Abstract
BACKGROUND
Previous results from our trial of early treatment with continuous positive airway pressure (CPAP) versus early surfactant treatment in infants showed no significant difference in the outcome of death or bronchopulmonary dysplasia. A lower (vs. higher) target range of oxygen saturation was associated with a lower rate of severe retinopathy but higher mortality. We now report longer-term results from our prespecified hypotheses.
METHODS
Using a 2-by-2 factorial design, we randomly assigned infants born between 24 weeks 0 days and 27 weeks 6 days of gestation to early CPAP with a limited ventilation strategy or early surfactant administration and to lower or higher target ranges of oxygen saturation (85 to 89% or 91 to 95%). The primary composite outcome for the longer-term analysis was death before assessment at 18 to 22 months or neurodevelopmental impairment at 18 to 22 months of corrected age.
RESULTS
The primary outcome was determined for 1234 of 1316 enrolled infants (93.8%); 990 of the 1058 surviving infants (93.6%) were evaluated at 18 to 22 months of corrected age. Death or neurodevelopmental impairment occurred in 27.9% of the infants in the CPAP group (173 of 621 infants), versus 29.9% of those in the surfactant group (183 of 613) (relative risk, 0.93; 95% confidence interval [CI], 0.78 to 1.10; P = 0.38), and in 30.2% of the infants in the lower-oxygen-saturation group (185 of 612), versus 27.5% of those in the higher-oxygen-saturation group (171 of 622) (relative risk, 1.12; 95% CI, 0.94 to 1.32; P = 0.21). Mortality was increased with the lower-oxygen-saturation target (22.1%, vs. 18.2% with the higher-oxygen-saturation target; relative risk, 1.25; 95% CI, 1.00 to 1.55; P = 0.046).
CONCLUSIONS
We found no significant differences in the composite outcome of death or neurodevelopmental impairment among extremely premature infants randomly assigned to early CPAP or early surfactant administration and to a lower or higher target range of oxygen saturation. (Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute; SUPPORT ClinicalTrials.gov number, NCT00233324.)
Extremely premature infants are at high risk for death and neurosensory or developmental impairment in early childhood. 1–3 The risk of neurodevelopmental impairment increases with decreasing gestational age and greater severity of illness. Neurodevelopmental impairment is often a consequence of neonatal complications.4–12 Although surfactant administration decreases the risk of death and bronchopulmonary dysplasia, randomized, controlled trials of various respiratory interventions have not shown significant reductions in mortality and morbidity or improvement in developmental outcomes.13–17 We previously reported results of the multicenter, randomized, controlled Surfactant, Positive Pressure, and Pulse Oximetry Randomized Trial (SUPPORT), which involved extremely premature infants (from 24 to 27 weeks of gestation); treatment with noninvasive continuous positive airway pressure (CPAP) shortly after birth, as compared with early intubation and surfactant administration, did not reduce rates of death or bronchopulmonary dysplasia or other major morbidity at 36 weeks of postmenstrual age.18
Although oxygen supplementation is necessary for survival in many preterm infants, several studies have shown that it increases the risk of retinopathy of prematurity,19 bronchopulmonary dysplasia,20,21 periventricular leukomalacia,22 and cerebral palsy.23 Results from SUPPORT showed no significant difference in the composite outcome of death before discharge or severe retinopathy of prematurity among infants randomly assigned to a lower target range of oxygen saturation (85 to 89%) versus a higher range (91 to 95%). However, in the lower-oxygen-saturation group, the risk of retinopathy of prematurity among infants who survived to discharge was decreased (8.6%, vs. 17.9% in the higher-oxygen-saturation group; relative risk, 0.52; 95% confidence interval [CI], 0.37 to 0.73; P<0.001) and the risk of death was increased (19.9% vs. 16.2%; relative risk, 1.27; 95% CI, 1.01 to 1.60; P = 0.04).24
We now report the results of our longer-term follow-up of the infants in this study, assessing whether early, noninvasive CPAP with a limited ventilation strategy, as compared with early surfactant administration, and a lower, as compared with higher, target range of oxygen saturation would each decrease the incidence of death before assessment at 18 to 22 months or neurodevelopmental impairment at 18 to 22 months of corrected age.
METHODS
STUDY DESIGN
SUPPORT was a randomized, controlled trial involving 1316 extremely preterm infants (gestational age, 24 weeks 0 days to 27 weeks 6 days) born between February 2005 and February 2009, who were enrolled at delivery at 20 centers in the United States participating in the Neonatal Research Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Permuted-block randomization was used, with stratification according to study center and gestational age (24 weeks 0 days to 25 weeks 6 days vs. 26 weeks 0 days to 27 weeks 6 days). Infants who were part of multiple births were randomly assigned, as a unit, to the same treatment group.
In the delivery room, the infants were randomly assigned to receive either CPAP immediately after delivery with a limited ventilation strategy, as described previously, if subsequent intubation was required, or intubation with surfactant administration within an hour after birth, followed by conventional ventilation.18 Using a 2-by-2 factorial design, we also randomly assigned participants to a target oxygen-saturation range of 85 to 89% (lower-oxygen-saturation group) or 91 to 95% (higher-oxygen-saturation group); we used pulse oximeters that were specially designed to maintain blinding (see the Supplementary Appendix, available with the full text of this article at NEJM.org).24
The procedures for enrollment, intervention, and data collection have been reported previously. 18,24 The trial was approved by the institutional review board at each participating site and by RTI International, which is the independent data coordinating center for the Neonatal Research Network. Written informed consent was obtained from the parent or guardian of each child before delivery. Two of the authors employed by RTI International vouch for the accuracy and completeness of the data and analyses reported, and the members of the SUPPORT subcommittee vouch for the fidelity of the trial to the study protocol (see the Supplementary Appendix).
ASSESSMENTS
At 18 to 22 months of corrected age, surviving infants underwent a comprehensive neurodevelopmental assessment performed by neurologic examiners and neurodevelopmental testers who were unaware of the treatment assignments and were evaluated annually for testing reliability. Cognitive function was assessed with the use of the Bayley Scales of Infant and Toddler Development, third edition (BSID-III); scores are assessed relative to a standardized mean (±SD) of 100±15, with higher scores indicating better performance.25 The modified Gross Motor Function Classification System (GMFCS) was used to classify gross-motor performance, with scores ranging from 0 (normal) to 5 (most impaired).26 Moderate-to-severe cerebral palsy was defined as a nonprogressive disorder with abnormal muscle tone in at least one arm or leg that was associated with abnormal control of movement or posture and a GMFCS score of 2 or higher. 27,28 Assessments of hearing impairment (defined as the inability to understand the oral directions of the examiner and to communicate, with or without hearing amplification) and visual impairment (defined as vision worse than 20/200) were based on examination and parental report.
Certified research staff collected demographic and neonatal-outcome data using standard definitions from the Neonatal Research Network. Demographic and outcome data included gestational age; birth weight; sex; status with respect to multiple gestation; race or ethnic group; and history of medical or surgical necrotizing enterocolitis (modified Bell’s stage ≥2, on a scale ranging from 1 to 3, with higher scores indicating greater severity of disease), intraventricular hemorrhage of grade 3 or 4 or periventricular leukomalacia, late-onset sepsis, retinopathy of prematurity, bronchopulmonary dysplasia (physiological), and use of postnatal glucocorticoids. Socioeconomic variables included health insurance status, maternal marital status, maternal educational level, household income, language spoken at home, and status with respect to whether the child was living with biologic parents. Socioeconomic data were updated during the 18-to-22-month visit; these data were used if data from the neonatal period were not available.
OUTCOMES
The prespecified primary composite outcome for this trial was death before assessment at 18 to 22 months or neurodevelopmental impairment at 18 to 22 months of corrected age. This composite outcome was selected because infants who died before 18 months of corrected age could not be classified as having neurodevelopmental impairment. Neurodevelopmental impairment was defined as any of the following: a cognitive composite score on the BSID-III of less than 70, a GMFCS score of 2 or higher, moderate or severe cerebral palsy, hearing impairment, or bilateral visual impairment. Other prespecified outcomes at 18 to 22 months of corrected age were death and neurodevelopmental impairment. Exploratory secondary outcomes included the individual components of the neurodevelopmental-impairment assessment, levels of cognitive delay, and a comparison of outcomes within the higher and lower gestational-age strata.
STATISTICAL ANALYSIS
The sample-size calculations were based on Neonatal Research Network data for infants born in the year 2000; the details have been reported previously. 18,24 Although the sample size for the study was estimated on the basis of hospital outcomes (i.e., death or bronchopulmonary dysplasia for the ventilation intervention, and death or retinopathy of prematurity for the oxygenation intervention), the final sample size was sufficient to detect an absolute reduction of 10 percentage points in the composite outcome of death or neurodevelopmental impairment, with the use of a two-sided significance level of 0.05, conservatively assuming an initial outcome rate of 55% in the surfactant and higher-oxygen-saturation groups and a 15% rate of loss to follow-up, as well as adjustment for familial clustering.
Data were entered on standard forms and were transmitted to RTI International, which stored, managed, and analyzed the data for the study. All analyses were performed according to the intention-to-treat principle. Unadjusted comparisons of demographic and birth characteristics between treatment groups were performed with the use of chi-square tests for categorical variables and t-tests for continuous variables. The primary analyses focused on the percentage of infants in each group for whom the primary composite outcome of death before assessment at 18 to 22 months or neurodevelopmental impairment at 18 to 22 months of corrected age could be assigned. Analysis of this and all other categorical outcomes was performed with the use of robust Poisson regression in a generalized-estimating-equation model to obtain adjusted relative risks with 95% confidence intervals. The denominator used to calculate the frequency of each outcome was the number of children for whom status with respect to that outcome was known. Continuous outcomes were analyzed with the use of mixed-effects linear models to obtain adjusted means and standard errors.
Analyses of all 18-to-22-month outcomes were adjusted, as prespecified, for gestational-age strata, study center, and familial clustering (because infants who were part of multiple births were assigned to the same treatment group). Tests were conducted for the presence of statistical interaction between the two interventions by adding an interaction term to the models. To test the effect of characteristics that differed between the groups of children with and without follow-up, a sensitivity analysis using multiple imputation was conducted, in which missing values for the primary outcome were imputed on the basis of the treatment assignment, perinatal characteristics, and in-hospital outcomes.29 Two-sided P values of less than 0.05 were considered to indicate statistical significance for all analyses; no adjustments were made for multiple comparisons.
RESULTS
CHARACTERISTICS OF THE PATIENTS
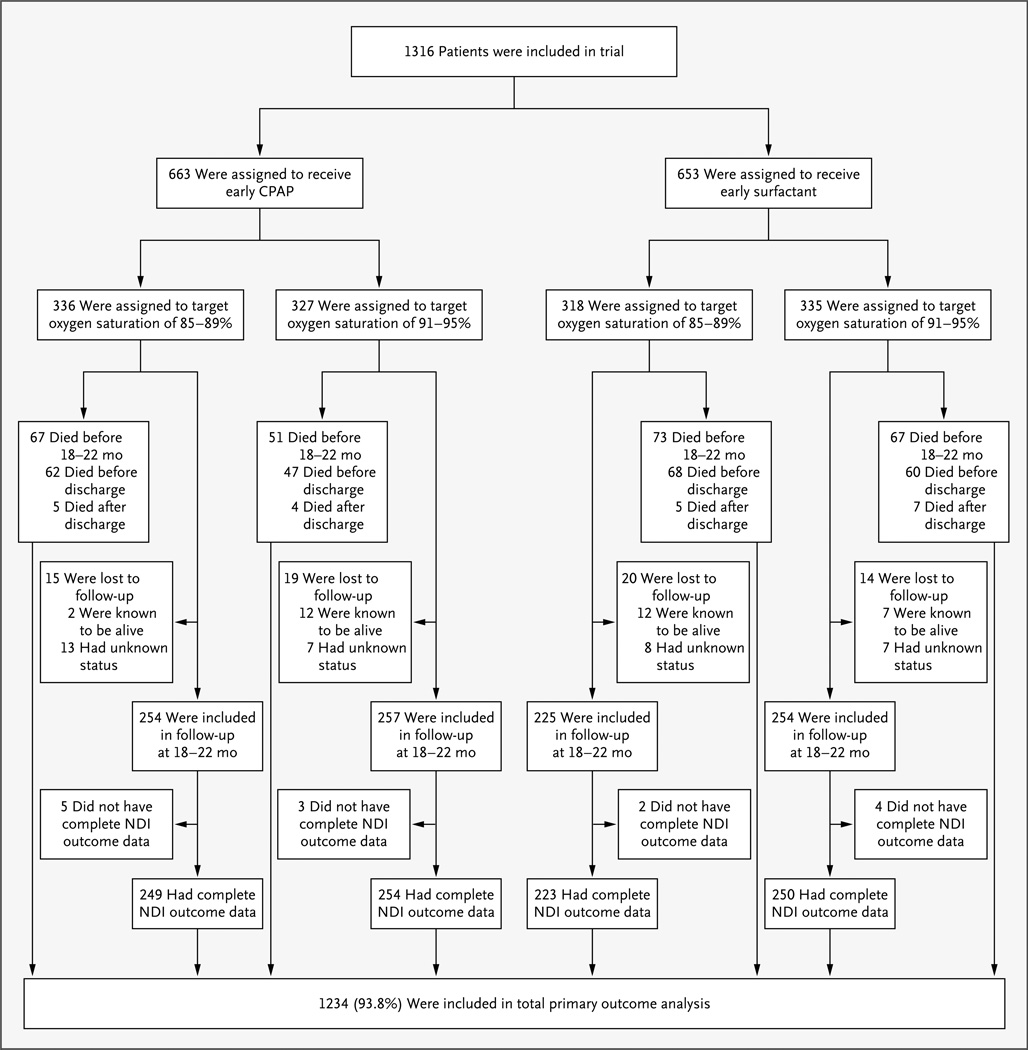
The primary composite outcome of death or neurodevelopmental impairment was determined for 93.8% of the children (1234 of 1316) enrolled in the trial (Fig. 1). A total of 258 children were known to have died before 18 to 22 months of age. Of the 68 children for whom a neurodevelopmental assessment was missing, 33 were known to be alive. A neurodevelopmental assessment was performed at 18 to 22 months of corrected age for 990 of 1058 children (93.6%). The presence or absence of neurodevelopmental impairment was determined for 98.6% of all children seen (976 of 990); 14 children had an incomplete evaluation that precluded the assignment of a neurodevelopmental-impairment status. The follow-up rate and the mean corrected age at neurodevelopmental assessment were similar for all treatment groups (Table 1).
Figure 1. Enrollment, Randomization, and Outcomes.
The primary composite outcome was determined for 93.8% of the enrolled infants. A total of 258 children were known to have died before 18 to 22 months of corrected age. Of the 68 children with a missing neurodevelopmental assessment, 33 were known to be alive. A neurodevelopmental assessment was performed at 18 to 22 months of corrected age for 990 of 1058 children (93.6%). The presence or absence of neurodevelopmental impairment (NDI) was determined for 98.6% of all children seen; 14 children had an incomplete evaluation that precluded the assignment of NDI status.
Table 1.
Demographic and Clinical Characteristics of the Follow-up Cohorts.*
| Characteristic | CPAP (N = 511) |
Surfactant (N = 479) |
Lower Oxygen Saturation (N = 479) |
Higher Oxygen Saturation (N = 511) |
|---|---|---|---|---|
| Birth weight — g | 849±186 | 852±193 | 858±186 | 844±192 |
| Gestational age at birth — wk | 26.3±1.1 | 26.3±1.1 | 26.3±1.1 | 26.2±1.0 |
| Small for gestational age — no. (%)† | 23 (4.5) | 32 (6.7) | 17 (3.5)‡ | 38 (7.4) |
| Male sex — no. (%) | 256 (50.1) | 266 (55.5) | 240 (50.1) | 282 (55.2) |
| Multiple birth — no. (%) | 138 (27.0) | 114 (23.8) | 124 (25.9) | 128 (25.0) |
| Maternal use of antenatal glucocorticoids — no. (%) | 493 (96.5) | 456 (95.2) | 462 (96.5) | 487 (95.3) |
| Cesarean section — no. (%) | 352 (68.9) | 315 (65.8) | 332 (69.3) | 335 (65.6) |
| Neonatal outcome — no./total no. (%)§ | ||||
| Severe retinopathy of prematurity | 62/479 (12.9) | 58/434 (13.4) | 38/442 (8.6)¶ | 82/471 (17.4) |
| Bronchopulmonary dysplasia‖ | 193/511 (37.8) | 187/479 (39.0) | 177/479 (37.0) | 203/511 (39.7) |
| Intraventricular hemorrhage of grade 3 or 4 or periventricular leukomalacia | 70/510 (13.7) | 46/478 (9.6) | 56/478 (11.7) | 60/510 (11.8) |
| Necrotizing enterocolitis | 56/511 (11.0)** | 30/479 (6.3) | 42/479 (8.8) | 44/511 (8.6) |
| Late-onset sepsis or meningitis | 167/511 (32.7) | 154/479 (32.2) | 155/479 (32.4) | 166/511 (32.5) |
| Use of postnatal glucocorticoids | 34/508 (6.7)** | 55/476 (11.6) | 41/477 (8.6) | 48/507 (9.5) |
| Corrected age at follow-up — mo | 19.9±2.4 | 20.1±2.7 | 19.9±2.4 | 20.2±2.7 |
Plus–minus values are means ±SD. There were no significant between-group differences, except as noted. Additional demographic characteristics of the cohorts are provided in Table S1 in the Supplementary Appendix. CPAP denotes continuous positive airway pressure.
Infants who were small for gestational age were defined as those with a birth weight in less than the 10th percentile.
P<0.01 for the comparison with the higher-oxygen-saturation group.
The comparisons of neonatal outcomes were adjusted for stratification factors (study center and gestational-age group) and familial clustering.
P<0.001 for the comparison with the higher-oxygen-saturation group.
Assessment for bronchopulmonary dysplasia was performed at 36 weeks of postmenstrual age.
P<0.05 for the comparison with the surfactant group.
As compared with the mothers of the 990 children who underwent a neurodevelopmental assessment at 18 to 22 months of corrected age, the mothers of the 68 children who did not undergo an assessment were less likely to be married (47% vs. 31%, P = 0.01) and more likely to have only public health insurance (52% vs. 69%, P = 0.008). No other demographic or neonatal characteristics differed significantly between the groups.
The demographic and clinical characteristics of the follow-up population are summarized in Table 1 and in Table S1 in the Supplementary Appendix. Almost all mothers received antenatal glucocorticoids. At follow-up, there were more children who were small for their gestational age and more children with severe retinopathy of prematurity in the higher-oxygen-saturation group than in the lower-oxygen-saturation group. As compared with the surfactant group, children in the CPAP group were more likely to have had necrotizing enterocolitis and less likely to have been exposed to postnatal glucocorticoids. A total of 32% of the infants in the CPAP group were intubated in the delivery room; 65% of the infants in the CPAP group received surfactant with limited ventilation.
PRIMARY OUTCOME
The frequency of the composite outcome of death or neurodevelopmental impairment did not differ significantly between the CPAP and surfactant groups or between the lower-oxygen-saturation and higher-oxygen-saturation groups at 18 to 22 months of corrected age (Tables 2 and 3). Mortality before neonatal discharge accounted for 92% of the overall mortality observed by 18 to 22 months. Mortality did not differ significantly between the CPAP and surfactant groups but remained significantly higher in the lower-oxygen-saturation group than in the higher-oxygen-saturation group. There were no significant differences in the primary outcome between treatment groups in subgroup analyses stratified according to gestational age at birth (Tables S2 and S3 in the Supplementary Appendix). The results of the sensitivity analysis using multiple imputations were virtually identical to the results of the analysis in which missing data were excluded (data not shown). There was no significant interaction between the two interventions with respect to the composite outcome of death or neurodevelopmental impairment or either of its components (P>0.70 for all comparisons).
Table 2.
Rates and Relative Risks of Death before Assessment at 18 to 22 Months or Neurodevelopmental Impairment at 18 to 22 Months of Corrected Age in the CPAP and Surfactant Groups.*
| Variable | CPAP | Surfactant | Adjusted Relative Risk (95% CI) |
P Value |
|---|---|---|---|---|
| number/total number (percent) | ||||
| Primary outcome determined | 621/663 (93.7) | 613/653 (93.9) | 1.00 (0.97–1.03) | 0.83 |
| Death or NDI | 173/621 (27.9) | 183/613 (29.9) | 0.93 (0.78–1.10) | 0.38 |
| Death before assessment at 18–22 mo of corrected age | 118/643 (18.4) | 140/638 (21.9) | 0.83 (0.67–1.04) | 0.10 |
| NDI | 55/503 (10.9) | 43/473 (9.1) | 1.16 (0.79–1.71) | 0.44 |
| BSID-III cognitive score <70† | 36/502 (7.2) | 36/472 (7.6) | 0.95 (0.61–1.50) | 0.84 |
| GMFCS score ≥2‡ | 26/511 (5.1) | 23/479 (4.8) | 0.98 (0.57–1.69) | 0.95 |
| Moderate or severe cerebral palsy | 21/511 (4.1) | 19/479 (4.0) | 0.93 (0.51–1.72) | 0.82 |
| Bilateral blindness | 4/511 (0.8) | 7/479 (1.5) | 0.53 (0.16–1.78) | 0.31 |
| Hearing impairment | 17/511 (3.3) | 7/479 (1.5) | 2.27 (0.96–5.37) | 0.06 |
Relative risks and P values were adjusted for stratification factors (study center and gestational-age group) and familial clustering; analyses of blindness were not adjusted for study center, owing to the small number of patients with this characteristic. NDI denotes neurodevelopmental impairment.
Scores on the Bayley Scales of Infant and Toddler Development, third edition (BSID-III), are assessed relative to a standardized mean (±SD) of 100±15, with higher scores indicating better performance.
Gross-motor function was assessed by means of the modified Gross Motor Function Classification System (GMFCS), with scores ranging from 0 to 5 and higher scores indicating greater impairment.
Table 3.
Rates and Relative Risks of Death before Assessment at 18 to 22 Months or Neurodevelopmental Impairment at 18 to 22 Months of Corrected Age in the Lower-Oxygen-Saturation and Higher-Oxygen-Saturation Groups.*
| Variable | Lower Oxygen Saturation | Higher Oxygen Saturation | Adjusted Relative Risk (95% CI) | P Value |
|---|---|---|---|---|
| number/total number (percent) | ||||
| Primary outcome determined | 612/654 (93.6) | 622/662 (94.0) | 1.00 (0.97–1.03) | 0.79 |
| Death or NDI | 185/612 (30.2) | 171/622 (27.5) | 1.12 (0.94–1.32) | 0.21 |
| Death before assessment at 18–22 mo of corrected age | 140/633 (22.1) | 118/648 (18.2) | 1.25 (1.00–1.55) | 0.046 |
| NDI | 45/472 (9.5) | 53/504 (10.5) | 0.87 (0.60–1.28) | 0.49 |
| BSID-III cognitive score <70 | 34/471 (7.2) | 38/503 (7.6) | 0.91 (0.58–1.43) | 0.69 |
| GMFCS score ≥2 | 26/479 (5.4) | 23/511 (4.5) | 1.17 (0.68–2.01) | 0.56 |
| Moderate or severe cerebral palsy | 20/479 (4.2) | 20/511 (3.9) | 1.00 (0.54–1.83) | >0.99 |
| Bilateral blindness | 5/479 (1.0) | 6/511 (1.2) | 0.90 (0.28–2.90) | 0.86 |
| Hearing impairment | 12/479 (2.5) | 12/511 (2.3) | 1.16 (0.54–2.49) | 0.70 |
Relative risks and P values were adjusted for stratification factors (study center and gestational-age group) and familial clustering; analyses of blindness were not adjusted for study center, owing to the small number of patients with this characteristic.
OTHER OUTCOMES
The incidences of the individual components of neurodevelopmental impairment (BSID-III cognitive composite score of <70, GMFCS score of ≥2, moderate or severe cerebral palsy, hearing impairment, and blindness) among surviving infants did not differ significantly between the CPAP and surfactant groups or between the lower-oxygen-saturation and higher-oxygen-saturation groups (Tables 2 and 3). Neither were there significant between-group differences in the individual components of neurodevelopmental impairment when the groups were stratified according to gestational age (Tables S2 and S3 in the Supplementary Appendix). However, in the lower-gestational-age stratum, mortality was higher in the surfactant group than in the CPAP group. Although the rates of severe retinopathy of prematurity and eye surgery were higher in the higher-oxygen-saturation group than in the lower-oxygen-saturation group, the rates of bilateral blindness, blindness of at least one eye, and other vision impairment did not differ significantly between the groups at 18 to 22 months of corrected age (Table 4). There were no significant differences between the CPAP and surfactant groups or between the lower-oxygen-saturation and higher-oxygen-saturation groups in the rates of the composite outcome of death or individual neurodevelopmental-impairment components (data not shown), mean cognitive composite scores on the BSID-III, or the percentage of infants with cognitive composite scores of less than 80 points or less than 85 points (Table S4 in the Supplementary Appendix). Of the 976 children who were evaluated at 18 to 22 months of corrected age, 583 (60%) had normal status with respect to neuromotor, neurosensory, and cognitive development (with normal cognitive development defined as a BSID-III cognitive composite score of ≥85 points).
Table 4.
Visual Outcome at 18 to 22 Months of Corrected Age in the Lower-Oxygen-Saturation and Higher-Oxygen-Saturation Groups.*
| Variable | Lower Oxygen Saturation |
Higher Oxygen Saturation |
Adjusted Relative Risk (95% CI) |
P Value |
|---|---|---|---|---|
| number/total number (percent) | ||||
| Strabismus | 46/478 (9.6) | 41/510 (8.0) | 1.20 (0.80–1.80) | 0.38 |
| Nystagmus | 22/479 (4.6) | 13/510 (2.5) | 1.81 (0.89–3.69) | 0.10 |
| Eyes track 180 degrees | 462/476 (97.1) | 493/507 (97.2) | 1.00 (0.98–1.02) | 0.93 |
| Corrective lenses for both eyes† | 21/468 (4.5) | 20/493 (4.1) | 1.15 (0.63–2.10) | 0.65 |
| Blind with some function in both eyes† | 3/450 (0.7) | 2/475 (0.4) | 1.57 (0.27–8.96) | 0.61 |
| Blind with no useful vision in both eyes† | 2/449 (0.4) | 4/477 (0.8) | 0.54 (0.10–2.96) | 0.48 |
| Other abnormal eye finding†‡ | 6/453 (1.3) | 12/485 (2.5) | 0.55 (0.21–1.46) | 0.23 |
| Blind in at least one eye | 5/479 (1.0) | 8/511 (1.6) | 0.67 (0.22–2.02) | 0.48 |
| Eye surgery performed§ | 31/477 (6.5) | 67/509 (13.2) | 0.53 (0.35–0.78) | 0.002 |
Relative risks and P values were adjusted for stratification factors (study center and gestational-age group) and familial clustering; analyses of blindness and other abnormal eye finding were not adjusted for study center, owing to the small numbers of patients with these characteristics.
The reference group for relative risk was the group of children with vision that appeared to be normal in both eyes.
Other abnormal eye finding was defined as an abnormality other than a condition requiring corrective lenses but not one severe enough for the child to be considered blind in that eye. Children whose eyes were classified in two different vision categories were included in the other-abnormal-eye-finding category.
Reasons for surgery are listed in Table S5 in the Supplementary Appendix.
DISCUSSION
In this large, multicenter trial involving very-high-risk, extremely premature infants, we found no significant difference in the primary composite follow-up outcome of death before assessment at 18 to 22 months or neurodevelopmental impairment at 18 to 22 months of corrected age between infants randomly assigned to treatment with early CPAP and those assigned to early intubation and surfactant administration or between those randomly assigned to the lower-oxygen-saturation group and those assigned to the higher-oxygen-saturation group. Mortality did not differ significantly between the CPAP and surfactant groups, and mortality remained significantly higher in the lower-oxygen-saturation group than in the higher-oxygen-saturation group — findings that are consistent with our earlier results. 18,24 There were no significant differences between the CPAP and surfactant groups or between the higher-oxygen-saturation and lower-oxygen-saturation groups with respect to the frequencies among surviving infants of neurodevelopmental impairment and its components, including severe cognitive impairment (BSID-III cognitive composite score, <70), moderate or severe cerebral palsy, moderate or severe motor impairment (GMFCS score, ≥2), hearing impairment, and bilateral blindness.
Recent trials have raised concern about using lower target ranges of oxygen saturation because of the possibility of increased mortality among extremely premature infants.21,30 In SUPPORT, the risk of death during the initial hospitalization was increased among neonates randomly assigned to the lower-oxygen-saturation group, as compared with those assigned to the higher-oxygen-saturation group, and among neonates in the lowest gestational-age stratum, mortality was increased in the surfactant group as compared with the CPAP group. As previously reported, the causes of death did not differ significantly between the lower-oxygen-saturation and higher-oxygen-saturation groups.24 Although significant differences in mortality persisted at 18 to 22 months of corrected age, these differences largely reflected the differences in mortality before hospital discharge. There are other ongoing studies of this matter that, once completed, could inform decisions.31
Severe retinopathy of prematurity may be associated with poor visual outcomes, even with treatment.32,33 In this study, infants in the lower-oxygen-saturation group who survived to discharge had a lower incidence of severe retinopathy of prematurity (8.6%, vs. 17.9% in the higher-oxygen-saturation group).24 Although eye surgery was significantly less frequent in the lower-oxygen-saturation group than in the higher-oxygen-saturation group, there were no significant between-group differences with respect to rates of unilateral and bilateral blindness, nystagmus, strabismus, or the use of corrective lenses. We did not collect detailed data on visual function at the 18-to-22-month visit.
The strengths of this study include the large initial sample, which provided sufficient power to detect a clinically significant difference in the prespecified outcome of death or neurodevelopmental impairment, and the high percentage of surviving infants who underwent a comprehensive, standardized neurodevelopmental evaluation at 18 to 22 months of corrected age.
The study also has several limitations. The requirement for antenatal consent, which is associated with enrollment bias, may limit generalizability. 34,35 In addition, the incidence of neurodevelopmental impairment in extremely premature infants in the present study was substantially lower than that previously reported by the Neonatal Research Network.36 The present study used the BSID-III for cognitive assessment, whereas previous Neonatal Research Network studies used an earlier edition, the BSID-II. Changes in the test design and standardization between the two editions may account for the lower incidence of neurodevelopmental impairment reported here.36 Although the BSID-III scores in this study were higher than those previously reported for extremely premature infants, there were no significant differences between the treatment groups in this study.
Another limitation is the fact that the reported follow-up results are based on a single visit at 18 to 22 months of corrected age; other disabilities may not be evident until later in childhood. A subcohort of the SUPPORT study will be followed at school age to evaluate the longer-term neurodevelopmental outcome. Also, in comparing several secondary outcomes between pairs of treatments in this factorial-design trial (early CPAP vs. early surfactant treatment and lower vs. higher target ranges of oxygen saturation), we made no adjustments for multiple comparisons; appropriate caution should therefore be used in interpreting the reported results. Finally, differences in the neurodevelopmental outcome may have been blunted by the smaller difference in oxygen saturation between the higher-oxygen-saturation and lower-oxygen-saturation groups than was planned.24
In conclusion, there were no significant differences in the composite outcome of death before assessment at 18 to 22 months or neurodevelopmental impairment at 18 to 22 months of corrected age between extremely preterm infants randomly assigned at delivery to early CPAP and those assigned to early intubation with surfactant administration or between infants assigned to lower oxygen saturation and those assigned to higher oxygen saturation. Early CPAP with a limited ventilation strategy can be considered as an alternative to early surfactant treatment, even in infants as immature as those at 24 weeks of gestational age. It is important to consider the risk of death or neurodevelopmental impairment when deciding on oxygen-saturation targets in extremely preterm infants. Because mortality remained lower in the higher-oxygen-saturation group at the time of follow-up and there were no adverse visual or neurodevelopmental problems, lower oxygen-saturation targets cannot be recommended in these extremely preterm infants.
Supplementary Material
Acknowledgments
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute.
APPENDIX
The authors’ affiliations are as follows: the Department of Pediatrics, University of California at San Diego, San Diego (Y.E.V., N.N.F., W.R.), and the Department of Pediatrics, Division of Neonatal and Developmental Medicine, Stanford University School of Medicine and Lucile Packard Children’s Hospital, Palo Alto (S.R.H.) — both in California; the Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.P.-C., W.A.C.); the Statistics and Epidemiology Unit, RTI International, Research Triangle Park (M.G.G.), the Department of Pediatrics, Duke University, Durham (R.F.G.), and Wake Forest University School of Medicine, Winston-Salem (T.M.O.) — all in North Carolina; the Department of Pediatrics, Rainbow Babies and Children’s Hospital, Case Western Reserve University, Cleveland (M.C.W., D.E.W.-C., N.S.N.), and the Department of Pediatrics, Cincinnati Children’s Hospital Medical Center and University of Cincinnati (K.S., K.Y.) — both in Ohio; the Department of Pediatrics, Women and Infants Hospital, Brown University, Providence, RI (A.R.L., B.R.V.); the Department of Pediatrics, Division of Neonatology, University of Utah School of Medicine, Salt Lake City (B.A.Y., R.G.F., A.B.); the Statistics and Epidemiology Unit, RTI International, Rockville (A.D.), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda (R.D.H.) — both in Maryland; the Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas (R.J.H.); the Department of Pediatrics, University of Texas Medical School at Houston, Houston (P.W.E.); the Department of Pediatrics, University of Iowa, Iowa City (M.J.A.); the Department of Pediatrics, Emory University School of Medicine and Children’s Healthcare of Atlanta, Atlanta (I.A.-C.); the Department of Pediatrics, Wayne State University, Detroit (A.P.); the Department of Pediatrics, Indiana University School of Medicine, Indianapolis (B.P., A.M.D.); the Department of Pediatrics, Division of Newborn Medicine, Floating Hospital for Children, Tufts Medical Center, Boston (E.C.M.); the Department of Pediatrics, Yale University School of Medicine, New Haven, CT (R.A.E.); the University of Miami Miller School of Medicine, Miami (C.R.B.); the University of New Mexico Health Sciences Center, Albuquerque (J.F.); and the Department of Pediatrics, University of Rochester Medical Center, Rochester, NY (G.J.M.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. N Engl J Med. 2000;343:378–384. doi: 10.1056/NEJM200008103430601. [DOI] [PubMed] [Google Scholar]
- 2.Mikkola K, Ritari N, Tommiska V, et al. Neurodevelopmental outcome at 5 years of age of a national cohort of extremely low birth weight infants who were born in 1996–1997. Pediatrics. 2005;116:1391–1400. doi: 10.1542/peds.2005-0171. [DOI] [PubMed] [Google Scholar]
- 3.Arpino C, Compagnone E, Montanaro ML, et al. Preterm birth and neurodevelopmental outcome: a review. Childs Nerv Syst. 2010;26:1139–1149. doi: 10.1007/s00381-010-1125-y. [DOI] [PubMed] [Google Scholar]
- 4.Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics. 2000;105:1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 5.Beaino G, Khoshnood B, Kaminski M, et al. Predictors of cerebral palsy in very preterm infants: the EPIPAGE prospective population-based cohort study. Dev Med Child Neurol. 2010;52(6):e119–e125. doi: 10.1111/j.1469-8749.2010.03612.x. [DOI] [PubMed] [Google Scholar]
- 6.Bassler D, Stoll BJ, Schmidt B, et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics. 2009;123:313–318. doi: 10.1542/peds.2008-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farooqi A, Hagglof B, Sedin G, Serenius F. Impact at age 11 years of major neonatal morbidities in children born extremely preterm. Pediatrics. 2011;127(5):e1247–e1257. doi: 10.1542/peds.2010-0806. [DOI] [PubMed] [Google Scholar]
- 8.Walsh MC, Morris BH, Wrage LA, et al. Extremely low birthweight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J Pediatr. 2005;146:798–804. doi: 10.1016/j.jpeds.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 9.Neubauer AP, Voss W, Kattner E. Outcome of extremely low birth weight survivors at school age: the influence of perinatal parameters on neurodevelopment. Eur J Pediatr. 2008;167:87–95. doi: 10.1007/s00431-007-0435-x. [DOI] [PubMed] [Google Scholar]
- 10.Laptook AR, O’Shea TM, Shankaran S, Bhaskar B. Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005;115:673–680. doi: 10.1542/peds.2004-0667. [DOI] [PubMed] [Google Scholar]
- 11.Kobaly K, Schluchter M, Minich N, et al. Outcomes of extremely low birth weight (<1 kg) and extremely low gestational age (<28 weeks) infants with bronchopulmonary dysplasia: effects of practice changes in 2000 to 2003. Pediatrics. 2008;121:73–81. doi: 10.1542/peds.2007-1444. [DOI] [PubMed] [Google Scholar]
- 12.Schulzke SM, Deshpande GC, Patole SK. Neurodevelopmental outcomes of very low-birth-weight infants with necrotizing enterocolitis: a systematic review of observational studies. Arch Pediatr Adolesc Med. 2007;161:583–590. doi: 10.1001/archpedi.161.6.583. [DOI] [PubMed] [Google Scholar]
- 13.Sinn JK, Ward MC, Henderson-Smart DJ. Developmental outcome of preterm infants after surfactant therapy: systematic review of randomized controlled trials. J Paediatr Child Health. 2002;38:597–600. doi: 10.1046/j.1440-1754.2002.00061.x. [DOI] [PubMed] [Google Scholar]
- 14.Marlow N, Greenough A, Peacock JL, et al. Randomised trial of high frequency oscillatory ventilation or conventional ventilation in babies of gestational age 28 weeks or less: respiratory and neurological outcomes at 2 years. Arch Dis Child Fetal Neonatal Ed. 2006;91:F320–F326. doi: 10.1136/adc.2005.079632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truffert P, Paris-Llado J, Escande B, et al. Neuromotor outcome at 2 years of very preterm infants who were treated with high-frequency oscillatory ventilation or conventional ventilation for neonatal respiratory distress syndrome. Pediatrics. 2007;119(4):e860–e865. doi: 10.1542/peds.2006-2082. [DOI] [PubMed] [Google Scholar]
- 16.Donohue PK, Gilmore MM, Cristofalo E, et al. Inhaled nitric oxide in preterm infants: a systematic review. Pediatrics. 2011;127(2):e414–e422. doi: 10.1542/peds.2010-3428. [DOI] [PubMed] [Google Scholar]
- 17.Barrington KJ, Finer N. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst Rev. 2010;12:CD000509. doi: 10.1002/14651858.CD000509.pub4. [DOI] [PubMed] [Google Scholar]
- 18.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362:1970–1979. doi: 10.1056/NEJMoa0911783. [Erratum, N Engl J Med 2010; 362:2235.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tin W, Milligan DW, Pennefather P, Hey E. Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Arch Dis Child Fetal Neonatal Ed. 2001;84:F106–F110. doi: 10.1136/fn.84.2.F106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saugstad OD. Surfactant therapy is still on the move. J Matern Fetal Neonatal Med. 2003;14:145–146. doi: 10.1080/jmf.14.3.145.146. [DOI] [PubMed] [Google Scholar]
- 21.Saugstad OD, Aune D. In search of the optimal oxygen saturation for extremely low birth weight infants: a systematic review and meta-analysis. Neonatology. 2011;100:1–8. doi: 10.1159/000322001. [DOI] [PubMed] [Google Scholar]
- 22.Haynes RL, Folkerth RD, Keefe RJ, et al. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62:441–450. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- 23.Collins MP, Lorenz JM, Jetton JR, Paneth N. Hypocapnia and other ventilation-related risk factors for cerebral palsy in low birth weight infants. Pediatr Res. 2001;50:712–719. doi: 10.1203/00006450-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 24.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–1969. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayley scales of infant and toddler development. 3rd ed. San Antonio, TX: Harcourt Assessment; 2006. [Google Scholar]
- 26.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 27.Amiel-Tison CGJ. Neurological development from birth to six years. Baltimore: Johns Hopkins University Press; 1998. [Google Scholar]
- 28.Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. [Erratum, Dev Med Child Neurol 2007; 49: 480.] [PubMed] [Google Scholar]
- 29.Kenward MG, Carpenter J. Multiple imputation: current perspectives. Stat Methods Med Res. 2007;16:199–218. doi: 10.1177/0962280206075304. [DOI] [PubMed] [Google Scholar]
- 30.Stenson B, Brocklehurst P, Tarnow-Mordi W. Increased 36-week survival with high oxygen saturation target in extremely preterm infants. N Engl J Med. 2011;364:1680–1682. doi: 10.1056/NEJMc1101319. [DOI] [PubMed] [Google Scholar]
- 31.Askie LM, Brocklehurst P, Darlow BA, Finer N, Schmidt B, Tarnow-Mordi W. NeOProM: Neonatal Oxygenation Prospective Meta-analysis Collaboration study protocol. BMC Pediatr. 2011;11:6. doi: 10.1186/1471-2431-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Stahl A, Hellstrom A, Smith LE. Current update on retinopathy of prematurity: screening and treatment. Curr Opin Pediatr. 2011;23:173–178. doi: 10.1097/MOP.0b013e3283423f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer EA, Hardy RJ, Dobson V, et al. 15-Year outcomes following threshold retinopathy of prematurity: final results from the Multicenter Trial of Cryotherapy for Retinopathy of Prematurity. Arch Ophthalmol. 2005;123:311–318. doi: 10.1001/archopht.123.3.311. [DOI] [PubMed] [Google Scholar]
- 34.Rich W, Finer NN, Gantz MG, et al. Enrollment of extremely low birth weight infants in a clinical research study may not be representative. Pediatrics. 2012;129:480–484. doi: 10.1542/peds.2011-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rich WD, Auten KJ, Gantz MG, et al. Antenatal consent in the SUPPORT trial: challenges, costs, and representative enrollment. Pediatrics. 2010;126(1):e215–e221. doi: 10.1542/peds.2009-3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vohr BR, Stephens BE, Higgins RD, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley Assessment of Outcomes. J Pediatr. 2012;161(2):222.e3–228.e3. doi: 10.1016/j.jpeds.2012.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.