Abstract
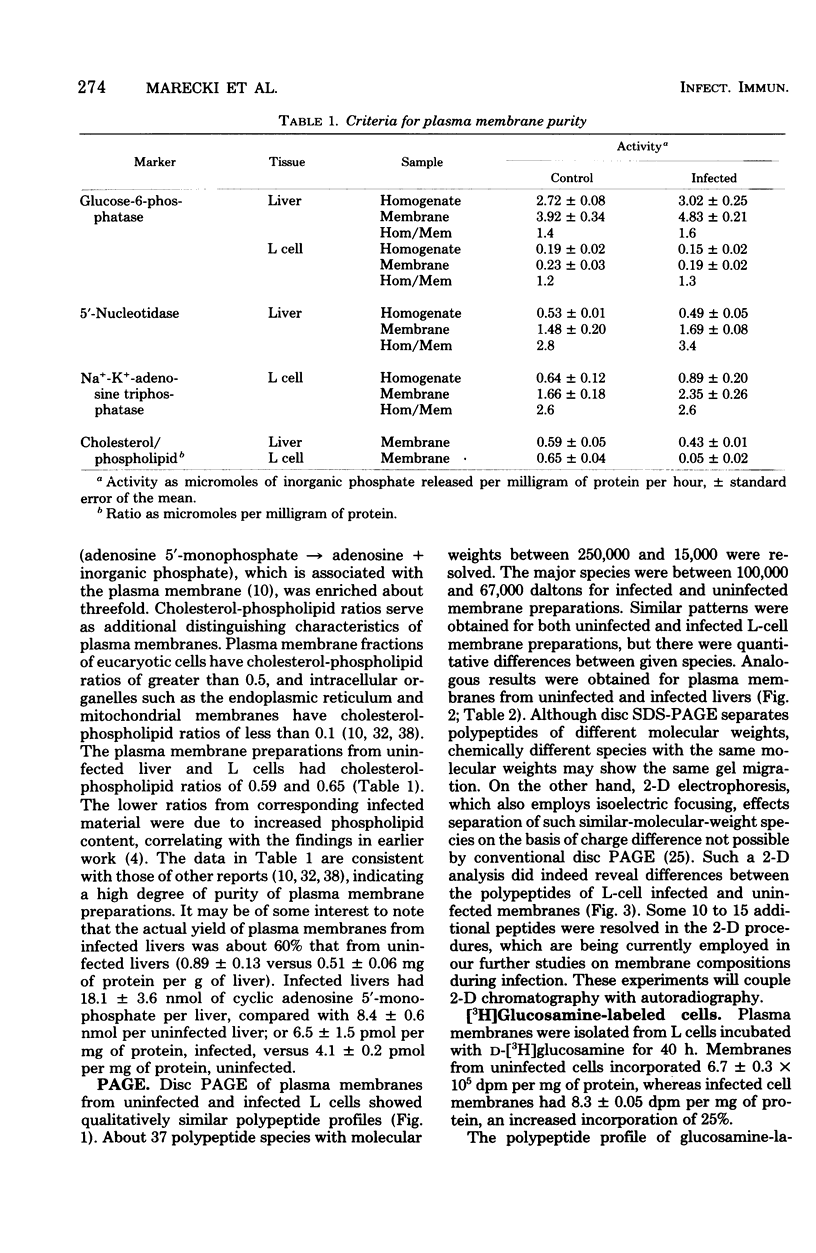
Changes in plasma membrane proteins of guinea pig liver and L-929 cells were studied during infection with Coxiella burnetii. Polypeptide species resolved by disc polyacrylamide gel electrophoresis with sodium dodecyl sulfate showed quantitative but no qualitative differences between uninfected and infected samples. When the O'Farrell technique of isoelectric focusing, followed by sodium dodecyl sulfate-slab gel polyacrylamide gel electrophoresis, was employed, additional polypeptides were resolved. Livers and L cells were labeled with [3H]-glucosamine. Infected livers incorporated less [3H]glucosamine in the membrane proteins than uninfected material, presumably indicating lower glycoprotein levels. Infected L-cell membranes incorporated greater amounts of [3H]glucosamine, and also were labeled to a greater extent than uninfected membranes, employing the [125I]lactoperoxidase technique. Uninfected L cells showed a greater agglutinability with concanavalin A than did infected cells. Infected livers had much greater levels of cyclic adenosine 3',5'-monophosphate. The data indicate changes in plasma membranes as a result of infection. Possible physiological consequences of membrane changes are discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Atkinson P. H., Summers D. F. Purification and properties of HeLa cell plasma membranes. J Biol Chem. 1971 Aug 25;246(16):5162–5175. [PubMed] [Google Scholar]
- Baca O. G., Paretsky D. Some physiological and biochemical effects of a Coxiella burneti lipopolysaccharide preparation on guinea pigs. Infect Immun. 1974 May;9(5):939–945. doi: 10.1128/iai.9.5.939-945.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik I. Z., Balshin M., Breuer W., Rothstein A. Pyridoxal phosphate. An anionic probe for protein amino groups exposed on the outer and inner surfaces of intact human red blood cells. J Biol Chem. 1975 Jul 10;250(13):5130–5136. [PubMed] [Google Scholar]
- Chandrasekhara N., Narayan K. A. Studies on liver plasma membranes of rats fed N-2-fluorenylacetamide. Cancer Res. 1970 Dec;30(12):2876–2880. [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Greenberg C. S., Glick M. C. Electrophoretic study of the polypeptides from surface membranes of mammalian cells. Biochemistry. 1972 Sep 26;11(20):3680–3685. doi: 10.1021/bi00770a003. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. I. Enzymatic iodination of mouse L cells. J Cell Biol. 1975 Feb;64(2):438–460. doi: 10.1083/jcb.64.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R. C., Brown J. C. Identification of a high molecular weight trans-membrane protein in mouse L cells. J Mol Biol. 1975 Oct 5;97(4):413–422. doi: 10.1016/s0022-2836(75)80051-9. [DOI] [PubMed] [Google Scholar]
- Hunt R. C., Brown J. C. Surface glycoproteins of mouse L cells. Biochemistry. 1974 Jan 1;13(1):22–28. doi: 10.1021/bi00698a004. [DOI] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn E. D. Current concepts of membrane structure and function. Fed Proc. 1969 Jan-Feb;28(1):6–11. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meezan E., Wu H. C., Black P. H., Robbins P. W. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. II. Separation of glycoproteins and glycopeptides by sephadex chromatography. Biochemistry. 1969 Jun;8(6):2518–2524. doi: 10.1021/bi00834a039. [DOI] [PubMed] [Google Scholar]
- Morrison M. The determination of the exposed proteins on membranes by the use of lactoperoxidase. Methods Enzymol. 1974;32:103–109. doi: 10.1016/0076-6879(74)32013-7. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- PARETSKY D., DOWNS C. M., SALMON C. W. SOME BIOCHEMICAL CHANGES IN THE GUINEA PIG DURING INFECTION WITH COXIELLA BURNETII. J Bacteriol. 1964 Jul;88:137–142. doi: 10.1128/jb.88.1.137-142.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paretsky D., Stueckemann J. Chemical and biochemical changes in subcellular fractions of guinea pig liver during infection with Coxiella burneti. J Bacteriol. 1970 May;102(2):334–340. doi: 10.1128/jb.102.2.334-340.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduslo J. F., Greenberg C. S., Glick M. C. Proteins exposed on the surface of mammalian membranes. Biochemistry. 1972 Jul 4;11(14):2616–2621. doi: 10.1021/bi00764a011. [DOI] [PubMed] [Google Scholar]
- Singer S. J. Molecular biology of cellular membranes with applications to immunology. Adv Immunol. 1974;19(0):1–66. doi: 10.1016/s0065-2776(08)60251-5. [DOI] [PubMed] [Google Scholar]
- Solyom A., Lauter C. J., Trams E. G. Plasma membranes from isolated liver cells. Biochim Biophys Acta. 1972 Aug 9;274(2):631–637. doi: 10.1016/0005-2736(72)90211-8. [DOI] [PubMed] [Google Scholar]
- Van Lenten L., Ashwell G. Studies on the chemical and enzymatic modification of glycoproteins. A general method for the tritiation of sialic acid-containing glycoproteins. J Biol Chem. 1971 Mar 25;246(6):1889–1894. [PubMed] [Google Scholar]
- Vaughan M., Pierce N. F., Greenough W. B., 3rd Stimulation of glycerol production in fat cells by cholera toxin. Nature. 1970 May 16;226(5246):658–659. doi: 10.1038/226658a0. [DOI] [PubMed] [Google Scholar]
- Weinstein D. B., Marsh J. B., Glick M. C., Warren L. Membranes of animal cells. IV. Lipids of the L cell and its surface membrane. J Biol Chem. 1969 Aug 10;244(15):4103–4111. [PubMed] [Google Scholar]
- Wu H. C., Meezan E., Black P. H., Robbins P. W. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. I. Glucosamine-labeling patterns in 3T3, spontaneously transformed 3T3, and SV-40-transformed 3T3 cells. Biochemistry. 1969 Jun;8(6):2509–2517. doi: 10.1021/bi00834a038. [DOI] [PubMed] [Google Scholar]



