Abstract
The notion that stress plays a role in the etiology of psychotic disorders, especially schizophrenia, is longstanding. However, it is only in recent years that the potential neural mechanisms mediating this effect have come into sharper focus. The introduction of more sophisticated models of the interplay between psychosocial factors and brain function has expanded our opportunities for conceptualizing more detailed psychobiological models of stress in psychosis. Further, scientific advances in our understanding of adolescent brain development have shed light on a pivotal question that has challenged researchers; namely, why the first episode of psychosis typically occurs in late adolescence/young adulthood. In this paper, we begin by reviewing the evidence supporting associations between psychosocial stress and psychosis in diagnosed patients as well as individuals at clinical high risk for psychosis. We then discuss biological stress systems and examine changes that precede and follow psychosis onset. Next, research findings on structural and functional brain characteristics associated with psychosis are presented; these findings suggest that normal adolescent neuromaturational processes may go awry, thereby setting the stage for the emergence of psychotic syndromes. Finally, a model of neural mechanisms underlying the pathogenesis of psychosis is presented and directions for future research strategies are explored.
Keywords: psychosis, prodrome, stress, hypothalamic–pituitary–adrenal (HPA) axis, brain development, clinical high risk
INTRODUCTION
Psychotic disorders are arguably the most devastating of all psychiatric illnesses, and there is now a clear scientific consensus that they involve both structural and functional brain abnormalities. Etiologic models of schizophrenia and other psychotic disorders have undergone significant changes in conjunction with advances in our scientific understanding of brain function and molecular genetics. As the complexities of brain and genetic mechanisms have become more apparent, our conceptualizations of psychosis have also increased in complexity. Nonetheless, stress has been an enduring element in theories and models of the etiology of psychosis, with perspectives on stress broadening to include both psychosocial and biological factors (Walker and Diforio, 1997; Walker et al., 2008). Thus the “diathesis-stress” model, which posits an interaction between preexisting vulnerability and stress, has maintained a central position in contemporary theories.
Although vulnerability to schizophrenia and other psychotic disorders is assumed to originate from genetic factors and abnormalities in fetal brain development, neuromaturational processes during adolescence are also posited to play an important role in the clinical expression of illness (Feinberg, 1982; Keshavan et al., 1994, 2005; Adams et al., 2000). This is because clinical onset of psychosis typically occurs in late adolescence/early adulthood and is generally conceptualized as a neurodevelopmental disorder (Brennan and Walker, 2001). The notion that adolescent neuromaturational processes are relevant to psychosis has gained ascendance in conjunction with an increasing research focus on the prodromal phase of psychosis. As described below, the prodrome is the period of functional decline and gradual onset of subclinical psychotic symptoms that precedes the first psychotic episode (Addington and Heinssen, 2012). It is viewed as an optimal period for identifying the mechanisms that give rise to psychosis, as well as the most plausible developmental period for future studies of preventive intervention. And most relevant to the present paper, adolescence is also a stage that is being increasingly recognized as a unique period with respect to stress sensitivity (Eiland and Romeo, 2012).
By way of background, it is important to note that recent advances in our understanding of genetic and environmental mechanisms conferring risk for psychosis do not appear to correspond with current nosological distinctions among psychotic disorders. Rather, evidence suggests that schizophrenia and other psychotic disorders share genetic (Craddock et al., 2009) and environmental risk factors, such as prenatal complications (Buka and Fan, 1999) and cannabis use (Moore et al., 2007). In addition, as described below, there is evidence that similar neurobiological processes are involved in the adverse effects of stress exposure on all diagnostic categories of psychosis.
In this paper, we discuss research on diagnosed psychotic patients, emphasizing studies that shed light on the emergence of psychosis by focusing on individuals who manifest clinical risk syndromes. We begin with an overview of research findings on the role of psychosocial stress and trauma in psychosis. Then we turn to the biological aspects of the stress response, with an emphasis on the hypothalamic–pituitary–adrenal (HPA) axis. As one of the primary neural systems governing the stress response, this system has been the focus of most research on biological aspects of stress in psychosis. We therefore examine its function and development, as well as the role it may be playing in psychotic disorders. While evidence indicates that the HPA axis is hyperactive in psychotic disorders, findings also suggest how it might be involved in the neuropathology underlying these illnesses. Specifically, activation of the HPA axis is postulated to contribute to the development of aberrant brain structural changes and to augment abnormal function of dopamine (DA) brain circuitry linked with the emergence of psychosis. Sensitivity to these effects may be amplified by early exposure to stress that sensitizes stress responsivity. Below we discuss a model of these mechanisms and offer suggestions for future research strategies.
PSYCHOSOCIAL STRESS AND PSYCHOSIS
Until relatively recently, the research examining associations between stress and psychosis has largely focused on stressful life events (e.g. loss of a family member, parental divorce, serious illness, birth of a child, etc.), with particular attention to events that are uncontrollable and relatively independent of the patient’s illness (Phillips et al., 2007). Cross-sectional studies have not provided consistent evidence that patients diagnosed with schizophrenia or other psychotic disorders experience more of these stressful life events than healthy or psychiatric controls (for reviews, see Phillips et al., 2007; Walker et al., 2008). While several longitudinal designs have revealed a significant increase in the number of life events preceding psychotic relapse (Malla et al., 1990; Hultman et al., 1997; Mondelli et al., 2010a), at least one study failed to replicate these findings (see Phillips et al., 2007).
There also appears to be a threshold effect, such that when the number of stressful life events exceeds the threshold, symptom onset or exacerbation occurs. For example, a longitudinal population study revealed that recent negative life events increased the risk of psychotic symptom presentation, but only in the group with exposure to ten or more negative events (Lataster et al., 2011). Further, the individual’s perception of the event as stressful, undesirable, and/or uncontrollable is also relevant (Horan et al., 2005; Renwick et al., 2009). This is illustrated in a study by Horan et al. (2005) in which schizophrenia patients actually reported lower rates of life events than healthy controls, yet they appraised both positive and negative life events as less controllable and more poorly managed, and rated positive life events as less desirable.
Psychosis also appears to be associated with greater emotional reactivity to stressors, as indexed by self-report measures of reactivity, arousability, and anxiety (Docherty et al., 2009). Furthermore, scores on emotional reactivity moderate the relationship between stressful life events and psychotic symptoms, such that life events were found to lead to symptom exacerbation primarily in patients who scored high in emotional reactivity (Docherty et al., 2009). Taken together, these results suggest that there are differences among psychotic patients and that their responses to stress should be taken in consideration in attempting to understand associations between stressful life events and psychosis.
More recently, some researchers have broadened the focus to examine the impact of minor stressors, or “daily hassles” (e.g. rushing to meet a deadline, transportation problems, etc.) on patients with psychoses. These studies have generally shown that patients with psychosis report a range of daily stressors and that ratings of self-reported daily stressors are positively correlated with psychotic, depressive, and anxious symptom severity (for review, see Phillips et al., 2007). Myin-Germeys and her research group in the Netherlands have utilized a novel prospective paradigm, the Experience Sampling Method (ESM), to assess the immediate impact of stressful experiences on the mood and symptom severity (Myin-Germeys et al., 2001, 2003, 2004, 2005; Habets et al., 2012). In the ESM, participants are trained to respond to random daytime signals for the completion of research measures. In studies by this group, participants recorded stressful experiences, as well as their appraisals of and reaction to them, multiple times a day for several consecutive days. Analyses indicated that patients and their first-degree relatives were more reactive to daily stressors than healthy controls, and that they manifested corresponding increases in psychotic symptom severity and negative affect. Such findings are consistent with evidence of the role of reactivity as a moderator of the association between stressful life events and psychosis (Docherty et al., 2009).
CHILDHOOD TRAUMA AND PSYCHOSIS
There is mounting evidence that experiencing childhood trauma, a particularly severe form of stress, renders individuals more vulnerable to developing psychosis later in life. Traumatic events include exposure to sexual, physical, or emotional abuse, physical and emotional neglect, and loss of a parent. Varese et al. (2012) conducted a meta-analysis of research on the association between childhood trauma and psychosis. This review included 41 studies of over 79,000 individuals, encompassing case-control, prospective/quasi-prospective, and population-based cross-sectional research designs. Despite wide variability in study design and measurement of both psychosis and childhood adversity, they found a significant relationship, represented by an odds ratio (OR) of 2.78. Analyses of prospective studies revealed that individuals who had experienced childhood adversity were nearly three times more likely to exhibit psychotic symptoms than individuals with no history of adversity (OR=2.75–2.99). Similarly, retrospective studies showed that patients with psychosis were more likely to report a history of childhood adversity than controls (OR=2.72). These associations remained significant when controlling for possible confounds such as urbanicity, gender, SES, genetic family history of mental illness, and cannabis or other drug use.
Consistent with studies using global measures of stressful life events, several population-based studies have shown that experiencing multiple childhood traumas and/or types of traumas further heightens psychosis risk (Janssen et al., 2004; Whitfield et al., 2005; Spauwen et al., 2006; Shevlin et al., 2008; Galletly et al., 2011; Saha et al., 2011). There is also an association between childhood trauma and the severity of the symptom and course of schizophrenia (for review, see Lysaker et al., 2005; Read et al., 2005; Schenkel et al., 2005; Alvarez et al., 2011; Burns et al., 2011; Heins et al., 2011; Ramsay et al., 2011). For example, severity and/or frequency of traumatic experiences have been found to be associated with severity of hallucinations and delusions (Schenkel et al., 2005; Burns et al., 2011; Heins et al., 2011). Furthermore, trauma exposure is related to mood symptom severity, such as depression and anxiety, in patients diagnosed with non-affective psychoses (Lysaker et al., 2005; Schenkel et al., 2005; Burns et al., 2011). Finally, with respect to illness course, there is evidence that patients with exposure to childhood trauma have an earlier onset of the disorder, earlier first hospitalization, and more hospital admissions than those with no trauma exposure (Schenkel et al., 2005; Alvarez et al., 2011).
STRESS SENSITIZATION
Taken together, the evidence that psychosis is associated with both increased reactivity to stress and greater exposure to childhood trauma has led some to raise the notion of stress sensitivity. Stress sensitization refers to the augmenting effects of stress/trauma exposure on subsequent behavioral and biological responses to stress (Grace, 2010; Valenti et al., 2011). Several studies of large samples have documented this effect at the behavioral level in humans. For example, while a report from a longitudinal study of a large cohort revealed that stress exposure was associated with increased risk of mood symptoms and appraisal of events as more stressful, the magnitude of these effects varied according to the individuals’ history of childhood trauma (McLaughlin et al., 2010). Specifically, recent stressors were associated with a 27.3% increase in risk of depression in the subsequent year among individuals who reported three or more traumatic childhood events vs. a 14.8% increase in risk among those with no childhood trauma. Similar results are reported by other investigators (Glaser et al., 2006; Wichers et al., 2009; Slavich et al., 2011; Stroud et al., 2011).
Two studies have provided preliminary evidence for a stress sensitization effect in psychosis. Using the ESM described above, Lardinois et al. (2011) found that psychotic patients with a history of childhood trauma exposure were more sensitive to daily stressors than patients with no trauma exposure, as indexed by significantly greater increases in both negative affect and psychotic symptoms in response to daily stress. Consistent with this, a prospective population-based study of adolescents and young adults found that the association between severe recent life stress and psychosis risk was stronger among individuals with a history of childhood adversity (Lataster et al., 2011).
Although the neural mechanisms underlying stress sensitization are unknown, it has been suggested that changes in the HPA axis and/or DA circuitry may be a factor. For example, research on rodents has shown that exposure to severe or persistent stress/trauma can heighten HPA reactivity and DA augmentation in response to subsequent stressors (Yuii et al., 2007; Grace, 2010; McLaughlin et al., 2010). These postulated mechanisms are discussed further below.
THE PRODROMAL STAGE OF PSYCHOSIS
If it is the case that stress can serve to trigger the expression of latent vulnerabilities to psychosis, then elucidating the mechanisms prior to the first episode has the greatest potential to inform approaches to preventive intervention. As noted above, the psychosis prodrome is characterized by deterioration in functioning and increasing symptoms that precede the onset of psychosis (Yung and McGorry, 1996). The duration of the prodromal phase can vary from months to years, with the characteristic signs including attenuated positive symptoms (e.g., unusual sensory experiences and ideations, suspiciousness, and disorganized thought), negative symptoms (e.g., social withdrawal, anhedonia, and decreased emotional experience and expression), and a range of “nonspecific” symptoms (e.g., anxiety, depression, and impaired attention) (Walker et al., 1993; Fuller et al., 2002; Lencz et al., 2004; Yung et al., 2004). Because many view the prodromal phase as affording the greatest opportunities for preventive intervention, structured interview measures for prospective assessment of prodromal features have been developed (Correll et al., 2010; Addington and Heinssen, 2012). These structured interviews yield symptom ratings and employ standard severity threshold criteria for designating prodromal status. The term clinical high-risk (CHR) is generally used to refer to those individuals who exhibit clinical signs conforming to the prodromal criteria designated in these measures. Most who meet CHR criteria are in distress and help-seeking, usually for nonspecific symptoms of mood disturbance and/or troubling ideations.
In the United States, the Structured Interview for Prodromal Syndromes (SIPS; Miller et al., 2002; McGlashan et al., 2010) has been the most widely used of the prospective measures, and, like similar measures, it enhances positive predictive power above the population prevalence for psychosis. It is estimated that among those who meet standardized criteria for CHR status based on the SIPS and other standardized measures of prodromal syndromes, the rate of conversion to psychosis is between 20% and 40% within two to four years (McFarlane, 2011). While this level of predictive power is superior to that obtained purely on the basis of family history of the disorder, or genetic high risk (GHR) status (Gottesman, 1991; Kendler and Gardner, 1997; Hans et al., 2004), ongoing longitudinal investigations are aimed at enhancing positive predictive power to a level that will justify attempts at preventive intervention. In fact, the North American Prodrome Longitudinal Study (NAPLS) project is examining both behavioral and biological factors that might be used in conjunction with clinical measures of prodromal syndromes to both enhance prediction and shed light on neural mechanisms (Addington et al., 2007; Cannon et al., 2008; Addington and Heinssen, 2012).
PSYCHOSOCIAL STRESS AND THE PRODROME
To better understand the impact of stress on the emergence of psychosis, researchers have begun to investigate the relation of psychosocial stress with the presentation and progression of the prodrome. Similar to the findings from research on psychosis, studies comparing CHR samples with healthy controls do not provide consistent support for more stressful life events in CHR samples. One study showed that CHR adolescents reported more total stressful life events than controls, as well as more independent and undesirable events (Tessner et al., 2011). However, another study found that CHR participants reported fewer stressful life events than controls, although these researchers only measured events over a 12-month period (Phillips et al., 2012). In these same studies, consistent with the findings of Horan et al. (2005) in their sample of schizophrenia patients, CHR patients also rated the events as significantly more distressing than did controls.
Similarly, research on daily stressors indicates that the number of reported daily stressors does not differ by diagnostic group, but CHR individuals report them as more stressful or upsetting than controls (Tessner et al., 2011; Phillips et al., 2012). These studies also show that self-reported chronic stress levels are associated with greater positive and depressive symptom severity in CHR patients. Finally, it appears that individuals who meet CHR criteria are more reactive to daily stress. A prospective study using ESM found that CHR subjects were more emotionally reactive to daily stressors than healthy controls, and they manifested a stress-linked exacerbation of symptoms comparable to that shown by patients with psychosis (Palmier-Claus et al., 2012).
Exposure to childhood trauma is also linked with the CHR syndrome. For example, Thompson et al. (2009) found that the rate of trauma exposure prior to the age of 18 in a sample of CHR participants in New York City was 97%, with 83% reporting physical abuse, 67% emotional abuse, and 27% sexual abuse. Similarly, Bechdolf et al. (2010) found a high prevalence of childhood trauma (70%) in a CHR sample, with Cox regression analyses revealing that sexual abuse significantly increased risk of conversion to psychosis (OR=2.96). In a subsample of 280 CHR participants from the first wave of NAPLS (Addington et al., 2007), only 15.7% reported experiencing childhood physical or sexual abuse (Addington et al., submitted for publication). Nevertheless, consistent with findings in patient groups (Schenkel et al., 2005; Burns et al., 2011; Heins et al., 2011), those with a history of abuse experienced more severe positive and affective symptoms than those with no child abuse history (Holtzman CW, Walker EF, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, McGlashan TH, Perkins DO, Seidman LJ, Tsuang M, Woods SW, unpublished data. Traumatic childhood experiences and risk for psychosis).
Taken together, the results of research on stress in CHR samples offer no consistent support for a higher occurrence of life events or daily stress. Yet, as is the case with diagnosed psychotic patients, CHR patients tend to experience stressful life events and daily stressors as more subjectively stressful. Further, the rate of self-reported childhood trauma exposure is higher in both CHR and psychotic samples, and there is evidence that this exposure leads to stress-sensitization in patients with psychosis. To date, there are no published reports on stress-sensitization in CHR samples or on the relation of psychosocial stress with conversion to psychosis in CHR subjects. Thus, further research is needed to determine whether stress and/or stress-sensitization are/is predictive of the onset of psychotic disorder.
As noted by Phillips et al. (2007), the results of research on stress and trauma should be interpreted with caution given the caveat that individual differences in personality and psychiatric vulnerabilities may influence both reports of stressful events and the likelihood of exposure to stress/trauma. Thus, rather than causing vulnerability to psychosis, stress/trauma exposure may be a consequence of vulnerability. While experimental studies on humans aimed at establishing causation are not an ethical option, there are recent findings from a study of monozygotic (MZ) twins that are relevant to this issue. Using the MZ twin differences approach, investigators obtained data on childhood adversity and subclinical “psychotic experiences” from both members of the twin pairs (Alemany et al., 2012). They found that within-pair twin differences in exposure to childhood adversity were significantly associated with corresponding differences in subclinical positive symptoms, suggesting that the relation between childhood adversity and symptoms is not solely attributable to genetically determined differences in vulnerability.
Together, the findings reviewed above raise intriguing questions about the nature of the stress response in individuals who are vulnerable to psychosis. Of course, ultimately, we must shift to the biological level of analysis in order to understand the impact of stress/trauma on the neuropathological process(es) that give(s) rise to psychosis. Whether the stressful event/trauma is real or imagined, if the perceived stressor activates the brain’s stress systems, it has the potential to alter brain function.
Research on biological response to stress in psychotic disorders, especially as compared with mood disorders, is limited in scope (Walker et al., 2008). A great deal more work is needed to determine whether and how the various neural systems that govern the stress response are dysfunctional in psychosis. Nonetheless, as described below, there is a rapidly accumulating body of research findings that suggest the HPA axis is a pivotal neural system mediating the adverse effects of stress on exposure on individuals at risk for psychosis. While a comprehensive review of the HPA axis is beyond the scope of this paper, in the sections below we provide a brief overview of the key elements to serve as a background for discussion of the research findings on psychosis.
THE HPA AXIS
The response of the HPA axis to stress entails a cascade of neurohormonal signaling (Jameison and Dinan, 2001; Stevens and White, 2010; Armario et al., 2012; Danese and McEwen, 2012; Hill and Tasker, 2012; Silverman and Sternberg, 2012). Following stress exposure, cortical input to corticotrophin-releasing hormone (CRH) neurons in the paraventricular nucleus (PVN) of the hypothalamus results in the release of CRH from axon terminals into pituitary circulation. CRH then stimulates secretion of adrenocorticotrophic hormone (ACTH) from the anterior pituitary gland into the bloodstream. ACTH, in turn, stimulates adrenal gland production of glucocorticoids (principally cortisol in primates and corticosterone in rodents) via type 2 melanocortin receptors (MC2-R), thereby initiating synthesis of cortisol which is released into circulation by diffusion. Glucocorticoids act on tissue throughout the body and cross the blood–brain barrier to act on multiple brain regions via both membrane and intracellular neuronal receptors. Homeostasis in the system is achieved, in part, through negative feedback via GRs; direct modulatory feedback is provided by hypothalamic and pituitary GRs, while indirect modulation is provided by limbic, thalamic, and frontal cortex receptors.
There are two types of GRs, mineralocorticoid receptors (MRs) and GRs, which are present throughout the limbic and paralimbic regions, including the hippocampus, amygdala and prefrontal cortex (Lupien et al., 2007; Oitzl et al., 2010). MRs have a six- to 10-fold higher affinity for endogenous glucocorticoids, whereas GRs have a higher affinity for synthetic glucocorticoids (e.g., dexamethasone (DEX) or prednisone) and are activated by higher levels of endogenous glucocorticoids in times of stress or pharmacologic challenge. In the hippocampus, GRs are primarily expressed in the dentate gyrus and CA1 and CA2 subregions, which are critical for cognitive function (Derijk and de Kloet, 2005; Walker et al., 2008; Oitzl et al., 2010). GRs are also present in the thalamus, septum, and PVN, as well as broadly throughout the prefrontal cortex and other cortical areas.
At rest, the majority of MRs are occupied, while only about 10% of GRs are bound to glucocorticoids. However, during stress or at the peak of the circadian cortisol rhythm (i.e., morning), MRs are saturated and increasing proportions of GRs become occupied (Stevens and White, 2010; Silverman and Sternberg, 2012). Both animal and human studies suggest that the relative proportion of MR to GR activation may be an important moderating factor in multiple brain processes, with the relation of receptor activation to brain function constituting an “inverted U” such that too much or too little can impair cognitive processes (Diamond et al., 1992; Lupien and McEwen, 1997; de Kloet et al., 1999; Lupien et al., 2002).
In addition to CRH, ACTH, cortisol, and numerous other messengers are involved in the HPA axis. For example, pro-opiomelanocortin (POMC), a prohormone, is the precursor for many bioactive peptides, including ACTH. ACTH is derived from POMC in the anterior pituitary, and POMC transcription is also subject to inhibition via feedback from activation of GR (Stevens and White, 2010). As the nature and the mechanisms of action of POMC and other precursor hormones are elucidated, we can expect to see more research on their role in psychosis. As described below, there is already some evidence that abnormalities in POMC are associated with psychotic disorders.
In response to persistent stress exposure, the HPA axis changes at multiple levels; this has primarily been documented in experimental animal research, but there is evidence that similar processes occur in humans. For instance, chronic elevations in ACTH can contribute to adrenal hyperplasia in humans, which can result in greater cortisol output in response to the same level of ACTH (Harvey and Sutcliffe, 2010; Oitzl et al., 2010). There are also changes at the level of the pituitary that may contribute to alterations in the ‘set-point’ of the HPA axis (Mondelli et al., 2008; Danese and McEwen, 2012; Krishnamurthy et al., 2012). Finally, changes in the negative feedback at multiple levels can result from glucocorticoid-induced compromises to brain structure (Damsted et al., 2011).
Chronic glucocorticoid exposure appears to have adverse effects on numerous brain regions. Sustained corticosterone elevations in rodents, including those due to chronic stress or corticosterone administration, are associated with the regression of synapses and a decrease in dendritic spines in hippocampal and prefrontal neurons (for reviews, see Bennett, 2008; Damsted et al., 2011; McEwen, 2012). Also, elevated glucocorticoids can suppress myelination, as well as affect calcium ion channels, structural protein levels in glial cells, and amino acid levels in the hippocampus – all of which can contribute to regional volumetric reductions (Belanoff et al., 2001; Damsted et al., 2011). Moreover, in rodent and primate hippocampus, persistent stress and glucocorticoid elevations lead to reduced neuronal excitability (Joels, 2001), impaired synaptic plasticity (McEwen, 2012), and reduced neurogenesis (Schoenfeld and Gould, 2012). The association in humans of increased cortisol levels with decreased hippocampal (Rao et al., 1989; Starkman et al., 1992; Lupien et al., 1998; Tessner et al., 2007) and cortical gray matter volume (Mainz et al., 2012) is consistent with these findings.
HPA activation is, of course, only one mediator of the biological stress response. Its activation trails the more rapid response by the sympathetic–adrenal–medullary (SAM) axis within the sympathetic nervous system, that involves stimulation of catecholamine release (Chrousos, 2009). Prefrontal cortex and limbic structures also contribute to the regulation of SAM function in response to stress.
As noted above, the present discussion focuses on the HPA axis, especially cortisol secretion, because it has received greater attention in research on psychosis. This is partially a consequence of the ease of measuring secretagogues involved in HPA function, especially cortisol, which can be assayed noninvasively and reliably in saliva (Walker et al., 2008). Further, research has revealed pervasive effects of glucocorticoids on brain structure and function, including hippocampal abnormalities that have been shown to be linked with psychosis (Mondelli et al., 2010a,b; Dranovsky and Leonardo, 2012). HPA function can, of course, be indexed in secretagogues other than cortisol, including via measurement of ACTH which, as described above, also plays a pivotal role in regulating HPA function in response to persistent stress (Papadimitriou and Priftis, 2009; Stevens and White, 2010). Compared to cortisol, however, ACTH has been relatively neglected in research on psychosis.
Glucocorticoids also interact with and modulate neurotransmitter systems in the brain, so their effects on brain function and circuitry can be pervasive (McEwen, 1998). For example, HPA axis activation, as indexed by heightened levels of cortisol, leads to the augmentation of DA levels in the human brain (Schatzberg et al., 1985; Wand et al., 2007). This is particularly relevant for psychosis because DA, more than any other neurotransmitter system, is implicated in the full spectrum of psychotic disorders (Howes and Kapur, 2009). Animal models indicate that glucocorticoid levels also affect serotonin (Belanoff et al., 2001; Heim and Binder, 2011), norepinephrine (Rastogi and Singhal, 2012), and glutamate in the cortex (Wolf, 2003; Bennett, 2008).
Finally, it is of relevance to note the normative developmental change in activation of the HPA axis in humans, as it parallels the gradual age-related increase in risk for psychotic disorders. Specifically, there is a post-pubertal increase in basal and stress-induced cortisol secretion that occurs in conjunction with advancing stages of puberty and appears to extend into the 3rd decade of life in humans (Lupien et al., 2002; Adam, 2006; Walker et al., 2010; Shirtcliff et al., 2011). This maturational increase in activity has been hypothesized to augment stress sensitivity in adolescence (Walker et al., 2008; Eiland and Romeo, 2012). To date, we are aware of no published studies on adolescent maturational changes in other indices of HPA function in humans, thus more research is needed to elucidate changes in CRH, ACTH, and other secretagogues. Such investigations are especially important in light of the evidence, described below, that HPA activity is involved in the expression of vulnerability for psychosis.
THE HPA AXIS AND PSYCHOSIS
There is a burgeoning literature on the relation between the HPA axis and psychosis. In a review of this literature, Walker et al. (2008) summarized several trends in the research findings that support this assumption, and subsequent research findings have converged on the same conclusions.
First, there is strong evidence that patients with psychosis, especially non-medicated patients, have higher baseline cortisol levels, and studies published subsequent to this review provide additional support for this conclusion (Garner et al., 2010; Kale et al., 2010; Venkatasubramanian et al., 2010). Although there are fewer studies of ACTH, largely due to limitations on its assay in saliva and urine, there is also evidence of elevated ACTH in psychotic patients (Ryan et al., 2004; Brunelin et al., 2008). In fact, both ACTH and the DA metabolite homovanillic acid (HVA) response to metabolic stressor are also elevated in psychotic patients relative to controls (Brunelin et al., 2008). Moreover, a recent postmortem study of pituitary glands from schizophrenia patients and controls revealed that the level of proACTH was elevated, and the POMC level showed a trend toward elevation in pituitaries from schizophrenia patients (Krishnamurthy et al., 2012).
Second, antipsychotic medications reduce cortisol and ACTH secretion, in addition to decreasing positive symptoms, and findings published subsequent to the above review support this effect as well (Venkatasubramanian et al., 2010).
Third, psychotic patients show HPA axis dysfunction in response to pharmacologic challenge, such as the DEX suppression test (DST). For example, administration of DEX, an exogenous glucocorticoid, typically leads to cortisol suppression due to the HPA axis negative feedback loop. DEX binds to GRs that, in turn, inhibit the expression of the POMC gene and, subsequently, the secretion of ACTH and cortisol. Thus, cortisol nonsuppression after the DST is an index of HPA axis dysfunction. In the DEX/CRH test, a more sensitive measure of HPA function, DEX is administered, then the following day CRH is administered, and ACTH and cortisol responses are measured. More pronounced ACTH and cortisol secretion in response to CRH reflect reduced glucocorticoid feedback regulation. The above review by Walker et al. (2008), and other reports (Owashi et al., 2008), have found higher post DST and DEX/CRH levels of cortisol and ACTH in patients with schizophrenia and affective psychoses than in healthy and depressed comparison groups. In many patients, this response is reversible with antipsychotic treatment (Ceskova et al., 2006).
Fourth, neuroimaging studies have consistently revealed volumetric reductions in both cortical and subcortical brain regions in psychosis patients, and comparisons of discordant MZ twins suggest that among the most pronounced reductions are those in the hippocampus. Again, more recent evidence supports this conclusion (Shepherd et al., 2012). Given the role of the hippocampus in modulating HPA activation, these results are also consistent with reports of an inverse correlation between baseline cortisol and hippocampal volume in psychosis (Mondelli et al., 2010b).
Fifth, the severity of most symptom dimensions, including positive, negative, and mood symptoms, tend to be positively related to cortisol levels in samples of patients with psychosis. Recent studies of first episode psychotic patients revealed significant associations of positive and negative symptom severity with cortisol levels (Garner et al., 2010; Belvederi Murri et al., 2012). Further, the magnitude of symptom severity reduction in response to antipsychotic medications has been linked with the magnitude of cortisol decrease (Mondelli et al., 2010a).
Finally, a causal role for HPA activity in triggering or exacerbating psychotic symptoms is supported by research on hypercortisolemia. For example, hypercortisolemia induced by administration of exogenous corticosteroids in high doses can trigger psychotic symptoms (Buchman, 2001; Warrington and Bostwick, 2006). Symptoms of hypercortisolemia-induced psychosis include pressured speech, hallucinations, delusions, and disorganized thought, which are often indistinguishable from the symptoms of psychotic disorders (Ling et al., 1981; Lewis and Smith, 1983; Wada et al., 2001). Indeed, disorders characterized by hypercortisolemia, such as Cushing’s syndrome, often involve psychotic symptoms that remit in conjunction with cortisol levels in response to treatments such as etomidate, an adrenal suppressant (Chan et al., 2011), mifepristone (van der Lely et al., 1991; Chu et al., 2001), and other treatments (Kelly et al., 2003). Interestingly, the risk for psychotic symptoms in Cushing’s, though elevated across the lifespan, is lower in childhood than in young adulthood.
Although there is relatively consistent evidence for elevated baseline HPA activity in psychosis, especially in non-medicated patients, findings from investigations of psychosocial stress-induced cortisol are mixed. Some studies using laboratory psychosocial stressors have found that psychotic patients show no difference or less cortisol increase after stress induction, both on (Jansen et al., 2000; Brenner et al., 2009) and off (van Venrooij et al., 2012) antipsychotic medications. The absence of an enhanced psychosocial stress-induced cortisol increase in psychosis, despite evidence for baseline elevations, may reflect several factors. First, there are ceiling effects on cortisol increments beyond heightened baseline levels (Crowley et al., 1993). Second, and perhaps more salient, after the onset of psychosis, the stress generated by the symptoms, especially distressing perceptions and ideations, may diminish the effect of any external psychosocial stress. Also, the psychosocial events that have the capacity for generating stress may become idiosyncratic. As discussed earlier, psychosis patients subjectively experience daily events as more distressing than healthy controls, even in the absence of increased stressor frequency.
It has been suggested that stress-sensitization may play a role in the elevated ‘set point’ of HPA activity observed in some psychotic patients. For example, Bennett (2008) reviews the neural mechanisms through which early stressful experiences can alter the set point of the HPA axis and lead to chronically higher glucocorticoid activity, abnormalities in neuronal connectivity, and increased risk for subsequent psychosis. It has been shown that HPA set point is influenced by environmental factors such as prenatal maternal stress and early postnatal stress (Maccari et al., 2003), and is also moderately heritable (van Hulle et al., 2012). Thus, as described below, genetic factors may also modulate biobehavioral sensitivity to stress.
In summary, several lines of research support an association between HPA axis function and psychosis. Specifically, there is an elevation in cortisol secretion for at least a subgroup of psychotic patients, which may have implications for symptom severity and course. More recently, the focus of some investigators has shifted to studies of the HPA axis in CHR subjects, with the goal of addressing the critical question of whether abnormalities in HPA function precede the onset of psychosis.
THE HPA AXIS AND CHR
Only a few studies to date have directly investigated indices of HPA axis functioning in individuals at risk for psychosis. Some of the earliest studies used small samples and yielded inconsistent results. For example, Thompson et al. (2007a) conducted a DEX/CRH test in 12 CHR patients, three of whom converted to psychosis during a two-year follow-up period. While the sample size did not allow for statistical analyses, the authors found that blood cortisol levels were similar in the three converters and nine non-converters, and non-converters reported more depression, anxiety, lifetime stressful events, and daily hassles than converters. This is contrary to past reports that typically find more severe stress, anxiety and depression among those who later convert (Yung et al., 2003; Addington and Heinssen, 2012).
However, in another report, this research group studied 23 CHR participants and found that baseline cortisol levels were positively correlated with the experience of daily hassles, but not with lifetime stressful events (Thompson et al., 2007b). Cortisol was also positively correlated with levels of depression and anxiety, but not with psychotic symptomatology, global psychopathology, or current global functioning. In addition to its small sample size these studies were limited by reliance on only one blood sample to measure cortisol, thus potentially reducing the reliability of the cortisol estimate (Dowd et al., 2009), and eliciting a stress response in anticipation of the blood draw (Jessop and Turner-Cobb, 2008).
More recent studies utilizing salivary cortisol measures and larger samples have yielded more consistent results. A study using salivary cortisol and a relatively large sample of CHR subjects (n = 56) and healthy controls revealed elevated cortisol in the CHR sample when compared to controls (Walker et al., 2010). Further, those CHR subjects who subsequently converted to a psychotic disorder (n = 14) had higher cortisol levels through the year preceding psychosis onset. This study utilized multiple measures of cortisol at each time point. A later study of salivary cortisol in a CHR sample revealed that basal cortisol was significantly higher in medication-free CHR patients when compared to both healthy controls and to CHR patients taking psychotropic medications (antipsychotics and/or antidepressants) (Sugranyes et al., 2012). Another report on the sample showed that cortisol was positively correlated with the severity of anxiety, suspiciousness and impaired stress tolerance in the CHR group (Corcoran et al., 2012). Finally, in the largest sample to date, an investigation of baseline cortisol levels from the ongoing second wave of NAPLS showed significantly higher baseline salivary cortisol in CHR subjects (n > 200) than healthy controls (n > 100) matched on age and sex (Walker et al., submitted). Cortisol levels were also positively, though modestly, correlated with ratings of positive, negative, disorganized, and general prodromal symptoms.
In a very important recent study of CHR and psychotic patients, investigators used PET to examine stress-induced cortisol and DA release as indexed by percent change in receptor binding potential between conditions (stress and control) in the limbic striatum (LST), associative striatum (AST), and sensorimotor striatum (SMST) (Mizrahi et al., 2012). The stressor was the MIST (Montreal imaging stress task), a challenging mental arithmetic task. Compared to healthy controls, CHR and psychotic patients had more pronounced DA response in the AST and SMST, as well as a greater cortisol response to the stressor. Further, there was a significant positive association between the increases in cortisol and DA. These findings converge with previous studies on the relation of glucocorticoids and DA in animal models (Cruz et al., 2012), as well as experimental research with healthy human subjects (Walker et al., 2008).
Finally, in one of the few studies of cortisol in GHR siblings, Collip et al. (2011) investigated stress, psychotic experiences, negative affect, and cortisol. Using ESM procedures, siblings and healthy controls were signaled randomly ten times per day over six days to complete survey forms and provide saliva samples. The authors report that siblings had higher cortisol levels than controls, and this difference persisted over the course of an expected diurnal decline in cortisol secretion. While the two groups did not report different frequencies or intensities of daily stressors, siblings had a larger cortisol increase following unpleasant events. Further, siblings also showed increased cortisol responses and negative affect in response to “momentary psychotic experiences,” while the control group did not.
In summary, while limited in number, the largest studies to date suggest that CHR patients, especially those who are nonmedicated, have elevated salivary cortisol and that those who convert to psychosis are characterized by the highest levels. Clearly, additional research is needed to address the pivotal issue of whether elevations in cortisol are preceding and triggering psychosis onset in some individuals, and, if so, what neural mechanisms are involved. Because HPA activity is associated with a range of physical and psychiatric disorders (Frodl and O’Keane, 2012), we must also address questions about the nature of the specific relation with psychosis. In particular, what preexisting brain vulnerability is modulated in its expression to yield the psychotic syndrome, as opposed to another disorder?
BRAIN DEVELOPMENT AND PSYCHOSIS
As noted above, a large body of research has documented a range of abnormalities in brain structure in schizophrenia and other psychoses (Shepherd et al., 2012). These include increased ventricular volume, decreased cortical gray matter, hippocampal (Adriano et al., 2012), and temporal volumes (Siever and Davis, 2004), and increased pituitary volume (Garner et al., 2005; Pariante et al., 2005; Mondelli et al., 2008; Takahashi et al., 2009). Recent systematic reviews of the literature on volumetric differences between psychotic patients and healthy controls conclude that there is a reduction in cortical gray matter volume in most regions and that these reductions characterize both first-episode and chronic patients (Arnone et al., 2009; Levitt et al., 2010). These abnormalities appear to increase over the course of illness, in at least some patients (Hulshoff Pol and Kahn, 2008). Further, studies of patients with onset of psychosis during adolescence indicate that gray matter volumetric reductions, relative to same-age healthy controls, are more pronounced the earlier the onset of the illness (Douaud et al., 2009). Finally, comparisons of MZ twins discordant for schizophrenia indicate that volumetric reductions are more pronounced in the affected co-twin, especially for the peri-hippocampal region, indicating that the brain abnormalities in psychotic patients are partially independent of genetic factors (van Haren et al., 2004; Borgwardt et al., 2010). While regional declines in brain volume are not specific to psychosis, and their implications for brain circuitry have not been elucidated, it is likely that they are indicators of underlying abnormalities in circuitry or “connectivity” that can give rise to psychotic symptoms (Várkuti et al., 2011).
More recently, volumetric reductions have been documented in CHR subjects, with greater reductions found in those who convert to psychosis (Puri, 2010; Smieskova et al., 2010; Fusar-Poli et al., 2011). For example, a meta-analysis of data from over 700 healthy controls and 800 CHR subjects revealed that the CHR group showed reduced gray matter volume in the parahippocampal/hippocampal regions, anterior cingulate, right superior temporal gyrus, left precuneus, left medial frontal gyrus, and right middle frontal gyrus (Fusar-Poli et al., 2011). Among the CHR subjects, those who later developed psychosis showed lower baseline gray matter volume in the right inferior frontal gyrus and in the right superior temporal gyrus. Additionally, consistent with evidence of heightened HPA activity preceding and following the onset of psychoses, the hippocampus appears to be a brain region with marked volumetric reduction in patients with psychotic disorders, as well as in those at elevated risk for psychosis (Witthaus et al., 2010).
Indeed, it has been suggested that the brain abnormalities associated with psychosis, as well as risk for a psychotic disorder, may partially reflect the adverse effects of persistent glucocorticoid elevations on brain structure (Walker et al., 2008). To date, we are aware of only one report on the relation of brain structure with indices HPA activity in psychosis; in a study using CT scans, higher rates of DST non-suppression were observed in psychotic patients with larger ventricular brain ratios (VBRs) which tend to be inversely correlated with volume (Rothschild et al., 1989). Given the evidence that adolescence is associated with increased HPA activity, as well as a normative decline in cortical gray matter volume (Peper et al., 2011), the role of HPA secretagogues in both normal adolescent neuromaturation and the etiology of psychosis is an important area for investigation.
THE HPA AXIS AND NEURAL MECHANISMS IN PSYCHOSIS
While there is compelling evidence that stress plays a role in the etiology of psychosis, it is also clear that a myriad of physical and psychiatric disorders can be triggered or exacerbated by stress (de Kloet, 2003). Thus increased activity of the HPA axis is a nonspecific risk factor for psychosis. Similarly, none of the volumetric brain abnormalities that have been observed in CHR and psychotic patients have been shown to be unique to psychosis: they are present, albeit often to a lesser degree, in patients with other psychiatric and neurological disorders that entail no psychotic symptoms. Therefore the structural brain changes that have been documented in psychosis must also be viewed as nonspecific factors. The ‘psychotogenic’ final common pathway(s) in the neuropathological process is(are), therefore, more likely at the level of brain circuitry and neurotransmitters. Moreover, given the heterogeneity in the clinical presentation of psychotic disorders, as well as the variability in associated biomarkers, it is likely that there are multiple such processes.
The pivotal question then becomes: how does activation of the HPA axis trigger the expression of the specific circuitry and neurotransmitters’ vulnerabilities associated with psychosis? In the following sections, we will consider the mechanisms that might be involved in stress exacerbation of some of the plausible neuropathological processes.
DA
As noted, hypotheses about DA dysregulation in psychosis have been prominent in the literature for decades (Howes and Kapur, 2009). Evidence supporting the role of DA comes from several lines of investigation. First, numerous PET studies have shown that the magnitude of DA receptor occupancy, particularly in the D2 subtype of receptors, is strongly associated with the clinical effectiveness of antipsychotic medications (Uchida et al., 2011). Second, there is now robust evidence of a dysregulation of presynaptic DA activity in schizophrenia that leads to excessive DA release in the striatum, especially the areas projecting to the AST where there is integration among cognitive and limbic cortical inputs (Miyake et al., 2011). Furthermore, recent evidence indicates that heightened striatal DA activity, including increased synthesis, is present in CHR individuals prior to the onset of psychosis (Howes et al., 2009; Bauer et al., 2012).
The relations among stress, HPA activity and neurotransmitter activity, especially DA, have been the subject of recent research. Although the mechanisms are not yet understood, it is clear that glucocorticoid secretion augments DA activity in certain brain regions (Dallman et al., 2004; Arnsten, 2011), especially the mesolimbic system (Marinelli et al., 2006). For example, in a PET investigation of healthy human subjects, exposure to a psychosocial stressor caused a significant release of DA in the ventral striatum, as indexed by a reduction in [11C] raclopride binding; the magnitude of the cortisol response was highly positively correlated with DA release in the ventral striatum (r = 0.78), consistent with a facilitating effect of cortisol on DA neuron firing (Pruessner et al., 2004). Conversely, animal research has shown that agents that suppress glucocorticoid secretion also reduce brain DA release in a manner similar to antipsychotics (Piazza et al., 1996). Finally, there is evidence that agents that enhance DA activity also heighten HPA activity and augment cortisol secretion in humans (Philippi et al., 2000; Locatelli et al., 2010). For example, bupropion, a DA reuptake inhibitor, increases cortisol release in healthy humans (Piacentini et al., 2004).
These and other findings document the synergistic relation between HPA activity and DA neurotransmission. They also suggest how both stress exposure and maturationally determined increases in cortisol secretion might trigger a neuropathological DA-driven process. Thus, when HPA activation augments striatal DA in at-risk individuals characterized by preexisting DA hyperactivity, a positive or regenerative feedback loop might ensue and lead to disruption in the functioning of mesolimbic and/or frontal circuits that can give rise to psychotic symptoms.
GABA and glutamate
Although less well documented than DA, other neurotransmitter systems have also been implicated in the pathogenesis of psychotic disorders. A comprehensive review of the research is beyond the scope of this paper; however, certain findings relevant to GABA and glutamate are noteworthy. For example, it has been suggested that dysfunction in GABA-mediated synaptic inhibition disrupts neural function in psychosis (Lodge and Grace, 2011). Thus, DA dysregulation may be secondary to abnormalities in GABA neurotransmission and resultant hyperactivity within subregions of the hippocampus. Enhanced ventral hippocampal activity is linked with both greater DA activity and positive symptoms, and preclinical studies indicate that modulation of a particular GABA receptor [alpha5GABA(A)] can decrease this aberrant DA signaling and associated behaviors. Further, these authors note that stress appears to augment ventral hippocampal function via the HPA axis and may thereby exacerbate or trigger psychotic symptoms.
Taking another perspective, some have implicated the activity of glutamate, an excitatory neurotransmitter, in the pathogenesis of psychosis; specifically it has been suggested that psychosis involves an abnormality in neurotransmission at N-methyl-d-aspartate (NMDA)-type glutamate receptors. Drawing on the established psychotomimetic effects of phencyclidine (PCP) and ketamine, both of which are NMDA receptor antagonists, some have suggested that NMDA receptor hypofunction can give rise to psychosis (Stone et al., 2007; Kantrowitz and Javitt, 2010). Indeed, administration of PCP and ketamine to healthy individuals often leads to cognitive deficits and symptoms characteristic of psychosis (for review, see Coyle, 2006). Furthermore, rodent studies have shown NMDA receptor hypofunction on GABAergic inhibitory neurons, resulting in increased levels of cortical glutamate, after treatment with PCP or ketamine. While glutamate is an excitatory neurotransmitter, there is also evidence that the NMDA receptor plays an indirect inhibitory role, in that it can suppress neural circuit activity via activation of inhibitory GABA interneurons. Further, the removal of the inhibitory influence of GABA results in glutamatergic excitotoxicity and cell damage and/or death in frontal and temporal cortices, which might explain the cortical hypoactivity observed in psychosis (Stone et al., 2007).
Rolls and Deco (2011) recently expanded on the theory that cortical hypoactivity results from reduced NMDA receptor function (Gonzalez-Burgos and Lewis, 2012 for review), thereby contributing to the cognitive and negative symptoms of schizophrenia. These authors explore how abnormalities in neuromaturational processes in late adolescence, such as synaptic pruning, decreases in gray matter volume, and changes in GABA-mediated inhibition and DA may contribute to psychosis onset by reducing the excitability of cortical neurons. Recent work with animal models of schizophrenia suggests that early stress can lead to glutamatergic dysfunction (Egerton et al., 2012). Clearly, more research is needed to identify the pathways through which HPA activation affects GABAergic and glutamatergic neurotransmission in individuals at risk for psychosis.
EPIGENETIC PROCESSES
It has been shown that glucocorticoids and other steroid hormones have slow emerging effects, which are assumed to be subserved by genomic mechanisms, as well as more rapid effects that are more likely to involve nongenomic mechanisms (Evanson et al., 2010; Groeneweg et al., 2011; McEwen, 2012; Strelzyk et al., 2012). Most of the relations between glucocorticoids and neurotransmitters described above are relatively acute effects that are measurable in the laboratory, in that they entail transient modulation of neurotransmitter synthesis, release, or receptor sensitivity, but do not necessarily involve changes in gene expression.
Epigenetic refers to modifications to the genome that affects gene expression without altering the DNA sequence. Glucocorticoids and other hormones can influence components of epigenetic processes, such as DNA methylation and histone modification, and thereby influence structural characteristics of the brain (Charmandari et al., 2003; Rodriguez-Waitkus et al., 2003; Wintermantel et al., 2005; Daufeldt et al., 2006; Kouzmenko et al., 2010; Nugent and McCarthy, 2011). These changes in gene expression influence the protein messengers and can therefore result in changes in brain structure that have the potential to be permanent.
Although our understanding of the relations among hormonal changes, gene expression, and brain structure/function is limited, there is accumulating evidence that gonadal and adrenal hormones play a critical role in triggering the expression of genes that govern brain maturation, especially during adolescence/young adulthood (Hyman, 1996; Thompson et al., 2000; Sisk and Zehr, 2005; Peper et al., 2010). Further, recent studies of regional brain volumes in twins have revealed significant age by heritability interactions, such that white matter volume heritability estimates increased with age through adolescence, indicating that the expression of genes governing white matter development increases during this developmental period (Wallace et al., 2006).
Researchers have also shown that mRNA levels for brain-derived neurotrophic factor (BDNF), which is present throughout the brain and promotes neuron survival and growth, increase significantly during adolescence/young adulthood, after which time they are relatively stable (Webster et al., 2002). The significant increase in BDNF mRNA during the young adult period coincides with the time when the frontal cortex matures both structurally and functionally. Belanoff et al. (2001) also note that glucocorticoids can reduce levels of BDNF and neurotrophin 3 (NT-3), both of which are implicated in the regulation of neuronal structure and function. Consistent with this, a recent study by Issa et al. (2010) found an inverse relationship between cortisol and BDNF levels in post-mortem brains of schizophrenia patients. Thus, in CHR individuals, elevated HPA activity through the course of adolescence may alter the expression of genes that modulate the activity of BDNF, thereby derailing important neuromaturational processes.
GENE–ENVIRONMENT INTERACTIONS
The notion of a gene–environment interaction assumes that effects of “environmental” factors, such as stress, will vary as a function of the individual’s genotype. Some epigenetic processes also entail gene–environment interactions, in that individuals vary in their susceptibility to certain epigenetic processes because of their genotype. Research on gene–environment interactions in psychosis is growing rapidly (e.g. van Winkel et al., 2008b; Korver et al., 2012). While some studies suggesting gene-stress interaction have used proxy measures for genetic vulnerability, such as a family history of psychosis, others have measured specific candidate gene polymorphisms.
One study of psychotic patients and their healthy biological siblings used ESM to measure daily stress reactions (Lataster et al., 2010). Findings indicated a familial clustering of increased stress-reactivity, suggesting both shared genetic and environmental influences underlying stress-reactivity in siblings and patients. A more recent neuroimaging study comparing schizophrenia patients and their biological siblings revealed that exposure to childhood trauma was associated with reduced cortical thickness in patients but not siblings (Habets et al., 2011), indicating that patients might be particularly sensitive to the deleterious effects of early life stress.
With respect to specific genes, evidence suggests that psychosocial stress may interact with single nucleotide polymorphisms (SNPs) on at least three genes implicated in the development of psychosis: namely, polymorphisms within the catechol-O-methyltransferase (COMT), BDNF, and neuregulin 1 (NR1) genes.
The COMT gene codes an enzyme that affects DA, norepinephrine, and epinephrine. Important for understanding its significance in psychosis is that COMT augments the breakdown of DA in the prefrontal cortex. Within the COMT gene a functional polymorphism involving a Met to Val substitution at codon 158 results in two common allelic variants, the valine (Val) and the methionine (Met) allele, that are associated with high and low enzyme activity, respectively (Lotta et al., 1995). The Val allele is associated with increased COMT activity and consequent reduction of DA neurotransmission in the frontal cortex, but it may also increase levels of mesolimbic (phasic) DA activity (Chen et al., 2004). A study of young men in military training revealed a significant interaction between COMT genotype and stress, with Val carriers showing the greatest increases in psychotic symptom severity in relation to stress exposure (Stefanis et al., 2007). A subsequent study showed that Val carriers also experienced more paranoia in response to event stress when compared with Met carriers (Simons et al., 2009).
In another report, however, researchers examined self-reported stress and symptoms in cannabis-using psychotic patients and healthy cannabis users and found that Met/Met patients showed the largest increases in psychotic symptoms and negative affect with increased stress (van Winkel et al., 2008a). This pattern of findings is consistent with the results of an investigation of the relation between COMT genotype and cortisol secretion in healthy and CHR adolescents (Walder et al., 2010). Findings from that study revealed higher cortisol levels for Met homozygotes (compared with heterozygotes and Val homozygotes) across a one-year period among Met carriers, suggesting that COMT genotype is associated with differences in cortisol secretion during adolescence. The inconsistent findings on the relation of COMT genotype with cortisol highlight the challenges of research on gene–environment interactions in humans, yet they also suggest the potential benefits of this research with respect to developing more targeted preventive interventions.
The gene that codes for BDNF has also been linked with stress sensitivity. BDNF is a neurotrophin that promotes the growth, differentiation, and survival of developing neurons (Poo, 2001). A common Val/Met SNP at position 66 in the BDNF gene was identified as a functional polymorphism. The Val variant is associated with higher neuronal BDNF secretory activity than the Met variant (Egan et al., 2003). A study of a large random sample (n = 533) from the general population revealed that Met carriers who were exposed to childhood abuse were more likely to report positive psychotic-like experiences than those with the Val/Val genotype (Alemany et al., 2011). Using a general population sample of twins, other investigators also found that BDNF Met carriers showed more social-stress-induced paranoia than those with the Val/Val genotype (Simons et al., 2009). Such findings support the notion that genetic factors play a role in determining stress sensitivity, and are consistent with findings presented above that indicate an inverse relation of cortisol with BDNF levels.
Because an NR1 polymorphism, the T/T genotype, has been linked with increased risk of psychosis, Keri et al. (2009) examined polymorphisms of the NR1 gene (SNP8NRG243177/r6994992) in relation to stress sensitivity. Schizophrenia patients and one of their family members participated in neutral and conflictual interactions while relatives’ critical comments and patients’ unusual ideations were recorded. Patients with the NR1 T/T genotype expressed more unusual thoughts than C-carriers (C/T and C/C) during conflict-related interactions, but not during neutral interactions.
Although the above findings on single candidate genes indicate that certain polymorphisms may confer heightened vulnerability to stress, it is important to note that the proportion of the variance accounted for by these interactions is modest, as would be expected based on the absence of any gene of large effect in the etiology of psychosis. More recently, investigators have begun to examine interactions among multiple genes in relation to stress sensitivity. For example, Peerbooms et al. (2012) tested the interaction between COMT Val158Met and another potential risk gene, the methylenetetrahydrofolate reductase (MTHFR) C677T gene, which has been shown to be linked with cognition in schizophrenia patients and healthy controls. They found that the MTHFR C677T genotype moderated the interaction between COMT Val158Met genotype and stress in patients, but not controls, such that patients with the MTHFR 677 T-allele and COMT Met/Met combination displayed the largest increases in psychotic symptoms in reaction to daily stress. Those with the MTHFR 677 C/C genotype showed no significant COMT Val158Met X stress interaction.
In summary, the number of published reports on gene-environment interactions in relation to stress and psychotic symptoms is limited, and there are not yet any consistent trends in the findings. Nonetheless, they illustrate the potential for identifying subgroups of individuals, especially CHR patients, who may be particularly sensitive to stress.
SUMMARY AND CONCLUSIONS
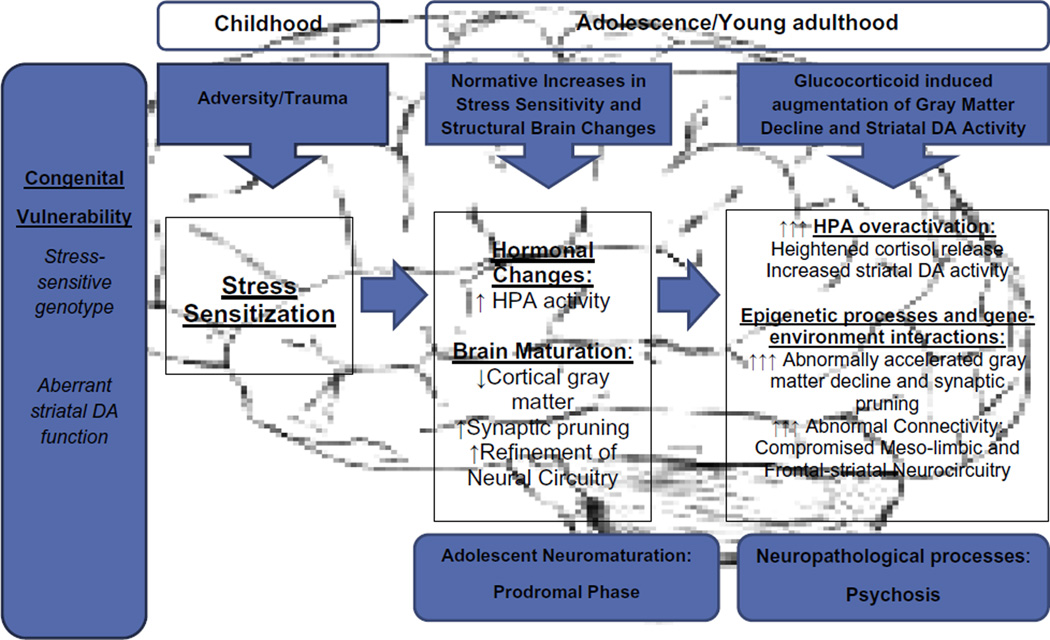
A multifaceted picture is beginning to emerge in our scientific understanding of stress and psychosis. Drawing on the above findings, a tentative model of the neural mechanisms is summarized in Fig. 1. The model is not assumed to be exhaustive of all potential mechanisms, nor is it postulated to apply to all cases of psychosis, as there may be an etiologic subtype of psychosis characterized by greater stress sensitivity. The model is, however, intended to suggest how stress might impact the developmental trajectory of individuals at risk for psychosis.
Fig. 1.
Stress and neurodevelopmental mechanisms in psychosis: hypothesized stages and processes.
Listed at the left of Fig. 1, are two congenital sources of vulnerability that are relevant to subsequent stress sensitivity in psychosis. Research on the first, stress-sensitive genotypes, has explored gene–environment interactions that influence individual differences in the biobehavioral response to stress. The work is in its infancy, and there are significant methodological challenges in identifying reliable gene–environment interactions (Walker et al., 2011). It is likely that a myriad of genes is involved, that their effects vary from small to large, that they interact in complex ways, and that they are nonspecific with regard to conferring risk for psychiatric disorder. Genes that influence secretagogues and receptors relevant to the HPA axis are among the promising targets for investigation.
The second hypothesized source of congenital vulnerability in Fig. 1, aberrant striatal DA function, is assumed to be more uniquely linked with risk for psychosis. The striatum is sensitive to prenatal insults, and DA function (e.g., synthesis, reuptake, receptor density, and sensitivity) in this region is partially genetically determined and shows marked developmental changes in humans, with evidence of greater activity in adolescence/early adulthood (Walker, 1994). Further, striatal DA abnormalities can affect circuits that govern a range of cognitive, affective, and motor functions, as the striatum is a critical element in mesolimbic and frontal circuitry, as well as motor circuits. The developmental trajectories of these circuits vary in conjunction with maturational changes in underlying connectivity, with some characterized by maturational prominence during the modal period of risk for psychosis onset (Walker, 1994; Várkuti et al., 2011).
Moving to the childhood stage in Fig. 1, the model reflects the increasing evidence of a relation between exposure to childhood trauma/adversity and risk for psychosis. As described above, there is evidence from research on humans that such exposure can result in stress-sensitization. Animal models indicate that this process is subserved by a sensitization of the HPA axis and DA systems. When combined with congenital vulnerability, stress-sensitization may be amplified and set the stage for the more pronounced biobehavioral sensitivity to stress observed in CHR patients, especially during the adolescent period (Eiland and Romeo, 2012).
As noted above, research has revealed normative increases in stress sensitivity, as well as changes in brain structure and function, through adolescence and extending into young adulthood. In Fig. 1, the onset of these normative changes is assumed to correspond with the period when prodromal signs often emerge. Subsequently, if a neuropathological process is triggered and superimposed on these normative changes, the result will be a more dramatic increase in HPA activity which, through genomic mechanisms, can lead to a more pronounced loss of gray matter volume, with associated disruptions in connectivity. Through nongenomic processes, increased HPA activity can also augment striatal DA activity in disrupted circuits, eventually giving rise to episodes of psychotic symptoms. As noted earlier, this has the potential to engender a positive feedback loop, as the synergistic effects of cortisol and DA ensue.
The process described here is, of course, just one of several plausible models for conceptualizing the role of stress and HPA activity in psychosis. Other neurotransmitter systems, including GABA and glutamate, are likely involved, and there are undoubtedly individual differences among patients in the confluence of factors that trigger psychotic symptoms. While space does not permit greater discussion of the numerous alternatives, other authors have speculated on some of them in detail (Phillips et al., 2007; Bennett, 2008; Grace, 2010; Lodge and Grace, 2011).
It is also important to acknowledge that childhood behavioral abnormalities and functional deficits are also associated with psychosis (Walker, 1994; Fuller et al., 2002; Schenkel et al., 2005). Childhood premorbid impairments span all functional domains: motor, cognitive, and socioemotional. Although such deficits are not observed in all individuals who develop a psychotic disorder, the fact that they are more common in children at risk is consistent with the notion that vulnerability is congenital, although typically latent prior to adolescence.
FUTURE DIRECTIONS
Assuming that pivotal neuropathological processes are occurring during the prodromal period in adolescence/young adulthood, longitudinal research efforts aimed at elucidating them, especially in their initial stages in CHR patients, is a high priority. Given the findings to date, the following issues are central for future research on stress and psychosis.
First, we need to conduct more comprehensive, longitudinal research on stress biomarkers, especially the HPA axis, in CHR individuals. We need a more detailed and dynamic picture of HPA secretagogues, including prohormones, CRH, and ACTH, as well as cortisol, and, eventually, the characteristics of their receptors throughout the brain. This will allow us to determine which point(s) in the HPA cascade is(are) aberrant in those who progress to psychosis. Further, indices of the SAM system should be examined, such as alpha amylase, to examine whether this stress system is elevated in CHR patients. With respect to brain measures, techniques should be expanded beyond structural MRI to include measures that index connectivity, such as diffusion tensor imaging (DTI). Although we are aware of no established ligands for use with PET to reliably estimate GR and MR in humans, peripheral blood mononuclear cells’ measures of GR receptor numbers can be used until ligands are available.
A second priority is the identification of precipitants of the brain changes that are detectable prior to psychosis onset. We need to examine the cross-sectional and longitudinal relations of biological indicators of stress systems with brain volumetric declines and changes in DA activity, given the evidence that both are observed prior to psychosis onset. The results of such research hold promise for shedding light on neural signaling processes that could provide leverage for preventive intervention.
Third, heterogeneity in biological stress sensitivity should be targeted in future research. Assuming there is genetic heterogeneity among CHR individuals that confers differential sensitivity to stress, further research on candidate genes and the relation of their polymorphisms with biological indicators of stress sensitivity may prove fruitful. As discussed earlier, several candidates have already been identified. Environmental factors, such as exposure to early trauma/adversity, may also contribute to etiologic heterogeneity via stress-sensitization, particularly in the adolescent period. In pursuing the goal of parsing etiologic subtypes, the application of analytic techniques such as cluster and taxonomic analyses, aimed at identifying stress-sensitive subgroup(s) should be considered.
Finally, while we are not yet at a point where any one specific preventive intervention is indicated by extant research findings, the above research will inform future directions. In research on Cushing’s syndrome, agents that dampen cortisol activity have been shown to have beneficial effects with respect to psychiatric symptoms, cognitive functions, and brain structure (Starkman et al., 1992; McEwen and Gianaros, 2011). For instance, administration of the glucocorticoid antagonist mifepristone to Cushing’s patients rapidly diminishes psychotic symptoms (van der Lely et al., 1991). Several glucocorticoid antagonists, including mifepristone, have also been examined in the treatment of patients with mood and psychotic disorders, with some promising results (Schatzberg and Lindley, 2008). However, the number of studies is small and they are characterized by confounds with psychotropic medications. Further, to our knowledge, none have targeted patients who manifest elevated pretreatment cortisol levels. Many would consider the rate of false positives (i.e., >50%) with current prospective measures of prodromal syndromes to be too high to justify intervention with available glucocorticoid antagonists, given their potential adverse side effects that could be more pronounced in young adults (Schatzberg and Lindley, 2008). Before proceeding with trials of glucocorticoid antagonists in CHR samples, more targeted research is needed on diagnosed psychotic patients with elevated cortisol levels. In the interim, effective psychotherapeutic approaches to dampening HPA activity have been reported (Tafet and Feder, 2011), and these are more appropriate for young CHR patients. Thus psychotherapeutic interventions might not only benefit CHR patients, but also serve as a probe to measure the consequences of reductions in biological indicators of stress for symptom levels, brain structure, and conversion to psychosis.
We should note one line of research on stress and psychosis that appears to hold less promise; namely, the utilization of laboratory psychosocial stress challenges. These paradigms have been very informative in studies of healthy participants, but have yielded inconsistent findings in research with psychotic and CHR patients. This may reflect diagnostic group differences in construal of the stress-inductions. The research reviewed in this paper indicates that CHR and psychotic patients differ from controls in their subjective stress reactions. It is likely that endogenous factors, such as distressing ideations and perceptions, are also contributing to their subjective distress and obscuring the effects of psychosocial stress induction. In contrast, pharmacologic challenges and within-subject studies using ESM, in conjunction with repeated measures of biomarkers, may have greater potential as paradigms for elucidating the behavioral and biological consequences of stress-system perturbation.
In summary, there has been significant progress in our understanding of the role of stress in risk for psychosis, and recent research with CHR samples has suggested important directions for future investigations. The next generation of research holds great promise for elucidating the biological mechanisms as well as potential preventive interventions. Though more work is needed, the payoff, in terms of reducing the number of young lives derailed by serious mental illness, is well worth the price.
Abbreviations
- ACTH
adrenocorticotrophic hormone
- AST
associative striatum
- BDNF
brain-derived neurotrophic factor
- CHR
clinical high-risk
- COMT
catechol-O-methyltransferase
- CRH
corticotrophin-releasing hormone
- DA
dopamine
- DEX
dexamethasone
- DST
dexamethasone suppression test
- ESM
Experience Sampling Method
- GHR
genetic high risk
- GRs
glucocorticoid receptors
- HPA
hypothalamic–pituitary–adrenal
- HVA
homovanillic acid
- Met
methionine
- MRs
mineralocorticoid receptors
- MTHFR
methylenetetrahydrofolate reductase
- MZ
monozygotic
- NAPLS
North American Prodrome Longitudinal Study
- NMDA
N-methyl-d-aspartate
- NR1
neuregulin 1
- OR
odds ratio
- PCP
phencyclidine
- POMC
pro-opiomelanocortin
- SAM
sympathetic–adrenal–medullary
- SIPS
Structured Interview for Prodromal Syndromes
- SMST
sensorimotor striatum
- Val
valine
REFERENCES
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adams J, Barone S, Jr, LaMantia A, Philen R, Rice DC, Spear L, Susser E. Workshop to identify critical windows of exposure for children’s health: neurobehavioral work group summary. Environ Health Perspect. 2000;108(Suppl. 3):535–544. doi: 10.1289/ehp.00108s3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Heinssen R. Prediction and prevention of psychosis in youth at clinical high risk. Ann Rev Clin Psychol. 2012;8:269–289. doi: 10.1146/annurev-clinpsy-032511-143146. [DOI] [PubMed] [Google Scholar]
- Addington J, Cadenhead KS, Cannon TD, Cornblatt B, McGlashan TH, Perkins DO, Seidman LJ, Tsuang M, Walker EF, Woods SW, Heinssen R. North American Prodrome Longitudinal Study: a collaborative multisite approach to prodromal schizophrenia research. Schizophr Bull. 2007;33(3):665–672. doi: 10.1093/schbul/sbl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Stowkowy J, Cadenhead KS, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Cannon TD. Early traumatic experiences in those at clinical high risk for psychosis. doi: 10.1111/eip.12020. (submitted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriano F, Caltagirone C, Spalletta G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. Neuroscientist. 2012;18:180–200. doi: 10.1177/1073858410395147. [DOI] [PubMed] [Google Scholar]
- Alemany S, Arias B, Aguilera M, Villa H, Moya J, Ibáñez M, Vossen H, Gastó C, Ortet G, Fañaná s L. Childhood abuse, the BDNFVal66Met polymorphism and adult psychotic-like experiences. Br J Psychiatry. 2011;199:38–42. doi: 10.1192/bjp.bp.110.083808. [DOI] [PubMed] [Google Scholar]
- Alemany S, Goldberg X, van Winkel R, Gastó C, Peralta V, Fañaná s L. Childhood adversity and psychosis: examining whether the association is due to genetic confounding using a monozygotic twin differences approach. Eur Psychiatry. 2012 doi: 10.1016/j.eurpsy.2012.03.001. (epub advance access) [DOI] [PubMed] [Google Scholar]
- Alvarez M-J, Osés A, Foguet Q, Solà J, Arrufat F-X. Prevalence and clinical impact of childhood trauma in patients with severe mental disorders. J Nerv Ment Dis. 2011;199(3):156–161. doi: 10.1097/NMD.0b013e31820c751c. [DOI] [PubMed] [Google Scholar]
- Armario A, Daviu N, Muñoz-Abellán C, Rabasa C, Fuentes S, Belda X, Nadal R. What can we know from pituitary–adrenal hormones about the nature and consequences of exposure to emotional stressors? Cell Mol Neurobiol. 2012;32(5):749–758. doi: 10.1007/s10571-012-9814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry. 2009;195(3):194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. Int J Dev Neurosci. 2011;29(3):215–223. doi: 10.1016/j.ijdevneu.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Praschak-Rieder N, Kasper S, Willeit M. Is dopamine neurotransmission altered in prodromal schizophrenia? Curr Pharm Des. 2012;18(12):1568–1579. doi: 10.2174/138161212799958611. [DOI] [PubMed] [Google Scholar]
- Bechdolf A, Thompson A, Nelson B, Cotton S, Simmons MB, Amminger GP, Leicester S, Francey SM, McNab C, Krstev J, Sidis A, McGorry PD, Yung AR. Experience of trauma and conversion to psychosis in an ultra-high-risk (prodromal) group. Acta Psychiatr Scand. 2010;121:377–384. doi: 10.1111/j.1600-0447.2010.01542.x. [DOI] [PubMed] [Google Scholar]
- Belanoff JK, Gross K, Yager A, Schatzberg AF. Corticosteroids and cognition. J Psychiatr Res. 2001;35:127–145. doi: 10.1016/s0022-3956(01)00018-8. [DOI] [PubMed] [Google Scholar]
- Belvederi Murri M, Pariante CM, Dazzan P, Hepgul N, Papadopoulos AS, Zunszain P, Di Forti M, Murray RM, Mondelli V. Hypothalamic–pituitary–adrenal axis and clinical symptoms in first-episode psychosis. Psychoneuroendocrinology. 2012;37(5):629–644. doi: 10.1016/j.psyneuen.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Bennett MR. Stress and anxiety in schizophrenia and depression: glucocorticoids, corticotrophin-releasing hormone and synapse regression. Aust N Z J Psychiatry. 2008;42:995–1002. doi: 10.1080/00048670802512073. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Picchioni MM, Ettinger U, Toulopoulou T, Murray R, McGuire PK. Regional gray matter volume in monozygotic concordant and discordant for schizophrenia. Biol Psychiatry. 2010;67(10):956–964. doi: 10.1016/j.biopsych.2009.10.026. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Walker EF. Vulnerability to schizophrenia risk factors in childhood and adolescence. In: Ingram RE, Price JM, editors. Vulnerability to psychopathology: risk across the lifespan. New York: The Guilford Pressp; 2001. pp. 476–498. [Google Scholar]
- Brenner K, Liu A, Laplante DP, Lupien S, Pruessner JC, Ciampi A, Joober R, King S. Cortisol response to a psychosocial stressor in schizophrenia: blunted, delayed, or normal? Psychoneuroendocrinology. 2009;34(6):859–868. doi: 10.1016/j.psyneuen.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Brunelin J, d’Amato T, van Os J, Cochet A, Suaud-Chagny M-F, Saoud M. Effects of acute metabolic stress on the dopaminergic and pituitary-adrenal axis activity in patients with schizophrenia, their unaffected siblings and controls. Schizophr Res. 2008;100:206–211. doi: 10.1016/j.schres.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol. 2001;33(4):289–294. doi: 10.1097/00004836-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Buka SL, Fan AP. Association of prenatal and perinatal complications with subsequent bipolar disorder and schizophrenia. Schizophr Res. 1999;39:113–119. doi: 10.1016/s0920-9964(99)00109-7. (discussion 160-111) [DOI] [PubMed] [Google Scholar]
- Burns JK, Jhazbhay K, Esterhuizen T, Emsley R. Exposure to trauma and the clinical presentation of first-episode psychosis in South Africa. J Psychiatr Res. 2011;45:179–184. doi: 10.1016/j.jpsychires.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceskova E, Kasparek T, Zoukova A, Prikryl R. Dexamethasone suppression test in first-episode schizophrenia. Neuro Endocrinol Lett. 2006;27(4):433–437. [PubMed] [Google Scholar]
- Chan LF, Vaidya M, Westphal B, Allgrove J, Martin L, Afshar F, Hindmarsh PC, Savage MO, Grossman AB, Storr HL. Use of intravenous etomidate to control acute psychosis induced by the hypercortisolaemia in severe paediatric Cushing’s disease. Horm Res Paediatr. 2011;75(6):441–446. doi: 10.1159/000324419. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Kino T, Souvatzoglou E, Chrousos GP. Pediatric stress: hormonal mediators and human development. Horm Res. 2003;59(4):161–179. doi: 10.1159/000069325. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska B, Halim N, Ma Q, Matsumoto M, Melhem S, Kolachana B, Hyde T, Herman M, Apud J, Egan M, Kleinman J, Weinberger D. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocr. 2009;5(7):374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Chu JW, Matthias DF, Belanoff J, Schatzberg A, Hoffman AR, Feldman D. Successful long-term treatment of refractory Cushing’s disease with high-dose mifepristone (RU 486) J Clin Endocrinol Metab. 2001;86:3568–3573. doi: 10.1210/jcem.86.8.7740. [DOI] [PubMed] [Google Scholar]
- Collip D, Nicolson NA, Lardinois M, Lataster T, van Os J, Myin-Germeys I. Daily cortisol, stress reactivity and psychotic experiences in individuals at above average genetic risk for psychosis. Psychol Med. 2011;41(11):2305–2315. doi: 10.1017/S0033291711000602. [DOI] [PubMed] [Google Scholar]
- Corcoran CM, Smith C, McLaughlin D, Auther A, Malaspina D, Cornblatt B. HPA axis function and symptoms in adolescents at clinical high risk for schizophrenia. Schizophr Res. 2012;135:170–174. doi: 10.1016/j.schres.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Hauser M, Auther AM, Cornblatt BA. Research in people with psychosis risk syndrome: a review of the current evidence and future directions. J Child Psychol Psychiatry. 2010;51:390–431. doi: 10.1111/j.1469-7610.2010.02235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26(4):365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, O’Donovan MC, Owen MJ. Psychosis genetics: modeling the relationship between schizophrenia, bipolar disorder, and mixed (or “schizoaffective”) psychoses. Schizophr Bull. 2009;35(5):482–490. doi: 10.1093/schbul/sbp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley S, Hindmarsh PC, Honour JW, Brook CG. Reproducibility of the cortisol response to stimulation with a low dose of ACTH(1-24): the effect of basal cortisol levels and comparison of low-dose with high-dose secretory dynamics. J Endocrinol. 1993;136:67–172. doi: 10.1677/joe.0.1360167. [DOI] [PubMed] [Google Scholar]
- Cruz FC, Marin MT, Leão RM, Planeta CS. Stress-induced cross-sensitization to amphetamine is related to changes in the dopaminergic system. J Neural Transm. 2012;119(4):415–424. doi: 10.1007/s00702-011-0720-8. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Strack AM, Scribner KS, Pecoraro N, La Fleur SE, Houshyar H, Gomez F. Chronic stress-induced effects of corticosterone on brain: direct and indirect. Ann N Y Acad Sci. 2004;1018:141–150. doi: 10.1196/annals.1296.017. [DOI] [PubMed] [Google Scholar]
- Damsted SK, Born AP, Paulson OB, Uldall P. Exogenous glucocorticoids and adverse cerebral effects in children. Eur J Paediatr Neurol. 2011;15(6):465–477. doi: 10.1016/j.ejpn.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behavr. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Daufeldt S, Klein R, Wildt L, Allera A. Membrane initiated steroid signaling (MISS): computational, in vitro and in vivo evidence for a plasma membrane protein initially involved in genomic steroid hormone effects. Mol Cell Endocrinol. 2006;246(1–2):42–52. doi: 10.1016/j.mce.2005.11.027. [DOI] [PubMed] [Google Scholar]
- de Kloet ER. Hormones, brain and stress. Endocr Regul. 2003;37(2):51–68. [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- DeRijk R, de Kloet ER. Corticosteroid receptor genetic polymorphisms and stress responsivity. Endocrine. 2005;28(3):263–270. doi: 10.1385/ENDO:28:3:263. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2(4):421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Docherty NM, St.-Hilaire A, Aakre JM, Seghers JP. Life events and high-trait reacitivity together predict psychotic symptom increases in schizophrenia. Schizophr Bull. 2009;35(3):638–645. doi: 10.1093/schbul/sbn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G, Mackay C, Andersson J, James S, Quested D, Ray MK, Connell J, Roberts N, Crow TJ, Matthews PM, Smith S, James A. Schizophrenia delays and alters maturation of the brain in adolescence. Brain. 2009;132(Pt 9):2437–2448. doi: 10.1093/brain/awp126. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Simanek AM, Aiello AE. Socio-economic status, cortisol and allostatic load: a review of the literature. Int J Epidemiol. 2009;38(5):1297–1309. doi: 10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranovsky A, Leonardo ED. Is there a role for young hippocampal neurons in adaptation to stress? Behav Brain Res. 2012;227(2):371–375. doi: 10.1016/j.bbr.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Egerton A, Fusar-Poli P, Stone JM. Glutamate and psychosis risk. Curr Pharm Des. 2012;18(4):466–478. doi: 10.2174/138161212799316244. [DOI] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.10.048. (epub advance access) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanson NK, Herman JP, Sakai RR, Krause EG. Nongenomic actions of adrenal steroids in the central nervous system. J Neuroendocrinol. 2010;22(8):846–861. doi: 10.1111/j.1365-2826.2010.02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Frodl T, O’Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis. 2012 doi: 10.1016/j.nbd.2012.03.012. (epub advance access) [DOI] [PubMed] [Google Scholar]
- Fuller R, Nopoulos P, Arndt S, O’Leary D, Ho B, Andreasen N. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry. 2002;159:1183–1189. doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Crescini A, Deste G, Kempton MJ, Lawrie S, McGuire P, Sacchetti E. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35:1175–1185. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Galletly C, van Hooff M, McFarlane A. Psychotic symptoms in young adults exposed to childhood trauma—a 20 year follow-up study. Schizophr Res. 2011;127:76–82. doi: 10.1016/j.schres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Garner B, Pariante CM, Wood SJ, Velakoulis D, Phillips L, Soulsby B, Brewer WJ, Smith DJ, Dazzan P, Berger GE, Yung AR, van den Buuse M, Murray R, McGorry PD, Pantelis C. Pituitary volume predicts future transition to psychosis in individuals at ultrahigh risk of developing psychosis. Biol Psychiatry. 2005;58:417–423. doi: 10.1016/j.biopsych.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Garner B, Phassouliotis C, Phillips LJ, Markulev C, Butselaar F, Bendall S, Yun Y, McGorry PD. Cortisol and dehydroepiandrosterone-sulphate levels correlate with symptom severity in first-episode psychosis. J Psychiatr Res. 2010;45(2):249–255. doi: 10.1016/j.jpsychires.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Glaser J, van Os J, Portegijis PJM, Myin-Germeys I. Childhood trauma and emotional reactivity to daily life stress in adult frequent attenders of general practitioners. J Psychosom Res. 2006;61(2):229–236. doi: 10.1016/j.jpsychores.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. NMDA receptor hypofunction, parvalbumin-positive neurons and cortical gamma oscillations in schizophrenia. Schizophr Bull. 2012;38(5):950–957. doi: 10.1093/schbul/sbs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II. Schizophrenia genesis: the origins of madness. New York, NY: WH Freeman & Company; 1991. [Google Scholar]
- Grace AA. Dopamine system dysregulation by the ventral subiculum as the common pathophysiological basis for schizophrenia psychosis, psychostimulant abuse, and stress. Neurotox Res. 2010;18(3–4):367–376. doi: 10.1007/s12640-010-9154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joëls M. Rapid nongenomic effects of corticosteroids and their role in the central stress response. J Endocrinol. 2011;209:153–167. doi: 10.1530/JOE-10-0472. [DOI] [PubMed] [Google Scholar]
- Habets P, Marcelis M, Gronenschild E, Drukker M, van Os J. Reduced cortical thickness as an outcome of differential sensitivity to environmental risks in schizophrenia. Biol Psychiatry. 2011;69:487–494. doi: 10.1016/j.biopsych.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Habets P, Collip D, Myin-Germeys I, Gronenschild E, van Bronswijk S, Hofman P, Lataster T, Lardinois M, Nicolson NA, van Os J, Marcelis M. Pituitary volume, stress reactivity, and genetic risk for psychotic disorder. Psychol Med. 2012;42(7):1523–1533. doi: 10.1017/S0033291711002728. [DOI] [PubMed] [Google Scholar]
- Hans SL, Auerbach JG, Styr B, Marcus J. Offspring of parents with schizophrenia: mental disorders during childhood and adolescence. Schizophr Bull. 2004;30:303–315. doi: 10.1093/oxfordjournals.schbul.a007080. [DOI] [PubMed] [Google Scholar]
- Harvey PW, Sutcliffe C. Adrenocortical hypertrophy: establishing cause and toxicological significance. J Appl Toxicol. 2010;30(7):617–626. doi: 10.1002/jat.1569. [DOI] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early-life stress and depression: review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Exp Neurol. 2011;233(1):102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heins M, Simons C, Lataster T, Pfeifer S, Versmissen D, Lardinois M. Childhood trauma and psychosis: a case-control and casesibling comparison across different levels of genetic liability, psychopathology, and type of trauma. Am J Psychiatry. 2011;168:1286–1294. doi: 10.1176/appi.ajp.2011.10101531. [DOI] [PubMed] [Google Scholar]
- Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic–pituitary–adrenal axis. Neuroscience. 2012;204:5–16. doi: 10.1016/j.neuroscience.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Ventura J, Nuechterlein KH, Subotnik KL, Hwang SS, Mintz J. Stressful life events in recent-onset schizophrenia: reduced frequencies and altered subjective appraisals. Schizophr Res. 2005;75:363–374. doi: 10.1016/j.schres.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Howes DO, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, Bramon-Bosch E, Valmaggia L, Johns L, Broome M, McGuire PK, Grasby PM. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34(2):354–366. doi: 10.1093/schbul/sbm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman CM, Wieselgren I, Oehman A. Relationships between social support, social coping and life events in the relapse of schizophrenic patients. Scand J Psychol. 1997;38:3–13. doi: 10.1111/1467-9450.00002. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Relevance of gene regulation to psychiatry. Am Psychiatr Press Rev Psychiatry. 1996;15:311–330. [Google Scholar]
- Issa G, Wilson C, Terry AV, Jr, Pillai A. An inverse relationship between cortisol and BDNF levels in schizophrenia: data from human postmortem and animal studies. Neurobiol Dis. 2010;39(3):327–333. doi: 10.1016/j.nbd.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Jameison K, Dinan TG. Glucocorticoids and cognitive function: from physiology to pathophysiology. Hum Psychopharmacol. 2001;16:293–302. doi: 10.1002/hup.304. [DOI] [PubMed] [Google Scholar]
- Jansen LMC, Gispen-de Wied CC, Kahn RS. Selective impairments in the stress response in schizophrenic patients. Psychopharmacology. 2000;149:319–325. doi: 10.1007/s002130000381. [DOI] [PubMed] [Google Scholar]
- Janssen I, Krabbendam L, Bak M, Hanssen M, Vollebergh W, de Graaf R, van Os J. Childhood abuse as a risk factor for psychotic experiences. Acta Psychiatr Scand. 2004;109:38–45. doi: 10.1046/j.0001-690x.2003.00217.x. [DOI] [PubMed] [Google Scholar]
- Jessop DS, Turner-Cobb JM. Measurement and meaning of salivary cortisol: a focus on health and disease in children. Stress. 2008;11(1):1–14. doi: 10.1080/10253890701365527. [DOI] [PubMed] [Google Scholar]
- Joels M. Corticosteroid actions in the hippocampus. J Neuroendocrinol. 2001;13:657–669. doi: 10.1046/j.1365-2826.2001.00688.x. [DOI] [PubMed] [Google Scholar]
- Kale A, Naphade N, Sapkale S, Kamaraju M, Pillai A, Joshi S, Mahadik S. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res. 2010;175(1–2):47–53. doi: 10.1016/j.psychres.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC. N-Methyl-D-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010;83:108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WF, Kelly MJ, Faragher B. A prospective study of psychiatric and psychological aspects of Cushing’s syndrome. Clin Endocrinol. 2003;45(6):715–720. doi: 10.1046/j.1365-2265.1996.8690878.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. The risk for psychiatric disorders in relatives of schizophrenic and control probands: a comparison of three independent studies. Psychol Med. 1997;27:411–419. doi: 10.1017/s003329179600445x. [DOI] [PubMed] [Google Scholar]
- Keri S, Kiss I, Seres I, Kelemen O. A polymorphism of the neuregulin 1 gene (SNP8NRG243177/rs6994992) affects reactivity to expressed emotion in schizophrenia. Am J Med Genet. 2009;150B(3):418–420. doi: 10.1002/ajmg.b.30812. [DOI] [PubMed] [Google Scholar]
- Keshavan M, Anderson S, Pettegrew J. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994;28:239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Keshavan M, Diwadkar V, Rosenberg D. Developmental biomarkers in schizophrenia and other psychiatric disorders: common origins, different trajectories? Epidemiol Psychiatr Soc. 2005;14:188–193. doi: 10.1017/s1121189x00007934. [DOI] [PubMed] [Google Scholar]
- Korver N, Quee PJ, Boos HB, Simons CJ, de Haan L. Genetic risk and outcome of psychosis (GROUP), a multisite longitudinal cohort study focused on gene–environment interaction; objectives, sample characteristics, recruitment, and assessment methods. Int J Methods Psychiatr Res. 2012;21(3):205–221. doi: 10.1002/mpr.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzmenko A, Ohtake F, Fujiki R, Kato S. Hormonal gene regulation through DNA methylation and demethylation. Epigenomics. 2010;2(6):765–774. doi: 10.2217/epi.10.58. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy D, Harris LW, Levin Y, Koutroukides TA, Rahmoune H, Pietsch S, Vanattou-Saifoudine N, Leweke FM, Guest PC, Bahn S. Metabolic, hormonal and stress-related molecular changes in post-mortem pituitary glands from schizophrenia subjects. World J Biol Psychiatry. 2012 doi: 10.3109/15622975.2011.601759. (epub advance access) [DOI] [PubMed] [Google Scholar]
- Lardinois M, Lataster T, Mengelers R, van Os J, Myin-Germeys I. Childhood trauma and increased stress sensitivity in psychosis. Acta Psychiatr Scand. 2011;123(1):28–35. doi: 10.1111/j.1600-0447.2010.01594.x. [DOI] [PubMed] [Google Scholar]
- Lataster T, Collip D, Lardinois M, Van Os J, Myin-Germeys I. Evidence for a familial correlation between increased reactivity to stress and positive psychotic symptoms. Acta Psychiatr Scand. 2010;122:395–404. doi: 10.1111/j.1600-0447.2010.01566.x. [DOI] [PubMed] [Google Scholar]
- Lataster J, Myin-Germeys I, Lieb R, Wittchen H-U, van Os J. Adversity and psychosis: a 10-year prospective study investigating synergism between early and recent adversity in psychosis. Acta Psychiatr Scand. 2011;125(5):388–399. doi: 10.1111/j.1600-0447.2011.01805.x. [DOI] [PubMed] [Google Scholar]
- Lencz T, Smith C, Auther A, Correll C, Cornblatt B. Nonspecific and attenuated negative symptoms in patients at clinical high-risk for schizophrenia. Schizophr Res. 2004;68:37–48. doi: 10.1016/S0920-9964(03)00214-7. [DOI] [PubMed] [Google Scholar]
- Levitt JJ, Bobrow L, Lucia D, Srinivasan P. A selective review of volumetric and morphometric imaging in schizophrenia. Curr Top Behav Neurosci. 2010;4:243–281. doi: 10.1007/7854_2010_53. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Smith RE. Steroid-induced psychiatric syndromes. A report of 14 cases and a review of the literature. J Affect Disord. 1983;5(4):319–332. doi: 10.1016/0165-0327(83)90022-8. [DOI] [PubMed] [Google Scholar]
- Ling MH, Perry PJ, Tsuang MT. Side effects of corticosteroid therapy: psychiatric aspects. Arch Gen Psychiatry. 1981;38:471–477. doi: 10.1001/archpsyc.1981.01780290105011. [DOI] [PubMed] [Google Scholar]
- Locatelli V, Bresciani E, Tamiazzo L, Torsello A. Central nervous system-acting drugs influencing hypothalamic–pituitary–adrenal axis function. Endocr Dev. 2010;17:108–120. doi: 10.1159/000262533. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Developmental pathology, dopamine, stress and schizophrenia. Int J Dev Neurosci. 2011;29(3):207–213. doi: 10.1016/j.ijdevneu.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J. Kinetics of human soluble and membranebound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry (Mosc) 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Lupien S, De Leon M, de Santi S, Convit A, Tarshish C, Nair NPV, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1(1):69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Wilkinson CW, Briere S, Menard C, Ng Ying Kin NM, Nair NP. The modulatory effects of corticosteroids on cognition: studies in young human populations. Psychoneuroendocrinology. 2002;27(3):401–416. doi: 10.1016/s0306-4530(01)00061-0. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. 2007;65:209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Lysaker PH, Beattie NL, Strasburger AM, Davis LW. Reported history of childhood sexual abuse in schizophrenia: associations with heightened symptom levels and poorer participation over four months in vocational rehabilitation. J Nerv Ment Dis. 2005;193(12):179–184. doi: 10.1097/01.nmd.0000188970.11916.76. [DOI] [PubMed] [Google Scholar]
- Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev. 2003;27(1–):119–127. doi: 10.1016/s0149-7634(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Mainz V, Schulte-Rüther M, Fink GR, Herpertz-Dahlmann B, Konrad K. Structural brain abnormalities in adolescent anorexia nervosa before and after weight recovery and associated hormonal changes. Psychosom Med. 2012;74(6):574–582. doi: 10.1097/PSY.0b013e31824ef10e. [DOI] [PubMed] [Google Scholar]
- Malla AK, Cortese L, Shaw TS, Ginsberg B. Life events and relapse in schizophrenia. A one year prospective study. Soc Psychiatry Psychiatr Epidemiol. 1990;25:221–224. doi: 10.1007/BF00782965. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Rudick CN, Hu XT, White FJ. Excitability of dopamine neurons: modulation and physiological consequences. CNS Neurol Dis Drug Targ. 2006;5(1):79–97. doi: 10.2174/187152706784111542. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The ever-changing brain: cellular and molecular mechanisms for the effects of stressful experiences. Dev Neurobiol. 2012;72(6):878–890. doi: 10.1002/dneu.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane WR. Prevention of the first episode of psychosis. Psychiatr Clin North Am. 2011;34:95–107. doi: 10.1016/j.psc.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan T, Walsh BC, Woods SW. The psychosis risk syndrome: handbook for diagnosis and follow-up. New York: Oxford University Press, New York; 2010. [Google Scholar]
- McLaughlin KA, Conron KJ, Koenen KC, Gilman SE. Childhood adversity, adult stressful life events, and risk of pastyear psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychol Med. 2010;40:1647–1658. doi: 10.1017/S0033291709992121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T, McGlashan T, Rosen J, Somjee L, Markovich P, Stein K, Woods S. Prospective diagnosis of the initial prodrome for schizophrenia based on the structured interview for prodromal syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- Miyake N, Thompson J, Skinbjerg M, Abi-Dargham A. Presynaptic dopamine in schizophrenia. CNS Neurosci Therapeut. 2011;17(2):104–109. doi: 10.1111/j.1755-5949.2010.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi R, Addington J, Rusjan PM, Suridjan I, Ng A, Boileau I, Pruessner JC, Remington G, Houle S, Wilson AA. Increased stress-induced dopamine release in psychosis. Biol Psychiatry. 2012;71(6):561–567. doi: 10.1016/j.biopsych.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Mondelli V, Dazzan P, Gabilondo A, Tournikioti K, Walshe M, Marshall N, Schulze KK, Murray RM, McDonald C, Pariante CM. Pituitary volume in unaffected relatives of patients with schizophrenia and bipolar disorder. Psychoneuroendocrinology. 2008;33(7):1004–1012. doi: 10.1016/j.psyneuen.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Mondelli V, Dazzan P, Hepgul N, Di Forti M, Aas M, D’Albenzio A, Di Nicola M, Fisher H, Handley R, Ries Marques T, Morgan C, Navari S, Taylor H, Papadopoulos A, Aitchison KJ, Murray RM, Pariante CM. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: the role of stress and of antipsychotic treatment. Schizophr Res. 2010a;116:234–242. doi: 10.1016/j.schres.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli V, Pariante CM, Navari S, Aas M, D’Albenzio A, Di Forti M, Handley R, Hepgul N, Reis Marques T, Taylor H, Papadopoulos AS, Aitchison KJ, Murray RM, Dazzan P. Higher cortisol levels are associated with smaller left hippocampal volume in firstepisode psychosis. Schizophr Res. 2010b;119:75–78. doi: 10.1016/j.schres.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, van Os J, Schwartz JE, Stone AA, Delespaul PA. Emotional reactivity to daily life stress in psychosis. Arch Gen Psychiatry. 2001;58:1137–1144. doi: 10.1001/archpsyc.58.12.1137. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, Krabbendam L, Delespaul P, van Os J. Do life events have their effects on psychosis by influencing the emotional reactivity to daily stress? Psychol Med. 2003;33:327–333. doi: 10.1017/s0033291702006785. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, Krabbendam L, Delespaul P, van Os J. Sex differences in emotional reactivity to daily life stress in psychosis. J Clin Psychiatr. 2004;65:805–809. doi: 10.4088/jcp.v65n0611. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, Delespaul P, van Os J. Behavioural sensitization to daily life stress in psychosis. Psychol Med. 2005;35:733–741. doi: 10.1017/s0033291704004179. [DOI] [PubMed] [Google Scholar]
- Nugent BM, McCarthy MM. Epigenetic underpinnings of developmental sex differences in the brain. Neuroendocrinology. 2011;93(3):150–158. doi: 10.1159/000325264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, Champagne DL, van der Veen R, de Kloet ER. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci Biobehav Rev. 2010;34(6):853–866. doi: 10.1016/j.neubiorev.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Owashi T, Otsubo T, Oshima A, Nakagome K, Higuchi T, Kamijim K. Longitudinal neuroendocrine changes assessed by dexamethasone/CRH and growth hormone releasing hormone tests in psychotic depression. Psychoneuroendocrinology. 2008;33(2):152–161. doi: 10.1016/j.psyneuen.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Palmier-Claus JE, Dunn G, Lewis SW. Emotional and symptomatic reactivity to stress in individuals at ultra-high risk of developing psychosis. Psychol Med. 2012;42(5):1003–1012. doi: 10.1017/S0033291711001929. [DOI] [PubMed] [Google Scholar]
- Papadimitriou A, Priftis KN. Regulation of the hypothalamicpituitary- adrenal axis. Neuroimmunomodulation. 2009;16(5):265–271. doi: 10.1159/000216184. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Dazzan P, Danese A, Morgan KD, Brudaglio F, Morgan C, Fearon P, Orr K, Hutchinson G, Pantelis C, Velakoulis D, Jones PB, Leff J, Murray RM. Increased pituitary volume in antipsychotic-free and antipsychotic-treated patients in the AEsop first-episode psychosis study. Neuropsychopharmacology. 2005;30(10):1923–1931. doi: 10.1038/sj.npp.1300766. [DOI] [PubMed] [Google Scholar]
- Peerbooms O, Rutten BPF, Collip D, Lardinois M, Lataster T, Thewissen V, Rad SM, Drukker M, Kenis G, van Os J, Myin-Germeys I, van Winkel R. Evidence that interactive effects of COMT and MTHFR moderate psychotic response to environmental stress. Acta Psychiatr Scand. 2012;125(3):247–256. doi: 10.1111/j.1600-0447.2011.01806.x. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, Delemarre-Van de Waal HA, Boomsma DI, Kahn RS, Hulshoff Pol HE. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2010;34(3):332. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Philippi H, Pohlenz J, Grimm W, Kollfer T, Schonberger W. Simultaneous stimulation of growth hormone, adrenocorticotropin and cortisol with L-dopa/L-carbidopa and propranololin children of short stature. Acta Paediatr. 2000;89(4):442–446. doi: 10.1080/080352500750028168. [DOI] [PubMed] [Google Scholar]
- Phillips LJ, Francey SM, Edwards J, McMurray N. Stress and psychosis: towards the development of new models of investigation. Clin Psychol Rev. 2007;27:307–317. doi: 10.1016/j.cpr.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Phillips LJ, Edwards J, McMurray N, Francey S. Comparison of experiences of stress and coping between young people at risk of psychosis and a non-clinical cohort. Behav Cogn Psychother. 2012;40:69–88. doi: 10.1017/S1352465811000397. [DOI] [PubMed] [Google Scholar]
- Piacentini MF, Meeusen R, Buyse L, De Schutter G, De Meirleir K. Hormonal responses during prolonged exercise are influenced by a selective DA/NA reuptake inhibitor. Br J Sports Med. 2004;38(2):129–133. doi: 10.1136/bjsm.2002.000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Barrot M, Rouge-Pont F, Marinelli M, Maccari S, Abrous DN, Simon H, Le Moal M. Suppression of glucocorticoid secretion and antipsychotic drugs have similar effects on the mesolimbic dopaminergic transmission. Proc Nat Acad Sci U S A. 1996;93(26):15445–15450. doi: 10.1073/pnas.93.26.15445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C] raclopride. J Neurosci. 2004;24(11):2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri BK. Progressive structural brain changes in schizophrenia. Expert Rev Neurother. 2010;10(1):33–42. doi: 10.1586/ern.09.142. [DOI] [PubMed] [Google Scholar]
- Ramsay CE, Gantt S, Broussard B, Compton MT. Clinical correlates of maltreatment and traumatic experiences in childhood and adolescence among predominantly African American, socially disadvantaged, hospitalized, first-episode psychosis patients. Psychiatr Res. 2011;188:343–349. doi: 10.1016/j.psychres.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VP, Krishnan RR, Goli V, Saunders WB, Ellinwood EH, Blazer DG, Nemeroff CB. Neuroanatomical changes and hypothalamic-pituitary-adrenal axis abnormalities. Biol Psychiatry. 1989;26:729–732. doi: 10.1016/0006-3223(89)90108-x. [DOI] [PubMed] [Google Scholar]
- Rastogi RB, Singhal RL. Evidence for the role of adrenocortical hormones in the regulation of noradrenaline and dopamine metabolism in certain brain areas. Br J Pharmacol. 2012;62(1):131–136. doi: 10.1111/j.1476-5381.1978.tb07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read J, van Os J, Morrison AP, Ross CA. Chilhood trauma, psychosis, and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112:330–350. doi: 10.1111/j.1600-0447.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- Renwick L, Jackson D, Turner N, Sutton M, Foley S, McWilliams S, Kinsella A, O’Callaghan E. Are symptoms associated with increased levels of perceived stress in first-episode psychosis? Int J Ment Health Nurs. 2009;18(3):186–194. doi: 10.1111/j.1447-0349.2009.00600.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Waitkus PM, Lafollette AJ, Ng BK, Zhu TS, Conrad HE, Glaser M. Steroid hormone signaling between Schwann cells and neurons regulates the rate of myelin synthesis. Ann N Y Acad Sci. 2003;1007:340–348. doi: 10.1196/annals.1286.033. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Deco G. A computational neuroscience approach to schizophrenia and its onset. Neurosci Biobehav Rev. 2011;35(8):1644–1653. doi: 10.1016/j.neubiorev.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Rothschild AJ, Benes F, Hebben N, Woods B, Luciana M, Bakanas E, Schatzberg AF. Relationships between brain CT scan findings and cortisol in psychotic and nonpsychotic depressed patients. Biol Psychiatry. 1989;26(6):565–575. doi: 10.1016/0006-3223(89)90081-4. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Sharifi N, Condren R, Thakore JH. Evidence of basal pituitary–adrenal overactivity in first episode, drug-naïve patients with schizophrenia. Psychoneuroendocrinology. 2004;29(8):1065–1070. doi: 10.1016/j.psyneuen.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Saha S, Varghese D, Slade T, Degenhardt L, Mills K, McGrath J, Scott J. The association between trauma and delusionallike experiences. Psychiatry Res. 2011;189(2):259–264. doi: 10.1016/j.psychres.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, Lindley S. Glucocorticoid antagonists in neuropsychiatric disorders. Eur J Pharmacol. 2008;583(2–3):358–364. doi: 10.1016/j.ejphar.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, Rothschild AJ, Langlais PJ, Bird ED, Cole JO. A corticosteroid/dopamine hypothesis for psychotic depression and related states. J Psychiatry Res. 1985;19:57–64. doi: 10.1016/0022-3956(85)90068-8. [DOI] [PubMed] [Google Scholar]
- Schenkel LS, Spaulding WD, DiLillo D, Silverstein SM. Histories of childhood maltreatment in schizophrenia: relationships with premorbid functioning, symptomatology, and cognitive deficits. Schizophr Res. 2005;76:273–286. doi: 10.1016/j.schres.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233(1):12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36(4):1342–1356. doi: 10.1016/j.neubiorev.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Shevlin M, Houston JE, Dorahy M, Adamson G. Cumulative traumas and psychosis: an analysis of the National Comorbidity Study and the British Psychiatric Morbidity Study. Schizophr Bull. 2008;34(1):193–199. doi: 10.1093/schbul/sbm069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Dev Psychobiol. 2011;54(5):493–502. doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever L, Davis K. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry. 2004;161:398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. 2012;1261(1):55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons CJ, Wichers M, Derom C, Thiery E, Myin-Germeys I, Krabbendam L, van Os J. Subtle gene–environment interactions driving paranoia in daily life. Genes Brain Behav. 2009;8(1):5–12. doi: 10.1111/j.1601-183X.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontiers Neuroendocrinol. 2005;26(3–4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Monroe SM, Gotlib IH. Early parental loss and depression history: associations with recent life stress in major depressive disorder. J Psychiatr Res. 2011;45(9):1146–1152. doi: 10.1016/j.jpsychires.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smieskova R, Fusar-Poli P, Allen P, Benfeldt K, Stieglitz RD, Drewe J, Radue EW, McGuire PK, Riecher-Rössler A, Borgwardt SJ. Neuroimaging predictors of transition to psychosis—a systematic review and meta-analysis. Neurosci Biobehav Rev. 2010;34(8):1206–1222. doi: 10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Spauwen J, Krabbendam L, Lieb R, Wittchen H-U, van Os J. Impact of psychological trauma on the development of psychotic symptoms: relationship with psychosis proneness. Br J Psychiatry. 2006;188:527–533. doi: 10.1192/bjp.bp.105.011346. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biol Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Stefanis NC, Henquet C, Avrampoulos D, Smyrnis N, Evdokimidis I, Myin-Germeys I, Stefanis CN, van Os J. COMT Val158Met moderation of stress-induced psychosis. Psychol Med. 2007;37:1651–1656. doi: 10.1017/S0033291707001080. [DOI] [PubMed] [Google Scholar]
- Stevens A, White A. ACTH: cellular peptide hormone synthesis and secretory pathways. Results Probl Cell Differ. 2010;50:63–84. doi: 10.1007/400_2009_30. [DOI] [PubMed] [Google Scholar]
- Stone JM, Morrison PD, Pilowsky LS. Glutamate and dopamine dysregulation in schizophrenia—a synthesis and selective review. J Psychopharmacol. 2007;21(4):440–452. doi: 10.1177/0269881106073126. [DOI] [PubMed] [Google Scholar]
- Strelzyk F, Hermes M, Naumann E, Oitzl M, Walter C, Busch HP, Richter S, Schächinger H. Tune it down to live it up? Rapid, non-genomic effects of cortisol on the human brain. J Neurosci. 2012;32(2):616–625. doi: 10.1523/JNEUROSCI.2384-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud CB, Davila J, Hammen C, Vrshek-Schallhorn S. Severe and nonsevere events in first onsets versus recurrences of depression: evidence for stress sensitization. J Abnorm Psychol. 2011;120(1):142–154. doi: 10.1037/a0021659. [DOI] [PubMed] [Google Scholar]
- Sugranyes G, Thompson JL, Corcoran CM. HPA-axis function, symptoms, and medication exposure in youths at clinical high risk for psychosis. J Psychiatr Res. 2012;46(11):1389–1393. doi: 10.1016/j.jpsychires.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafet GE, Feder DJ. Efficacy of cognitive therapy in the regulation of hypothalamic-pituitary-adrenal activity in patients with generalized anxiety disorder. Eur Psychiatry. 2011;26(Suppl. 1):1904. doi: 10.3758/cabn.5.1.37. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Velakoulis D, Lorenzetti V, Soulsby B, Zhou S-Y, Nakamura K, Seto H, Kurachi M, Pantelis C. Increased pituitary volume in schizophrenia spectrum disorders. Schizophr Res. 2009;108:114–121. doi: 10.1016/j.schres.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Tessner KD, Walker EF, Dhruv SH, Hochman K, Hamann S. The relation of cortisol levels with hippocampus volumes under baseline and challenge conditions. Brain Res. 2007;1179:70–78. doi: 10.1016/j.brainres.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Tessner KD, Mittal V, Walker EF. Longitudinal study of stressful life events and daily stressors among adolescents at high risk for psychotic disorders. Schizophr Bull. 2011;37(2):432–441. doi: 10.1093/schbul/sbp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404(6774):190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- Thompson KN, Berger G, Phillips LJ, Komesaroff P, Purcell R, McGorry PD. HPA axis functioning associated with transition to psychosis: combined DEX/CRH test. Journal of Psychiatr Res. 2007a;41:446–450. doi: 10.1016/j.jpsychires.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Thompson KN, Phillips LJ, Komesaroff P, Yuen HP, Wood SJ, Pantelis C, Velakoulis D, Yung AR, McGorry PD. Stress and HPA-axis functioning in young people at ultra high risk for psychosis. J Psychiatr Res. 2007b;41:561–569. doi: 10.1016/j.jpsychires.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Thompson JL, Kelly M, Kimhy D, Harkavy-Friedman JM, Khan S, Messinger JW, Schobel S, Goetz R, Malaspina D, Corcoran C. Childhood trauma and prodromal symptoms among indivduals at clinical high risk for psychosis. Schizophr Res. 2009;108:176–181. doi: 10.1016/j.schres.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida H, Takeuchi H, Graff-Guerrero A, Suzuki T, Watanabe K, Mamo DC. Dopamine D2 receptor occupancy and clinical effects: a systematic review and pooled analysis. J Clin Psychopharmacol. 2011;31(4):497–502. doi: 10.1097/JCP.0b013e3182214aad. [DOI] [PubMed] [Google Scholar]
- Valenti O, Lodge DJ, Grace AA. Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci. 2011;31(11):4280–4289. doi: 10.1523/JNEUROSCI.5310-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lely AJ, Foeken K, van der Mast RC, Lamberts SW. Rapid reversal of acute psychosis in the Cushing syndrome with the cortisol-receptor antagonist mifepristone (RU 486) Ann Intern Med. 1991;114:143–144. doi: 10.7326/0003-4819-114-2-143. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Picchioni MM, McDonald C, Marshall N, Davis N, Ribchester T, Hulshoff Pol HE, Sharma T, Sham P, Kahn RS, Murray R. A controlled study of brain structure in monozygotic twins concordant and discordant for schizophrenia. Biol Psychiatry. 2004;56(6):454–461. doi: 10.1016/j.biopsych.2004.06.033. [DOI] [PubMed] [Google Scholar]
- van Hulle CA, Shirtcliff EA, Lemery-Chalfant K, Hill H, Goldsmith Genetic and environmental influences on individual differences in cortisol level and circadian rhythm in middle childhood. Horm Behav. 2012;62(1):36–42. doi: 10.1016/j.yhbeh.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Venrooij J, Fluitman S, Lijmer JG, Kavelaars A, Heijnen CJ, Westenberg H, Kahn RS, Gispen-de Wied CC. Impaired neuroendocrine and immune response to acute stress in medication-naive patients with a first episode of psychosis. Schizophr Bull. 2012;38(2):272–279. doi: 10.1093/schbul/sbq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Winkel R, Henquet C, Rosa A, Papiol S, Fañanás L, De Hert M, Peuskens J, van Os J, Myin-Germeys I. Evidence that the COMT(Val158Met) polymorphism moderates sensitivity to stress in psychosis: an experience-sampling study. Am J Med Genet B Neuropsychiatr Genet. 2008a;147:10–17. doi: 10.1002/ajmg.b.30559. [DOI] [PubMed] [Google Scholar]
- van Winkel R, Stefanis NC, Myin-Germeys I. Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene–stress interaction. Schiz Bull. 2008b;34(6):1095–1105. doi: 10.1093/schbul/sbn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, Read J, van Os J, Bentall RP. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38(4):661–671. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Várkuti B, Cavusoglu M, Kullik A, Schiffler B, Veit R, Yilmaz Ö, Rosenstiel W, Braun C, Uludag K, Birbaumer N, Sitaram R. Quantifying the link between anatomical connectivity, gray matter volume and regional cerebral blood flow: an integrative MRI study. PloS One. 2011;6(4):e14801. doi: 10.1371/journal.pone.0014801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubramanian G, Chittiprol S, Neelakantachar N, Shetty T, Gangadhar BN. Effects of antipsychotic treatment on insulin-like growth factor-1 and cortisol in schizophrenia: a longitudinal study. Schizophr Res. 2010;119(1–3):131–137. doi: 10.1016/j.schres.2010.01.033. [DOI] [PubMed] [Google Scholar]
- Wada K, Yamada N, Sato T, Suzuki H, Miki M, Lee Y, Akiyama K, Kuroda S. Corticosteroid-induced psychotic and mood disorders: diagnosis defined by DSM-IV and clinical pictures. Psychosomatics. 2001;42:461–466. doi: 10.1176/appi.psy.42.6.461. [DOI] [PubMed] [Google Scholar]
- Walder DJ, Trotman HD, Cubells JF, Brasfield J, Tang Y-L, Walker EF. Catechol-O-methyltransferase modulation of cortisol secretion in psychiatrically at-risk and healthy adolescents. Psychiatr Genet. 2010;20(4):166–170. doi: 10.1097/YPG.0b013e32833a1ff3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, Grimes K, Davis D, Smith A. Childhood precursors of schizophrenia: facial expressions of emotion. Am J Psychiatry. 1993;150:1654–1660. doi: 10.1176/ajp.150.11.1654. [DOI] [PubMed] [Google Scholar]
- Walker EF. Developmentally moderated expressions of the neuropathology underlying schizophrenia. Schizophr Bull. 1994;20(3):453–480. doi: 10.1093/schbul/20.3.453. [DOI] [PubMed] [Google Scholar]
- Walker E, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104(4):667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Ann Rev Clin Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Walker EF, Brennan PA, Esterberg M, Brasfield J, Pearce B, Compton MT. Longitudinal changes in cortisol secretion and conversion to psychosis in at-risk youth. J Abnorm Psychol. 2010;119(2):401–408. doi: 10.1037/a0018399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, Trotman H, Brasfield JL, Esterberg M, Larson M. Stress hormones, genes, and neuronal signaling in adolescence. The perfect storm for vulnerability to psychosis. In: Kendler K, Jaffe S, Romer D, editors. The Dynamic Genome and Mental Health. New York: Oxford University Press; 2011. [Google Scholar]
- Wallace GL, Schmitt JE, Lenroot R, Viding E, Ordaz S, Rosenthal MA, Malloy EA, Clasen LS, Kendler KS, Neale MC, Giedd JN. A pediatric twin study of brain morphometry. J Child Psychol Psychiatry. 2006;47(10):987–993. doi: 10.1111/j.1469-7610.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- Wand GS, Oswald LM, McCaul ME, Wong DF, Johnson E, Zhou Y, Kuwabara H, Kumar A. Association of amphetamineinduced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology. 2007;32:2310–2320. doi: 10.1038/sj.npp.1301373. [DOI] [PubMed] [Google Scholar]
- Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361–1367. doi: 10.4065/81.10.1361. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Weikert CS, Herman MM, Kleinman JE. BDNF mRNA expression during postnatal development, maturation and aging of the human prefrontal cortex. Brain Res. 2002;139(2):139–150. doi: 10.1016/s0165-3806(02)00540-0. [DOI] [PubMed] [Google Scholar]
- Whitfield CL, Dube SR, Felitti VJ, Anda RF. Adverse childhood experiences and hallucinations. Child Abuse Negl. 2005;29(7):797–810. doi: 10.1016/j.chiabu.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Wichers M, Schrijvers D, Geschwind N, Jacobs N, Myin-Germeys I, Thiery E, Derom C, Sabbe B, Peeters F, Delespaul P, van Os J. Mechanisms of gene-environment interactions in depression: evidence that genes potentiate multiple sources of adversity. Psychol Med. 2009;39:1077–1086. doi: 10.1017/S0033291708004388. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Berger S, Greiner EF, Schutz G. Evaluation of steroid receptor function by gene targeting in mice. J Steroid Biochem Mol Biol. 2005;93(2–5):107–112. doi: 10.1016/j.jsbmb.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Witthaus H, Mendes U, Brüne M, Ozgürdal S, Bohner G, Gudlowski Y, Kalus P, Andreasen N, Heinz A, Klingebiel R, Juckel G. Hippocampal subdivision and amygdalar volumes in patients in an at-risk mental state for schizophrenia. J Psychiatry Neurosci. 2010;35(1):33–40. doi: 10.1503/jpn.090013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT. HPA axis and memory. Best Pract Res Clin Endocrinol Metab. 2003;17(2):287–299. doi: 10.1016/s1521-690x(02)00101-x. [DOI] [PubMed] [Google Scholar]
- Yuii K, Suzuki M, Kurachi M. Stress sensitization in schizophrenia. Ann N Y Acad Sci. 2007;1113:276–290. doi: 10.1196/annals.1391.013. [DOI] [PubMed] [Google Scholar]
- Yung AR, McGorry PD. The initial prodrome in psychosis: descriptive and qualitative aspects. Aust N Z J Psychiatry. 1996;30:587–599. doi: 10.3109/00048679609062654. [DOI] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, Pan Yuen H, Francey SM, McFarlane CA, Hallgren M, McGorry PD. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res. 2003;60(1):21–32. doi: 10.1016/s0920-9964(02)00167-6. [DOI] [PubMed] [Google Scholar]
- Yung A, Phillips L, Yuen H, McGorry P. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67:131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]