Abstract
Heme oxygenase (HO) is a stress protein and has been suggested to participate in defense mechanisms against agents that may induce oxidative injury such as metals, endotoxin, heme/hemoglobin, and various cytokines. Overexpression of HO in cells might therefore protect against oxidative stress produced by certain of these agents, specifically heme and hemoglobin, by catalyzing their degradation to bilirubin, which itself has antioxidant properties. We report here the successful in vitro transfection of rabbit coronary microvessel endothelial cells with a functioning gene encoding the human HO enzyme. A plasmid containing the cytomegalovirus promoter and the human HO cDNA complexed to cationic liposomes (Lipofectin) was used to transfect rabbit endothelial cells. Cells transfected with human HO exhibited an approximately 3.0-fold increase in enzyme activity and expressed a severalfold induction of human HO mRNA as compared with endogenous rabbit HO mRNA. Transfected and nontransfected cells expressed factor VIII antigen and exhibited similar acetylated low-density lipoprotein uptake (two important features that characterize endothelial cells) with > 85% of cells staining positive for each marker. Moreover, cells transfected with the human HO gene acquired substantial resistance to toxicity produced by exposure to recombinant hemoglobin and heme as compared with nontransfected cells. The protective effect of HO overexpression against heme/hemoglobin toxicity in endothelial cells shown in these studies provides direct evidence that the inductive response of human HO to such injurious stimuli represents an important tissue adaptive mechanism for moderating the severity of cell damage produced by these blood components.
Full text
PDF

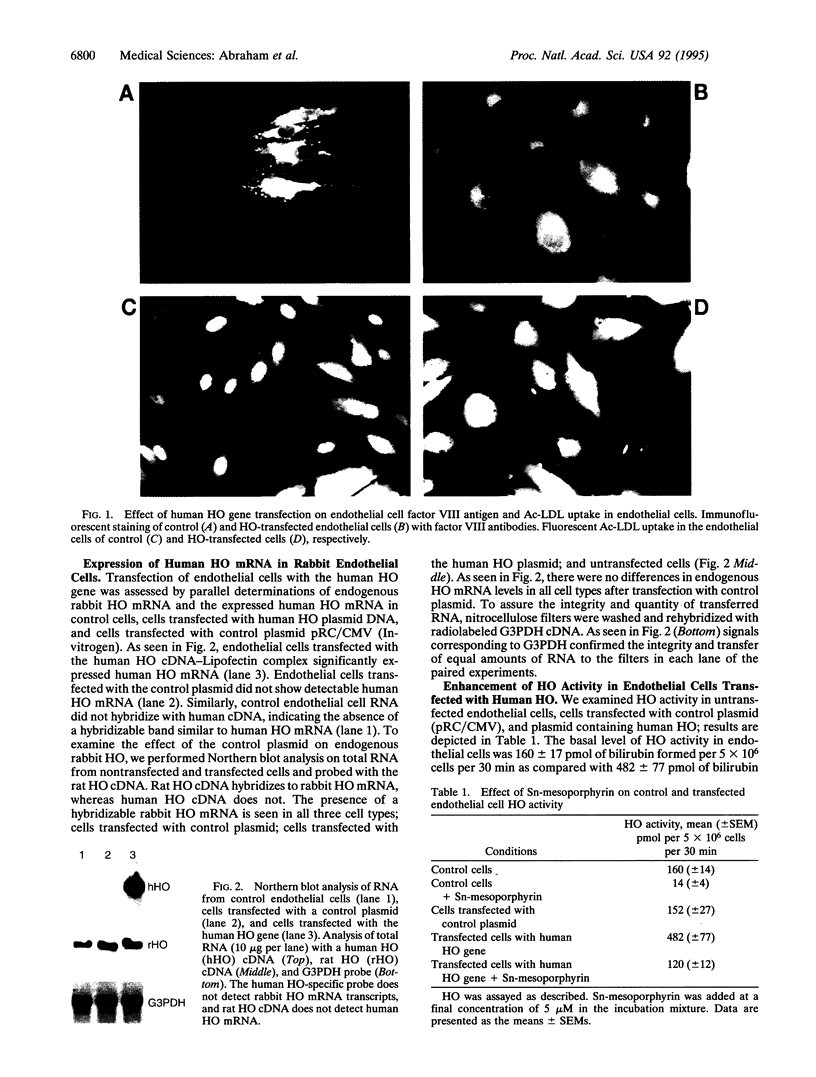
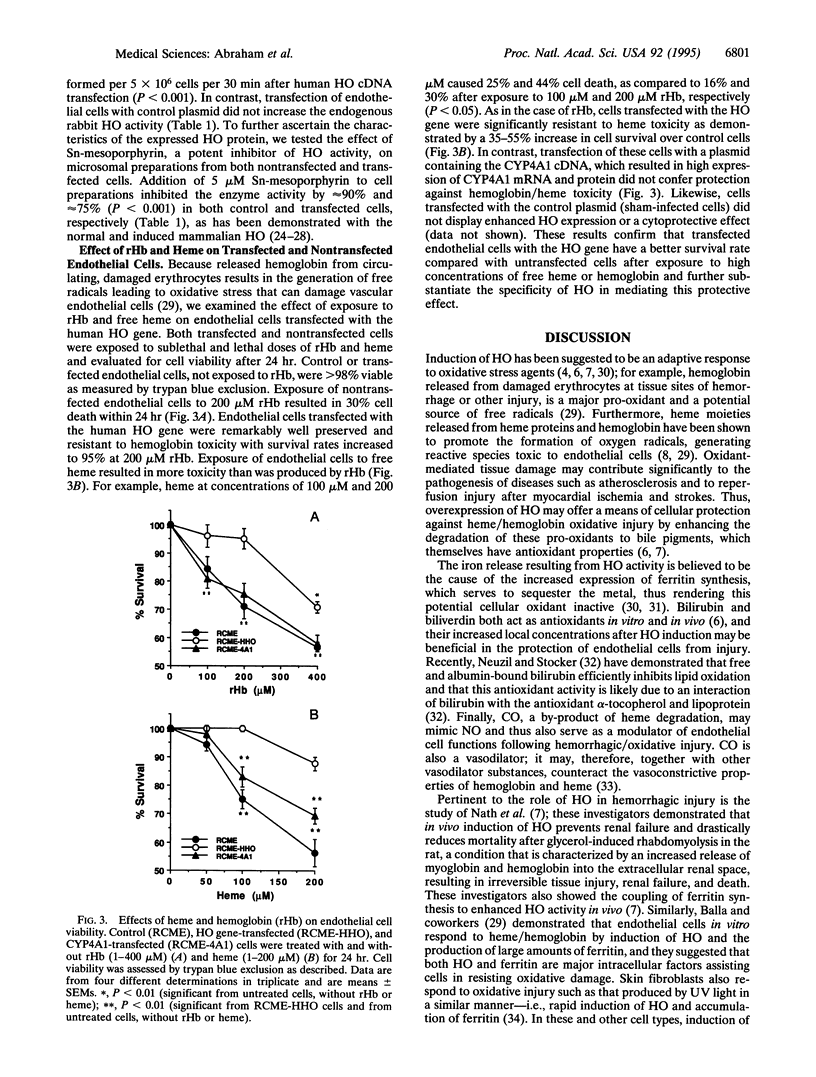

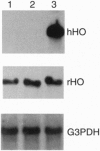
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N. G., Lin J. H., Schwartzman M. L., Levere R. D., Shibahara S. The physiological significance of heme oxygenase. Int J Biochem. 1988;20(6):543–558. doi: 10.1016/0020-711x(88)90093-6. [DOI] [PubMed] [Google Scholar]
- Alam J., Den Z. Distal AP-1 binding sites mediate basal level enhancement and TPA induction of the mouse heme oxygenase-1 gene. J Biol Chem. 1992 Oct 25;267(30):21894–21900. [PubMed] [Google Scholar]
- Balla J., Jacob H. S., Balla G., Nath K., Eaton J. W., Vercellotti G. M. Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9285–9289. doi: 10.1073/pnas.90.20.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. A., Bloomer J. R. The enzymatic defect in variegate prophyria. Studies with human cultured skin fibroblasts. N Engl J Med. 1980 Apr 3;302(14):765–769. doi: 10.1056/NEJM198004033021401. [DOI] [PubMed] [Google Scholar]
- Cantoni L., Rossi C., Rizzardini M., Gadina M., Ghezzi P. Interleukin-1 and tumour necrosis factor induce hepatic haem oxygenase. Feedback regulation by glucocorticoids. Biochem J. 1991 Nov 1;279(Pt 3):891–894. doi: 10.1042/bj2790891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond G. S., Galbraith R. A., Sardana M. K., Kappas A. Reduction of the C2 and C4 vinyl groups of Sn-protoporphyrin to form Sn-mesoporphyrin markedly enhances the ability of the metalloporphyrin to inhibit in vivo heme catabolism. Arch Biochem Biophys. 1987 May 15;255(1):64–74. doi: 10.1016/0003-9861(87)90294-3. [DOI] [PubMed] [Google Scholar]
- Drummond G. S., Rosenberg D. W., Kihlström-Johanson A. C., Kappas A. Effects of tin-porphyrins on developmental changes in hepatic cytochrome P450 content, selected P450-dependent drug-metabolizing enzyme activities and brain glutathione levels in the newborn rat. Pharmacology. 1989;39(5):273–284. doi: 10.1159/000138610. [DOI] [PubMed] [Google Scholar]
- Erzurum S. C., Lemarchand P., Rosenfeld M. A., Yoo J. H., Crystal R. G. Protection of human endothelial cells from oxidant injury by adenovirus-mediated transfer of the human catalase cDNA. Nucleic Acids Res. 1993 Apr 11;21(7):1607–1612. doi: 10.1093/nar/21.7.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jornot L., Junod A. F. Variable glutathione levels and expression of antioxidant enzymes in human endothelial cells. Am J Physiol. 1993 May;264(5 Pt 1):L482–L489. doi: 10.1152/ajplung.1993.264.5.L482. [DOI] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989 Jan;86(1):99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutty G., Hayden B., Osawa Y., Wiggert B., Chader G. J., Kutty R. K. Heme oxygenase: expression in human retina and modulation by stress agents in a human retinoblastoma cell model system. Curr Eye Res. 1992 Feb;11(2):153–160. doi: 10.3109/02713689209000066. [DOI] [PubMed] [Google Scholar]
- Landaw S. A., Drummond G. S., Kappas A. Targeting of heme oxygenase inhibitors to the spleen markedly increases their ability to diminish bilirubin production. Pediatrics. 1989 Dec;84(6):1091–1096. [PubMed] [Google Scholar]
- Lavrovsky Y., Schwartzman M. L., Levere R. D., Kappas A., Abraham N. G. Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5987–5991. doi: 10.1073/pnas.91.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C. S., Chapman G. D., Gammon R. S., Muhlestein J. B., Bauman R. P., Stack R. S., Swain J. L. Direct in vivo gene transfer into the coronary and peripheral vasculatures of the intact dog. Circulation. 1991 Jun;83(6):2007–2011. doi: 10.1161/01.cir.83.6.2007. [DOI] [PubMed] [Google Scholar]
- Llesuy S., Evelson P., González-Flecha B., Peralta J., Carreras M. C., Poderoso J. J., Boveris A. Oxidative stress in muscle and liver of rats with septic syndrome. Free Radic Biol Med. 1994 Apr;16(4):445–451. doi: 10.1016/0891-5849(94)90121-x. [DOI] [PubMed] [Google Scholar]
- McCoubrey W. K., Jr, Ewing J. F., Maines M. D. Human heme oxygenase-2: characterization and expression of a full-length cDNA and evidence suggesting that the two HO-2 transcripts may differ by choice of polyadenylation signal. Arch Biochem Biophys. 1992 May 15;295(1):13–20. doi: 10.1016/0003-9861(92)90481-b. [DOI] [PubMed] [Google Scholar]
- Mitani K., Fujita H., Sassa S., Kappas A. Heat shock induction of heme oxygenase mRNA in human Hep 3B hepatoma cells. Biochem Biophys Res Commun. 1989 Nov 30;165(1):437–441. doi: 10.1016/0006-291x(89)91089-9. [DOI] [PubMed] [Google Scholar]
- Nabel E. G., Plautz G., Nabel G. J. Site-specific gene expression in vivo by direct gene transfer into the arterial wall. Science. 1990 Sep 14;249(4974):1285–1288. doi: 10.1126/science.2119055. [DOI] [PubMed] [Google Scholar]
- Nath K. A., Balla G., Vercellotti G. M., Balla J., Jacob H. S., Levitt M. D., Rosenberg M. E. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992 Jul;90(1):267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzil J., Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem. 1994 Jun 17;269(24):16712–16719. [PubMed] [Google Scholar]
- Paller M. S., Jacob H. S. Cytochrome P-450 mediates tissue-damaging hydroxyl radical formation during reoxygenation of the kidney. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7002–7006. doi: 10.1073/pnas.91.15.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzardini M., Terao M., Falciani F., Cantoni L. Cytokine induction of haem oxygenase mRNA in mouse liver. Interleukin 1 transcriptionally activates the haem oxygenase gene. Biochem J. 1993 Mar 1;290(Pt 2):343–347. doi: 10.1042/bj2900343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara S., Müller R. M., Taguchi H. Transcriptional control of rat heme oxygenase by heat shock. J Biol Chem. 1987 Sep 25;262(27):12889–12892. [PubMed] [Google Scholar]
- Shibahara S., Yoshizawa M., Suzuki H., Takeda K., Meguro K., Endo K. Functional analysis of cDNAs for two types of human heme oxygenase and evidence for their separate regulation. J Biochem. 1993 Feb;113(2):214–218. doi: 10.1093/oxfordjournals.jbchem.a124028. [DOI] [PubMed] [Google Scholar]
- Stocker R. Induction of haem oxygenase as a defence against oxidative stress. Free Radic Res Commun. 1990;9(2):101–112. doi: 10.3109/10715769009148577. [DOI] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Taketani S., Kohno H., Yoshinaga T., Tokunaga R. The human 32-kDa stress protein induced by exposure to arsenite and cadmium ions is heme oxygenase. FEBS Lett. 1989 Mar 13;245(1-2):173–176. doi: 10.1016/0014-5793(89)80215-7. [DOI] [PubMed] [Google Scholar]
- Valaes T., Petmezaki S., Henschke C., Drummond G. S., Kappas A. Control of jaundice in preterm newborns by an inhibitor of bilirubin production: studies with tin-mesoporphyrin. Pediatrics. 1994 Jan;93(1):1–11. [PubMed] [Google Scholar]
- Verma A., Hirsch D. J., Glatt C. E., Ronnett G. V., Snyder S. H. Carbon monoxide: a putative neural messenger. Science. 1993 Jan 15;259(5093):381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- Vile G. F., Basu-Modak S., Waltner C., Tyrrell R. M. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vile G. F., Tyrrell R. M. Oxidative stress resulting from ultraviolet A irradiation of human skin fibroblasts leads to a heme oxygenase-dependent increase in ferritin. J Biol Chem. 1993 Jul 15;268(20):14678–14681. [PubMed] [Google Scholar]