Abstract
In this issue of Blood, Wu et al describe the molecular function of HFE, the gene most commonly mutated in hereditary hemochromatosis (HH).1 HH is the most frequent genetic disorder of the Western world. The authors show that HFE prevents ubiquitination and proteasomal degradation of bone-morphogenetic protein (BMP) receptor type I (Alk3), thereby increasing expression of this receptor on the cell surface of hepatocytes. As a consequence, transcription of the iron-hormone hepcidin is activated.
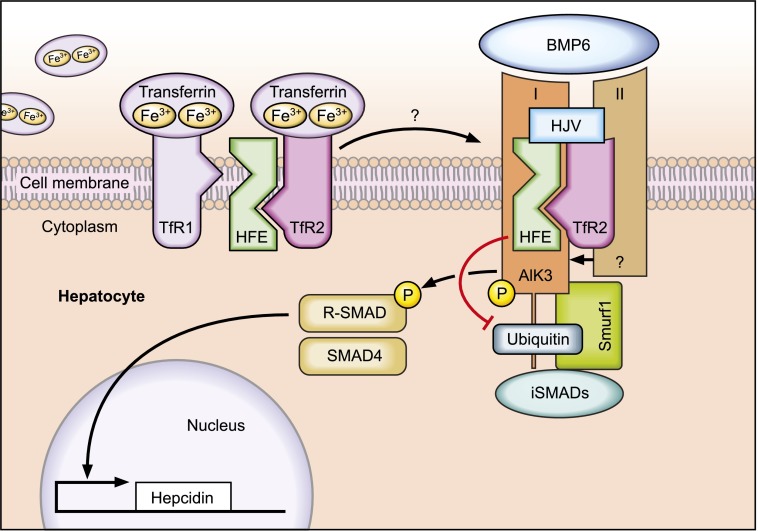
Regulated protein-protein interactions among HFE, TfR2, HJV (proteins mutated in HH), BMP receptors, and BMP ligands play a critical role in the “sensing” of transferrin-bound Fe to control hepcidin expression in hepatocytes. HFE binds to BMP receptor type I (Alk3) to prevent its ubiquitination and proteasomal degradation. As a result, expression of ALK3 is increased on the cell surface, activating BMP/SMAD signaling and hepcidin transcription. Professional illustration by Tom Webster, Lineworks, Inc.
HH is a potentially fatal disorder hallmarked by dietary iron hyperabsorption, hyperferremia, and tissue iron overload. Complications include liver cirrhosis, cancer, diabetes, heart failure, and arthritis that can be prevented by phlebotomy. All family studies identified homozygous missense mutations of the HFE gene (C282Y) occurring in the most common HH subtype.2 Wild-type HFE encodes a ubiquitously expressed major histocompatibility complex class 1–like molecule (MHC-1). Of note, the aforementioned point mutation inhibits binding of β2-microglobulin (β2M) and HFE cell-surface expression. Although HFE was discovered almost 20 years ago, its molecular function remained unknown.
Less common but clinically more severe forms of HH are caused by mutations in hemojuvelin (HJV), transferrin receptor 2 (TfR2), or hepcidin (HAMP). Most probably, regulated protein/protein interactions among the membrane proteins HFE, TfR2, and HJV in hepatocytes integrate signals elicited by the concentration of iron-bound transferrin and hepatocytic iron stores, which ultimately control the expression of hepcidin. This iron hormone is secreted by hepatocytes and binds to the iron exporter ferroportin to suppress dietary iron uptake and iron release from iron-recycling macrophages. These HH subtypes show unphysiologic low hepcidin levels, which explains increased dietary iron uptake and systemic iron accumulation in these patients.3
The mechanism by which iron-bound transferrin is sensed by hepatocytes to control hepcidin transcription is not fully understood. It was shown that HFE binds to TfR14 and that this association is inversely correlated to transferrin-iron saturation. If serum transferrin-iron levels increase, HFE is displaced from TfR1 to permit HFE interaction with TfR2.5 This ultimately triggers activation of hepcidin transcription. More recently, a multiprotein complex consisting of HFE, TfR2, and HJV was shown to form on the hepatocyte’s plasma membrane.6 The dynamics of these interactions remain unclear.
HJV is a glycophosphatidylinositol (GPI)-anchored protein that acts as a BMP co-receptor, thereby activating hepcidin transcription via the BMP-SMAD signaling cascade. BMP6, which is activated by increased iron levels, is the endogenous ligand for HJV.7,8 Binding of BMPs to the BMP receptor consisting of BMP type I and BMP type II causes activation of the kinase domain of BMP type II receptors (BMPRII, ActRIIa, ActRIIb), and phosphorylation of the BMP type I receptors (Alk2 and/or Alk3). Alk2 and Alk3 are 2 hepatic BMP type I receptors that are critical to maintain hepcidin expression.9 In turn, activated BMP type I receptors phosphorylate receptor-associated SMAD proteins (R-SMADs). These signaling molecules transfer together with SMAD4 to the hepatocyte nucleus to induce hepcidin transcription. Importantly, the BMP/SMAD signaling pathway is impaired in patients with HFE-HH,10 as well as in homozygous Hfe-deficient mice, suggesting a crucial role of HFE in BMP/SMAD signaling.
The present study by Wu et al provides novel and critical molecular insights into how HFE connects to BMP/SMAD signaling. In their elegant work, this group shows that HFE binds to the BMP type I receptor Alk3 in both cultured hepatocytes and mouse liver. Upon binding, Alk3 protein is stabilized on the hepatocyte’s cell surface. By applying pharmacologic inhibitors, the authors show that HFE binding to ALK3 diminishes polyubiquitination of Alk3 and thus its degradation via the proteasome. Consistent with this finding in cultured cells, Alk3 protein levels are reduced in mice lacking HFE, whereas Alk3 mRNA levels are not altered. The authors further analyze how the 2 most common polymorphisms in the HFE gene (C282Y and H63D) affect BMP/SMAD signaling. Indeed, both HFE variants are able to interact with Alk3 but failed to increase Alk3 protein levels as detected on the cell surface of hepatocytes. However, the underlying mechanisms differ: although the H63D variant failed to inhibit Alk3 ubiquitination, the HFE C282Y mutant protected ALK3 from ubiquitination, similar to wild-type HFE. The authors speculate that the HFE C282Y mutant protein that does not reach the cell membrane sequesters Alk3 inside cells, thereby preventing Alk3 from trafficking to the cell surface.
Future work will need to address the mechanism of how HFE inhibits Alk3 ubiquitination and whether it interferes with a complex formed between the Smad ubiquitin regulatory factor (Smurf)1, BMP type I receptors, and the inhibitory Smads 6 and 7 (iSMADs). In addition, the impact of TfR2 in BMP/SMAD signaling, as well as the dynamics of complex formation involved in the sensing of systemic iron levels needs to be unraveled. Nevertheless, the present paper represents a milestone in the understanding of iron regulation and might even have an impact on drug development to treat HH by pharmacologically regulating ubiquitination of Alk3.
Footnotes
Conflict-of-interest disclosure: M.U.M. received consulting fees from Novartis.
REFERENCES
- 1.Wu X-g, Wang Y, Wu Q, et al. HFE interacts with the BMP type I receptor ALK3 to regulate hepcidin expression. Blood. 2014;124(8):1335–1343. doi: 10.1182/blood-2014-01-552281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feder JN, Gnirke A, Thomas W, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13(4):399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 3.Bridle KR, Frazer DM, Wilkins SJ, et al. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet. 2003;361(9358):669–673. doi: 10.1016/S0140-6736(03)12602-5. [DOI] [PubMed] [Google Scholar]
- 4.Bennett MJ, Lebrón JA, Bjorkman PJ. Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor. Nature. 2000;403(6765):46–53. doi: 10.1038/47417. [DOI] [PubMed] [Google Scholar]
- 5.Goswami T, Andrews NC. Hereditary hemochromatosis protein, HFE, interaction with transferrin receptor 2 suggests a molecular mechanism for mammalian iron sensing. J Biol Chem. 2006;281(39):28494–28498. doi: 10.1074/jbc.C600197200. [DOI] [PubMed] [Google Scholar]
- 6.D’Alessio F, Hentze MW, Muckenthaler MU. The hemochromatosis proteins HFE, TfR2, and HJV form a membrane-associated protein complex for hepcidin regulation. J Hepatol. 2012;57(5):1052–1060. doi: 10.1016/j.jhep.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Andriopoulos B, Jr, Corradini E, Xia Y, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41(4):482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41(4):478–481. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 9.Steinbicker AU, Bartnikas TB, Lohmeyer LK, et al. Perturbation of hepcidin expression by BMP type I receptor deletion induces iron overload in mice. Blood. 2011;118(15):4224–4230. doi: 10.1182/blood-2011-03-339952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan JD, Ryan E, Fabre A, Lawless MW, Crowe J. Defective bone morphogenic protein signaling underlies hepcidin deficiency in HFE hereditary hemochromatosis. Hepatology. 2010;52(4):1266–1273. doi: 10.1002/hep.23814. [DOI] [PubMed] [Google Scholar]