Abstract
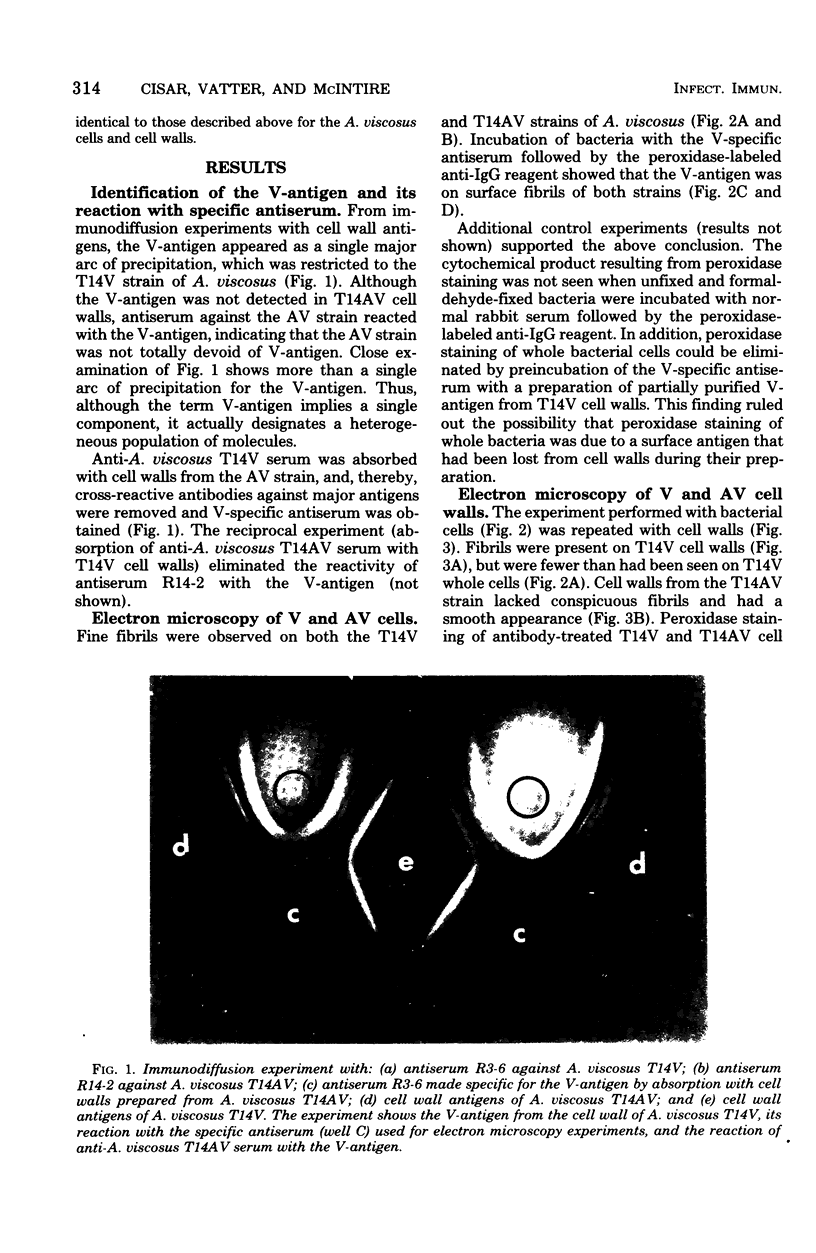
Actinomyces viscosus T14V is virulent (V) for monoinfected rats, causing periodontal disease and bone loss, whereas, A. viscosus T14AV, a mutant strain, is avirulent (AV). Surface antigens from the T14V and T14AV strains were prepared by lysozyme digestion of cell walls and were compared by immunodiffusion against antisera to T14V and T14AV whole cells. The V-associated antigen (V-antigen) was detected readily in the T14V, but not readily in the T14AV cell wall extract. Antiserum specific for the V-antigen was prepared by absorbing anti-A. viscosus T14V serum with cell walls from the T14AV strain. This antiserum was used in the indirect peroxidase-labeled antibody technique to localize the V-antigen on the bacterial cell surface at the ultrastructural level. With whole bacterial cells, the V-antigen was found on fine fibrils and was detected in both the T14V and T14AV strains. The presence of V-antigen on the AV strain was supported by the demonstration of antibodies against the V-antigen in anti-A. viscosus T14AV serum. Examination of isolated bacterial cell walls revealed a greater amount of fibrils and V-antigen on the T14V cell wall than on the T14AV cell wall. The data suggest that the presence of V-antigen represents a quantitative rather than a qualitative difference between the V and the AV strains of A. viscosus T14. Samples of human plaque were examined, and the V-antigen was found to be a specific marker for the fibril-containing layer of certain plaque bacteria, which are probably strains of A. viscosus or A. naeslundii.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ellen R. P., Balcerzak-Raczkowski I. B. Interbacterial aggregation of Actinomyces naeslundii and dental plaque streptococci. J Periodontal Res. 1977 Jan;12(1):11–20. doi: 10.1111/j.1600-0765.1977.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Engel D., Clagett J., Page R., Williams B. Mitogenic activity of Actinomyces viscosus. I. Effects on murine B and T lymphocytes, and partial characterization. J Immunol. 1977 Apr;118(4):1466–1471. [PubMed] [Google Scholar]
- Girard A. E., Jacius B. H. Ultrastructure of Actinomyces viscosus and Actinomyces naeslundii. Arch Oral Biol. 1974 Jan;19(1):71–79. doi: 10.1016/0003-9969(74)90228-3. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hammond B. F., Steel C. F., Peindl K. S. Antigens and surface components associated with virulence of Actinomyces viscosus. J Dent Res. 1976 Jan;55:A19–A25. doi: 10.1177/002203457605500111011. [DOI] [PubMed] [Google Scholar]
- Hirata A. A., Emerick A. J., Boley W. F. Hepatitis B virus antigen detection by reverse passive hemagglutination. Proc Soc Exp Biol Med. 1973 Jul;143(3):761–763. doi: 10.3181/00379727-143-37408. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Fitzgerald R. J., Stanley H. R. Plaque formation and periodontal pathology in gnotobiotic rats infected with an oral actinomycete. Am J Pathol. 1965 Dec;47(6):1157–1167. [PMC free article] [PubMed] [Google Scholar]
- Jordan H. V., Hammond B. F. Filamentous bacteria isolated from human root surface caries. Arch Oral Biol. 1972 Sep;17(9):1333–1342. doi: 10.1016/0003-9969(72)90166-5. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Keyes P. H., Bellack S. Periodontal lesions in hamsters and gnotobiotic rats infected with actinomyces of human origin. J Periodontal Res. 1972;7(1):21–28. doi: 10.1111/j.1600-0765.1972.tb00627.x. [DOI] [PubMed] [Google Scholar]
- Lai C. H., Listgarten M. A., Rosan B. Immunoelectron microscopic identification and localization of Streptococcus sanguis with peroxidase-labeled antibody: localization of Streptococcus sanguis in intact dental plaque. Infect Immun. 1975 Jan;11(1):200–210. doi: 10.1128/iai.11.1.200-210.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. H., Listgarten M. A., Rosan B. Immunoelectron microscopic identification and localization of Streptococcus sanguis with peroxidase-labeled antibody: localization of surface antigens in pure cultures. Infect Immun. 1975 Jan;11(1):193–199. doi: 10.1128/iai.11.1.193-199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J. Levan and levansucrase of Actinomyces viscosus. Infect Immun. 1977 Feb;15(2):518–526. doi: 10.1128/iai.15.2.518-526.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M. Galloylglucoses of low molecular weight as mordant in electron microscopy. I. Procedure, and evidence for mordanting effect. J Cell Biol. 1976 Sep;70(3):608–621. doi: 10.1083/jcb.70.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M. Galloylglucoses of low molecular weight as mordant in electron microscopy. II. The moiety and functional groups possibly involved in the mordanting effect. J Cell Biol. 1976 Sep;70(3):622–633. doi: 10.1083/jcb.70.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S., Hubersak C., Propas D. Induction of periodontal destruction in gnotobiotic rats by a human oral strain of Actinomyces naeslundii. Arch Oral Biol. 1970 Oct;15(10):993–995. doi: 10.1016/0003-9969(70)90095-6. [DOI] [PubMed] [Google Scholar]






