Abstract
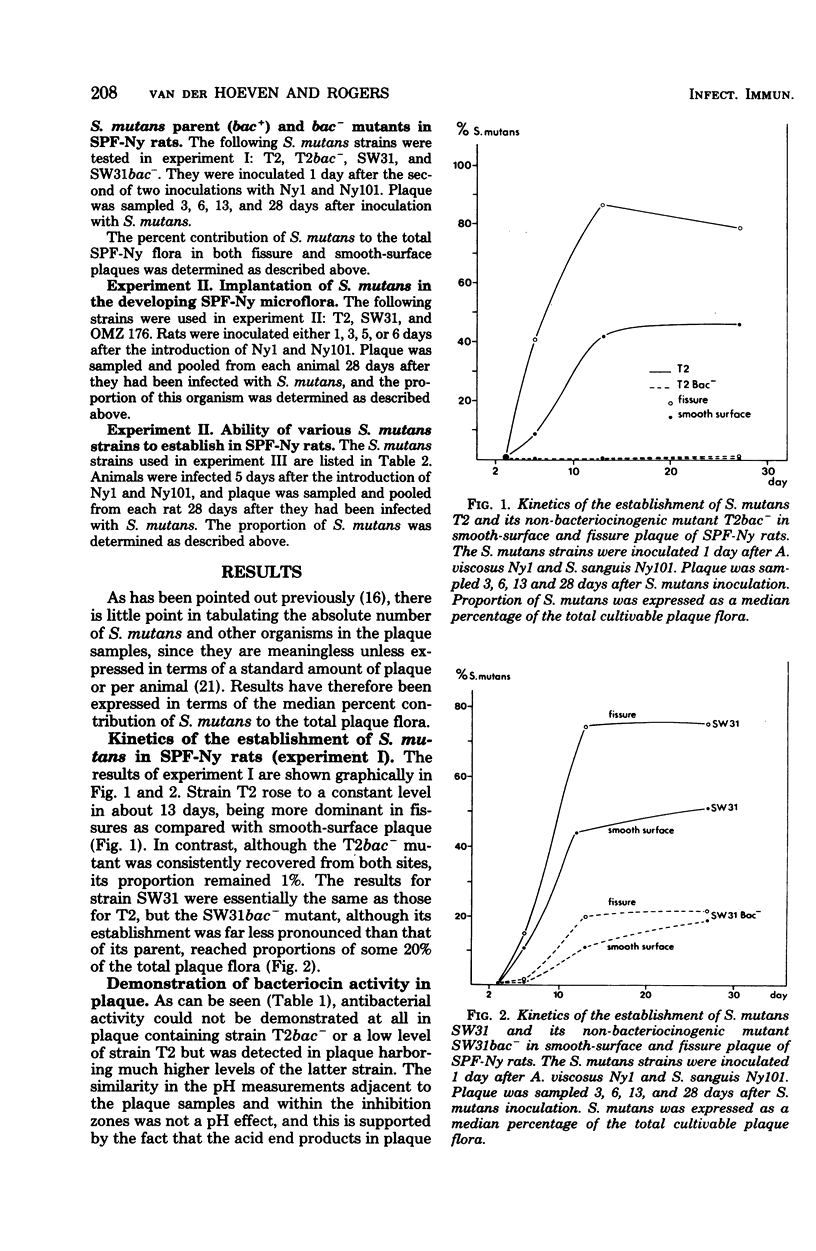
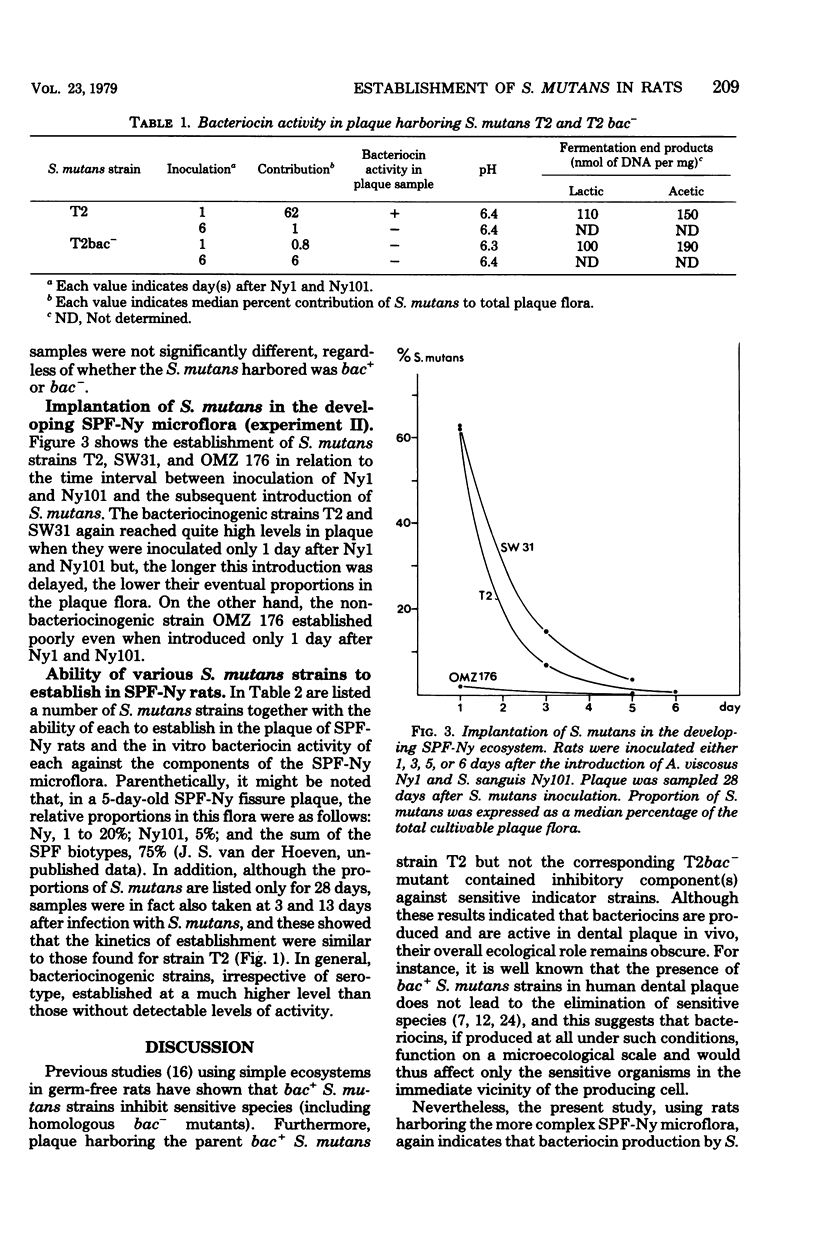
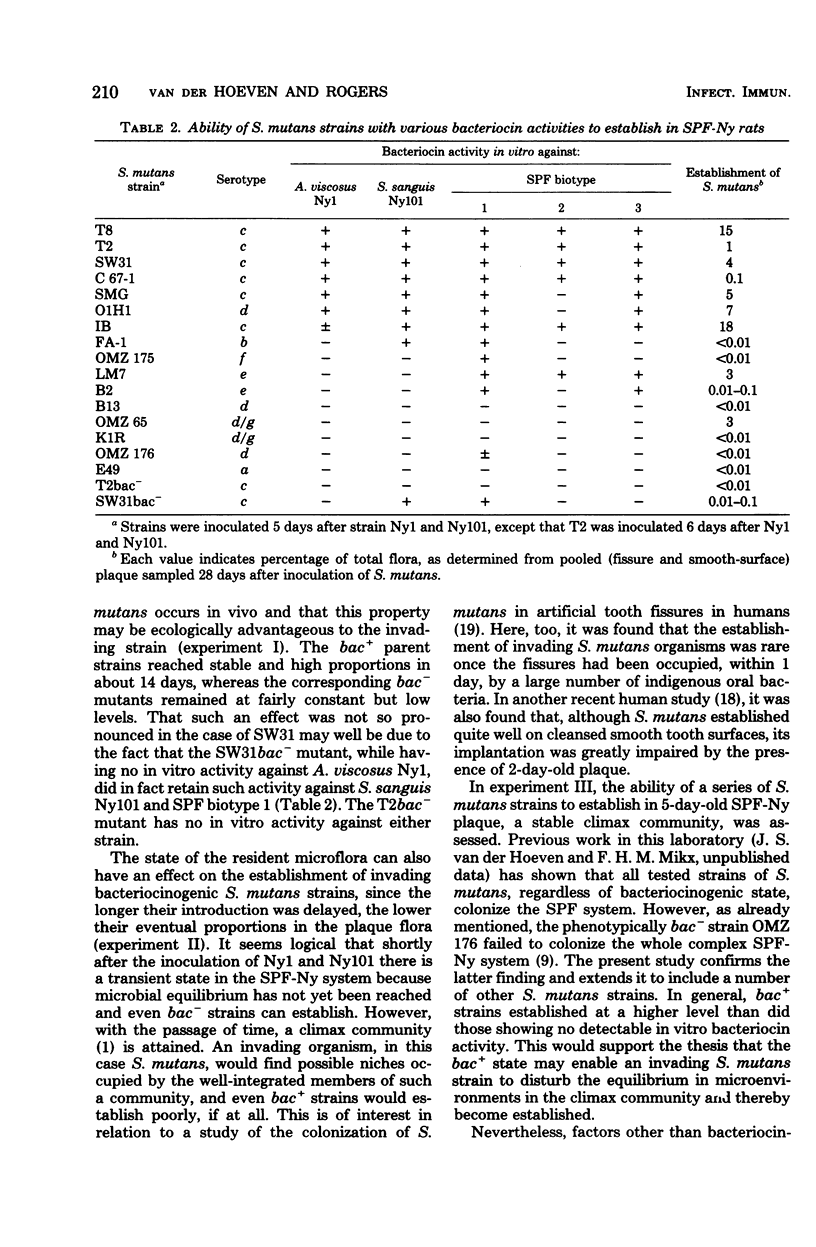
The outcome of the experimental implantation of Streptococcus mutans strains in humans and animals is unpredictable, and neither success nor failure can be explained. It seems logical to assume that, apart from dietary and host factors, the characteristics of the S. mutans strain involved and those of the resident plaque microflora are important in colonization. For example, previous work in this laboratory suggested that bacteriocin production accounts at least in part for the establishment of an invading bacterium in a microbial ecosystem. In the present study, a complex specific pathogen-free Ny plaque ecosystem was obtained by the inoculation of specific pathogen-free rats with Actinomyces viscosus Ny1 and S. sanguis Ny101, and the establishment of S. mutans in such rats was then examined. It was found that bacteriocinogenic (bac+) strains generally colonized in much higher proportions than non-bacteriocinogenic (bac−) strains. Moreover, the longer the delay in introducing S. mutans, the poorer was its establishment. Shortly after inoculation of strains Ny1 and Ny101, there is probably a transient state in which microbial equilibrium has not been reached, but later the specific pathogen-free Ny system attains a stable climax community which more strongly resists invaders. The ability of a number of S. mutans strains to establish in such a climax community was then examined, and it was found that bac+ strains generally established at a higher level than did bac− strains. In summary, it was concluded that, although the bac+ state is an important property in the successful invasion of a plaque by S. mutans, the stability of the resident microflora is also an important factor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Carlsson J., Söderholm G., Almfeldt I. Prevalence of Streptococcus sanguis and Streptococcus mutans in the mouth of persons wearing full-dentures. Arch Oral Biol. 1969 Mar;14(3):243–249. doi: 10.1016/0003-9969(69)90226-x. [DOI] [PubMed] [Google Scholar]
- Edman D. C., Keene H. J., Shklair I. L., Hoerman K. C. Dental floss for implantation and sampling of Streptococcus mutans from approximal surfaces of human teeth. Arch Oral Biol. 1975 Feb;20(2):145–148. doi: 10.1016/0003-9969(75)90171-5. [DOI] [PubMed] [Google Scholar]
- Hamada S., Ooshima T. Production and properties of bacteriocins (mutacins) from Streptococcus mutans. Arch Oral Biol. 1975 Oct;20(10):641–648. doi: 10.1016/0003-9969(75)90131-4. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Englander H. R., Engler W. O., Kulczyk S. Observations on the implantation and transmission of Streptococcus mutans in humans. J Dent Res. 1972 Mar-Apr;51(2):515–518. doi: 10.1177/00220345720510024501. [DOI] [PubMed] [Google Scholar]
- Kelstrup J., Gibbons R. J. Inactivation of bacteriocins in the intestinal canal and oral cavity. J Bacteriol. 1969 Sep;99(3):888–890. doi: 10.1128/jb.99.3.888-890.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasse B., Edwardsson S., Svensson I., Trell L. Implantation of caries-inducing streptococci in the human oral cavity. Arch Oral Biol. 1967 Feb;12(2):231–236. doi: 10.1016/0003-9969(67)90042-8. [DOI] [PubMed] [Google Scholar]
- Mikx F. H., Van Der Hoeven J. S., Plasschaert A. J., König K. G. Effect of Actinomyces viscosus on the establishment and symbiosis of Streptococcus mutans and Streptococcus sanguis in SPF rats on different sucrose diets. Caries Res. 1975;9(1):1–20. doi: 10.1159/000260138. [DOI] [PubMed] [Google Scholar]
- Mikx F. H., van der Hoeven J. S., Plasschaert A. J., König K. G. Establishment and symbiosis of Actinomyces viscosus, Streptococcus sanguis and Streptococcus mutans in germ-free Osborne-Mendel rats. Caries Res. 1976;10(2):123–132. doi: 10.1159/000260196. [DOI] [PubMed] [Google Scholar]
- Rogers A. H. Bacteriocin patterns of strains belonging to various serotypes of Streptococcus mutans. Arch Oral Biol. 1976;21(4):243–249. doi: 10.1016/0003-9969(76)90042-x. [DOI] [PubMed] [Google Scholar]
- Rogers A. H. Bacteriocin production and susceptibility among strains of Streptococcus mutans grown in the presence of sucrose. Antimicrob Agents Chemother. 1974 Nov;6(5):547–550. doi: 10.1128/aac.6.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A. H. Bacteriocin types of Streptococcus mutans in human mouths. Arch Oral Biol. 1975 Dec;20(12):853–858. doi: 10.1016/0003-9969(75)90066-7. [DOI] [PubMed] [Google Scholar]
- Rogers A. H. Bacteriocinogeny and the properties of some bacteriocins of Streptococcus mutans. Arch Oral Biol. 1976;21(2):99–104. doi: 10.1016/0003-9969(76)90079-0. [DOI] [PubMed] [Google Scholar]
- Rogers A. H. Effect of the medium on bacteriocin production among strains of Streptococcus mutans. Appl Microbiol. 1972 Aug;24(2):294–295. doi: 10.1128/am.24.2.294-295.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A. H., van der Hoeven J. S., Mikx F. H. Inhibition of Actinomyces viscosus by bacteriocin-producing strains of Streptococcus mutans in the dental plaque of gnotobiotic rats. Arch Oral Biol. 1978;23(6):477–483. doi: 10.1016/0003-9969(78)90080-8. [DOI] [PubMed] [Google Scholar]
- Ruangsri P., Orstavik D. Effect of the acquired pellicle and of dental plaque on the implantation of Streptococcus mutans on tooth surfaces in man. Caries Res. 1977;11(4):204–210. doi: 10.1159/000260269. [DOI] [PubMed] [Google Scholar]
- Svanberg M., Loesche W. J. The salivary concentration of Streptococci mutans and Streptococci sanguis and their colonization of artificial tooth fissures in man. Arch Oral Biol. 1977;22(7):441–447. doi: 10.1016/0003-9969(77)90125-x. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Upeslacis V. N., Jordan H. V., Skobe Z., Green D. B. Role of sucrose in colonization of Streptococcus mutans in conventional Sprague-Dawley rats. J Dent Res. 1976 Mar-Apr;55(2):202–215. doi: 10.1177/00220345760550020801. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Upeslacis V. N. Studies of the mechanism of sucrose-associated colonization of Streptococcus mutans on teeth of conventional rats. J Dent Res. 1976 Mar-Apr;55(2):216–222. doi: 10.1177/00220345760550020901. [DOI] [PubMed] [Google Scholar]
- Weerkamp A., Bongaerts-Larik L., Vogels G. D. Bacteriocins as factors in the in vitro interaction between oral streptococci in plaque. Infect Immun. 1977 Jun;16(3):773–780. doi: 10.1128/iai.16.3.773-780.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houte J., Burgess R. C., Onose H. Oral implantation of human strains of Streptococcus mutans in rats fed sucrose or glucose diets. Arch Oral Biol. 1976;21(9):561–564. doi: 10.1016/0003-9969(76)90023-6. [DOI] [PubMed] [Google Scholar]
- van der Hoeven J. S., Franken H. C., Camp P. J., Dellebarre C. W. Analysis of bacterial fermentation products by isotachophoresis. Appl Environ Microbiol. 1978 Jan;35(1):17–23. doi: 10.1128/aem.35.1.17-23.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


