Abstract
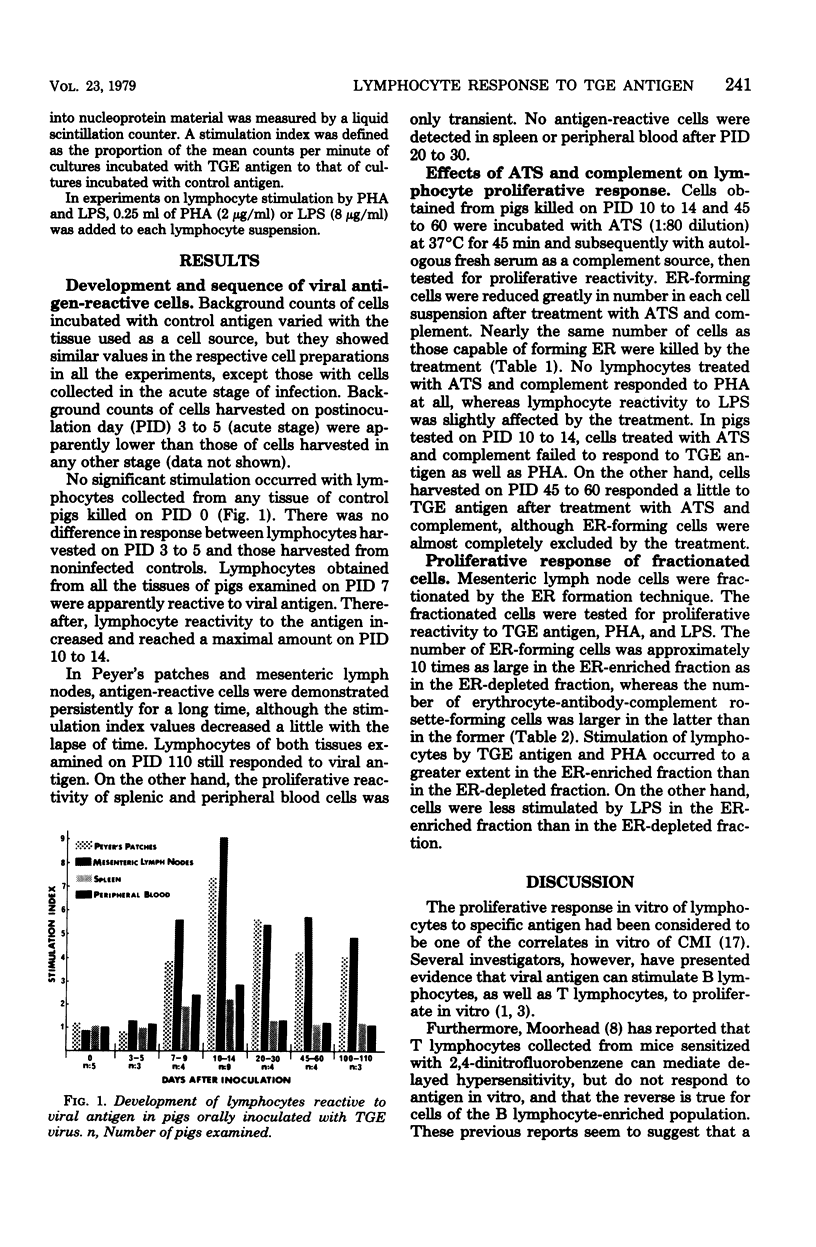
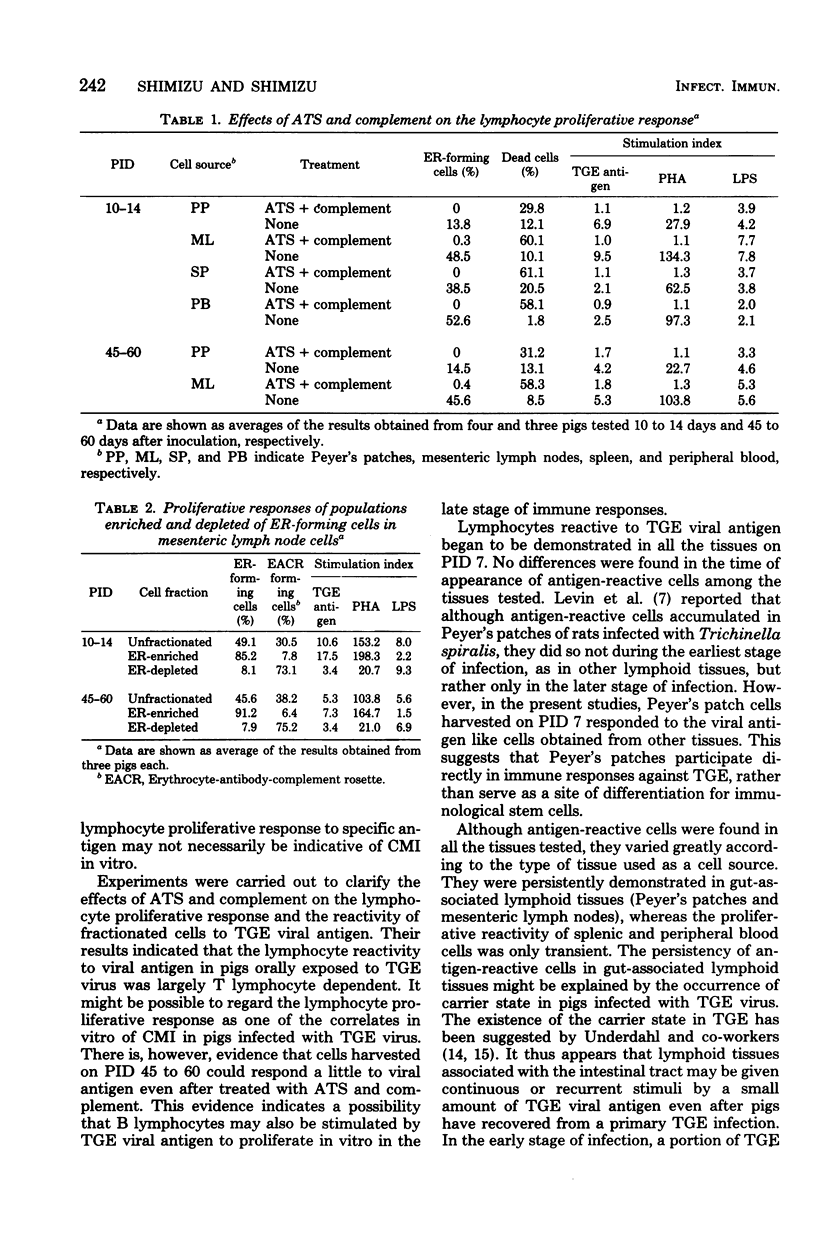
Development and sequence of lymphocytes reactive to viral antigen in Peyer's patches, mesenteric lymph nodes, spleen, and peripheral blood of pigs orally inoculated with transmissible gastroenteritis virus were investigated by a lymphocyte proliferative assay. Lymphocytes reactive to the viral antigen were first detected in all the tissues of pigs tested on postinoculation day 7. Thereafter, they increased in proliferative reactivity and reached a maximal amount on postinoculation days 10 to 14. Antigen-reactive cells were persistently demonstrated in Peyer's patches and mesenteric lymph nodes for at least 110 days after inoculation, although lymphocytes decreased a little in reactivity to the viral antigen with the lapse of time. On the other hand, splenic and peripheral blood cells were found to have only transient proliferative reactivity. No antigen-reactive cells were detected in spleen or peripheral blood after postinoculation days 20 to 30. Lymphocytes decreased remarkably in reactivity to the viral antigen and phytohemagglutinin when treated with anti-porcine thymocyte serum and complement. Their reactivity to lipopolysaccharides was hardly affected by the treatment. Cells harvested on postinoculation days 45 to 60, however, responded a little to the viral antigen even after they were treated with anti-porcine thymocyte serum and complement. Lymphocytes reactive to the viral antigen and phytohemagglutinin belonged mainly to the erythrocyte rosette-forming cell fraction, whereas those reactive to lipopolysaccharides were mostly found in the rosette-nonforming cell fraction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler W. H., Rabinowitz S. G. Host defenses during primary Venezuelan equine encephalomyelitis virus infection in mice. II. In vitro methods for the measurement and qualitation of the immune response. J Immunol. 1973 May;110(5):1354–1362. [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971 Jul 1;134(1):188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfenbein G. J., Rosenberg G. L. In vitro proliferation of rabbit bone marrow-derived and thymus-derived lymphocytes in response to vaccinia virus. Cell Immunol. 1973 Jun;7(3):516–521. doi: 10.1016/0008-8749(73)90216-5. [DOI] [PubMed] [Google Scholar]
- Frederick G. T., Bohl E. H. Local and systemic cell-mediated immunity against transmissible gastroenteritis, an intestinal viral infection of swine. J Immunol. 1976 Apr;116(4):1000–1004. [PubMed] [Google Scholar]
- HAELTERMAN E. O., HUTCHINGS L. M. Epidemic diarrheal disease of viral origin in newborn swine. Ann N Y Acad Sci. 1956 Aug 10;66(1):186–190. doi: 10.1111/j.1749-6632.1956.tb40119.x. [DOI] [PubMed] [Google Scholar]
- Harada K., Furuuchi S., Kumagai T., Sasahara J. Pathogenicity, immunogenicity and distribution of transmissible gastroenteritis virus in pigs. Natl Inst Anim Health Q (Tokyo) 1969 Winter;9(4):185–192. [PubMed] [Google Scholar]
- Levin D. M., Ottesen E. A., Reynolds H. Y., Kirkpatrick C. H. Cellular immunity in Peyer's patches of rats infected with Trichinella spiralis. Infect Immun. 1976 Jan;13(1):27–30. doi: 10.1128/iai.13.1.27-30.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhead J. W. Tolerance and contact sensitivity to DNFA in mice. VIII. Identification of distinct T cell subpopulations that mediate in vivo and in vitro manifestations of delayed hypersensitivity. J Immunol. 1978 Jan;120(1):137–144. [PubMed] [Google Scholar]
- Shimizu M., Pan I. C., Hess W. R. T and B lymphocytes in porcine blood. Am J Vet Res. 1976 Mar;37(3):309–317. [PubMed] [Google Scholar]
- Shimizu M., Shimizu Y. Micro-indirect hemagglutination test for detection of antibody against transmissible gastroenteritis virus of pigs. J Clin Microbiol. 1977 Aug;6(2):91–95. doi: 10.1128/jcm.6.2.91-95.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprino P. J., Morilla A., Ristic M. Intestinal immune response of feeder pigs to infection with transmissible gastroenteritis virus. Am J Vet Res. 1976 Feb;37(2):171–175. [PubMed] [Google Scholar]
- Tajima M. Morphology of transmissible gastroenteritis virus of pigs. A possible member of coronaviruses. Brief report. Arch Gesamte Virusforsch. 1970;29(1):105–108. doi: 10.1007/BF01253886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underdahl N. R., Mebus C. A., Stair E. L., Rhodes M. B., McGill L. D., Twiehaus M. J. Isolation of transmissible gastroenteritis virus from lungs of market-weight swine. Am J Vet Res. 1974 Sep;35(9):1209–1216. [PubMed] [Google Scholar]
- Underdahl N. R., Mebus C. A., Torres-Medina A. Recovery of transmissible gastroenteritis virus from chronically infected experimental pigs. Am J Vet Res. 1975 Oct;36(10):1473–1476. [PubMed] [Google Scholar]
- Woods R. D. Leukocyte migration-inhibition procedure for transmissible gastroenteritis viral antigens. Am J Vet Res. 1977 Aug;38(8):1267–1269. [PubMed] [Google Scholar]


