Abstract
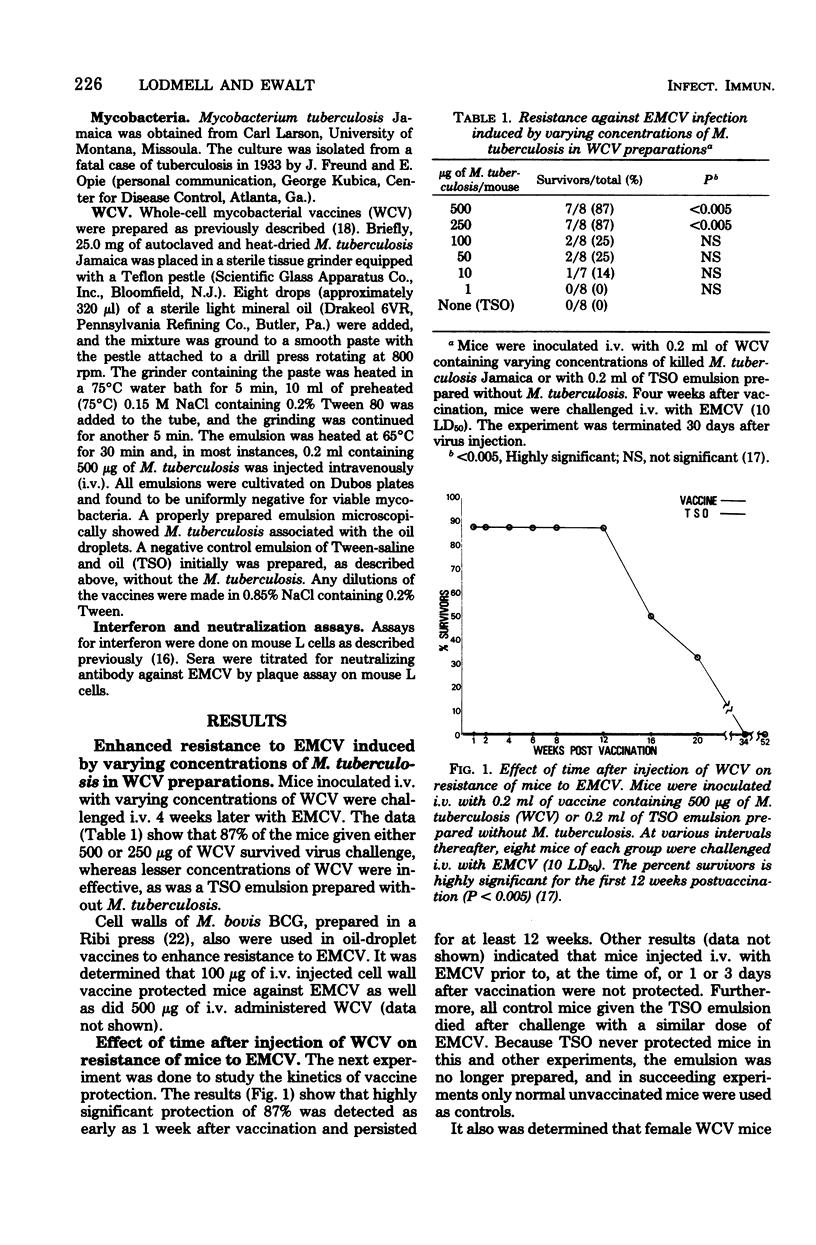
Female C57B1/10 mice injected intravenously (i.v.) with nonviable Mycobacterium tuberculosis Jamaica cells associated with oil-droplet emulsions (WCV) were highly resistant to the i.v. injection of encephalomyocarditis virus (EMCV). Resistance to infection (87% survival) was detected from 1 week to at least 12 weeks after injection of WCV. Mice vaccinated i.v. also were resistant to intraperitoneal, subcutaneous, or intramuscular virus challenge, but were not resistant to intracranial challenge. Mice vaccinated intraperitoneally also were resistant to virus infection, whereas WCV administered intramuscularly or subcutaneously did not protect mice from virus injected by any route. Less than 50% of WCV mice that survived virus challenge possessed serum anti-EMCV-neutralizing antibody (less than 1:10), and none had detectable (less than 1:10) serum interferon. Interferon was not detected in sera of WCV mice from 4 to 144 h after i.v. injection of EMCV. Studies concerning the effects of WCV on EMCV infection suggest that mice may be protected by mechanisms that inhibit early viral replication and spread of virus to the central nervous system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. G., Mudd S. Protection of mice against vaccinia virus by bacterial infection and sustained stimulation with specific bacterial antigens. Infect Immun. 1973 Jan;7(1):62–67. doi: 10.1128/iai.7.1.62-67.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEKIERKUNST A., SULITZEANU D. Induced resistance of mice to infection with Brucella abortus 2308 through vaccination with BCG. Nature. 1958 Sep 27;182(4639):883–884. doi: 10.1038/182883a0. [DOI] [PubMed] [Google Scholar]
- Baker M. B., Larson C. L., Ushijima R. N., Anderson F. D. Resistance of female mice to vaginal infection induced by Herpesvirus hominis type 2: effects of immunization with Mycobacterium bovis, intravenous injection of specific Herpesvirus hominis type 2 antiserum, and a combination of these procedures. Infect Immun. 1974 Dec;10(6):1230–1234. doi: 10.1128/iai.10.6.1230-1234.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold D. S., Keleti G., Youngner J. S. Antiviral activity of Brucella abortus preparations; separation of active components. Infect Immun. 1976 Mar;13(3):763–767. doi: 10.1128/iai.13.3.763-767.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLEDHILL A. W., REES R. J. Effect of a primary tuberculous infection on the resistance of male and female mice to Ectromelia. Nature. 1960 Aug 20;187:703–704. doi: 10.1038/187703b0. [DOI] [PubMed] [Google Scholar]
- Gorhe D. S. Inhibition of multiplication of foot and mouth disease virus in adult mice pretreated with Freund's complete adjuvant. Nature. 1967 Dec 23;216(5121):1242–1244. doi: 10.1038/2161242a0. [DOI] [PubMed] [Google Scholar]
- HOWARD J. G., BIOZZI G., HALPERN B. N., STIFFEL C., MOUTON D. The effect of Mycobacterium tuberculosis (BCG) infection on the resistance of mice to bacterial endotoxin and Salmonella enteritidis infection. Br J Exp Pathol. 1959 Jun;40(3):281–290. [PMC free article] [PubMed] [Google Scholar]
- Kelly M. T. Activation of guinea pig macrophages by cell walls of Mycobacterium bovis, strain BCG. Cell Immunol. 1976 Oct;26(2):254–263. doi: 10.1016/0008-8749(76)90369-5. [DOI] [PubMed] [Google Scholar]
- Kern E. R., Glasgow L. A., Overall J. C., Jr Antiviral activity of an extract of Brucella abortus: induction of interferon and immunopotentiation of host resistance. Proc Soc Exp Biol Med. 1976 Jul;152(3):372–376. doi: 10.3181/00379727-152-39399. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Hirt H. M., Munk K. Protection against herpes simplex virus infection in mice by Corynebacterium parvum. Infect Immun. 1977 Apr;16(1):9–11. doi: 10.1128/iai.16.1.9-11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamensans A., Chedid L., Lederer E., Rosselet J. P., Gustafson R. H., Spencer H. J., Ludwig B., Berger F. M. Enhancement of immunity against murine syngeneic tumors by a fraction extracted from non-pathogenic mycobacteria. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3656–3660. doi: 10.1073/pnas.72.9.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson C. L., Ushijima R. N., Karim R., Baker M. B., Baker R. E. Herpesvirus hominis type 2 infections in rabbits: effect of prior immunization with attenuated Mycobacterium bovis (BCG) cells. Infect Immun. 1972 Oct;6(4):465–468. doi: 10.1128/iai.6.4.465-468.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodmell D. L., Ewalt L. C., Notkins A. L. Inhibition of vaccinia virus replication in skin of tuberculin-sensitized animals challenged with PPD. J Immunol. 1976 Nov;117(5 PT2):1757–1761. [PubMed] [Google Scholar]
- Lodmell D. L., Notkins A. L. Cellular immunity to herpes simplex virus mediated by interferon. J Exp Med. 1974 Sep 1;140(3):764–778. doi: 10.1084/jem.140.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. J., Ribi E. E., Azuma I., Zbar B. Biologically active components from mycobacterial cell walls. II. Suppression and regression of strain-2 guinea pig hepatoma. J Natl Cancer Inst. 1974 Jan;52(1):103–111. doi: 10.1093/jnci/52.1.103. [DOI] [PubMed] [Google Scholar]
- Miller S. D., Zarkower A. Alterations of murine immunologic responses after silica dust inhalation. J Immunol. 1974 Nov;113(5):1533–1543. [PubMed] [Google Scholar]
- Morahan P. S., Kern E. R., Glasgow L. A. Immunomodulator-induced resistance against herpes simplex virus. Proc Soc Exp Biol Med. 1977 Apr;154(4):615–620. doi: 10.3181/00379727-154-39730. [DOI] [PubMed] [Google Scholar]
- Remington J. S., Merigan T. C. Resistance to virus challenge in mice infected with protozoa or bacteria. Proc Soc Exp Biol Med. 1969 Sep;131(4):1184–1188. doi: 10.3181/00379727-131-34066. [DOI] [PubMed] [Google Scholar]
- Ribi E., Anacker R. L., Barclay W. R., Brehmer W., Harris S. C., Leif W. R., Simmons J. Efficacy of mycobacterial cell walls as a vaccine against airborne tuberculosis in the Rheusus monkey. J Infect Dis. 1971 May;123(5):527–538. doi: 10.1093/infdis/123.5.527. [DOI] [PubMed] [Google Scholar]
- SULITZEANU D., BEKIERKUNST A., GROTO L., LOEBEL J. Studies on the mechanism of non-specific resistance to Brucella induced in mice by vaccination with BCG. Immunology. 1962 Jan;5:116–128. [PMC free article] [PubMed] [Google Scholar]
- Shayegani M., Mudd S. Lack of detectable circulating interferon in mice protected against vaccinia virus by induction and elicitation with bacterial systems. Infect Immun. 1973 Jan;7(1):117–118. doi: 10.1128/iai.7.1.117-118.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni E., Bekierkunst A. Nonspecific resistance against infection with Salmonella typhi and Salmonella typhimurium induced in mice by cord factor (trehalose-6,6'-dimycolate) and its analogues. Infect Immun. 1976 Nov;14(5):1125–1129. doi: 10.1128/iai.14.5.1125-1129.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Keleti G., Feingold D. S. Antiviral activity of an ether-extracted nonviable preparation of Brucella abortus. Infect Immun. 1974 Dec;10(6):1202–1206. doi: 10.1128/iai.10.6.1202-1206.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- duBuy H. Effect of silica on virus infections in mice and mouse tissue culture. Infect Immun. 1975 May;11(5):996–1002. doi: 10.1128/iai.11.5.996-1002.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


