveolin-2 is transported to the nucleus in response to insulin where it can alter the interaction between ERK and lamin A/C and modulate the activation of transcription factors and cell proliferation pathways (77–79). Caveolin-2 does not contain a nuclear localization signal and a nuclear import receptor for caveolins has not been identified. However, tyrosines 19 and 27 in the N terminus of caveolin-2 are required for insulin-induced nuclear targeting (77, 79). On insulin stimulation, caveolin-2 is transported through a retrograde pathway from the Golgi to the inner nuclear membrane (11). Rab6-GTP and microtubules mediate the movement of caveolin-2 from the Golgi to the ER. Caveolin-2 is then imported from the outer nuclear membrane, which is contiguous with the ER, to the inner nuclear membrane through interaction with gp210 in the nuclear pore. At the inner nuclear membrane, caveolin-2 can prevent heterochromatin assembly by reducing histone methylation and can promote ERK-mediated transcriptional activation of Elk-1 and STAT3, which induce a mitogenic response to insulin (11).
Caveolin is a tumor suppressor and is thought to influence gene regulation by binding DNA. The caveolin-1 gene is deleted in several types of cancers; decreased levels of caveolin-1 are found in breast and ovarian cancers (80–82). Fibroblasts transformed with the v-ABL and H-ras oncogenes down-regulate expression of caveolin-1 and lack morphological caveolae (83). Reintroduction of caveolin-1 into these transformed fibroblasts or re-expression of caveolin-1in human breast cancer cells inhibits tumor cell growth (84, 85). Caveolin is thought to regulate the cell cycle and tumor progression through modulation of signaling by the Ras/MAPK pathway, which can be influenced by its localization in plasma membrane caveolae. Other evidence suggests that caveolin also exerts its control at the transcriptional level (82). Endogenously or ectopically expressed caveolin-1 localizes to the nuclei of ovarian cancer cells and primarily associates with the nuclear matrix in high molecular weight complexes (86). This could indicate that soluble caveolin is transported into the nucleus to regulate gene expression, although no import pathway has been identified. Caveolin-1 in the nucleus can bind the promoters and down-regulate expression of cyclin D1 and the folate receptor, genes involved in the control of proliferation (86).
Endosome, lysosome, and peroxisome
The function of caveolin in the regulation of signal transduction and lipid transport extends to other organelles such as endosomes, lysosomes, and peroxisomes. In liver cells the endosome is required for down-regulation of the EGF receptor (87) and insulin binding stimulates trafficking within the endocytic compartment (88). In hepatic cells, MAPK signal transduction pathway components (Ras, Raf-1, Mek, Mek-P, and MAPK) are localized to caveolin-enriched domains at the cell surface (12). Early endosomes contain constitutively active Raf-1 and Mek-P; it is hypothesized that caveolin-1 is responsible for trafficking these proteins from the plasma membrane to the endosome as a means to regulate basal activity of this signal transduction pathway (12).
Although caveolin can be targeted to lysosomes for degradation, it also functions in cholesterol trafficking from lysosomes. Lysosomes are a source of lipoprotein-derived cholesterol and caveolin-1 normally moves to and from the cytoplasmic surface of lysosomes during intracellular cholesterol trafficking (13). Caveolin-1 reversibly accumulates on lysosomal membranes when cholesterol homeostasis is perturbed (89) or when cells are serum-starved and intralysosomal pH increases (13). At least a portion of the caveolin-1 returns to the plasma membrane on reversal of such perturbations.
Peroxisome-resident proteins, 70 kDa peroxisomal membrane protein (PMP70), and the peroxins, Pex13p and Pex14p, are associated with lipid rafts in peroxisomal membranes and depletion of cholesterol leads to defective sorting of the peroxisomal enzyme catalase (14). Caveolin-1 colocalizes with these proteins in peroxisomal lipid rafts of hepatocytes but is not required for peroxisome biogenesis (14). In hepatocytes peroxisomes are required for functions that include bile acid biosynthesis and β-oxidation of very long chain fatty acids (90, 91). Therefore, caveolin may be involved in transporting lipids or other substrates required for these metabolic activities to and from peroxisomes.
Lipid droplets
Caveolin can regulate cellular lipid homeostasis through its trafficking to lipid droplets. Caveolin localizes to the surface of intracellular lipid droplets (15, 48, 92), but how this occurs is not clear. Caveolin may enter lipid droplets through the ER, from plasma membrane caveolae, or as a soluble lipid-associated protein, depending on the cell type and stimulus (93, 94). For example, targeting of caveolin-1 to adipocyte lipid droplets involves caveolae internalization, which can be blocked by inhibitors of the endocytic pathway (51). The presence of caveolin in lipid droplets was initially thought to be an artifact resulting from the accumulation of high levels of caveolin in the ER, but endogenous caveolins can move in and out of lipid droplets (94). Caveolin localizes to lipid droplets in response to the intracellular accumulation of lipids; in regenerating liver, caveolin relocates from the plasma membrane to newly formed lipid droplets, suggesting a role for caveolin in lipid transport from these organelles (94). Additionally, a dominant-negative form of caveolin accumulates irreversibly in lipid droplets and causes an intracellular cholesterol imbalance (48, 95). Caveolin-1 also regulates the composition of the lipid droplet surface through protein-protein interaction and phospholipid remodeling, which assists in lipid droplet size expansion (93). Several of the proteins that require caveolin-1 expression for lipid droplet association are also found in caveolae, thus implicating a plasma membrane origin of lipid droplet caveolin (93).
CONCLUSION
As shown above, considerable evidence supports the idea that caveolin proteins regulate signaling and function at various intracellular sites. However, many questions remain: What is the role of multiple isoforms of caveolin in single cell types? In studies of various caveolin KO mice, loss of one isoform (e.g., caveolin-3 in cardiac myocytes) completely eliminates the expression of caveolae. Other isoforms of caveolin exist in the deficient cells that do not compensate for this loss, suggesting that certain caveolins have nonredundant roles in an isoform-specific manner, at least in certain cell types. Such roles for particular caveolins may prove to be important in pathophysiology. Also, caveolins have been shown to undergo numerous post-translational modifications (e.g., phosphorylation, SUMOylation, palmitoylation, ubiquitination) (96–100). What is the impact of modified caveolins on intracellular localization and function and is caveolin protein structure important in the localization and function? The absence of a crystal structure of caveolin has slowed understanding of its structure-function relationships. Many studies reveal that the plasma membrane can influence intracellular organelles and change the localization of caveolin. How are such changes orchestrated among the different compartments? Is caveolin acting as a chaperone to protect cells? It is possible that caveolin interaction with the cytoskeleton (101, 102) and with proteins such as integrins (103) may be critical to trafficking and localization to various subcellular sites. What are the precise roles of caveolins in regulating intracellular function? Does this regulation derive from influences of caveolin on metabolism at various cellular sites (10, 102, 104) to ultimately impact whole cell and organ function? Are unique subcellular, organelle-specific proteomes associated with caveolins that dictate homeostatic and pathophysiologic responses? The potential impact of further understanding of the mechanisms for the function of caveolins in the ER, Golgi, mitochondria, nucleus, and other intracellular sites is high. Moreover, it is intriguing to imagine that future efforts may involve the manipulation of caveolin proteins at specific organelles within the cell as novel therapeutic approaches to alter cellular function in health and disease.
Acknowledgments
The authors thank Drs. Piyush Patel and Ingrid Niesman for help with summary figure and compilation of electron microscopy, respectively.
This work was supported by grants from AP Giannini Foundation (H.N.F.), U.S. National Institute of Health HL091071 (H.H.P.), HL107200 (H.H.P.), HL066941 (D.M.R., H.H.P.), and HL115933 (D.M.R.), and VA Merit BX001963 (H.H.P.) and BX000783 (D.M.R.).
Footnotes
- Cav
- caveolin
- CSD
- caveolin scaffolding domain
- EGF
- epidermal growth factor
- eNOS
- endothelial nitric oxide synthase
- ER
- endoplasmic reticulum
- GPCR
- G-protein coupled receptor
- KO
- knockout
- MAM
- mitochondrial associated membranes
- mPTP
- mitochondrial permeability transition pore
- PDGF
- platelet derived growth factor
- PKA
- protein kinase A
- ROS
- reactive oxygen species
- SPR
- signal recognition particle
- TGF-β
- transforming growth factor-β
- TRPC
- transient receptor potential channel
- VEGF
- vascular endothelial growth factor
REFERENCES
- 1. Palade G. (1953) Fine structure of blood capillaries. J. Appl. Phys. 24, 1424 [Google Scholar]
- 2. Williams T. M., Lisanti M. P. (2004) The caveolin proteins. Genome Biol. 5, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song K. S., Scherer P. E., Tang Z., Okamoto T., Li S., Chafel M., Chu C., Kohtz D. S., Lisanti M. P. (1996) Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J. Biol. Chem. 271, 15160–15165 [DOI] [PubMed] [Google Scholar]
- 4. Head B. P., Patel H. H., Tsutsumi Y. M., Hu Y., Mejia T., Mora R. C., Insel P. A., Roth D. M., Drummond J. C., Patel P. M. (2008) Caveolin-1 expression is essential for N-methyl-D-aspartate receptor-mediated Src and extracellular signal-regulated kinase 1/2 activation and protection of primary neurons from ischemic cell death. FASEB J. 22, 828–840 [DOI] [PubMed] [Google Scholar]
- 5. Patel H. H., Murray F., Insel P. A. (2008) Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu. Rev. Pharmacol. Toxicol. 48, 359–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tagawa A., Mezzacasa A., Hayer A., Longatti A., Pelkmans L., Helenius A. (2005) Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J. Cell Biol. 170, 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smart E. J., Ying Y. S., Conrad P. A., Anderson R. G. (1994) Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J. Cell Biol. 127, 1185–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gkantiragas I., Brugger B., Stuven E., Kaloyanova D., Li X. Y., Lohr K., Lottspeich F., Wieland F. T., Helms J. B. (2001) Sphingomyelin-enriched microdomains at the Golgi complex. Mol. Biol. Cell 12, 1819–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li W. P., Liu P., Pilcher B. K., Anderson R. G. (2001) Cell-specific targeting of caveolin-1 to caveolae, secretory vesicles, cytoplasm or mitochondria. J. Cell Sci. 114, 1397–1408 [DOI] [PubMed] [Google Scholar]
- 10. Fridolfsson H. N., Kawaraguchi Y., Ali S. S., Panneerselvam M., Niesman I. R., Finley J. C., Kellerhals S. E., Migita M. Y., Okada H., Moreno A. L., Jennings M., Kidd M. W., Bonds J. A., Balijepalli R. C., Ross R. S., Patel P. M., Miyanohara A., Chen Q., Lesnefsky E. J., Head B. P., Roth D. M., Insel P. A., Patel H. H. (2012) Mitochondria-localized caveolin in adaptation to cellular stress and injury. FASEB J. 26, 4637–4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeong K., Kwon H., Lee J., Jang D., Hwang E. M., Park J. Y., Pak Y. (2012) Rab6-mediated retrograde transport regulates inner nuclear membrane targeting of caveolin-2 in response to insulin. Traffic 13, 1218–1233 [DOI] [PubMed] [Google Scholar]
- 12. Pol A., Calvo M., Enrich C. (1998) Isolated endosomes from quiescent rat liver contain the signal transduction machinery. Differential distribution of activated Raf-1 and Mek in the endocytic compartment. FEBS Lett. 441, 34–38 [DOI] [PubMed] [Google Scholar]
- 13. Mundy D. I., Li W. P., Luby-Phelps K., Anderson R. G. (2012) Caveolin targeting to late endosome/lysosomal membranes is induced by perturbations of lysosomal pH and cholesterol content. Mol. Biol. Cell 23, 864–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woudenberg J., Rembacz K. P., van den Heuvel F. A., Woudenberg-Vrenken T. E., Buist-Homan M., Geuken M., Hoekstra M., Deelman L. E., Enrich C., Henning R. H., Moshage H., Faber K. N. (2010) Caveolin-1 is enriched in the peroxisomal membrane of rat hepatocytes. Hepatology 51, 1744–1753 [DOI] [PubMed] [Google Scholar]
- 15. Ostermeyer A. G., Paci J. M., Zeng Y., Lublin D. M., Munro S., Brown D. A. (2001) Accumulation of caveolin in the endoplasmic reticulum redirects the protein to lipid storage droplets. J. Cell Biol. 152, 1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Monier S., Parton R. G., Vogel F., Behlke J., Henske A., Kurzchalia T. V. (1995) VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol. Biol. Cell 6, 911–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Monier S., Dietzen D. J., Hastings W. R., Lublin D. M., Kurzchalia T. V. (1996) Oligomerization of VIP21-caveolin in vitro is stabilized by long chain fatty acylation or cholesterol. FEBS Lett. 388, 143–149 [DOI] [PubMed] [Google Scholar]
- 18. Hayer A., Stoeber M., Bissig C., Helenius A. (2010) Biogenesis of caveolae: stepwise assembly of large caveolin and cavin complexes. Traffic 11, 361–382 [DOI] [PubMed] [Google Scholar]
- 19. Pol A., Martin S., Fernandez M. A., Ingelmo-Torres M., Ferguson C., Enrich C., Parton R. G. (2005) Cholesterol and fatty acids regulate dynamic caveolin trafficking through the Golgi complex and between the cell surface and lipid bodies. Mol. Biol. Cell 16, 2091–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hill M. M., Bastiani M., Luetterforst R., Kirkham M., Kirkham A., Nixon S. J., Walser P., Abankwa D., Oorschot V. M., Martin S., Hancock J. F., Parton R. G. (2008) PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell 132, 113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu L., Brown D., McKee M., Lebrasseur N. K., Yang D., Albrecht K. H., Ravid K., Pilch P. F. (2008) Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell. Metab. 8, 310–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu L., Pilch P. F. (2008) A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J. Biol. Chem. 283, 4314–4322 [DOI] [PubMed] [Google Scholar]
- 23. Okamoto T., Schlegel A., Scherer P. E., Lisanti M. P. (1998) Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J. Biol. Chem. 273, 5419–5422 [DOI] [PubMed] [Google Scholar]
- 24. Couet J., Li S., Okamoto T., Ikezu T., Lisanti M. P. (1997) Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J. Biol. Chem. 272, 6525–6533 [DOI] [PubMed] [Google Scholar]
- 25. Garcia-Cardena G., Martasek P., Masters B. S., Skidd P. M., Couet J., Li S., Lisanti M. P., Sessa W. C. (1997) Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J. Biol. Chem. 272, 25437–25440 [DOI] [PubMed] [Google Scholar]
- 26. Feron O., Belhassen L., Kobzik L., Smith T. W., Kelly R. A., Michel T. (1996) Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J. Biol. Chem. 271, 22810–22814 [DOI] [PubMed] [Google Scholar]
- 27. Shaul P. W., Smart E. J., Robinson L. J., German Z., Yuhanna I. S., Ying Y., Anderson R. G., Michel T. (1996) Acylation targets endothelial nitric-oxide synthase to plasmalemmal caveolae. J. Biol. Chem. 271, 6518–6522 [DOI] [PubMed] [Google Scholar]
- 28. Ju H., Zou R., Venema V. J., Venema R. C. (1997) Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J. Biol. Chem. 272, 18522–18525 [DOI] [PubMed] [Google Scholar]
- 29. Bucci M., Gratton J. P., Rudic R. D., Acevedo L., Roviezzo F., Cirino G., Sessa W. C. (2000) In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat. Med. 6, 1362–1367 [DOI] [PubMed] [Google Scholar]
- 30. Maniatis N. A., Shinin V., Schraufnagel D. E., Okada S., Vogel S. M., Malik A. B., Minshall R. D. (2008) Increased pulmonary vascular resistance and defective pulmonary artery filling in caveolin-1-/- mice. Am. J. Physiol. Lung. Cell. Mol. Physiol. 294, L865–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roth D. M., Patel H. H. (2011) Role of caveolae in cardiac protection. Pediatr. Cardiol. 32, 329–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hausenloy D. J., Yellon D. M. (2007) Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail. Rev. 12, 217–234 [DOI] [PubMed] [Google Scholar]
- 33. Peart J. N., Headrick J. P. (2009) Clinical cardioprotection and the value of conditioning responses. Am. J. Physiol. Heart Circ. Physiol. 296, H1705–1720 [DOI] [PubMed] [Google Scholar]
- 34. Krajewska W. M., Maslowska I. (2004) Caveolins: structure and function in signal transduction. Cell. Mol. Biol. Lett. 9, 195–220 [PubMed] [Google Scholar]
- 35. Patel H. H., Tsutsumi Y. M., Head B. P., Niesman I. R., Jennings M., Horikawa Y., Huang D., Moreno A. L., Patel P. M., Insel P. A., Roth D. M. (2007) Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. FASEB J. 21, 1565–1574 [DOI] [PubMed] [Google Scholar]
- 36. Razani B., Zhang X. L., Bitzer M., von Gersdorff G., Bottinger E. P., Lisanti M. P. (2001) Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J. Biol. Chem. 276, 6727–6738 [DOI] [PubMed] [Google Scholar]
- 37. Di Guglielmo G. M., Le Roy C., Goodfellow A. F., Wrana J. L. (2003) Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 5, 410–421 [DOI] [PubMed] [Google Scholar]
- 38. Del Pozo M. A., Balasubramanian N., Alderson N. B., Kiosses W. B., Grande-Garcia A., Anderson R. G., Schwartz M. A. (2005) Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat. Cell Biol. 7, 901–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marchiando A. M., Shen L., Graham W. V., Weber C. R., Schwarz B. T., Austin J. R., 2nd, Raleigh D. R., Guan Y., Watson A. J., Montrose M. H., Turner J. R. (2010) Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J. Cell Biol. 189, 111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Orlichenko L., Weller S. G., Cao H., Krueger E. W., Awoniyi M., Beznoussenko G., Buccione R., McNiven M. A. (2009) Caveolae mediate growth factor-induced disassembly of adherens junctions to support tumor cell dissociation. Mol. Biol. Cell 20, 4140–4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gervasio O. L., Whitehead N. P., Yeung E. W., Phillips W. D., Allen D. G. (2008) TRPC1 binds to caveolin-3 and is regulated by Src kinase - role in Duchenne muscular dystrophy. J. Cell Sci. 121, 2246–2255 [DOI] [PubMed] [Google Scholar]
- 42. Schubert W., Frank P. G., Razani B., Park D. S., Chow C. W., Lisanti M. P. (2001) Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J. Biol. Chem. 276, 48619–48622 [DOI] [PubMed] [Google Scholar]
- 43. Minshall R. D., Tiruppathi C., Vogel S. M., Malik A. B. (2002) Vesicle formation and trafficking in endothelial cells and regulation of endothelial barrier function. Histochem. Cell. Biol. 117, 105–112 [DOI] [PubMed] [Google Scholar]
- 44. Hu G., Vogel S. M., Schwartz D. E., Malik A. B., Minshall R. D. (2008) Intercellular adhesion molecule-1-dependent neutrophil adhesion to endothelial cells induces caveolae-mediated pulmonary vascular hyperpermeability. Circ. Res. 102, e120–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Minshall R. D., Sessa W. C., Stan R. V., Anderson R. G., Malik A. B. (2003) Caveolin regulation of endothelial function. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L1179–1183 [DOI] [PubMed] [Google Scholar]
- 46. Sun Y., Hu G., Zhang X., Minshall R. D. (2009) Phosphorylation of caveolin-1 regulates oxidant-induced pulmonary vascular permeability via paracellular and transcellular pathways. Circ. Res. 105, 676–685, 615 p following 685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Murata M., Peranen J., Schreiner R., Wieland F., Kurzchalia T. V., Simons K. (1995) VIP21/caveolin is a cholesterol-binding protein. Proc. Natl. Acad. Sci. U. S. A. 92, 10339–10343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pol A., Luetterforst R., Lindsay M., Heino S., Ikonen E., Parton R. G. (2001) A caveolin dominant negative mutant associates with lipid bodies and induces intracellular cholesterol imbalance. J. Cell Biol. 152, 1057–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fielding P. E., Fielding C. J. (1995) Plasma membrane caveolae mediate the efflux of cellular free cholesterol. Biochemistry 34, 14288–14292 [DOI] [PubMed] [Google Scholar]
- 50. Meshulam T., Simard J. R., Wharton J., Hamilton J. A., Pilch P. F. (2006) Role of caveolin-1 and cholesterol in transmembrane fatty acid movement. Biochemistry 45, 2882–2893 [DOI] [PubMed] [Google Scholar]
- 51. Le Lay S., Hajduch E., Lindsay M. R., Le Liepvre X., Thiele C., Ferre P., Parton R. G., Kurzchalia T., Simons K., Dugail I. (2006) Cholesterol-induced caveolin targeting to lipid droplets in adipocytes: a role for caveolar endocytosis. Traffic 7, 549–561 [DOI] [PubMed] [Google Scholar]
- 52. Liu P., Li W. P., Machleidt T., Anderson R. G. (1999) Identification of caveolin-1 in lipoprotein particles secreted by exocrine cells. Nat. Cell Biol. 1, 369–375 [DOI] [PubMed] [Google Scholar]
- 53. Bosch M., Mari M., Herms A., Fernandez A., Fajardo A., Kassan A., Giralt A., Colell A., Balgoma D., Barbero E., Gonzalez-Moreno E., Matias N., Tebar F., Balsinde J., Camps M., Enrich C., Gross S. P., Garcia-Ruiz C., Perez-Navarro E., Fernandez-Checa J. C., Pol A. (2011) Caveolin-1 deficiency causes cholesterol-dependent mitochondrial dysfunction and apoptotic susceptibility. Curr. Biol. 21, 681–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. DeGrella R. F., Simoni R. D. (1982) Intracellular transport of cholesterol to the plasma membrane. J. Biol. Chem. 257, 14256–14262 [PubMed] [Google Scholar]
- 55. Urbani L., Simoni R. D. (1990) Cholesterol and vesicular stomatitis virus G protein take separate routes from the endoplasmic reticulum to the plasma membrane. J. Biol. Chem. 265, 1919–1923 [PubMed] [Google Scholar]
- 56. Smart E. J., Ying Y., Donzell W. C., Anderson R. G. (1996) A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J. Biol. Chem. 271, 29427–29435 [DOI] [PubMed] [Google Scholar]
- 57. Lange Y. (1994) Cholesterol movement from plasma membrane to rough endoplasmic reticulum. Inhibition by progesterone. J. Biol. Chem. 269, 3411–3414 [PubMed] [Google Scholar]
- 58. Conrad P. A., Smart E. J., Ying Y. S., Anderson R. G., Bloom G. S. (1995) Caveolin cycles between plasma membrane caveolae and the Golgi complex by microtubule-dependent and microtubule-independent steps. J. Cell Biol. 131, 1421–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Munro S. (1998) Localization of proteins to the Golgi apparatus. Trends Cell Biol. 8, 11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sasai K., Ikeda Y., Ihara H., Honke K., Taniguchi N. (2003) Caveolin-1 regulates the functional localization of N-acetylglucosaminyltransferase III within the Golgi apparatus. J. Biol. Chem. 278, 25295–25301 [DOI] [PubMed] [Google Scholar]
- 61. Razani B., Lisanti M. P. (2001) Caveolin-deficient mice: insights into caveolar function human disease. J. Clin. Invest. 108, 1553–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hayashi T., Rizzuto R., Hajnoczky G., Su T. P. (2009) MAM: more than just a housekeeper. Trends Cell Biol. 19, 81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sano R., Annunziata I., Patterson A., Moshiach S., Gomero E., Opferman J., Forte M., d'Azzo A. (2009) GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol. Cell. 36, 500–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Razani B., Combs T. P., Wang X. B., Frank P. G., Park D. S., Russell R. G., Li M., Tang B., Jelicks L. A., Scherer P. E., Lisanti M. P. (2002) Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J. Biol. Chem. 277, 8635–8647 [DOI] [PubMed] [Google Scholar]
- 65. Cohen A. W., Razani B., Wang X. B., Combs T. P., Williams T. M., Scherer P. E., Lisanti M. P. (2003) Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am. J. Physiol. Cell. Physiol. 285, C222–C235 [DOI] [PubMed] [Google Scholar]
- 66. Cohen A. W., Razani B., Schubert W., Williams T. M., Wang X. B., Iyengar P., Brasaemle D. L., Scherer P. E., Lisanti M. P. (2004) Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes 53, 1261–1270 [DOI] [PubMed] [Google Scholar]
- 67. Asterholm I. W., Mundy D. I., Weng J., Anderson R. G., Scherer P. E. (2012) Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1. Cell. Metab. 15, 171–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mastrodonato M., Calamita G., Rossi R., Mentino D., Bonfrate L., Portincasa P., Ferri D., Liquori G. E. (2011) Altered distribution of caveolin-1 in early liver steatosis. Eur. J. Clin. Invest. 41, 642–651 [DOI] [PubMed] [Google Scholar]
- 69. Cohen A. W., Schubert W., Brasaemle D. L., Scherer P. E., Lisanti M. P. (2005) Caveolin-1 expression is essential for proper nonshivering thermogenesis in brown adipose tissue. Diabetes 54, 679–686 [DOI] [PubMed] [Google Scholar]
- 70. Horikawa Y. T., Panneerselvam M., Kawaraguchi Y., Tsutsumi Y. M., Ali S. S., Balijepalli R. C., Murray F., Head B. P., Niesman I. R., Rieg T., Vallon V., Insel P. A., Patel H. H., Roth D. M. (2011) Cardiac-specific overexpression of caveolin-3 attenuates cardiac hypertrophy and increases natriuretic peptide expression and signaling. J. Am. Coll. Cardiol. 57, 2273–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tsutsumi Y. M., Horikawa Y. T., Jennings M. M., Kidd M. W., Niesman I. R., Yokoyama U., Head B. P., Hagiwara Y., Ishikawa Y., Miyanohara A., Patel P. M., Insel P. A., Patel H. H., Roth D. M. (2008) Cardiac-specific overexpression of caveolin-3 induces endogenous cardiac protection by mimicking ischemic preconditioning. Circulation 118, 1979–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Moroianu J., Riordan J. F. (1994) Nuclear translocation of angiogenin in proliferating endothelial cells is essential to its angiogenic activity. Proc. Natl. Acad. Sci. U. S. A. 91, 1677–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rakowicz-Szulczynska E. M., Rodeck U., Herlyn M., Koprowski H. (1986) Chromatin binding of epidermal growth factor, nerve growth factor, and platelet-derived growth factor in cells bearing the appropriate surface receptors. Proc. Natl. Acad. Sci. U. S. A. 83, 3728–3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ferrara N., Davis-Smyth T. (1997) The biology of vascular endothelial growth factor. Endocr. Rev. 18, 4–25 [DOI] [PubMed] [Google Scholar]
- 75. Feng Y., Venema V. J., Venema R. C., Tsai N., Behzadian M. A., Caldwell R. B. (1999) VEGF-induced permeability increase is mediated by caveolae. Invest. Ophthalmol. Vis. Sci. 40, 157–167 [PubMed] [Google Scholar]
- 76. Feng Y., Venema V. J., Venema R. C., Tsai N., Caldwell R. B. (1999) VEGF induces nuclear translocation of Flk-1/KDR, endothelial nitric oxide synthase, and caveolin-1 in vascular endothelial cells. Biochem. Biophys. Res. Commun. 256, 192–197 [DOI] [PubMed] [Google Scholar]
- 77. Kwon H., Jeong K., Pak Y. (2009) Identification of pY19-caveolin-2 as a positive regulator of insulin-stimulated actin cytoskeleton-dependent mitogenesis. J. Cell. Mol. Med. 13, 1549–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kwon H., Jeong K., Hwang E. M., Park J. Y., Pak Y. (2011) A novel domain of caveolin-2 that controls nuclear targeting: regulation of insulin-specific ERK activation and nuclear translocation by caveolin-2. J. Cell. Mol. Med. 15, 888–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kwon H., Jeong K., Hwang E. M., Park J. Y., Hong S. G., Choi W. S., Pak Y. (2009) Caveolin-2 regulation of STAT3 transcriptional activation in response to insulin. Biochim. Biophys. Acta 1793, 1325–1333 [DOI] [PubMed] [Google Scholar]
- 80. Engelman J. A., Zhang X. L., Galbiati F., Lisanti M. P. (1998) Chromosomal localization, genomic organization, and developmental expression of the murine caveolin gene family (Cav-1, -2, and -3). Cav-1 and Cav-2 genes map to a known tumor suppressor locus (6-A2/7q31). FEBS Lett. 429, 330–336 [DOI] [PubMed] [Google Scholar]
- 81. Fiucci G., Ravid D., Reich R., Liscovitch M. (2002) Caveolin-1 inhibits anchorage-independent growth, anoikis and invasiveness in MCF-7 human breast cancer cells. Oncogene 21, 2365–2375 [DOI] [PubMed] [Google Scholar]
- 82. Bagnoli M., Tomassetti A., Figini M., Flati S., Dolo V., Canevari S., Miotti S. (2000) Downmodulation of caveolin-1 expression in human ovarian carcinoma is directly related to alpha-folate receptor overexpression. Oncogene 19, 4754–4763 [DOI] [PubMed] [Google Scholar]
- 83. Koleske A. J., Baltimore D., Lisanti M. P. (1995) Reduction of caveolin and caveolae in oncogenically transformed cells. Proc. Natl. Acad. Sci. U. S. A. 92, 1381–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Engelman J. A., Wykoff C. C., Yasuhara S., Song K. S., Okamoto T., Lisanti M. P. (1997) Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J. Biol. Chem. 272, 16374–16381 [DOI] [PubMed] [Google Scholar]
- 85. Lee S. W., Reimer C. L., Oh P., Campbell D. B., Schnitzer J. E. (1998) Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene 16, 1391–1397 [DOI] [PubMed] [Google Scholar]
- 86. Sanna E., Miotti S., Mazzi M., De Santis G., Canevari S., Tomassetti A. (2007) Binding of nuclear caveolin-1 to promoter elements of growth-associated genes in ovarian carcinoma cells. Exp. Cell. Res. 313, 1307–1317 [DOI] [PubMed] [Google Scholar]
- 87. Jackle S., Runquist E. A., Miranda-Brady S., Havel R. J. (1991) Trafficking of the epidermal growth factor receptor and transferrin in three hepatocytic endosomal fractions. J. Biol. Chem. 266, 1396–1402 [PubMed] [Google Scholar]
- 88. Baass P. C., Di Guglielmo G. M., Authier F., Posner B. I., Bergeron J. J. (1995) Compartmentalized signal transduction by receptor tyrosine kinases. Trends Cell Biol. 5, 465–470 [DOI] [PubMed] [Google Scholar]
- 89. Butler J. D., Blanchette-Mackie J., Goldin E., O'Neill R. R., Carstea G., Roff C. F., Patterson M. C., Patel S., Comly M. E., Cooney A., et al. (1992) Progesterone blocks cholesterol translocation from lysosomes. J. Biol. Chem. 267, 23797–23805 [PubMed] [Google Scholar]
- 90. Lazarow P. B. (1978) Rat liver peroxisomes catalyze the beta oxidation of fatty acids. J. Biol. Chem. 253, 1522–1528 [PubMed] [Google Scholar]
- 91. Pedersen J. I., Gustafsson J. (1980) Conversion of 3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholestanoic acid into cholic acid by rat liver peroxisomes. FEBS Lett. 121, 345–348 [DOI] [PubMed] [Google Scholar]
- 92. Fujimoto T., Kogo H., Ishiguro K., Tauchi K., Nomura R. (2001) Caveolin-2 is targeted to lipid droplets, a new “membrane domain” in the cell. J. Cell Biol. 152, 1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Blouin C. M., Le Lay S., Eberl A., Kofeler H. C., Guerrera I. C., Klein C., Le Liepvre X., Lasnier F., Bourron O., Gautier J. F., Ferre P., Hajduch E., Dugail I. (2010) Lipid droplet analysis in caveolin-deficient adipocytes: alterations in surface phospholipid composition and maturation defects. J. Lipid Res. 51, 945–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pol A., Martin S., Fernandez M. A., Ferguson C., Carozzi A., Luetterforst R., Enrich C., Parton R. G. (2004) Dynamic and regulated association of caveolin with lipid bodies: modulation of lipid body motility and function by a dominant negative mutant. Mol. Biol. Cell 15, 99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Van Meer G. (2001) Caveolin, cholesterol, and lipid droplets? J. Cell Biol. 152, F29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fuhs S. R., Insel P. A. (2011) Caveolin-3 undergoes SUMOylation by the SUMO E3 ligase PIASy: sumoylation affects G-protein-coupled receptor desensitization. J. Biol. Chem. 286, 14830–14841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kirchner P., Bug M., Meyer H. (2013) Ubiquitination of the N-terminal region of caveolin-1 regulates endosomal sorting by the VCP/p97 AAA-ATPase. J. Biol. Chem. 288, 7363–7372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hayer A., Stoeber M., Ritz D., Engel S., Meyer H. H., Helenius A. (2010) Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J. Cell Biol. 191, 615–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Glenney J. R., Jr., Soppet D. (1992) Sequence and expression of caveolin, a protein component of caveolae plasma membrane domains phosphorylated on tyrosine in Rous sarcoma virus-transformed fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 89, 10517–10521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dietzen D. J., Hastings W. R., Lublin D. M. (1995) Caveolin is palmitoylated on multiple cysteine residues. Palmitoylation is not necessary for localization of caveolin to caveolae. J. Biol. Chem. 270, 6838–6842 [DOI] [PubMed] [Google Scholar]
- 101. Head B. P., Patel H. H., Insel P. A. (2014) Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim. Biophys. Acta 1838, 532–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Niesman I. R., Zemke N., Fridolfsson H. N., Haushalter K. J., Levy K., Grove A., Schnoor R., Finley J. C., Patel P. M., Roth D. M., Head B. P., Patel H. H. (2013) Caveolin isoform switching as a molecular, structural, and metabolic regulator of microglia. Mol. Cell. Neurosci. 56, 283–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Boscher C., Nabi I. R. (2013) Galectin-3- and phospho-caveolin-1-dependent outside-in integrin signaling mediates the EGF motogenic response in mammary cancer cells. Mol. Biol. Cell 24, 2134–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Shiroto T., Romero N., Sugiyama T., Sartoretto J. L., Kalwa H., Yan Z., Shimokawa H., Michel T. (2014) Caveolin-1 is a critical determinant of autophagy, metabolic switching, and oxidative stress in vascular endothelium. PLoS One 9, e87871. [DOI] [PMC free article] [PubMed] [Google Scholar]


