Abstract
An age-dependent increase in the resistance of BALB/c mice to induction of focal necrotic hepatitis by herpes simplex virus type 2 was demonstrated. In 3-week-old mice inoculated intraperitoneally with virus, numerous necrotic foci developed in the liver. As the mice matured, the number of lesions declined until the age of 8 weeks, when no further increase in resistance appeared. Corresponding to this, the virus titers of livers and spleens of 3-week-old mice were higher than in 8-week-old animals throughout the infection, and the infection was apparently terminated in these organs of the adult mice by day 5. In vitro infection of peritoneal macrophages from 3-week-old and 8-week-old mice showed that this age-related resistance was concomitant with an increased restriction of virus replication in peritoneal macrophages from adult mice. Since, furthermore, the resistance of adult mice could be abolished by intravenous inoculation of the macrophage-toxic agent silica before infection, and since adoptive transfer of 2 X 10(6) syngeneic macrophages from adult mice to young ones conferred to the latter a resistance comparable to that of the adult mice, it is concluded that macrophage maturation is responsible for the age-dependent resistance seen in this infection.
Full text
PDF
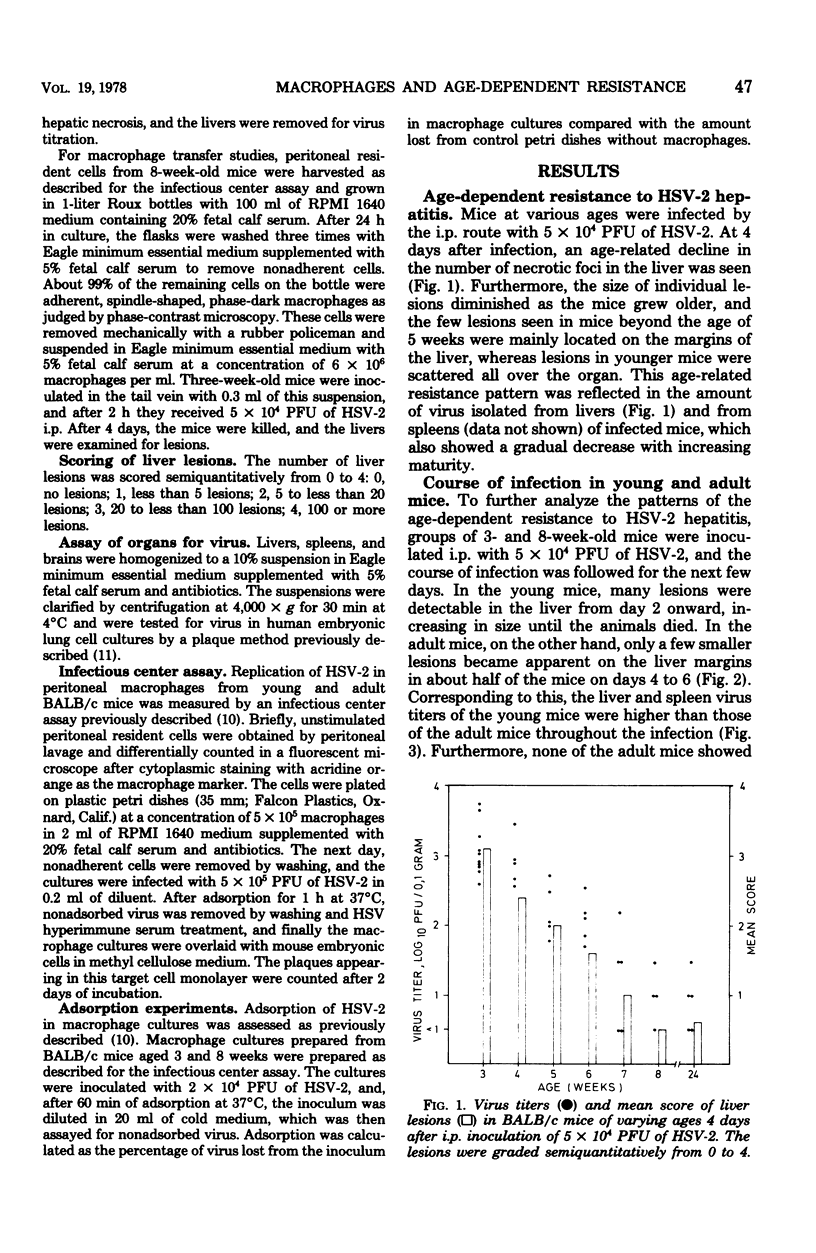
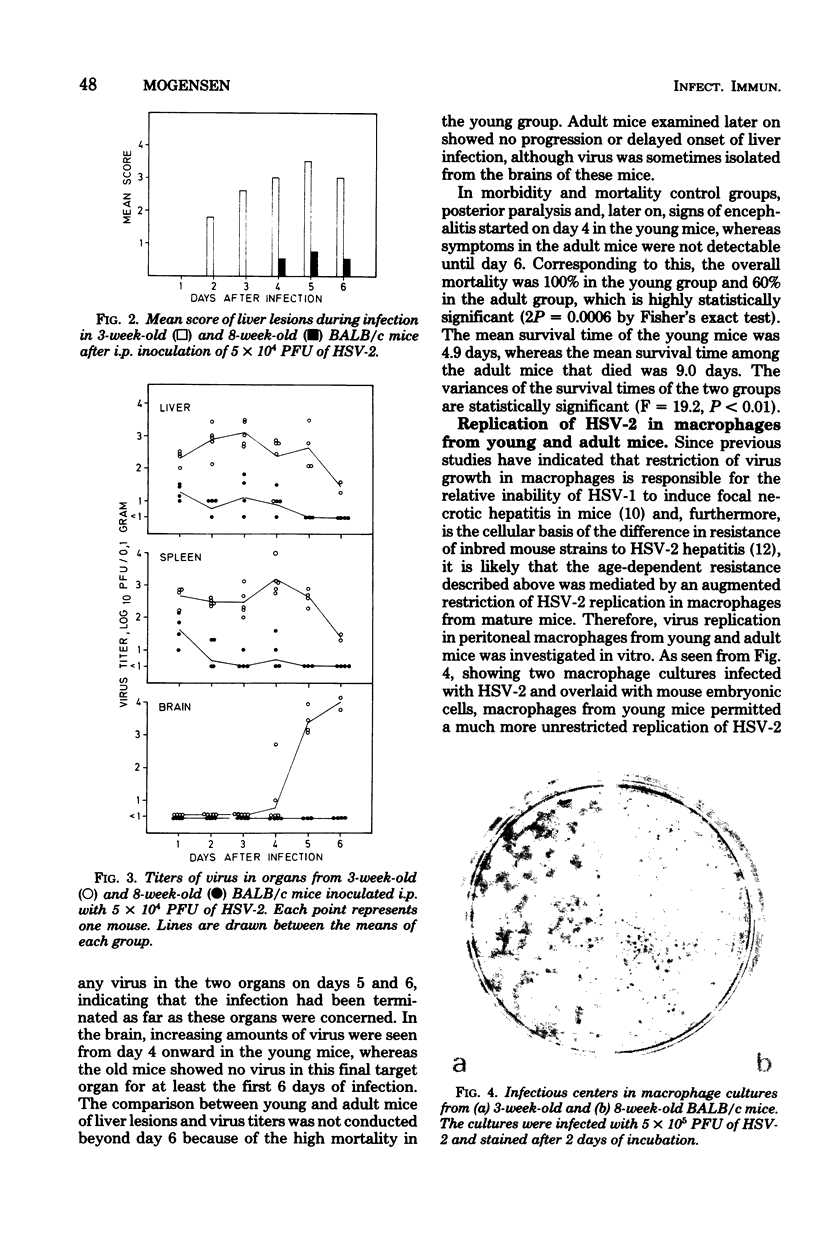
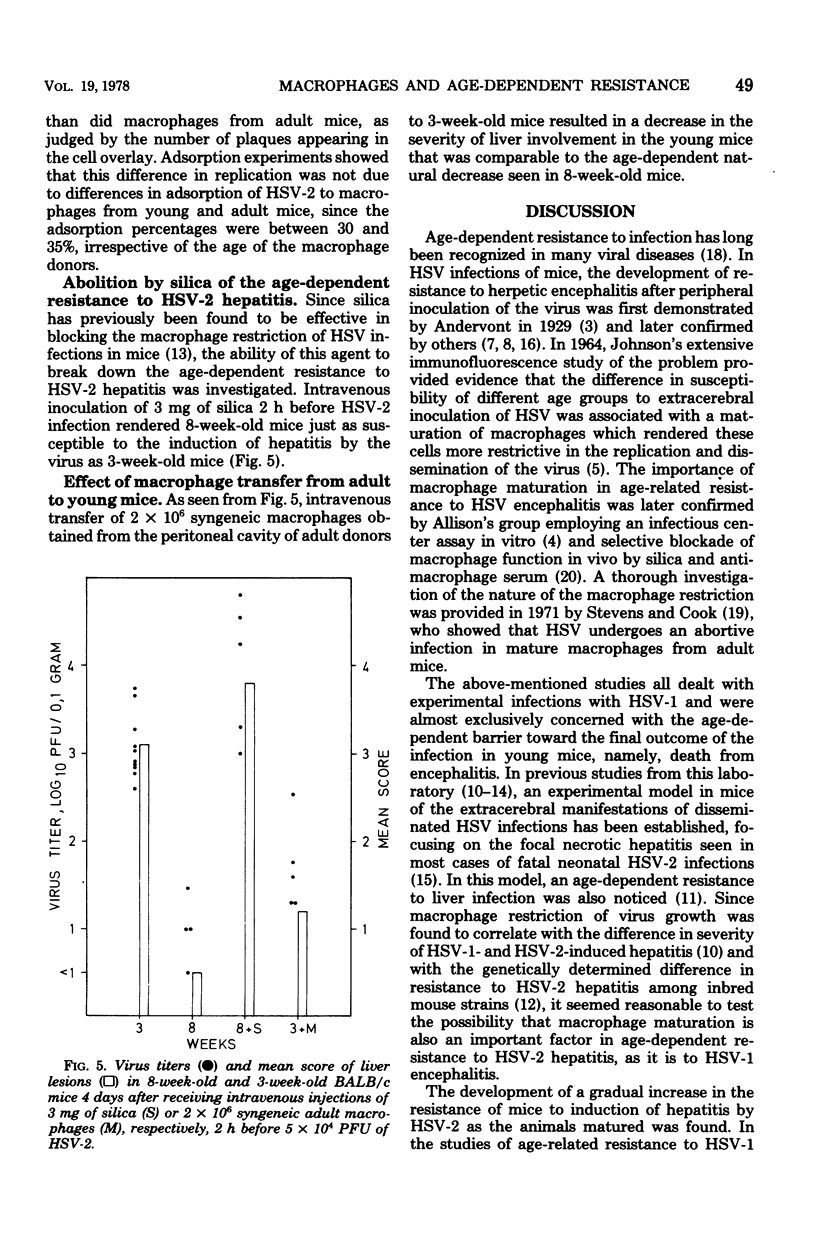

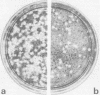
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSEL R. W., MONACO L., MARCHISIO M. A. THE SPECIFICITY OF THE CYTOTOXIC ACTION OF SILICA--A STUDY IN VITRO. Br J Exp Pathol. 1963 Aug;44:351–364. [PMC free article] [PubMed] [Google Scholar]
- KILBOURNE E. D., HORSFALL F. L., Jr Studies of herpes simplex virus in newborn mice. J Immunol. 1951 Oct;67(4):321–329. [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C., Andersen H. K. Effect of silica on the pathogenic distinction between herpes simplex virus type 1 and 2 hepatitis in mice. Infect Immun. 1977 Aug;17(2):274–277. doi: 10.1128/iai.17.2.274-277.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C. Biological conditions influencing the focal necrotic hepatitis test for differentiation between herpes simplex virus types 1 and 2. Acta Pathol Microbiol Scand B. 1976 Jun;84(3):154–158. [PubMed] [Google Scholar]
- Mogensen S. C. Genetics of macrophage-controlled resistance to hepatitis induced by herpes simplex virus type 2 in mice. Infect Immun. 1977 Aug;17(2):268–273. doi: 10.1128/iai.17.2.268-273.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C., Teisner B., Andersen H. K. Focal necrotic hepatitis in mice as a biological marker for differentiation of Herpesvirus hominis type 1 and type 2. J Gen Virol. 1974 Oct;25(1):151–155. doi: 10.1099/0022-1317-25-1-151. [DOI] [PubMed] [Google Scholar]
- Mogensen S. Role of macrophages in hepatitis induced by Herpes simplex virus types 1 and 2 in mice. Infect Immun. 1977 Mar;15(3):686–691. doi: 10.1128/iai.15.3.686-691.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias A. J., Alford C. A., Korones S. B. Infection of the newborn with herpesvirus hominis. Adv Pediatr. 1970;17:185–226. [PubMed] [Google Scholar]
- RUCHMAN I. Virulence of strains of herpes simplex virus for mice. Proc Soc Exp Biol Med. 1954 Aug-Sep;86(4):649–652. doi: 10.3181/00379727-86-21191. [DOI] [PubMed] [Google Scholar]
- SIGEL M. M. Influence of age on susceptibility to virus infections with particular reference to laboratory animals. Annu Rev Microbiol. 1952;6:247–280. doi: 10.1146/annurev.mi.06.100152.001335. [DOI] [PubMed] [Google Scholar]
- Selgrade M. K., Osborn J. E. Role of macrophages in resistance to murine cytomegalovirus. Infect Immun. 1974 Dec;10(6):1383–1390. doi: 10.1128/iai.10.6.1383-1390.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]